Abstract
Protein kinase C (PKC) plays an important role in the regulation of glioma growth; however, the identity of the specific isoform and mechanism by which PKC fulfills this function remain unknown. In this study, we demonstrate that PKC activation in glioma cells increased their progression through the cell cycle. Of the six PKC isoforms that were present in glioma cells, PKC α was both necessary and sufficient to promote cell cycle progression when stimulated with phorbol 12-myristate 13-acetate. Also, decreased PKC α expression resulted in a marked decrease in cell proliferation. The only cell cycle-regulatory molecule whose expression was rapidly altered and increased by PKC α activity was the cyclin-cyclin-dependent kinase (CDK) inhibitor p21Waf1/Cip1. Coimmunoprecipitation studies revealed that p21Waf1/Cip1 upregulation was accompanied by an incorporation of p21Waf1/Cip1 into various cyclin-CDK complexes and that the kinase activity of these complexes was increased, thus resulting in cell cycle progression. Furthermore, depletion of p21Waf1/Cip1 by antisense strategy attenuated the PKC-induced cell cycle progression. These results suggest that PKC α activity controls glioma cell cycle progression through the upregulation of p21Waf1/Cip1, which facilitates active cyclin-CDK complex formation.
Protein kinase C (PKC) is a multigene family of phospholipid-dependent serine-threonine kinases which plays a central role in signal transduction and has been implicated in a wide range of physiological or abnormal cellular functions, such as cell growth, transformation, and differentiation. The 12 members of the PKC family known so far are divided into three groups based on their requirements for activation (for reviews, see references 39 and 41). The conventional PKCs α, β1, β2, and γ require Ca2+, diacylglycerol, and phosphatidylserine for full activation. The novel PKCs δ, ɛ, η, θ, ν, and μ do not require Ca2+ for activation. Finally, the atypical PKCs ζ and ι/λ are both Ca2+ and diacylglycerol insensitive. Conventional and novel PKC isoforms are activated by the tumor-promoting phorbol esters, while the atypical isoforms are not. The distribution of PKC isoforms is both tissue specific and cell type specific. Also, the roles of a specific PKC isoform can be different from one cell type to another. The difference in function of the various PKC isozymes in cells is thought to be mainly due to a tight control of their subcellular localization, by a set of anchoring proteins, and substrate availability (reviewed in reference 24).
Malignant gliomas are the most common brain neoplasms and are the second highest cause of death from neurological diseases after stroke. High-grade gliomas, glioblastoma multiforme, have a very poor prognosis, with less than 10% of patients surviving beyond 2 years. Although classical anticancer therapies are ineffective, it was recently shown that PKC inhibitors such as tamoxifen, at PKC-inhibitory concentrations, produced a 40% response rate in patients with recurrent malignant gliomas (3, 11, 37). The use of PKC inhibitors in clinical trials for patients with gliomas stems from our previous observation that PKC is dysregulated in gliomas (reviewed in reference 5). Moreover, PKC inhibitors could reduce glioma cell proliferation by over 90% (4, 6, 10, 43). Specific inhibition of PKC α, using an antisense oligonucleotide strategy, inhibited U87 glioma cell growth in vitro (1) and in a mouse model in vivo (12, 49). Also, the use of a PKC α-specific ribozyme blocked glioma cell growth (45). Collectively, the data suggest that PKC plays an important role in the regulation of glioma cell proliferation, although the identity of the PKC isoform and the mechanisms by which PKC accomplish these functions remain to be clarified.
In this study, we have characterized the pattern of PKC isozyme expression and activation in several glioma cell lines and assessed which of these could be responsible for increasing the proliferation rate of glioma cells. Our results indicate that the α isoform of PKC controls proliferation and positively regulates cell cycle progression in glioma cells through the upregulation of p21Waf1/Cip1; the latter was found in ternary cyclin–cyclin-dependent kinase (CDK)–p21 complexes with increased kinase activity, thus facilitating cell cycle progression. Moreover, our data from analyses using antisense for p21 indicate that p21Waf1/Cip1 upregulation is required for the PKC-induced cell cycle progression.
MATERIALS AND METHODS
Tissue culture.
The human glioma cell lines used were U251N, U373, A172, U178, and U563 (10). Cells were grown in minimal essential medium containing 10% fetal bovine serum, 0.1 mM nonessential amino acids, 0.1% dextrose, 2 μg of penicillin-streptomycin per ml, 1 mM sodium pyruvate, and 2 mM l-glutamine. All medium constituents were from Gibco-BRL.
Western blotting.
Cells were lysed in digitonin-Triton lysis buffer (20 mM Tris [pH 7.5], 2 mM EGTA, 2 mM EDTA, 0.5 mg of digitonin per ml, 1% Triton X-100) supplemented with 10 mM NaF, 4 mM phenylmethylsulfonyl fluoride, leupeptin (10 μg/ml), aprotinin (10 μg/ml), pepstatin A (10 μg/ml), and 10 mM sodium orthovanadate and scraped from the culture dish with a cell scraper. Lysates were homogenized for 10 s at 6,000 rpm in a homogenizer (Brinkman). Protein concentration was determined by the Bio-Rad protein assay (Bradford method). For detection of PKC isoforms in crude cell lysates, 100 μg of protein per well was loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel. For detection of the various cell cycle-regulatory proteins, 40 μg of protein was loaded per well. Proteins were transferred onto polyvinylidene difluoride membranes (Immobilon; Millipore) for 2 h at 350 mA. Membranes were blocked in phosphate-buffered saline (PBS) containing 0.5% Tween 20 and 10% milk overnight. Enhanced chemiluminescence (Amersham) was used for immunodetection.
Antibodies.
Rabbit polyclonal antisera for PKC α, γ, ɛ, δ, and ζ were a gift from N. Groome (Oxford, England), as was the mouse monoclonal hybridoma supernatant specific for PKC β2. Antibodies for PKC μ, ι, η (rabbit), and θ (mouse monoclonal) were from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against PKC β1 and p34cdc2 were from Calbiochem. Mouse monoclonal antibodies against PKC α (clone 3) and p21Waf1 (clone 70) were from Transduction Laboratories. Antibodies for p21 (C-19), CDK4 (C-22), CDK6 (C-21), CDK2 (M-2), cdc25B (C-20), cyclin A (H-432), cyclin B1 (H-433), and cyclin D1 (H-295) are all rabbit polyclonal antibodies from Santa Cruz Biotechnology; the p27 (F-8) antibody is a mouse monoclonal antibody from the same source. Secondary antibodies (sheep anti-mouse horseradish peroxidase conjugated and goat anti-rabbit horseradish peroxidase conjugated) were from Jackson ImmunoResearch Laboratories.
RT-PCR.
Total RNA was extracted using TRIZOL reagent (Gibco-BRL). Integrity of RNA was checked by agarose gel electrophoresis and ethidium bromide staining. One microgram of RNA was used as a template for each reverse transcriptase (RT)-mediated PCR (RT-PCR). The reverse transcription step was carried at 50°C for 15 min using avian myeloblastosis virus RT (Gibco-BRL). PCR (50 cycles, to maximize detection) was performed as follows: denaturation for 45 s, annealing (63°C for PKC γ; 62°C for PKC α; 58°C for PKC β1 and β2) for 60 s, and elongation at 72°C for 90 s. PCR products were analyzed by agarose (2%) gel electrophoresis and ethidium bromide staining. Primer sequences for PKC α, β1, β2, and γ were described previously (50).
Flow cytometry.
Cells were trypsinized, rinsed once in PBS, and resuspended in 300 μl of PBS. One milliliter of absolute ethanol was then added. Samples were kept at 4°C. Fixed cells were pelleted and resuspended in PBS containing RNase A (50 μg/ml) and propidium iodide (50 μg/ml) and then incubated for 30 min at 37°C. Cell cycle analysis was done on a FACScan (Becton Dickinson).
Immunofluorescence.
Glioma cells were seeded in culture dishes containing glass coverslips and allowed to grow for at least 24 h. Cells were fixed in 70% ethanol, rinsed three times in PBS, and incubated for 5 min in PBS containing 1 μg of propidium iodide per ml. After three rinses in PBS, coverslips were mounted onto glass slides. Observation was done on a Leica DMRBE microscope, and images were acquired using a Spot charge-coupled device camera.
Subcellular fractionation.
Cells were partitioned into soluble (cytosolic) and particulate fractions, using a method adapted from Frey et al. (17). Briefly, cells were lysed in digitonin lysis buffer (as described above but without Triton X-100) and homogenized for 10 s at 6,000 rpm. Digitonin-soluble (cytosolic) and insoluble (particulate) fractions were separated by ultracentrifugation at 100,000 × g (29,000 rpm) for 45 min at 4°C. Supernatant was collected and formed the cytosolic fraction. The pellet was resuspended in digitonin buffer containing 1% Triton X-100, incubated on ice for 30 min, and cleared by centrifugation for 10 min at 10,000 × g at 4°C. Proteins were quantified by the Bio-Rad protein assay. Samples were subjected to SDS-PAGE as described above; 30 μg of protein was loaded per well.
Antisense PKC α and p21Waf1/Cip1.
Full-length cDNAs for human PKC α or p21Waf1/Cip1 were subcloned in antisense orientation into a pREP9 episomal vector (Invitrogen). The antisense construct or control vector (pREP9) was transfected in U251N cells using the conventional calcium phosphate method; individual clones were selected using G418 (Calbiochem) at 400 μg/ml and isolated with cloning rings (Bellco Glass). Clones were screened by Western blotting for decreased expression of the protein of interest. Transfected cells were maintained permanently under selection pressure.
Evaluation of the growth rate of PKC α transfectants.
To measure the growth rate of the isolated clones, 25,000 cells were plated in 1 ml of feeding medium per well in 24-well plates. Four days later, cells were trypsinized, and the entire content of each well (in 500 μl of PBS) was then transferred to vials (each containing 9.5 ml of PBS) and counted in a Z2 Coulter Counter (3-μm gate). The resultant values obtained represented the total number of cells per well. Results were analyzed using one-way analysis of variance with Bonferroni multiple comparisons.
Multiprobe RPA.
The in vitro transcription kit for probe synthesis, RNase protection assay (RPA; Riboquant) kit, and probe sets hCC-1, hCYC-1, and hCC-2 were from Pharmingen. The experimental procedure was done as described by the manufacturer; 5 μg of RNA was used per sample.
Coimmunoprecipitation.
Cyclins A, B1, and D1 were immunoprecipitated using monoclonal antibodies conjugated to agarose beads (BF683-AC, GNS-AC, and HD11-AC, respectively) (Santa Cruz Biotechnology) that do not interfere with kinase activity. p21 immunoprecipitations were carried out using agarose-conjugated polyclonal anti-p21 (C-19) antibodies from the same source. Immunoprecipitation and kinase assays were performed as described by Matsushime et al. (38). Semiconfluent U251N glioma cells were scraped at the appropriate time point in 1 ml of immunoprecipitation buffer (IP buffer; 50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.1% Tween 20, 10% glycerol; complemented with 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM sodium orthovanadate, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin A per ml, and 1 mM phenylmethylsulfonyl fluoride [PMSF]). The cells were lysed on ice for 30 min with vortexing every 5 min. Lysates were clarified by centrifugation at 10,000 × g for 10 min at 4°C. Protein concentration was determined by the Bio-Rad protein assay. In order to start with an equivalent amount of material, 250 or 500 μg of proteins (volumes were adjusted to 500 μl with IP buffer) was used for each immunoprecipitation. Immunoprecipitations were carried in a sequential manner because of the large amount of proteins required for each time point, as well as for better consistency in results, each cyclin being sequentially immunoprecipitated from the same cellular extract. Protein extracts were incubated with 10 μg of the indicated primary antibodies for 1 h at 4°C. The immunoprecipitated complexes were then washed three times with 1 ml of IP buffer and once with kinase buffer (see below). Half of the samples were submitted to Western blotting; the other half was subjected to kinase assay. For kinase assays, control immunoprecipitations with protein G-coated agarose beads only were carried and processed similarly to samples.
Cyclin-CDK complex kinase assay.
The above immunoprecipitated complexes were resuspended in 40 μl of kinase buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 2.5 mM EGTA, 0.1 mM sodium orthovanadate). Five μCi of [γ-32P]ATP (3,000 Ci/mmol; NEN) and 2 μg of histone H1 or 1 μg of Rb fragment (46 kDa) (769; Santa Cruz Biotechnology), as substrate, were added. The reaction mixture was incubated for 30 min at 30°C. Samples were boiled in SDS sample buffer and subjected to SDS-PAGE; the dried gels were autoradiographed on Kodak Blue XB-1 film.
RESULTS
Glioma cell lines express the PKC isoforms α, δ, ɛ, η, μ, and ζ.
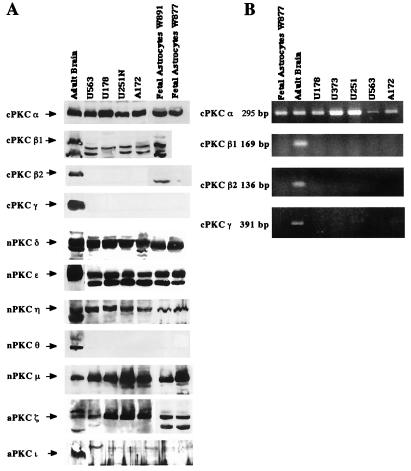
Using Western blot analysis, we determined that PKCs α, δ, ɛ, η, μ, and ζ were expressed in all four human glioma cells (U251N, U178, U563, and A172) tested, while the isoforms β1, β2, γ, ι, and θ could not be detected (Fig. 1A). RT-PCR, using conventional PKC isoform-specific primers, confirmed the lack of isoforms β1, β2, and γ (Fig. 1B). It is of note that the four glioma cell lines tested exhibited the same pattern of expression. A similar expression pattern was observed in two human fetal astrocyte primary cultures. Such a similarity between glioma cells and astrocytes is not surprising since glioma cells are commonly thought to arise from cells of the astrocytic lineage. In view of the similarities in PKC isoform expression by all four glioma cell lines, subsequent experiments focused on the U178 and U251N glioma lines.
FIG. 1.
Human glioma cell lines express the PKC isoforms α, δ, ɛ, η, μ, and ζ. (A) Expression of 11 PKC isoforms was examined in four different human glioma cell lines (U251N, U178, U563, and A172) and in two human fetal astrocyte primary cultures (64) by Western blotting using isoform-specific PKC antibodies as noted in Materials and Methods. The PKC species (and molecular masses) were α (82 kDa), β1 (80 kDa), β2 (80 kDa), γ (80 kDa), δ (78 kDa), ɛ (90 kDa), η (78 kDa), θ (79 kDa), μ (115 kDa), ι (74 kDa), and ζ (72 kDa). Protein extract from adult human brain was used as a positive control. Equal amounts of protein (100 μg) were loaded in each well. (B) Expression of the four conventional PKC isoforms (α, β1, β2, and γ) was analyzed by RT-PCR using isoform-specific primers in five human glioma cell lines (U251N, U178, U563, U373, and A172) and one human fetal astrocyte primary culture. RNA extracts from human adult brain were used as a positive control.
PKC activation with a phorbol ester increases progression of human glioma cells through the cell cycle.
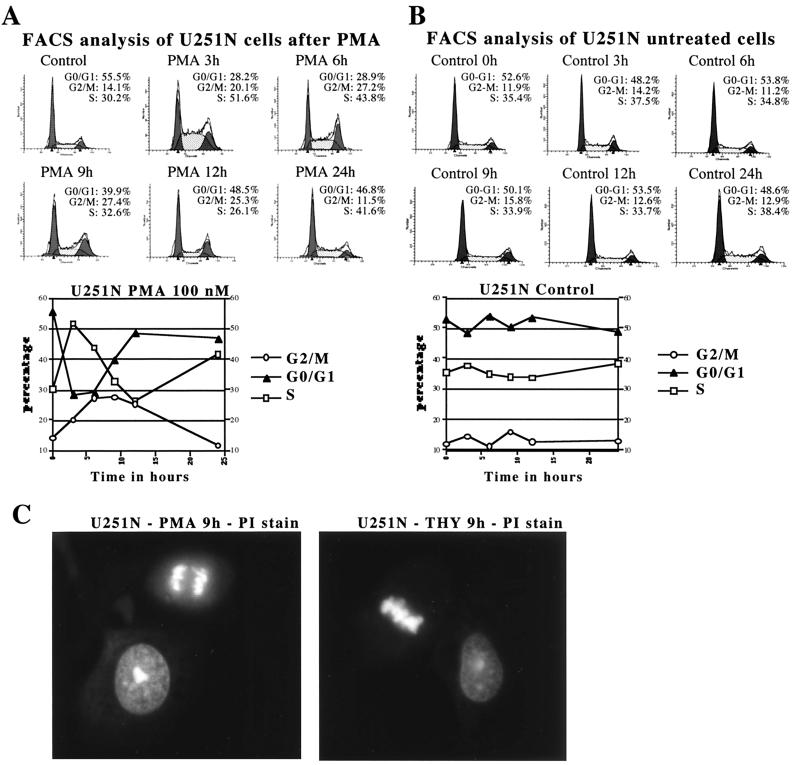
Previous work had shown that PKC inhibitors dramatically affect the growth rate of glioma cells (4, 6, 10, 43); however, the identity of the PKC isoform involved remains unclear. To establish which isoform(s) of PKC was potentially involved, we tested the effect of a phorbol ester, phorbol 12-myristate 13-acetate (PMA), a potent activator of conventional and novel PKC isoforms, on the cell cycle progression of the human glioma cell line U251N. Upon PMA (100 nM) addition, U251N cells rapidly entered S phase (Fig. 2A), with a corresponding drop of the G0-G1 content. This was followed by a marked progression into the G2-M phases of the cell cycle between 6 and 12 h of treatment. By 24 h of treatment, the distribution of the cells between the different phases of the cell cycle was very similar to that of the control cells. Similar results were obtained using the human glioma cell lines U178, A172, and U563 (data not shown). Control cells, treated only with vehicle (dimethyl sulfoxide), showed no significant change in distribution between the different phases of the cell cycle during the 24-h time course examined (Fig. 2B). To confirm that cells were not blocked in the G2 phase, propidium iodide-stained U251N cells grown on glass coverslips were analyzed by immunofluorescence microscopy at various times following PMA or vehicle treatment. Cells at all stages of mitosis could be observed in both phorbol ester-treated cells (Fig. 2C) and vehicle-treated cells, indicating that the cells were progressing normally through mitosis. The percentage of cells showing a mitotic appearance (condensed chromatin and chromosome alignment) correlated with the G2-M content determined by flow cytometry analysis in both PMA-treated and vehicle-treated cells (data not shown). Therefore, PKC activation seems to play a role in the regulation of cell cycle progression.
FIG. 2.
PKC activation with phorbol ester increases progression of human glioma cells through S and the G2-M phases of the cell cycle. (A) Flow cytometry analysis of PMA-treated U251N cells. (B) Flow cytometry analysis of untreated U251N cells. Asynchronously growing U251N cells were treated with 100 nM PMA or with vehicle only, collected at various time points, and stained with propidium iodide. For each side scatter plot, the y axis is the number of cells, while the x axis is the DNA content. Values from each scatter plot are graphed below panels A and B. Similar results after PMA treatment were obtained in over 10 independent experiments. (C) Immunofluorescence of cellular DNA stained with propidium iodide (PI) showing cells in interphase or at different stages of mitosis. U251N cells were grown on glass coverslips for 24 h, treated either with PMA or thymeleatoxin for 9 h, and then fixed.
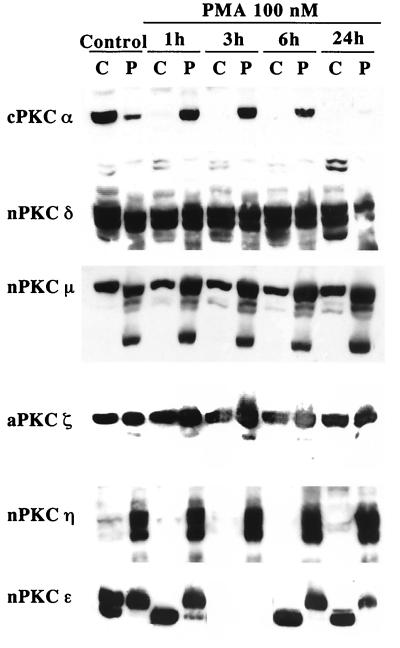
To identify the isoform of PKC responsible for the increased progression of the cells through the cell cycle, we monitored the effect of PMA on the subcellular localization of the six PKC isoforms expressed in glioma cells. Translocation of PKC from the cytosol to the membrane is a hallmark of its activation (41). Upon PMA treatment, only PKCs α and ɛ were translocated from the cytosolic to the particulate fraction (Fig. 3); PKC δ, η, μ, and ζ remained unaffected by PMA. PKC α was totally downregulated by proteolytic degradation by 24 h of treatment, while PKC ɛ was still present, and translocated, in the cells at that time. Collectively, the data indicate that of the six PKC isoforms expressed in glioma cells, only PKC α and ɛ were significantly activated by PMA stimulation.
FIG. 3.
PKC α and ɛ are the only isoforms translocated by PMA in glioma cells. Shown is Western blot analysis of the subcellular distribution between cytosolic and membrane fractions of the six PKC isoforms expressed in glioma cells following phorbol ester treatment using isoform-specific antibodies. U251N protein extracts collected at various times following PMA treatment were fractionated into cytosolic (C) and particulate (P) fractions; 30 μg of protein was loaded in each well. Only PKCs α and ɛ were translocated in response to PMA. Similar distribution following PMA treatment was also obtained for the U178 glioma cell line (data not shown). Note that in the doublet obtained for PKC ɛ, only the upper band (90 kDa) is the active form of the enzyme.
PKC α is necessary and sufficient to increase progression through the cell cycle.
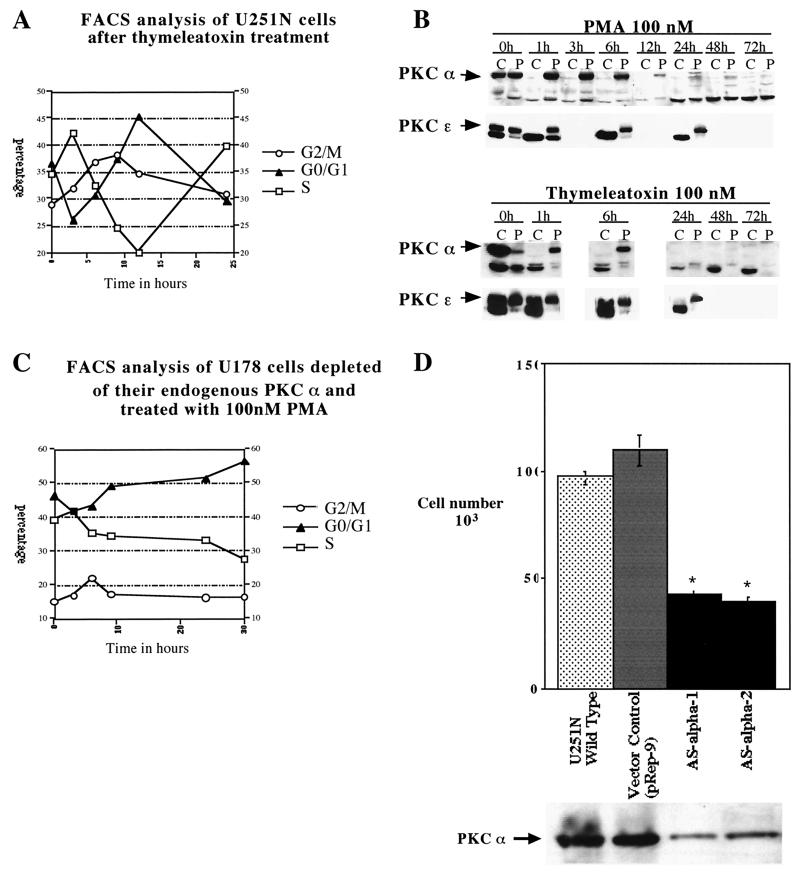
To differentiate between PKC α and ɛ, we used the conventional isoform-specific PKC agonist thymeleatoxin (27, 44); in glioma cells, thymeleatoxin should activate only PKC α, since it is the only conventional PKC isoform expressed in those cells. Flow cytometry analysis of thymeleatoxin (100 nM)-treated U251N cells (Fig. 4A) revealed a cell cycle progression profile similar to that obtained with PMA (Fig. 2A). Similar results using thymeleatoxin were obtained with the glioma cell lines U178, A172, and U563 (data not shown). Western blot analysis confirmed that PKC α was specifically translocated (and activated) by thymeleatoxin, whereas PKC ɛ remained unaffected (Fig. 4B). In addition, the increased progression of glioma cells through the cell cycle correlated with the time frame of activation or translocation of PKC α. The analysis of later time points revealed that PKC α was still downregulated at 48 and 72 h following either PMA or thymeleatoxin treatment (Fig. 4B).
FIG. 4.
PKC α is necessary and sufficient to increase cell cycle progression. (A) Flow cytometry analysis of U251N glioma cells after thymeleatoxin treatment. Asynchronously growing U251N cells were stimulated with thymeleatoxin (100 nM), collected at various time points, and stained with propidium iodide for DNA content analysis. Similar results were obtained in over 10 independent experiments. (B) Western blot analysis of the subcellular localization of PKCs α and ɛ upon PMA or thymeleatoxin treatment between 0 and 72 h. PKC α was rapidly (within 1 h) translocated to the membrane by both PMA and thymeleatoxin and downregulated by 24 h; the protein remained undetectable at 72 h of treatment. On the other hand, PKC ɛ was translocated only upon PMA addition and was not downregulated at later time points. U251N protein extracts collected at various times following PMA or thymeleatoxin treatment were fractionated into cytosolic (C) and particulate (P) fractions; 30 μg of protein was loaded in each well. (C) Flow cytometry analysis of U178 glioma cells depleted of their endogenous PKC α by 48 h of PMA treatment and restimulated with PMA (time zero to 30 h). This shows the requirement for PKC α to be present in order to increase cell cycle progression in glioma cells. Similar results were obtained using thymeleatoxin to deplete PKC α and to restimulate the cells (data not shown). (D) PKC α regulates the growth rate of glioma cells. Twenty-five thousand cells were seeded for each cell line (U251N, control vector, ASα1, and ASα2). Four days later, cells were counted using a Coulter Counter; numbers are displayed in the top panel. Each clone was analyzed in quadruplicates. Results were analyzed using a one-way analysis of variance with Bonferroni multiple comparisons. ∗, P < 0.0001. Western blot using a monoclonal anti-PKC α antibody (Transduction Laboratories) (below) shows the endogenous PKC α level in each clone; 100 μg of protein was loaded in each well.
Further confirmation of the specific role of PKC α in the regulation of cell cycle progression of glioma cells was provided by PKC α depletion experiments. U178 (or U251N [data not shown]) human glioma cells were pretreated with PMA for 48 h in order to deplete the cells of their endogenous PKC α. Cells were then restimulated with 100 nM PMA, and their distribution between the different phases of the cell cycle was analyzed between 0 and 30 h following restimulation by flow cytometry (Fig. 4C). In the absence of a detectable level of PKC α, there was no significant change in the cell cycle progression of glioma cells following PMA stimulation over the 30-h time course (Fig. 4C), unlike the case with cells containing PKC α (Fig. 2A and B).
The role of PKC α in cell proliferation was further addressed using an antisense strategy to partially deplete glioma cells of their endogenous PKC α. Equally seeded cultures of the different clones were grown for 4 days and counted, giving a direct reading of their growth rate. Two antisense PKC α clones, ASα1 and ASα2, exhibited a growth rate of less than half the rate of the wild-type U251N or empty vector-transfected cells (Fig. 4B), thus indicating that PKC α levels are directly proportional to the basal proliferation rate of glioma cells.
Altogether, the data strongly suggest that PKC α-specific activation is necessary and sufficient for the increased progression of human glioma cells through the cell cycle induced by PKC agonists such as PMA or thymeleatoxin. Moreover, our data indicate that PKC α directly controls glioma cell proliferation, as decreased PKC α expression correlates with decreased proliferation.
p21Waf1/Cip1 is upregulated following PKC α activation.
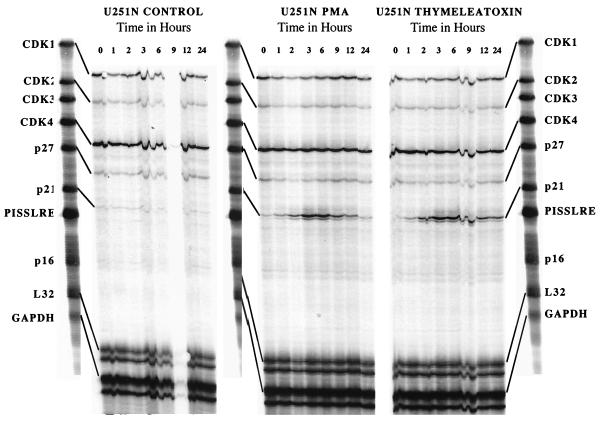
To evaluate the molecular mechanism by which PKC α induces cell cycle progression, we examined the transcript levels of several cell cycle-regulatory proteins by multiprobe RPA. Using the probe set hCC-1, we determined that p21 mRNA was strongly and rapidly upregulated (Fig. 5) between 1 and 12 h following PMA or thymeleatoxin treatment, with a maximum increase at 3 and 6 h. Other mRNA (CDK1/cdc2, CDK2, CDK4, and p27) levels remained unaffected by PKC α activation. CDK3 and PISSLRE (a cdc2-related kinase acting at G2/M) (31) mRNAs could not be detected in this assay. Using the probe set hCYC-1 and hCC-2 (data not shown), we determined that the mRNA levels of p130, Rb, p107, p53, p27, p18, and cyclins A, B, C, D1, and D3 were not affected by PKC activation. Transcripts for p57, p19, p16, p15/p14, cyclin D2, and cyclin A1 could not be detected using this assay.
FIG. 5.
p21Waf1 mRNA is upregulated by PKC α activity, as determined by multiprobe RPA of U251N glioma cells RNA extracts in untreated (control), PMA-treated, or thymeleatoxin-treated cells at various time points; 5 μg of RNA was used for each reaction. RPA using the hCC-1 probe set shows a marked upregulation of p21 mRNA between 1 and 12 h following PMA or thymeleatoxin addition. p16 and CDK3 mRNAs were undetectable at all times. No change was detected in the various CDK mRNAs levels. These results are representative of three independent experiments.
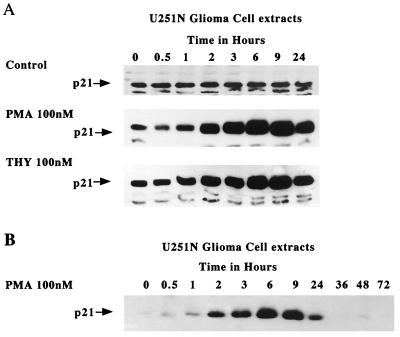
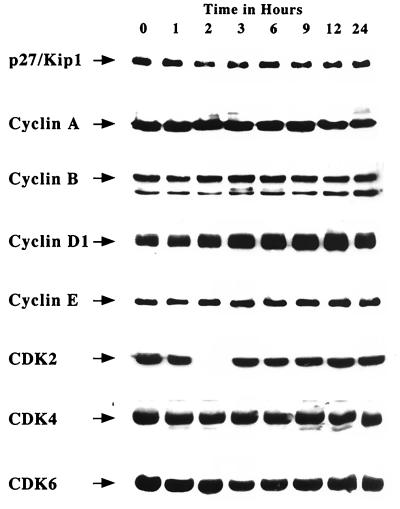
Consistent with a change at the mRNA level, the p21 protein was also upregulated (Fig. 6). In untreated U251N cells, p21 protein level remained constant, while in cells treated either with PMA or thymeleatoxin, p21 was strongly upregulated by 2 h, with a maximum at 9 h after treatment. The expression level of p21 was monitored over a 72-h period following PMA treatment of U251N cells; Fig. 6B shows that p21 induction is transient and correlates with the time frame of PKC α activation. Western blot analyses were also performed for a variety of other cell cycle regulators. In correspondence with the RNA results, protein levels of p27Kip1, cyclins A, B, D1, and E, and CDK2, CDK4, and CDK6 were not altered from controls (Fig. 7). These data indicate that the CKI p21Waf1/Cip1 is the only cell cycle-regulatory molecule upregulated following PKC α activation.
FIG. 6.
Upregulation of the p21Waf1 protein following PKC α activation. (A) Western blot analysis of U251N glioma cells treated with PMA (100 nM) or thymeleatoxin (100 nM) or untreated shows a strong upregulation of the p21 protein following PMA or thymeleatoxin treatment. Gels show representative results of three independent experiments. (B) p21 levels over a 72-h period following PMA treatment of U251N cells; 40 μg of protein was loaded per well. Note that the exposure time for panel B was shorter than that for panel A to better assess the magnitude of p21 induction, thus explaining the apparently low p21 levels at 0, 36, 48, and 72 h.
FIG. 7.
Western blot analysis of various cell cycle-regulatory proteins following PMA treatment of U251N glioma cells. The data obtained by RPA were confirmed at the protein level. PMA treatment did not alter the protein levels of various cell cycle regulators; 60 μg of proteins was loaded per well. Results are representative of three independent experiments.
p21Waf1/Cip1 upregulation is associated with the formation of active ternary cyclin-CDK-p21 complexes.
Although the upregulation of p21 would appear paradoxical, a number of reports have shown that p21 can be upregulated during cell proliferation (23, 34, 36, 40, 42) and that p21 protein can act as an assembly and activity-promoting factor for cyclin-CDK complexes (8, 30). To establish whether p21 was associated with active cyclin-CDK complexes, we performed coimmunoprecipitations using cyclin-specific antibodies, and kinase assays, at various times following PKC activation.
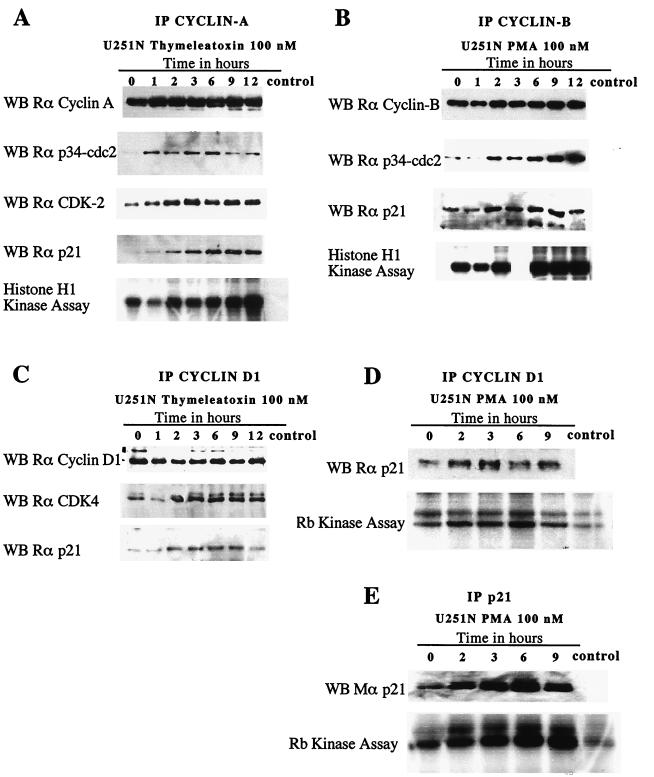
Upon PKC activation, a rapid (within 1 h) and sustained (up to 12 h) association of the immunoprecipitated cyclin with its CDK partner(s) and with p21, to form a ternary complex, was observed (Fig. 8A to C); the amount of cyclin immunoprecipitated remained constant throughout the experiment. We ensured that we were not working in the presence of saturating amounts of the respective cyclins. Thus, changes in the amount of CDK and p21 coimmunoprecipitated, as well as the changes in the kinase activity of the complex, are not attributable to variations of the amount of cyclin immunoprecipitated. Kinase assays were performed on the immunoprecipitates, using histone H1 as a substrate for cyclin A and cyclin B complexes (Fig. 8A and B) and Rb as a substrate for cyclin D1 complexes (Fig. 8D). There is an apparent correlation between the formation of the ternary cyclin-CDK-p21 complexes and the increased kinase activity of these complexes. Changes in kinase activity of cyclin E immunoprecipitates were not significant (data not shown).
FIG. 8.
Increased association of p21Waf1/Cip1 with cyclin-CDK complexes following thymeleatoxin or PMA stimulation of U251N glioma cells. The formation of a ternary complex was accompanied by an increase in the kinase activity of the complexes. (A to D) Immunoprecipitates of cyclins A, B, and D1 at various times following thymeleatoxin or PMA stimulation of U251N cells were subjected to SDS-PAGE and blotted for each cyclin. They show that approximately equal amounts of cyclins were present in the cells throughout the duration of the experiment. Western blots (WB) for the CDK partners and p21 show the amount of protein coimmunoprecipitated along with the cyclin, as a ternary complex (Rα corresponds to rabbit antibody against the relevant target). For each immunoprecipitation (IP), the kinase activity of each complex was measured by its ability to phosphorylate histone H1 or Rb in vitro. The cyclin B-associated kinase activity at 3 h was not measured. As expected, an increasing amount of p21 was immunoprecipitated following PMA stimulation (E) p21 was detected using a monoclonal anti-p21 antibody (Transduction Laboratories). The p21-associated kinase activity appears correlated to the amount of p21 immunoprecipitated. Western blots and kinase assay results are representative of three independent experiments. The control lane in each panel refers to extracts subjected to protein G-coated agarose beads immunoprecipitation only.
To confirm that p21-containing cyclin-CDK complexes could exhibit a kinase activity, reciprocal immunoprecipitation of p21Waf1/Cip1 at various times following PMA stimulation were performed (Fig. 8E), and the Rb-kinase activity coimmunoprecipitated with p21 was measured. Figure 8E shows an increasing amount of p21 immunoprecipitated following PKC stimulation in glioma cells, in agreement with the upregulation of the protein observed previously (Fig. 6); the p21-associated kinase activity increased correspondingly. These results suggest that p21 associates with cyclin-CDK complexes and that this is accompanied by an increased kinase activity of these complexes.
p21Waf1/Cip1 upregulation is required for the PKC-induced cell cycle progression.
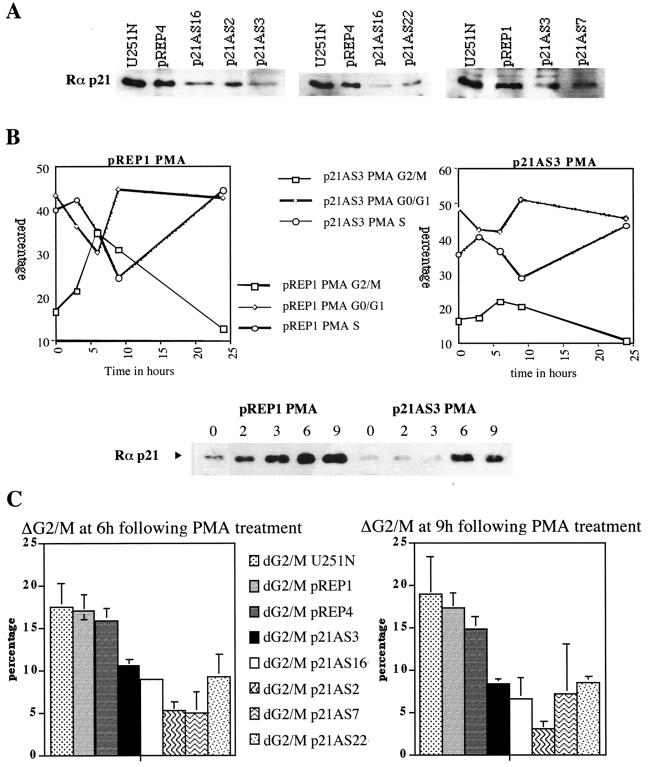
To establish the requirement of p21 upregulation in PKC α-induced cell cycle progression, we used an antisense approach to decrease the p21 protein level and to prevent, at least partially, its upregulation. The p21 cDNA was cloned in antisense orientation in the episomal vector pREP9 and transfected in U251N glioma cells; the endogenous p21 protein level of several clones is shown (Fig. 9A). Upon PMA treatment, the induction of progression through G2/M is markedly reduced compared to the control vector (Fig. 9B), however, the initial entry into S phase still occurs. The induction of the p21Waf1/Cip1 protein is clearly reduced compared to empty vector-transfected cells (Fig. 9B). Figure 9C shows the percentage of cells induced to progress through G2/M in wild-type and empty vector-transfected cells and in five individual antisense p21 clones. In response to PMA treatment, antisense p21-overexpressing cells exhibit a marked reduction in the percentage of cells induced to progress through G2/M (ΔG2/M) compared to wild-type or empty vector-transfected cells. The average inhibition of progression for the five p21 antisense clones compared to the three control lines is 57%. This result suggests that p21 upregulation is required for PKC α-induced cell cycle progression.
FIG. 9.
p21Waf1/Cip1 upregulation is required for PKC-induced cell cycle progression. (A) Endogenous p21 protein level in the wild-type and empty vector-transfected cells (pREP1 and -4) and in p21 antisense-transfected cells (p21AS); 50 μg of protein was loaded per well. (B) Flow cytometry analysis of pREP1 and p21AS3 clones following PMA treatment. There is a marked reduction in the number of p21AS3 cells induced to progress through G2/M compared to empty vector-transfected cells (pREP1). For Western blot analysis of the p21 protein (bottom), extracts were collected at the same time as the flow cytometry samples. (C) Flow cytometry analysis of wild-type, empty vector-transfected, and p21 antisense-transfected cells at 6 and 9 h following PMA treatment. p21AS clones exhibit a marked reduction of the number of cells induced to progress through G2/M. ΔG2/M = % G2/M PMA treated − % G2/M control. Each bar is the mean of three independent experiments; the standard error of the mean for each cell line is plotted on the graph.
DISCUSSION
PKC plays a major role in the regulation of cell growth and differentiation; its specific function depends on the cellular context, localization, and substrate availability (reviewed in reference 24). In a number of cell systems, it has been shown that either activation or inhibition of PKC could influence cell cycle progression by a variety of mechanisms (reviewed in references 16 and 33).
PKC plays a role in the regulation of cell cycle progression in glioma cells.
We have found that PKC activation by phorbol esters increased glioma cell progression through the cell cycle. This is consistent with previous data showing the correlation between PKC activity and glioma cell proliferation (10) and the clinical results that inhibitors of PKC may have efficacy in patients with gliomas (3, 11, 37). Moreover, we have determined that PKC α activation is necessary and sufficient to increase cell cycle progression of glioma cells. We have also shown that the expression level of PKC α directly correlates with the proliferation rate of glioma cells.
Recently, PKC has been associated with regulation of cell cycle progression (reviewed in references 16 and 33) either during the G1-to-S progression or during the G2/M transition. PKC has been shown to regulate G1 progression through the modulation of CDK activity, either by modifying cyclin or CDK expression levels or by modifying the expression of the cyclin-CDK inhibitors (CKIs). In Swiss 3T3 cells, phorbol esters accelerate growth factor-induced cell cycle entry and progression into S phase by elevating cyclin D1 levels and downregulating p27Kip1 expression (35). Predominantly, however, PKC plays an inhibitory role in cell cycle progression. PMA treatment of IMR-90 cells resulted in G1 arrest due to the downregulation of CDK7 and cyclin H, thus preventing the activation of CDK2 (19). Overexpression of PKC η caused delayed entry into S phase and prolonged the G1 phase in NIH 3T3 cells; this correlated with increased expression of p21Cip1 and p27Kip1, decreased CDK2 associated activity, and decreased Rb phosphorylation (32). In intestinal epithelial cells, PKC α-specific activation resulted in G1 arrest and delayed transit through S and G2/M phases through an upregulation of p21 and p27, resulting in hypophosphorylation of Rb (17). It was also demonstrated that PMA-induced G1 arrest could be mediated via phosphorylation of p53 by PKC and activation of p53 DNA binding (13). Increasing evidence also implicate PKC in the inhibition of the G2/M transition, often by modulating cdc2 (CDK1) activity by influencing the expression levels of cdc25 or the CKIs. Growth arrest in G2/M following PMA treatment was observed in Demel melanoma cells (2), U937 leukemia cells (20), and vascular endothelial cells (29). This growth inhibition correlated with the downregulation of cyclin B and/or cdc25, thus causing the inhibition of cdc2 kinase activity. Also, PKC β2 activity was shown to be required for the G2/M transition in HL60 cells, by acting as a lamin B kinase; lamin B phosphorylation is required for nuclear envelope breakdown to occur at the onset of mitosis (48). Thus, in contrast to most cell types, the activation of PKC, and specifically PKC α, in glioma cells facilitates progression of cells through the cell cycle.
p21Waf1/Cip1 is upregulated by PKC α activation in glioma cells.
To elucidate the mechanism by which PKC increased glioma cell cycle progression, we analyzed the expression of various cell cycle-regulatory proteins following PKC activation. The only cell cycle-regulatory protein upregulated by PKC activity was p21Cip1/Waf1. A number of studies have reported the PKC-induced upregulation of p21 in other cell types, but these were associated with cell cycle block, unlike the case for glioma cells reported here (2, 17, 20, 52). PKC-induced p21 upregulation was shown to be p53 independent in some cases and to occur in cells expressing a mutant p53 (51). It is very likely the case in this report since the U251N glioma cell line expresses a mutant, transcriptionally inactive form of p53, and no change in p53 amounts, at least at the mRNA level, was detected at any time (data not shown). Jung et al. (26) reported that p21 expression was consistently elevated in human glioma specimens, independently of the presence of a functional p53. In view of our results, the high p21 levels in gliomas could be a consequence of the high PKC enzyme activity in these cells.
p21Waf1/Cip1 associates with cyclin-CDK complexes, resulting in increased kinase activity.
Our results indicate that p21 upregulation was accompanied by an increase in ternary cyclin-CDK-p21 complex formation and by an increase in their associated kinase activity. Moreover, the upregulation of p21 appeared to be required for the PKC-induced cell cycle progression. Our results are supported by the findings that p21 upregulation could occur in response to mitogenic signals (23, 34, 36, 40, 42). More particularly, p21 upregulation was required to promote proliferation of myeloid cells following steel factor and granulocyte-macrophage colony-stimulating factor stimulation, as bone marrow cells from p21−/− mice could not be induced to proliferate following such stimulation (36). Other reports have suggested a role for p21 as an assembly factor for cyclin-CDK complexes: Zhang et al. (53) showed that p21 could be associated with both catalytically active and inactive cyclin-CDK complexes (cyclin A-CDK2 and cyclin B-Cdc2) and proposed a model according to which the stoichiometry of p21 was critical to allow or inhibit kinase activity. When one p21 molecule was binding to cyclin-CDK, the complex was catalytically active, while binding of several p21 subunits inhibited the complex. LaBaer et al. (30) showed that p21 could function as an assembly- and activity-promoting factor for cyclin D1-CDK4, cyclin D3-CDK4, and cyclin E-CDK2 complexes when p21 levels were below a certain threshold, after which the presence of excess p21 became inhibitory. Cheng et al. (8) have shown compelling evidence for the roles played by p21 and p27 in the regulation of cyclin-CDK complex assembly and activity. In their study (8), mouse embryo fibroblasts deficient for both p21 and p27 fail to assemble detectable levels of cyclin D-CDK4 complexes and to efficiently target cyclin D to the nucleus. Both the assembly and activity of cyclin D-CDK4 complexes could be restored by reintroducing either p21 or p27 in those cells (8).
The expression of a number of cell cycle regulatory proteins is altered in gliomas. Several CDKs are overexpressed in a large subset of gliomas and glioma cell lines (7, 9, 21, 46). On the other hand, mRNAs for p57, p19, p16, and p15 could not be detected in U251N cells (data not shown). The INK4a (encoding p16 and p19) and INK4b (encoding p15) genes have previously been reported to be either deleted, mutated, or hypermethylated (thus preventing transcriptional activity) in a wide range of tumors, including gliomas (22, 46, 47). One may speculate that due to the abundance of various cyclins and CDKs, and lack of several CKIs in glioma cells, the upregulated levels of p21Waf1/Cip1 induced by PKC activation do not become inhibitory; p21 molecules are “mopped up” by cellular cyclins and CDKs, resulting in increased assembly of active cyclin-CDK-p21 complexes and leading to increased cell cycle progression. Our findings raise the possibility that p21Waf1/Cip1 may be an active player in the pathology of glioma cells and participate to maintain a hyperproliferative state in those cells. Furthermore, it was reported recently that the p21 protein was elevated in 50% of cases of patients with astrocytomas, especially within the higher grades (28), and that p21 expression was associated with a shorter overall survival in patients with gliomas (28).
Another attractive hypothesis that could account for the absence of inhibition of cyclin-CDK complexes by p21 is the existence of a class of p21-binding proteins that could modulate its inhibitory activity. The human papillomavirus type 16 E7 oncoprotein can interact with p21Waf1/Cip1 and abrogate p21-mediated inhibition of cyclin A- and E-associated kinase activities (18, 25). Another oncoprotein, SET, was found to bind directly to p21 and to reverse the inhibition of cyclin E-CDK2 complexes (15). Thus, one could speculate that the absence of inhibition of p21-containing cyclin-CDK complexes could be due to the presence of one or more of these proteins that can modulate the inhibitory activity of p21Waf1/Cip1.
The transformed phenotype of glioma cells appears to be a complex interplay of numerous factors: aberrations in signaling cascades due to growth factor receptor amplification or mutations; increased PKC activity; absence of several tumor suppressor proteins; and the aberrant timing of cyclin expression (14), overexpression of several CDKs and cyclins, and abundance of p21, thus resulting in deregulated growth control.
In summary, our results suggest that in glioma cells, PKC α activation upregulates the p21Waf1/Cip1 protein, which is incorporated into active ternary cyclin-CDK-p21, thus facilitating cell cycle progression. p21Waf1/Cip1 may represent an important therapeutic target to control the growth rate of glioma cells.
ACKNOWLEDGMENTS
We gratefully thank Stephen Robbins and Karl Riabowol for critical reading of this manuscript and useful discussions. We thank Lorie Robertson from the Flow Cytometry Laboratory for technical expertise.
This work was supported by the Brain Tumor Foundation of Canada. A.B. is a Research Student of the National Cancer Institute of Canada supported with funds provided by the Terry Fox Run. V.W.Y. is a Medical Research Council of Canada scientist and a senior scholar of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Ahmad S, Mineta T, Martuza R L, Glazer R I. Antisense expression of protein kinase C alpha inhibits the growth and tumorigenicity of human glioblastoma cells. Neurosurgery. 1994;35:904–909. doi: 10.1227/00006123-199411000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Buffolino P, Coppock D L. Regulation of the cell cycle at the G2/M boundary in metastatic melanoma cells by 12-O-tetradecanoyl phorbol-13-acetate (TPA) by blocking p34cdc2 kinase activity. Exp Cell Res. 1998;242:381–390. doi: 10.1006/excr.1997.3911. [DOI] [PubMed] [Google Scholar]
- 3.Baltuch G H, Shenouda G, Langleben A, Villemure J H. High dose tamoxifen in the treatment of recurrent high-grade glioma: a report of clinical stabilization and tumor regression. Can J Neurol Sci. 1993;20:168–170. doi: 10.1017/s0317167100047788. [DOI] [PubMed] [Google Scholar]
- 4.Baltuch G H, Dooley N P, Couldwell W T, Yong V W. Staurosporine differentially inhibits glioma versus non-glioma cell lines. J Neurooncol. 1993;16:141–147. doi: 10.1007/BF01324701. [DOI] [PubMed] [Google Scholar]
- 5.Baltuch G H, Dooley N P, Villemure J-G, Yong V W. Protein kinase C and growth regulation of malignant gliomas. Can J Neurol Sci. 1995;22:264–271. doi: 10.1017/s0317167100039457. [DOI] [PubMed] [Google Scholar]
- 6.Begemann M, Kashimawo S A, Choi Y J A, et al. Inhibition of the growth of glioblastomas by CPG41251, an inhibitor of protein kinase C, and by phorbol ester tumor promoter. Clin Cancer Res. 1996;2:1017–1030. [PubMed] [Google Scholar]
- 7.Burns K L, Ueki K, Jhung S L, Koh J, Louis D N. Molecular genetics correlates of p16, CDK4, and Rb immunochemistry in glioblastomas. J Neuropathol Exp Neurol. 1998;57:122–130. doi: 10.1097/00005072-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cheng M, Olivier P, Dielh J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello J F, Plass C, Arap W, Chapman V M, Held W A, Berger M S, Huang H J S, Cavenee W K. Cyclin dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res. 1997;57:1250–1254. [PubMed] [Google Scholar]
- 10.Couldwell W T, Antel J P, Yong V W. Protein kinase C activity correlates with the growth rate of malignant gliomas. II. Effect of glioma mitogens and modulators of PKC. Neurosurgery. 1992;31:717–724. doi: 10.1227/00006123-199210000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Couldwell W T, Hinton D R, Surnock A A, DeGiorgio C M, Weiner L P, Apuzzo M L J, Masri L, Weiss M H. Treatment of recurrent malignant gliomas with chronic oral high dose tamoxifen. Clin Cancer Res. 1996;2:619–622. [PubMed] [Google Scholar]
- 12.Dean N, McKay R, Miraglia L, Howard R, Cooper S, Giddings J, Nicklin P, Meister L, Ziel R, Geiger T, Muller M, Fabbro D. Inhibition of growth of human tumor cell lines in nude mice by an antisense oligonucleotide inhibitor of protein kinase C alpha expression. Cancer Res. 1996;56:3499–3507. [PubMed] [Google Scholar]
- 13.Delphin C, Baudier J. The protein kinase C activator, phorbol ester, cooperates with wild type p53 species in ras transformed embryo fibroblasts growth arrest. J Biol Chem. 1994;269:29579–29587. [PubMed] [Google Scholar]
- 14.Dirks P B, Hubbard S L, Murakami M, Rutka J T. Cyclin and cyclin-dependent kinase expression in human astrocytoma cell lines. J Neuropathol Exp Neurol. 1997;56:291–300. doi: 10.1097/00005072-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Estanyol J M, Jaumont M, Casanovas O, Rodriguez-Vilarrupla A, Agell N, Bachs O. The protein SET regulates the inhibitory effect of p21/Cip1 on cyclin-E-cyclin dependent kinase-2 activity. J Biol Chem. 1999;274:33161–33165. doi: 10.1074/jbc.274.46.33161. [DOI] [PubMed] [Google Scholar]
- 16.Fishman D D, Segal S, Livneh E. The role of protein kinase C in G1 and G2/M phases of the cell cycle. Int J Oncol. 1998;12:181–186. doi: 10.3892/ijo.12.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Frey M R, Saxon M L, Zhao X, Rollins A, Evans S S, Black J D. Protein kinase C mediated cell cycle arrest involves induction of p21Waf1/Cip1 and p27Kip1 and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 18.Funk J O, Waga S, Harry J B, Espling E, Stillman B, Galloway D A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada K, Takuwa N, Zhou W, Kumada M, Takuwa Y. Protein kinase C inhibits the CAK-CDK2 cyclin dependent kinase cascade and G1/S cell cycle progression in human diploid fibroblasts. Biochim Biophys Acta. 1993;1310:149–156. doi: 10.1016/0167-4889(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 20.Hass R, Gunji H, Hirano M, Weichselbaum R, Kufe D. Phorbol ester induced monocytic differentiation is associated with G2 delay and down regulation of cdc25 expression. Cell Growth Differ. 1993;4:159–166. [PubMed] [Google Scholar]
- 21.He J, Olson J J, James C D. Lack of p16INK4 or retinoblastoma protein (Rb), or amplification associated overexpression of CDK4 is observed in distinct subsets of malignant glial tumors and cell lines. Cancer Res. 1995;55:4833–4836. [PubMed] [Google Scholar]
- 22.Herman J G, Jen J, Merlo A, Baylin S B. Hypermethylation associated inactivation indicates a tumor suppressor role for p15/INK4b1. Cancer Res. 1996;56:722–727. [PubMed] [Google Scholar]
- 23.Hiyama H, Iavarone A, Reeves S A. Regulation of the CDK inhibitor p21 gene during cell cycle progression is under the control of the transcription factor E2F. Oncogene. 1998;16:1513–1523. doi: 10.1038/sj.onc.1201667. [DOI] [PubMed] [Google Scholar]
- 24.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 25.Jones D L, Alani R M, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J M, Bruner J M, Ruan S, Langford L A, Kyritsis A P, Kobayashi T, Levin V A, Zhang W. Increased levels of p21/Waf1/Cip1 in human brain tumors. Oncogene. 1995b;11:2021–2028. [PubMed] [Google Scholar]
- 27.Kazanietz M G, Areces L B, Bahador A, Mischak H, Goodnight J, Mushinski J F, Blumberg P M. Characterization of ligand and substrate specificity for the calcium-dependent and calcium independent protein kinase C isozymes. Mol Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- 28.Korkolopoulou P, Kouzelis K, Christodoulou P, Papanikolaou A, Thomas-Tsagli E. Expression of retinoblastoma gene product and p21/Waf1/Cip1 protein in gliomas: correlations with proliferation markers, p53 expression and survival. Acta Neuropathol. 1998;95:617–624. doi: 10.1007/s004010050848. [DOI] [PubMed] [Google Scholar]
- 29.Kosaka C, Sasaguri T, Ishida A, Ogata J. Cell cycle arrest in G2 phase induced by phorbol ester and diacylglycerol in vascular endothelial cells. Am J Physiol. 1996;270:C170–C178. doi: 10.1152/ajpcell.1996.270.1.C170. [DOI] [PubMed] [Google Scholar]
- 30.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 31.Li S, MacLachlan T K, De Luca A, Claudio P P, Condorelli G, Giordano A. The cdc-2 related kinase, PISSLRE, is essential for cell growth and acts in G2 phase of the cell cycle. Cancer Res. 1995;55:3992–3995. [PubMed] [Google Scholar]
- 32.Livneh E, Shimon T, Bechor E, Doki Y, Schieren I, Weinstein I B. Linking protein kinase C to the cell cycle: ectopic expression of PKC eta in NIH-3T3 cells alters the expression of cyclins and CDK inhibitors and induces adipogenesis. Oncogene. 1996;12:1545–1555. [PubMed] [Google Scholar]
- 33.Livneh E, Fishman D D. Linking protein kinase C to cell cycle control. Eur J Biochem. 1997;248:1–9. doi: 10.1111/j.1432-1033.1997.t01-4-00001.x. [DOI] [PubMed] [Google Scholar]
- 34.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 35.Mann D J, Higgins T, Jones N C, Rozengurt E. Differential control of cyclins D1 and D3 and the CDK inhibitor p27/Kip1 by diverse signaling pathways in Swiss 3T3 cells. Oncogene. 1997;14:1759–1766. doi: 10.1038/sj.onc.1201134. [DOI] [PubMed] [Google Scholar]
- 36.Mantel C, Luo Z, Canfield J, Braun S, Deng C, Broxmeyer H E. Involvement of p21Cip1 and p27Kip1 in the molecular mechanisms of Steel Factor induced proliferative synergy in vitro and of p21Cip1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 37.Mastronardi L, Puzzilli F, Couldwell W T, Farah J O, Lunardi P. Tamoxifen and carboplatin combinational treatment of high-grade gliomas. J Neurooncol. 1998;38:59–68. doi: 10.1023/a:1005968724240. [DOI] [PubMed] [Google Scholar]
- 38.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellor H, Parker P J. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michieli P, Chedid M, Lin D, Pierce J H, Mercer W E, Givol D. Induction of WAF1/CIP1 by a p53 independent pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 41.Newton A C. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 42.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G R, Roberts J M. Interleukin-2 mediated elimination of the p27Kip1 cyclin dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 43.Pollack I F, Kawecki S. The effect of Calphostin C, a potent photodependent protein kinase C inhibitor, on the proliferation of glioma cells in vitro. J Neurooncol. 1997;31:255–266. doi: 10.1023/a:1005729626354. [DOI] [PubMed] [Google Scholar]
- 44.Ryves W J, Evans A T, Olivier A R, Parker P J, Evans F J. Activation of the PKC isotypes alpha, beta 1, gamma, delta, and epsilon by phorbol esters of different biological activities. FEBS Lett. 1991;288:5–9. doi: 10.1016/0014-5793(91)80989-g. [DOI] [PubMed] [Google Scholar]
- 45.Sioud M, Sorensen D R. A nuclease-resistant protein kinase C alpha ribozyme blocks glioma cell growth. Nat Biotechnol. 1998;16:556–561. doi: 10.1038/nbt0698-556. [DOI] [PubMed] [Google Scholar]
- 46.Sonoda Y, Yoshimoto T, Sekiya T. Homozygous deletion of the MTS1/p16 and MTS2/p15 genes and amplification of the CDK4 gene in glioma. Oncogene. 1995;11:2145–2149. [PubMed] [Google Scholar]
- 47.Srivenugopal K S, Ali-Osman F. Deletions and rearrangements inactivate the p16/INK4 gene in human glioma cells. Oncogene. 1996;12:2029–2034. [PubMed] [Google Scholar]
- 48.Thompson L J, Fields A P. Beta2 protein kinase C is required for the G2/M transition of cell cycle. J Biol Chem. 1996;271:15045–15053. doi: 10.1074/jbc.271.25.15045. [DOI] [PubMed] [Google Scholar]
- 49.Yazaki T, Ahmad S, Chablavi A, Zylber-Katz E, Dean N M, Rabkin S D, Martuza R L, Glazer R I. Treatment of glioblastoma U-87 by systemic administration of an antisense protein kinase C-alpha phosphorothioate oligodeoxynucleotide. Mol Pharmacol. 1996;50:236–242. [PubMed] [Google Scholar]
- 50.Yong V W, Dooley N P, Noble P G. Protein kinase C in cultured adult human oligodendrocytes: a potential role for isoform alpha as a mediator of process outgrowth. J Neurosci Res. 1994;39:83–96. doi: 10.1002/jnr.490390111. [DOI] [PubMed] [Google Scholar]
- 51.Zeng Y-X, El-Deiry W S. Regulation of p21/Waf1/Cip1 expression by p53 independent mechanisms. Oncogene. 1996;12:1557–1564. [PubMed] [Google Scholar]
- 52.Zezula J, Sexl V, Hutter C, Karel A, Schutz W, Freissmuth M. The cyclin-dependent kinase inhibitor p21cip1 mediates the growth inhibitory effect of phorbol esters in human venous endothelial cells. J Biol Chem. 1997;272:29967–29974. doi: 10.1074/jbc.272.47.29967. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Hannon G H, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]