Abstract
Background
Acute respiratory distress syndrome (ARDS) is a major complication of COVID-19 and is associated with high mortality and morbidity. We aimed to assess whether intravenous immunoglobulins (IVIG) could improve outcomes by reducing inflammation-mediated lung injury.
Methods
In this multicentre, double-blind, placebo-controlled trial, done at 43 centres in France, we randomly assigned patients (1:1) receiving invasive mechanical ventilation for up to 72 h with PCR confirmed COVID-19 and associated moderate-to-severe ARDS to receive either IVIG (2 g/kg over 4 days) or placebo. Random assignment was done with a web-based system and was stratified according to the participating centre and the duration of invasive mechanical ventilation before inclusion in the trial (<12 h, 12–24 h, and >24–72 h), and treatment was administered within the first 96 h of invasive mechanical ventilation. To minimise the risk of adverse events, the IVIG administration was divided into four perfusions of 0·5 g/kg each administered over at least 8 hours. Patients in the placebo group received an equivalent volume of sodium chloride 0·9% (10 mL/kg) over the same period. The primary outcome was the number of ventilation-free days by day 28, assessed according to the intention-to-treat principle. This trial was registered on ClinicalTrials.gov, NCT04350580.
Findings
Between April 3, and October 20, 2020, 146 patients (43 [29%] women) were eligible for inclusion and randomly assigned: 69 (47%) patients to the IVIG group and 77 (53%) to the placebo group. The intention-to-treat analysis showed no statistical difference in the median number of ventilation-free days at day 28 between the IVIG group (0·0 [IQR 0·0–8·0]) and the placebo group (0·0 [0·0–6·0]; difference estimate 0·0 [0·0–0·0]; p=0·21). Serious adverse events were more frequent in the IVIG group (78 events in 22 [32%] patients) than in the placebo group (47 events in 15 [20%] patients; p=0·089).
Interpretation
In patients with COVID-19 who received invasive mechanical ventilation for moderate-to-severe ARDS, IVIG did not improve clinical outcomes at day 28 and tended to be associated with an increased frequency of serious adverse events, although not significant. The effect of IVIGs on earlier disease stages of COVID-19 should be assessed in future trials.
Funding
Programme Hospitalier de Recherche Clinique.
Introduction
Globally, more than 133 million patients have been infected by SARS-CoV-2, and more than 2·9 million have died from COVID-19.1 Acute respiratory distress syndrome (ARDS) is one of the most severe complications of COVID-19; it is associated with increased mortality, prolonged invasive mechanical ventilation, increased length of stay in an intensive care unit or in hospital,2 and long-term disability.3
COVID-19-associated ARDS results from both the viral infection and its accompanying inflammatory response.4 In cases where antiviral therapies did not have a benefit, some anti-inflammatory treatments have been shown to reduce the severity of COVID-19-associated pneumonia.5 For example, dexamethasone reduced 28-day mortality in patients with COVID-19 receiving invasive mechanical ventilation by 12·1%, and tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, might have benefits on organ failure.6, 7, 8 However, despite these advances, mortality related to COVID-19-associated ARDS remains as high as 30–40%, prompting the assessment of other immunomodulatory approaches.6, 8, 9
Research in context.
Evidence before this study
Mortality of patients receiving mechanical ventilation for COVID-19-associated acute respiratory distress syndrome (ARDS) ranges from 30% to 40%; corticosteroids and tocilizumab have been shown to reduce mortality, suggesting that immune system modulation could improve outcomes. Retrospective studies indicate that intravenous immunoglobulins (IVIG) could reduce mortality in patients receiving mechanical ventilation with COVID-19-associated ARDS. However, IVIG are costly, liable to shortage and associated with various side-effects. Therefore, we did a randomised trial to assess whether IVIG improve outcomes in patients receiving invasive mechanical ventilation for COVID-19-associated moderate-to-severe ARDS. We searched PubMed and the ClinialTrials database for Articles and trials from Jan 01, 2019, to Oct 11, 2021, using the search terms “COVID-19” and “intravenous immunoglobulins“. No studies evaluating the effects of IVIG on patients with COVID-19 associated moderate to severe ARDS were identified.
Added value of the study
Conversely to the results suggested by the available retrospective studies, our study shows that IVIG administration within 96 h of invasive mechanical ventilation in patients with COVID-19-associated moderate-to-severe ARDS did not modify the number of ventilation-free days at day 28 and tended to be associated with more serious adverse events, although the difference was not significant. This study provides the highest level of evidence against the use of IVIG in the COVID-19.
Implications of all the available evidence
IVIG should not be administered to patients with COVID-19-associated ARDS outside of the clinical trial setting; instead IVIG use should be spared for other inflammatory diseases.
SARS-CoV-2 replicates in bronchial cells and pneumocytes, inducing a local inflammatory reaction that spreads to the lung and triggers the local recruitment of immune cells and activated lymphocytes during the acute phase immune response.4 Intravenous immunoglobulins (IVIGs) have various immune modulatory properties that are theoretically relevant in COVID-19.10 In addition to IVIG scavenging the complement system and cytokines, they also stimulate the proliferation of regulatory T cells and restore their suppressive functions, thereby reducing the activation of innate immune cells and effector T cells.11 Of note, IVIG are commonly prescribed for post-viral endotheliitis, as seen in Kawasaki disease.12, 13 Furthermore, IVIG have been reported to be safe in patients who are critically ill, with treatment associated with a low rate of acute renal failure and thromboembolism.14 Retrospective observational studies have suggested that IVIG decrease mortality and duration of invasive mechanical ventilation in COVID-19-associated ARDS.15, 16, 17, 18, 19 Furthermore, it has been reported in China that about a third of patients with COVID-19 and ARDS have been treated with IVIG, without the specific effect of IVIG being evaluated.20, 21, 22 However, because of the cost and scarcity of IVIGs, which is currently indicated in various autoimmune and inflammatory diseases, showing the efficacy and safety of IVIG in patients who are critically ill with COVID-19 is needed to explore IVIG therapy as a viable treatment option.23
Therefore, we aimed to assess the efficacy, safety, and immunomodulatory effects of IVIG in patients admitted to an intensive care unit for moderate-to-severe ARDS associated with COVID-19.
Methods
Study design and participants
ICAR was a phase 3, double-blind, randomised, multicentre, placebo-controlled study, done in 43 centres in France (appendix 1 pp 2–4), evaluating the effect of IVIG in patients hospitalised with COVID-19-associated moderate or severe ARDS requiring mechanical ventilation. This investigator-initiated trial was designed in collaboration with the sponsor—Groupe Hospitalier Universitaire Paris Psychiatrie et Neurosciences, Paris, France—and was overseen by an Independent Data Monitoring Committee. The design of ICAR was published in a futility interim analysis study done in August, 2020, on 50 patients by the Independent Data Monitoring Committee defined upon a protocol amendment (protocol amendment 3; June 11, 2020; appendix 2 p 113).24
Critically ill patients (≥18 years) with COVID-19, confirmed by a positive PCR test, admitted to the intensive care unit were eligible for inclusion if they required invasive mechanical ventilation for moderate-to-severe ARDS, according to the Berlin Definition criteria.25 Patients had to be enrolled in the study within 72 h after starting invasive mechanical ventilation. Exclusion criteria were acute renal failure at admission, defined as plasma creatinine above 354 μmol/L, an increase in plasma creatinine baseline concentration by three-times or more, a diuresis of less than 0·3 mL/kg over the last 24 h, or anuria over the last 12 h; pregnancy; immunoglobulin A deficit; allergy to IVIG; and participation in another intervention trial. The patients received standard care according to the policy of each site, particularly regarding the use of corticosteroids and supportive care. Written informed consent, in accordance with local legislation, was obtained from all patients or their surrogates. If written informed consent could not be obtained, patients were included because the study was considered an emergency research by the institutional review board; consent was obtained as soon as the patients were able to provide it.
The trial was centrally approved by the Paris X ethics committee and has been done in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
Randomisation and masking
Randomisation was done with a web-based system. Trial group designation was concealed and the randomisation group was electronically sent to the centre's pharmacy. Patients were assigned (1:1) to receive either IVIG (IVIG group) or placebo (placebo group). Random assignment was stratified according to the participating centre and the duration of invasive mechanical ventilation before inclusion in the trial (<12 h, 12–24 h, and >24–36 h). The protocol was amended to extend the last temporal category to 72 h (protocol amendment 1, May 4, 2020; appendix 2 p 96).
Trial participants, care providers, and outcome assessors were masked to patient assignment. The double-blinding was provided by each hospital pharmacy, using opaque sleeves and tubing to conceal the product administered. To preserve the masking, research nurses supervised the administration and were asked not to disclose whether IVIG or placebo was infused. Masking was removed in the event of an adverse effect that could be attributed to IVIG or placebo and upon the responsible investigator's approval. The statisticians who analysed the data were masked to group assignment.
Procedures
CLAYRIG (Laboratoire Français du fractionnement et des biotechnologies, Les Ulis, France), a saccharose and maltose free IVIG, was administered for a total dose of 2 g/kg. IVIG infusion had to start before the end of the 96 h after the onset of invasive mechanical ventilation. To minimise the risk of adverse events, IVIG administration was divided into four perfusions of 0·5g/kg each given over at least 8 h over 4 days. Patients in the placebo group had to receive an equivalent volume of sodium chloride 0·9% (10 mL/kg), over the same period. The protocol was amended to extend the time for starting the IVIG administration from 72 h to 96 h after onset of invasive mechanical ventilation (protocol amendment 1, May 4, 2020; appendix 2 p 96).
Efficacy was evaluated on day 28, and patients were followed for 90 days. Assessments (administered treatments, ventilation parameters PaO2:FiO2 ratio, ventilator weaning trials, and sequential organ failure score) were done daily throughout the intensive care unit stay until day 28. If the patient was discharged from the intensive care unit but still in hospital, a visit was scheduled on days 14 and 28, and if the patient left the hospital, a telephone interview was done at day 90 by an investigator to collect primary and secondary outcome data. Baseline characteristics were collected upon admission to the intensive care unit. COVID-19 treatments administered between 7 days before enrolment up and day 2 after randomisation were considered concomitant.
Outcomes
The primary outcome was the number of ventilator-free days at day 28, defined as the number of days between the last extubation day and day 28. In the case of death before day 28, the score was zero. The primary outcome composite components were as time-to-event censored at day 28, within a competing risk framework; therefore, on day 28, the secondary efficacy endpoints of mortality, the proportion of patients who were extubated, and the duration of invasive mechanical ventilation were collected as subcomponents of the primary endpoint, measured according to the intention-to-treat population.
The key secondary outcomes were the sequential organ failure assessment score at day 14 and day 28; the occurrence of grade 3 or 4 adverse events or serious adverse events attributed to IVIG; the time to intensive care unit or hospital discharge; the clinical status at day 28 and day 90 as assessed by the seven-category ordinal scale; 90-day mortality; and lung injury score at day 28.26 The main exploratory secondary outcomes were occurrence of pulmonary embolism and nosocomial pneumonia within the first 28 days, cytokines concentrations (IL-6, TNF-α, and IL-13), and circulating lymphocytes populations at admission, days 7, 14, and 28. All were meausred in the intention-to-treat population.
Adverse events were coded using the MedDRA (version 22) coding dictionary; each adverse event was electronically reported to the sponsor and monitored until its complete resolution. The adverse events considered related to IVIG are included in appendix 1 (p 22). The number of deaths due to an adverse event and study discontinuation due to an adverse event were recorded. The safety analysis included all patients who received at least one dose of the study drug.
Statistical analysis
At the time of study design and according to previous studies on ARDS before the COVID-19 pandemic, we assumed that the mean number of days without invasive mechanical ventilation would be 10 days (SD 6 days) in the placebo group and 15 days (SD 6 days) in the IVIG group.27, 28 With an assumed 28-day mortality rate of 50% in the placebo group and 40% in the IVIG group,27, 28 the mean number of days without invasive mechanical ventilation was expected to be 9 days in the placebo group and 5 days in the IVIG group. A reduction in the mortality rate of 10% and a 5-day period of invasive mechanical ventilation resulting in a 4-day increase of ventilator-free days at day 28 was considered clinically relevant.
Because of the uncertainty about the assumption of normality of the distributions, the non-parametric Wilcoxon-Mann-Whitney test (U-test) was used for sample size estimation. Considering a power of 90% and a bilateral α risk level of 5%, 69 participants were required in each group. An interim analysis for futility was done after 50 (25 per group) patients completed the 28-day follow-up; as a result of this analysis the Independent Data Monitoring Committee reported that patient recruitment should continue without protocol modification. Subgroup analyses were stratified according to time to randomisation, age (≥60 years), and body-mass index (≥30 kg/m2)
The clustered Wilcoxon rank-sum test (Rosner-Glynn-Lee method) stratified by centre and invasive mechanical ventilation duration was used for the primary analysis of the principal endpoint.29 The hypothesis of equality of treatment groups for ventilator-free days was tested at a two-sided significance level of 0·05, adjusted for the interim analysis.
The Hodges-Lehmann estimator was used to assess the difference in median and relative CI for all quantitative outcomes. Unadjusted and adjusted odds ratios were used to test the risk of in-hospital death at 28 and 90 days.
The log-rank test and Kaplan-Meier plot were used for time-to-event analysis (survival at 28 days and 90 days, intensive care unit stay, and hospital discharge). A non-prespecified analysis of the effect of IVIG on the main outcome in patients who received corticosteroids was done. A subgroup analysis of patients with a BMI of 30 or more or aged 65 years or older of the main and secondary outcomes was done. Finally a sensitivity analysis was done on the per-protocol population. All other statistical analyses were done in the intention-to-treat population using SPSS (version 26.0) and RStudio (version 1.3.1093; appendix 1 p 8). This trial is registered on ClinicalTrials.gov, NCT04350580.
Role of the funding source
Neither the funder of the study nor the organisation providing the study drug had a role in study design, data collection, data analysis, data interpretation, or writing of the report. The study sponsor participated in the trial, collected the data, and did the analysis.
Results
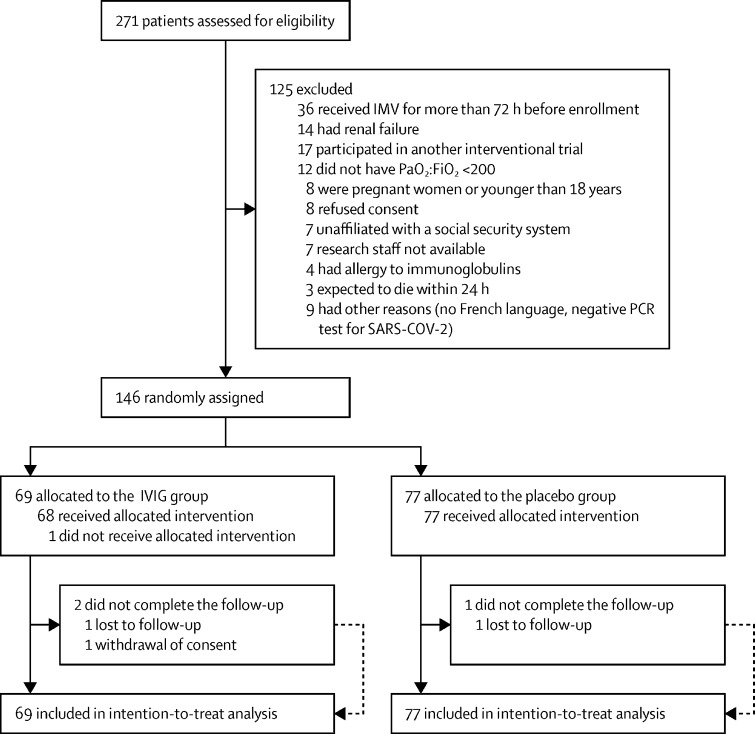
Between April 3, and Oct 20, 2020, 271 patients were assessed for eligibility from 27 active recruiting centres. 146 patients (43 [29%] women) were eligible for inclusion and randomly assigned: 69 (47%) patients to the IVIG group and 77 (53%) to the placebo group. 66 (96%) patients completed the trial in the IVIG group compared with 76 (99%) patients in the placebo group (figure 1 ). One patient who was assigned to receive IVIG did not receive the treatment drug, and was transferred to emergency care at a tertiary centre for extracorporeal life support. The median follow-up time was 90 days (IQR 20–90).
Figure 1.
Trial profile
FiO2=fraction of inspired oxygen. IVIG=intravenous immunoglobulin. IMV=invasive mechanical ventilation. PaO2=partial pressure of arterial oxygen in mm Hg.
Baseline demographic and disease characteristics were similar between both groups (table 1 ; appendix 1 pp 10–13). The mean age was 65·1 years (SD 12·2) in the IVIG group and 66·5 years (9·3) years in the placebo group. The median time between symptom onset and initiation of invasive mechanical ventilation was 8 days (6·0–11·0) in the IVIG group and 8 days (6·0–12·5) in the placebo group. The severity of critical illness and ARDS was similar between the two groups, as indicated by the absence of difference for the simplified acute physiology score II, sequential organ failure assessment score, lung compliance, and PaO2:FiO2 ratio upon admission (table 1). The ventilator settings did not differ between the two groups, nor did the use of corticosteroids and tocilizumab (table 1). The median dose of IVIG was 2 g/kg (IQR 1·9–2·0) over 4 days (appendix 1 p 20).
Table 1.
Baseline demographic and disease characteristics
| IVIG group (n=69) | Placebo group (n=77) | ||
|---|---|---|---|
| Demographics and comorbidities | |||
| Sex | |||
| Male | 49 (71%) | 54 (70%) | |
| Female | 20 (29%) | 23 (30%) | |
| Age | |||
| Age (years) | 65·1 (12·2) | 66·5 (9·3) | |
| Patients 65 years or older | 38 (55%) | 49 (64%) | |
| Body-mass index (kg/m2) | 30·9 (5·75) | 30·2 (6·20) | |
| Median Charlson Comorbidity score* | 3 (1–4) | 3 (2–4) | |
| Performance status inferior or equal to 1 | 45 (65%) | 60 (78%) | |
| COVID-19 course | |||
| Time between symptom onset and initiation of invasive mechanical ventilation in days | 8 (6·0–11·0) | 8 (6·0–12·5) | |
| Time between initiation of invasive mechanical ventilation and random assignment | |||
| <12 h | 23 (33%) | 31 (40%) | |
| 12–24 h | 23 (33%) | 23 (30%) | |
| >24–72 h | 23 (33%) | 23 (30%) | |
| Simplified acute physiology score II† | 41·0 (32·0–50·0) | 39·0 (31·5–50·0) | |
| Critical illness and acute respiratory distress syndrome severity | |||
| Sequential organ failure assessment score | 6 (4·0–8·0) | 6 (3·0–8·0) | |
| Kidney disease improving global outcome score | 0 (0–0) | 0 (0–0) | |
| Vasopressor support | 36 (52%) | 35 (45%) | |
| Lung injury score‡ | 3·0 (2·7–3·3) | 3·0 (3·0–3·5) | |
| PaO2 :FiO2 ratio | 125 (96–155) | 110 (80–153) | |
| Lung compliance (mL/cm H2O)§ | 32·5 (29·0–36·0) | 29·5 (26·0–33·0) | |
| Radiological score (number of quadrant[s] with alveolo-interstitial opacities) | 4 (2–4) | 4 (3–4) | |
| Acute respiratory distress syndrome management | |||
| Tidal volume (mL/kg of predicted body weight) | 6·2 (5·6–6·7) | 6·2 (5·8–6·6) | |
| Positive end expiratory pressure (cm H2 O) | 12 (9·8–14·0) | 12 (10·0–14·0) | |
| Inspiratory plateau pressure (cm H2 O) | 24 (23–26) | 25 (24–26) | |
| Laboratory value | |||
| Lymphocyte count (×109/L) | 0·67 (0·28) | 0·56 (0·28) | |
| Platelet count (×109/L) | 292 (136) | 278 (109) | |
| Plasma C-reactive protein concentration (μg/mL) | 164 (91) | 160 (90) | |
| COVID-19 treatment before and 2 days after random assignment | |||
| Corticosteroid | 49 (71%) | 55 (71%) | |
| Tocilizumab | 5 (7%) | 7 (9%) | |
| Antibiotics | 56 (81%) | 65 (84%) | |
Data are n (%), median (IQR), or mean (SD). FiO2 =fraction of inspired oxygen. IVIG=intravenous immunoglobulins. PaO2 =partial pressure of arterial oxygen in mm Hg.
Higher Charlson Comorbidity score indicates more comorbidities.
Scores range from 0 to 163, with higher scores indicating greater severity of illness.
When values were missing for one of the four components of the lung injury score, it was considered to be 0 and the mean was realised.
Lung compliance is calculated as the measured tidal volume divided by (plateau pressure in cm H2 O minus total positive end expiratory pressure in cm H2 O).
The median number of ventilator-free days at day 28 was 0·0 (IQR 0·0–8·0) in the IVIG group and 0·0 (0·0–6·0) in the placebo group (with a difference estimate between the medians of 0·0 [95% CI 0·0–0·0]; p=0·21; table 2 ; figure 2 ). The mortality rate at day 28 did not differ between the two groups (24 [35%] of 69 patients in the IVIG group vs 20 [26%] of 77 patients in the placebo group; unadjusted odds ratio 1·52 [0·75–3·09]; p=0·25; table 2). The proportion of extubated patients at day 28 and the median duration of invasive mechanical ventilation were also similar between the two groups (table 2).
Table 2.
Primary and secondary outcomes
| IVIG group (n=69) | Placebo group (n=77) | Difference (95% CI)* | Unadjusted odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Median number of ventilation-free days at 28 days | 0·0 (0·0 to 8·0) | 0·0 (0·0 to 6·0) | 0·0 (0·0 to 0·0) | .. | 0·21† | |
| Mean number of ventilation-free days at 28 days | 6·7 (4·6 to 8·8) | 7·0 (4·9 to 9·2) | 0·5 (−3·5 to 2·5) | .. | .. | |
| Competing risk | ||||||
| Death at 28 days | 24 (35%) | 20 (26%) | .. | 1·52 (0·75 to 3·09) | 0·25 | |
| Patients receiving mechanical ventilation at day 28 | 15 (22%) | 22 (29%) | .. | 1·44 (0·67 to 3·07) | 0·35 | |
| Median time to last extubation (days)‡ | 12·5 (8·0 to 18·0) | 9·5 (7·0 to 18·0) | 1·0 (−3·0 to 4·0) | .. | 0·38 | |
| Prespecified secondary outcomes | ||||||
| Median lung injury score at day 28§ | 2·8 (2·4 to 3·1) | 2·7 (2·2 to 3·1) | 0·0 (−0·5 to 0·5) | .. | 0·60 | |
| Median sequential organ failure assessment score at day 28¶ | 7 (3 to 10) | 6 (4 to 10) | 1 (−3 to 2) | .. | 0·65 | |
| Median clinical ordinal score at 28 days‖ | 3 (1·0 to 4·0) | 3 (1·0 to 5·0) | 0 (−1·0 to 0·0) | .. | 0·47 | |
| Median clinical ordinal score at 90 days** | 1 (1·0 to 1·0) | 1 (1·0 to 1·0) | 0·0 (0·0 to 0·0) | .. | 0·56 | |
| Death at day 90 | 28 (41%) | 31 (40%) | .. | 1·01 (0·52 to 1·97) | 0·97 | |
| Median time to ICU discharge in days** | 21 (15·0 to 27·0) | 21 (13·0 to 29·0) | 1·0 (−6·0 to 7·0) | .. | 0·74 | |
| Median time to hospital discharge in days** | 34 (29·0 to 46·0) | 39 (27·0 to 49·0) | −2·0 (−11·0 to 8·0) | .. | 0·84 | |
Data are n (%), mean (95% CI), or median (IQR). ICU=intensive care unit. IVIG=intravenous immunoglobulins. Adjusted ORs for age, sex, body-mass index.
If not specified data are Hodges-Lehmann median difference.
Clustered Wilcoxon rank sum test using Rosner-Glynn-Lee method stratified according to the centre and invasive mechanical ventilation duration at randomisation day.
Only in survivors extubated within day 28.
When values were missing for one of the 4 components of the lung injury score, it was considered to be 0 and the mean was realised.
When values were missing for one organ of the sequential organ failure assessment score, the organ was considered free of failure.
Only in survivors at day 28.
Only in survivors at day 90.
Figure 2.
Cumulative distribution of ventilation-free days (A), and Kaplan-Meier curves of patients who were extubated (B), and probability of survival (C)
IVIG=intravenous immunoglobulin.
The sequential organ failure assessment and the lung injury scores at day 14 (appendix 1 p 14) and day 28 (table 2) were not statistically different between the two groups, whereas lung compliance was significantly lower in the IVIG group at day 14 (appendix 1 p 14). The day 28 and day 90 seven-category clinical ordinal scale between the IVIG and placebo groups were similar (table 2; appendix 1 pp 16–17) as were the proportion of patients discharged (16 [37%] of 43 patients in the IVIG group vs 19 [37%] of 52 patients in the placebo group) and the median length of intensive care unit and hospital stays (table 2).
The number of adverse events was similar between the two groups (152 events in the IVIG group vs 154 in the placebo group; table 3 ). There was a non-statistically significantly higher number of serious adverse events in the IVIG group (78 events in the IVIG group vs 47 events in the placebo group; table 3; appendix 1 pp 22–23). 22 (32%) patients in the IVIG group had at least one serious adverse event compared with 15 (20%) patients in the placebo group (p=0·089) Three adverse events led to unmasking in the placebo group. There was no difference in the occurrence of ventilator acquired pneumonia between the two groups. However, ten (15%) patients in the IVIG group had deep vein thrombosis compared with three (4%) in the placebo group. Additionally, four of the ten patients in the IVIG group had pulmonary embolism compared with one patients in the placebo group (table 3; appendix 1 pp 22–23).
Table 3.
Adverse events in the safety population
| IVIG group (n=68) | Placebo group (n=76) | ||
|---|---|---|---|
| Any adverse events | 152 | 154 | |
| Patients with at least one adverse event | 51 (75%) | 54 (71%) | |
| Any serious adverse event | 78 | 47 | |
| Patients with at least one serious adverse event* | 22 (32%) | 15 (20%) | |
| Patients with adverse events of special interest | |||
| Ventilator-associated pneumonia† | 28 (41%) | 29 (38%) | |
| Catheter-related infection | 10 (15%) | 8 (11%) | |
| Other infection | 1 (1%) | 3 (4%) | |
| Septic shock | 7 (10%) | 5 (7%) | |
| Acute kidney injury‡ | 15 (22%) | 16 (21%) | |
| Renal replacement therapy | 4 (6%) | 5 (7%) | |
| Deep vein thrombosis or pulmonary embolism§ | 10 (15%) | 3 (4%) | |
| Other¶ | 46 (68%) | 30 (39%) | |
Data are n or n (%). The full detailed list of serious adverse events is available in appendix 1 (p 22) IVIG=intravenous immunoglobulins.
Fisher exact test p value=0·089.
Ventilator-acquired pneumonia was defined by investigators at each centre.
Acute kidney injury was defined according to the Kidney Disease Global Improvement group.
Deep vein thrombosis confirmed by an echography; pulmonary embolism was diagnosed with CT or clinically suspected.
Two patients with A+ blood group developed an immunological haemolysis with elution positive assay in the intravenous immunoglobulins group.
The prespecified subgroup analyses (according to time to randomisation, age, and body-mass index) were consistent with the main result and did not show any beneficial effect of IVIG (figure 3 ; appendix 1 p 18). A post-hoc analysis of mortality showed no statistically significant interaction between the IVIG and corticosteroid administration or body-mass index for the unadjusted mortality odds ratios at day 28 and day 90 (appendix 1 pp 18, 31–32). The sensitivity analysis done on the per-protocol population for both the primary outcome and competing events confirmed the non-significant treatment effect shown by the intention-to-treat analysis (appendix 1 p 19).
Figure 3.
Forest plot of the subgroup analysis of ventilator-free days
Mean difference is reported for the main outcome of ventilation-free days at day 28 for all the patients and for invasive mechanical ventilation time at randomisation, age, survival at day 7, body-mass index, corticosteroid administration, and in the per-protocol population. All subgroup analyses were prespecified except the analysis of patients receiving corticosteroids, which was not prespecified in the initial protocol. BMI=body-mass index.
The median plasma concentration of IL-13 was higher in the IVIG group (7·0 pg/mL [IQR 5·7 to 8·4]) than in the placebo cohort on day 7 (2·7 pg/mL [IQR 1·7 to 3·7]; median difference −4·4 [–6·1 to −2·6]), whereas both IL-6 and TNF-α concentrations were similar between the two groups at all timepoints (appendix 1 p 24). On day 28, the proportion of circulating CD4 T cells that were regulatory T lymphocytes was higher in patients treated in the IVIG group (6% [IQR 5 to 7]) compared with patients in the placebo group (4% [3 to 5]; median difference 2 [IQR 1 to 4]; appendix 1 p 25). Additionally, the proportion of circulating memory T CD4 cells at day 28 was higher in the IVIG group (62% [IQR 60 to 78]) than in the placebo group (41% [33 to 53]; median difference 24 [IQR 7 to 35]; appendix 1 p 25).
Discussion
In this multicentre, randomised, placebo-controlled, phase 3, clinical trial, IVIG did not significantly reduce ventilator-free days at day 28 in patients admitted to the intensive care unit for COVID-19-associated moderate-to-severe ARDS. Across the whole population—including patients older than 65 years, patients who were obese (body-mass index ≥30 kg/m2), and those who had received corticosteroid—IVIG had no effect on the duration of invasive mechanical ventilation and mortality, the two components of the primary endpoint.
Both duration of invasive mechanical ventilation and mortality rate were lower than expected on the basis of available data at the time of the ICAR design (end of March, 2020), which could have lowered the power of this study. However, the decrease in invasive mechanical ventilation duration and mortality indicates that the efficiency of care for patients with COVID-19-associated ARDS has dramatically improved since the start of the pandemic, highlighting the commitment of the participating centres to seek the most up to date treatments. The administration of corticosteroids probably had a beneficial effect on clinical outcomes, including contributing to a reduction in invasive mechanical ventilation duration and overall mortality. Our data suggests that IVIG and corticosteroids do not produce any synergistic effects in COVID-19-associated ARDS. However, one can argue that our study was underpowered and a 4-day reduction in ventilator-free days was too ambitious, suggesting that a benefit from IVIG could not definitively be ruled out.
We hypothesised a reduction in invasive mechanical ventilation duration of 5 days and a 10% mortality rate; we expected a treatment benefit that would legitimise the large-scale use of IVIG considering their cost and shortage. Despite the results showing the absence of benefits of IVIG in patients with COVID-19-associated ARDS, our data might have an effect on public health: IVIG use should be reserved for inflammatory or autoimmune diseases, such as Kawasaki or chronic polyneuropathy. Of note, it has been reported that up to 30% of patients with COVID-19 have been treated with IVIG.20, 21, 22
The limitations of IVIG administration in terms of clinical benefits cannot be ascribed to differences in ARDS severity or management between the two therapeutic groups, including ventilator setting and use of corticosteroids, neuromuscular blocking drugs, prone position, and nitric oxide. Therefore, the absence of an effect of IVIG might result from some detrimental immunological effects. We found that IVIG increased the plasma concentration of IL-13,30 which has been reported to contribute to lung fibrosis by promoting bronchial epithelium inflammation and inadequate repair processes in primitive lung fibrosis.31 We also found that IVIGs were associated with a non-signficant trend towards more frequent serious adverse events, of note thrombotic events that have been mainly reported to the IVIG-induced hyperproteinaemia. The administration of IVIG in four infusions of at least 8 h probably resulted in hyperviscosity, accounting for the higher number of patients reporting thrombosis in the IVIG group.
We have only included patients with COVID-19-associated ARDS who needed invasive mechanical ventilation. However, the criteria for invasive mechanical ventilation changed during the 6-month study period (ie, from April to October, 2020). In this time, non-invasive mechanical ventilation and high-flow nasal oxygen became increasingly used to avoid or delay invasive mechanical ventilation. Therefore, our results can be extended to the population of patients with COVID-19 who require non-invasive mechanical ventilation or high-flow nasal oxygen for moderate-to-severe ARDS. Of note, the median time to initiate invasive mechanical ventilation was 8 days (IQR 6·0–11·0) in the ICAR trial, which is similar to the delay reported in other studies.2, 9 In terms of COVID-19 course and ARDS severity and management, the ICAR cohort is representative of the general population of patients with COVID-19 who developed ARDS, as shown in large observational studies and clinical trials.2, 9, 32
Another possible schedule for administration of IVIG is for it to be administered at different timepoints over the course of COVID-19-associated respiratory failure. We cannot rule out the possibility that IVIG could prevent progression to ARDS if given earlier (eg, in patients with COVID-19-associated pneumonia). Conversely, we found that IVIG induced an increase in circulating regulatory and memory CD4 T lymphocytes at day 28 that could promote the tissue repair processes through a type 2 immune response.33, 34 This finding suggests that IVIG might be beneficial at the recovery phase of ARDS.
Our study shows that IVIG did not significantly improve outcomes and tended to be associated with more adverse events in patients with COVID-19-associated moderate-to-severe ARDS receiving invasive mechanical ventilation. Therefore, IVIG should not be used in this population but reserved for other diseases. The benefit of IVIGs at an earlier stage of COVID-19 related pneumonia should be addressed in future studies.
Data sharing
Anonymous participant data will be available upon completion of the clinical trial and publication of the completed study results upon request to the corresponding author. Proposals will be reviewed and approved by the sponsor, researchers, and staff, on the basis of scientific merit and absence of competing interests. Once the proposal has been approved, data can be transferred through a secure online platform after signing a data access agreement and a confidentiality agreement.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported by Groupe Hospitalier Universitaire Paris Psychiatrie et Neurosciences as the sponsor. This study was funded by a grant from the French ministry of Health (ICAR-PHRC 2020 COVID-19-20-0052) and Laboratoire Français du fractionnement et des Biotechnologies supplied the intravenous immunoglobulins for free. Biological samples were obtained from the Biological Resource Centre NeuroSciences Psychiatriques et Neurologiques, Groupe Hospitalier Universitaire Paris Psychiatrie et Neurosciences, in charge of centralising and managing biological data collection. We acknowledge the use of the Biological Resource Centre NSPN, GHU Paris Psychiatrie et Neurosciences Biobank (BB-0033-00026). We acknowledge the Société Française d'Anesthésie-Réanimation Research Network for supporting the study. We also acknowledge Yvonne Adebola involved as medical writer of the manuscript. We would like also to thank the Groupe Hospitalier Universitaire Paris and its Delegation for Clinical Research and Innovation. We thank Vivianne Awassi, Naoual Khalfi, Yuan Chen, Patricia Wawa, Hayet Sid Idris, and Aurélien Delas. We thank Dylan Messeca for his help in figure drawing and Camille Legouy for her critical reading. We thank Michel Wolff for his counseling role. We also thank the patients and their families for their participation in the study. Finally, we would like to thank Guillaume Turc, Raphael Porcher, Antoine Roquilly, and Franck Verdonk who were members of the data and safety monitoring board.
Contributors
TS, AM, KS, RLM, PA, and BL conceptualised the study. AM and TS coordinated the study. RLM curated the data. AM, TS, MJ, CLa, BL, RLM, and PA did the formal analysis. AM acquired the funding. AM, MJ, BrM, SS, JZ, GM, FS, LA, PC, GM, CB, PA, JR, CV, CS, JA, CG, CLe, BR, PM, SDR, P-LD, CS, AS, MG, BeM, SG, ChL, BL, AM, and KS contributed to the investigation. PA and RLM developed the methods. KS was the project administrator. AM and KS supervised the study. AM. TS, RLM, and KS wrote, reviewed, and edited the original draft. RLM, KS, AM, and TS verified the data. All authors were involved in organisation, coordination, conduct, and technical support of the study; collected data; critically reviewed and approved the manuscript; had full access to all data in the study, vouch for the integrity, accuracy, and completeness of the analysis and its fidelity to the study protocol, and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2021. https://covid19.who.int
- 2.COVID-ICU Group Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouthon L, Kaveri SV, Spalter SH, et al. Mechanisms of action of intravenous immune globulin in immune-mediated diseases. Clin Exp Immunol. 1996;104(suppl 1):3–9. [PubMed] [Google Scholar]
- 11.Kaufman GN, Massoud AH, Dembele M, Yona M, Piccirillo CA, Mazer BD. Induction of regulatory T cells by intravenous immunoglobulin: a bridge between adaptive and innate immunity. Front Immunol. 2015;6:469. doi: 10.3389/fimmu.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 14.Alejandria MM, Lansang MAD, Dans LF, Mantaring JB., 3rd Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD001090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9 doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esen F, Özcan PE, Orhun G, et al. Effects of intravenous immunoglobulin on the course of severe covid-19: results from a retrospective data analysis of a patient cohort in turkey treated with or without octagam®. Research Squared. 2021 doi: 10.21203/rs.3.rs-73448/v1. published online Sept 15. (preprint). [DOI] [Google Scholar]
- 19.Cao W, Liu X, Hong K, et al. High-Dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.627844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. J Emerg Med. 2020;58:711–712. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle JG. COVID-19 and the Threat to Immunoglobulin Availability. 2020. https://primaryimmune.org/news/covid-19-and-threat-immunoglobulin-availability
- 24.Mazeraud A, Gonçalves B, Aegerter P, et al. Effect of early treatment with polyvalent immunoglobulin on acute respiratory distress syndrome associated with SARS-CoV-2 infections (ICAR trial): study protocol for a randomized controlled trial. Trials. 2021;22:170. doi: 10.1186/s13063-021-05118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 26.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 28.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 29.Rosner B, Glynn RJ, Lee M-LT. Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics. 2003;59:1089–1098. doi: 10.1111/j.0006-341x.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 30.Tjon ASW, van Gent R, Jaadar H, et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J Immunol. 2014;192:5625–5634. doi: 10.4049/jimmunol.1301260. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd CM, Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat1604. [DOI] [PubMed] [Google Scholar]
- 34.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous participant data will be available upon completion of the clinical trial and publication of the completed study results upon request to the corresponding author. Proposals will be reviewed and approved by the sponsor, researchers, and staff, on the basis of scientific merit and absence of competing interests. Once the proposal has been approved, data can be transferred through a secure online platform after signing a data access agreement and a confidentiality agreement.