Abstract
Background and Purpose
In this study we evaluated the relationship between amyloid-beta (Aβ) deposition and 3 aspects of sleep quality in a group of clinically diagnosed Alzheimer's disease (AD) patients.
Methods
We used self-report questionnaires to assess the quality of sleep using 3 previously established surveys: the Glasgow Sleep Effort Scale (GSES), the Pittsburgh Sleep Quality Index (PSQI), and the Morningness-Eveningness Questionnaire (MEQ). These questionnaires focused on the sleep effort, sleep efficiency, and circadian rhythm patterns of each participant. Also, we evaluated the regional distribution of Aβ in the brain by amyloid positron emission tomography-computed tomography (PET-CT) standardized uptake value ratios (SUVRs) in healthy normal (HN), mild cognitive impairment (MCI), and AD dementia groups. The MCI and AD dementia groups were combined to form the group with cognitive impairment due to AD (CIAD).
Results
GSES and MEQ scores differed significantly between the HN, MCI, and AD dementia groups (p<0.037), whereas PSQI scores were similar across the groups (p=0.129). GSES and MEQ scores also differed between the HN and CIAD groups (p<0.018). Circadian rhythm scores positively correlated with amyloid PET-CT SUVR in posterior cingulate cortices (p<0.049).
Conclusions
Sleep effort and abnormal shifts in circadian rhythm were more significant in the CIAD group than in the HN group. At the same time, HN subjects had minimal sleep disturbance, irrespective of clinical status. Thus, alterations in circadian rhythm may be indicative of neurodegeneration due to Aβ deposition.
Keywords: Alzheimer's Disease, Amyloid Plaques, Sleep, Mild Cognitive Impairment, Sleep Quality
INTRODUCTION
Alzheimer's disease (AD) is one of the most prevalent neurodegenerative diseases.1 Although it is associated with various clinical risk factors, recent studies have suggested a possible correlation between sleep disruption and the outbreak of AD. AD patients often complain about fragmented sleep or struggling to fall asleep.2,3,4
Aggregation of insoluble amyloid-beta (Aβ) peptide in various brain regions is a hallmark of AD.5 Clinical studies have reported the association between Aβ burden and poor sleep.2,6,7,8 In addition, reduced glymphatic cerebrospinal fluid (CSF) inflow into the brain during awakening results in an insufficient exchange of CSF with interstitial fluid, raising the risk that neurodegenerative disease may occur.3 Poor sleep quality detected by self-reported sleep parameters showed a close relationship with amyloid burden in various cohorts.9,10
Amnestic mild cognitive impairment (MCI) is thought to represent a preclinical stage of AD.11,12 MCI patients revealed physiological sleep abnormalities than did controls; these included less time in slow-wave sleep and lower delta and theta power during sleep, which may interfere with sleep-dependent memory consolidation.13 However, although it is well known that the cognitive decline in MCI can cause AD, the role of sleep in amnestic MCI and its relationship to the Aβ burden is not well understood.14,15,16
In this study we investigated whether sleep quality—measured by self-report sleep questionnaires—differs between healthy normal subjects (HN) and patients with MCI or AD dementia itself. Moreover, we tried to find a correlation between sleep quality and patterns of Aβ aggregation in different brain regions.
METHODS
Participants
We prospectively recruited patients from 2016 to 2018 at a tertiary university hospital. All tests and interviews were done with the patient's or a guardian's informed consent (IRB No. HY 2016-12-029). We distributed the participants into HN (n=21), MCI (Amnestic or non-amnestic MCI; n=49), and AD dementia (n=26) groups. HN subjects were validated by neuropsychological testing, magnetic resonance imaging (MRI), and an amyloid positron emission tomography-computed tomography (PET-CT) scan to assess normal amyloid deposition throughout the brain. The MCI and AD dementia groups were collectively assigned to the group with cognitive impairment due to AD (CIAD). We classified MCI patients according to the diagnostic scheme of MCI, which is also known to be the preclinical phase of AD.17 We additionally sorted out MCI patients with positive amyloid deposition by amyloid PET-CT. We selected AD dementia patients based on the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association Alzheimer's criteria.18 For dementia screening, we examined the participants using the standard Korean version of the Mini-Mental State Examination and assigned Korean Clinical Dementia Rating Sum of Box (CDR-SB) scores.19,20 We evaluated structural criteria for diagnosis by analyzing MRI.
Sleep questionnaire
We asked patients to take 3 different questionnaires about their sleep quality. The Glasgow Sleep Effort Scale (GSES), the Pittsburgh Sleep Quality Index (PSQI), and the Morningness-Eveningness Questionnaire (MEQ), are self-report questionnaires to assess the quality of sleep. These questionnaires focused on each participant's sleep effort, sleep efficiency, and circadian rhythm patterns. We used the Korean translation of each questionnaire. The 7-item GSES measures the level of anxiety triggered by not being able to control sleep habits.21 The PSQI measures various aspects of an individual's sleep quality in the preceding 4 weeks, including sleep duration and how sleep problems affect lifestyle.22 The MEQ identifies each participant's circadian-rhythm type by considering their preferred wake-up and bedtime, as well as how they felt during their most active hours of the day.23
Amyloid PET-CT imaging
Each participant underwent amyloid PET-CT imaging with fluorine-18 (18F) florbetaben. Images were acquired 90 minutes after intravenous injection of 300±3.75 MBq 18F-florbetaben. The parameters for image acquisition were as follows: matrix size=336×336; Gaussian filter, full-width at half-maximum=2.0; zoom=2.0; and 1 bed per 20 minutes. Three-dimensional images were reconstructed with CT-based attenuation correction and 6 iteration steps with 8 subsets. We used the Syngo.via server and client software v.04.01.0000.0001 (Siemens Healthcare GmbH, Erlangen, Germany) to obtain mean standardized uptake value (SUVmean) on bilateral frontal, temporal, parietal, and posterior cingulate cortices (PCC) of the brain using its automatic recognition and drawing feature for those 8 different regions as the volume of interest. Then, we calculated each SUV ratio (SUVR) for the 8 brain regions with cerebellar SUVmean as the reference in a hemisphere-dependent fashion (e.g., left frontal SUVmean compared to left cerebellar SUVmean).24 we compared these SUVRs across the 3 clinical groups (the HN, MCI, and AD dementia groups).
Data analysis
We used descriptive statistics to analyze the background of participants in each group. We used a 1-way analysis of variance test and Kruskal-Wallis test to detect the presence of statistically significant differences in age, sex, and education level across the clinical groups. We also used the Kruskal-Wallis test to evaluate the statistical significance of sleep questionnaire scores across the groups. We assessed the difference between the HN group and the CIAD group by the Mann-Whitney U test. Finally, we evaluated the relationship between sleep quality and the abundance of Aβ plaques by using Spearman's Rho tests. We analyzed data using IBM SPSS statistics v.22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of the study population
Of the 96 elderly participants in this study (mean age: 68.2±10.7 years), 21.9% were considered to be HN, 51.0% to have MCI, and 27.1% to have AD dementia; 46% were male and 54% were female (n=96). Demographic information of each group is described in Table 1. The groups showed statistically significant differences in age, education level, and sex ratio (p<0.001, p<0.001, and p=0.025, respectively). MMSE and CDR-SB differed significantly between the groups (p<0.001), consistent with AD clinical criteria.
Table 1. Sex, age, education level, and MMSE and CDR-SB scores in the study population*.
| Variables | HN | MCI | AD dementia | p-value |
|---|---|---|---|---|
| Sex ratio (male:female) | 15:6 | 20:29 | 9:17 | 0.025† |
| Age (yr) | 60±9.33 | 68±10.04 | 75±8.39 | <0.001† |
| Education (yr) | 20±4.75 | 12±5.89 | 10±5.61 | <0.001† |
| MMSE | 29±1.80 | 26±3.44 | 19±6.41 | <0.001† |
| CDR-SB | 0.12±0.27 | 2.77±4.73 | 4.5±5.24 | 0.004† |
Data are shown as mean±standard deviation.
MMSE: Mini-Mental State Examination, CDR-SB: Korean Clinical Dementia Rating Sum of Box, HN: healthy normal, MCI: mild cognitive impairment, AD: Alzheimer's disease.
*Total number of patients: 96 (Hanyang University Seoul Hospital); †Mean difference is significant at the 0.05 level.
Sleep quality in study subjects
Table 2 presents the correlations of the 3 sleep questionnaires between the different groups. GSES and MEQ scores differed significantly between the groups of HN, MCI, and AD dementia subjects (p=0.037 and p=0.005, respectively). In contrast, there were no differences in PSQI scores (Fig. 1). A comparison of mean scores in each of the 3 tests between the groups showed that HN subjects had the lowest mean scores; MCI patients had moderate scores, and AD dementia patients had the highest scores (GSES: 1.762±1.79, 3.306±3.20, and 4.885±4.79, respectively; PSQI: 4.571±1.69, 5.286±3.12, and 7.115±4.58, respectively; and MEQ: 54.048±5.71, 59.306±8.79, and 60.653±6.67, respectively). When participants were sorted into 2 groups based on the amyloid burden, GSES and MEQ scores still showed statistically significant differences, whereas PSQI scores did not (Fig. 2).
Table 2. Significance of differences in results from 3 sleep questionnaires among clinical groups.
| Variables | HN | MCI | AD dementia | p-value* | CIAD | p-value† |
|---|---|---|---|---|---|---|
| GSES | 1.762±1.79 | 3.306±3.20 | 4.885±4.79 | 0.037* | 3.853±3.87 | 0.018† |
| PSQI | 4.571±1.69 | 5.286±3.12 | 7.115±4.58 | 0.129 | 5.920±3.76 | 0.333 |
| MEQ | 54.048±5.71 | 59.306±8.79 | 60.653±6.67 | 0.005* | 59.773±8.10 | 0.002† |
Data are shown as mean±standard deviation.
GSES: Glasgow Sleep Effort Scale, PSQI, Pittsburgh Sleep Quality Index, MEQ: Morningness-Eveningness Questionnaire, HN: healthy normal, MCI: mild cognitive impairment, AD: Alzheimer's disease, CIAD: cognitive impairment due to Alzheimer's disease.
*Kruskal Wallis test (grouping variable: HN, MCI, and AD dementia). Mean difference is significant at the 0.05 level; †Mann-Whitney U test (grouping variable: HN, CIAD). Mean difference is significant at the 0.05 level.
Fig. 1. The mean score of sleep effort, sleep disturbance, and the types of circadian rhythm differed in the stages of AD. (A) GSES mean scores of the normal, MCI, and AD groups increased as indicated: 1.762±1.79; 3.306±3.20; 4.885±4.79, respectively. (B) PSQI mean scores showed the same trend as did GSES: 4.429±1.50, 5.286±3.12, 7.154±4.54, respectively. (C) MEQ mean scores of normal, MCI, and AD groups showed lower scores in normal subjects, but higher score in the MCI and AD groups (m=54.048±5.71, 59.306±8.79, and 60.653±6.67, respectively).
HN: healthy normal, MCI: mild cognitive impairment, AD: Alzheimer's disease, GSES: Glasgow Sleep Effort Scale, PSQI: Pittsburgh Sleep Quality Index, MEQ: Morningness-Eveningness Questionnaire.
Fig. 2. The levels of sleep effort and sleep disturbance were higher in the CIAD group than in the healthy group. (A) GESE mean scores of healthy and CIAD group were 1.762±1.79 and 3.853±3.87, respectively; a similar trend was exhibited in PSQI mean score, 4.429±1.50 and 5.933±3.75, respectively. (B) MEQ mean scores of the healthy and CIAD groups were 54.048±5.705 and 59.773±8.10, respectively.
GSES: Glasgow Sleep Effort Scale, PSQI: Pittsburgh Sleep Quality Index, MEQ: Morningness-Eveningness Questionnaire, CIAD: cognitive impairment due to Alzheimer's disease.
Relationship between sleep quality and Aβ accumulation
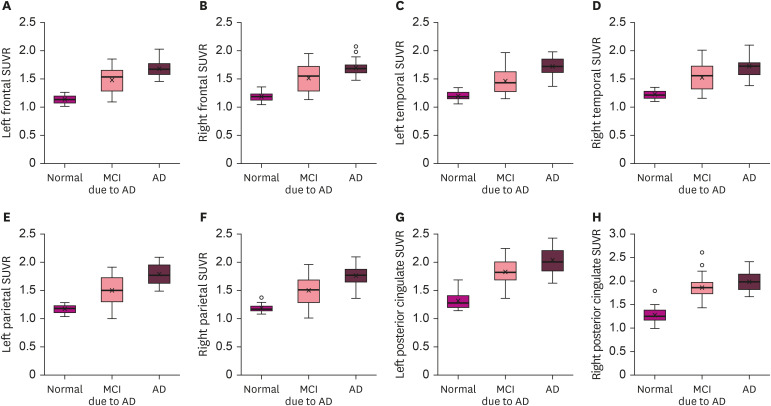
SUVRs derived from the amyloid PET-CT results differed between the HN, MCI, and AD dementia groups (p<0.038; Fig. 3). In addition, we tried to find out the correlation between individual sleep questionnaire scores and each SUVR for different brain regions. Although not all questionnaire scores were statistically correlated to brain scan results, there was a weak correlation between MEQ scores and Aβ deposition within the bilateral PCC (p<0.05) (Table 3).
Fig. 3. Regional differences in SUVR in the brains of HN subjects, MCI, and AD patients. The 8 regional SUVR scores showed an increasing trend from HN to MCI to AD. (A-H) SUVRs measured in the left (A) and right (B) frontal lobes; left (C) and right (D) temporal lobes; left (E) and right (F) parietal lobes; and left (G) and right (H) posterior cingulate cortices.
SUVR: standardized uptake value ratio, MCI: mild cognitive impairment, AD: Alzheimer's disease, HN: healthy normal.
Table 3. Spearman's rho (ρ) correlation between sleep questionnaire scores and beta-amyloid deposition in different brain regions.
| Variables | Frontal lobe | Temporal lobe | Parietal lobe | Cingulate cortex | |||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | ||
| GSES | |||||||||
| ρ | 0.166 | 0.117 | 0.196 | 0.173 | 0.134 | 0.090 | 0.179 | 0.161 | |
| Significance (2-tailed) | 0.106 | 0.257 | 0.056 | 0.091 | 0.194 | 0.384 | 0.082 | 0.118 | |
| PSQI | |||||||||
| ρ | 0.086 | 0.057 | 0.121 | 0.087 | 0.124 | 0.111 | 0.034 | 0.052 | |
| Significance (2-tailed) | 0.407 | 0.582 | 0.240 | 0.401 | 0.227 | 0.283 | 0.741 | 0.616 | |
| MEQ | |||||||||
| ρ | 0.219 | 0.201 | 0.199 | 0.194 | 0.240 | 0.197 | 0.261* | 0.275† | |
| Significance (2-tailed) | 0.052 | 0.059 | 0.052 | 0.059 | 0.068 | 0.054 | 0.010 | 0.007 | |
GSES: Glasgow Sleep Effort Scale, PSQI: Pittsburgh Sleep Quality Index, MEQ: Morningness-Eveningness Questionnaire.
*Correlation is significant at the 0.05 level (2-tailed); †Correlation is significant at the 0.01 level (2-tailed).
DISCUSSION
The extracellular aggregation of Aβ peptide is a hallmark of AD; it leads to neurodegeneration and sleep disorders.7 A previous study reported that shorter sleep duration and poorer sleep quality are associated with greater Aβ burden among community-dwelling older adults.9 An association of sleep and the clearance of Aβ plaques has previously been established; it was partly explained by the glymphatic system's action to clear the waste proteins during sleep.3 We hypothesized that neurodegeneration due to Aβ deposition could be related to the circadian rhythm.
We found that patients with AD dementia reported higher stress and anxiety resulting from sleeplessness than did HN subjects. In addition, the abnormal early-morning awakenings were frequent in the AD dementia subjects, as is consistent with the previous study, demonstrating that circadian-rhythm changes are expected in AD patients.25
Significant differences in GSES scores between the HN, MCI, and AD dementia groups suggest that individuals without AD pathology had no difficulty falling asleep. In contrast, AD dementia patients reported higher stress and anxiety when they had no control over falling asleep. The same trend was observed in the score between the HN group and the CIAD, suggesting that the presence of clinically diagnosed AD pathology is associated with greater sleep effort. Because sleep is an involuntary physiological process, any “efforts to sleep” are likely to fail and exacerbate insomnia for AD dementia patients.21 This problem may contribute to the etiology of AD, resulting in a rapid progression of the disease.7
Morningness-eveningness scores measured by MEQ differed significantly between HN, MCI and AD dementia subjects, indicating that each group is characterized by distinct sleep patterns. Most of the HN subjects showed an intermediate-type sleep pattern, whereas MCI and AD dementia patients were of the morning type. Disruptions in circadian rhythm have been apparent among AD dementia patients, suggesting that the deposition of Aβ peptide in specific brain regions might affect circadian-rhythm regulation even at preclinical stages.25,26,27 Our findings support this model, because patients that experience neuropathological changes show a shift of the circadian cycle to the morning type.
Although past studies showed that AD dementia patients experienced some sleep quality changes in sleep duration and frequent discontinuation of sleep, our data showed that the 3 different groups (HN subjects, MCI, and AD dementia patients) had relatively similar sleep quality as measured by PSQI.7,28,29 Longer sleep latency was associated with a more significant Aβ burden in prefrontal areas in asymptomatic middle-aged participants and older adults. The negative correlation between nocturnal awakenings and gray-matter volume in the insular region was also reported.10 Moreover, lower self-reported sleep quality was associated with greater Aβ burden and lower volume in brain areas relevant in aging and AD.10 PSQI generally covers various categories of sleep quality, which might have generated an inconclusive result. Failing to show significance in the composite measure of sleep efficiency in PSQI does not detract from the fact that specific surveys, such as of sleep effort and circadian rhythm, showed statistically significant differences.
We observed a positive correlation between MEQ scores and regional differences in SUVRs from the amyloid PET-CT scans. Increased levels of insoluble Aβ neurofibrils in the PCC may be the indicative marker of the abnormal function of the sleep-wake cycle, which often occurs with fragmented sleep.26,28 Our findings are biologically relevant, since the anatomical coordinates of Aβ deposits and circadian alterations were ascertained in clinically diagnosed AD dementia patients. The PCC is part of the medial prefrontal system, which represents a novel brain system of the default-mode network.30 Some studies have shown that the brain's default- network activity was lowered during the awake time, whereas PET images showed a high metabolism rate in the PCC in the rested brain.31,32 Imbalance in regulating the brain's default system attributable to the posterior cingulate hypometabolism can be consistent with our finding that abnormal sleep-cycle shifting proven by MEQ correlates with the degree of PCC affected by Aβ deposits. This finding can support the previous study revealing the association between shorter sleep duration and Aβ burden.9 Future studies would be valuable to reveal the correlation of alteration in circadian rhythm and molecular pathomechanisms caused by Aβ depositions.
There are several limitations to this study. First, we did not consider covariates, such as age, education years, and sex, which showed statistically significant differences between the 3 groups. The bias of having older individuals in the AD dementia group was anticipated because of the known association between AD progression and aging.33,34 In addition, education level in the HN group was higher than in the AD dementia group, as is consistent with the reported indirect relationship between educational level and incidence of AD.34 Sex distribution was not as significant as the age or the education level.
Second, we used only subjective measures of sleep quality (self-report sleep questionnaires). Moreover, subjective data collected in patients with cognitive deficits can arouse doubt about reliability. Including polysomnography or actigraphy in the assessment could have provided a more objective measure of sleep disruption or sleep/wake patterns. Also, we relied solely on amyloid PET-CT scans to diagnose AD pathology. Analyses of insoluble Aβ peptide and tau protein levels in the cerebral spinal fluid may have been more accurate in measuring AD pathology.
Next, a longitudinal study could be used to observe the worsening of sleep quality in AD dementia groups. Also, we did not measure the individual progression of sleep problems but focused on a cross-sectional study using the 3 groups in PSQI analysis. We analyzed only the final scores of the PSQI to measure poor sleep from each group. The difference in data analysis would have resulted in the opposite conclusion, since the previous study classified PSQI into 7 different sections, analyzing these scores individually.7
Last, the 3 administered tests (GSES, PSQI, and MEQ) had different score ranges; normalization of the scores is required to improve statistical analyses combined with SUVRs from PET-CT images.
Despite these limitations, our results suggest that alterations in circadian rhythm may have a close relationship with neurodegeneration due to Aβ burden. Increased sleep effort is a co-occurring problem in the preclinical and clinical stage of AD. Also, disturbance in circadian rhythm increases with Aβ plaque abundance in specific regions of the brain. The mean scores from each questionnaire indicated that AD dementia patients are more likely to experience poor-quality sleep than are HN subjects. We identified the correlation between circadian rhythm and the level of Aβ in the PCC, indicating a possible explanation for the altered sleep patterns of AD dementia patients. The biological significance of the disturbed circadian cycle observed in AD dementia patients can be further addressed by examining the production of hormones that regulate the sleep cycle and their relationship to excessive Aβ aggregation in specific brain regions.
Footnotes
Funding: This research was supported by a grant from the Eisai Korea.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim HJ.
- Data curation: Kim HJ.
- Formal analysis: Kang J, Choi HJ, Kim HJ.
- Funding acquisition: Kim HJ.
- Investigation: Kang J, Kim HJ.
- Methodology: Kang J, Kim HJ.
- Project administration: Kang J, Issacs GD, Kim HJ.
- Resources: Kang J, Kim HJ.
- Software: Kang J.
- Supervision: Sung W, Issacs GD, Kim HJ.
- Validation: Sung W, Kim HJ.
- Visualization: Kang J, Sung W.
- Writing - original draft: Kang J, Kim HJ.
- Writing - review & editing: Sung W, Issacs GD, Kim HJ.
References
- 1.Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargos EF, Pandolfi MB, Dias MP, Quintas JL, Guimarães RM, Nóbrega OT. Incidence of sleep disorders in patients with Alzheimer disease. Einstein (Sao Paulo) 2011;9:461–465. doi: 10.1590/S1679-45082011AO2145. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP, LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucey BP, Bateman RJ. Amyloid-β diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging. 2014;35(Suppl 2):S29–S34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, et al. The relationship between sleep quality and brain amyloid burden. Sleep. 2016;39:1063–1068. doi: 10.5665/sleep.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 13.Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolova LG, Thompson PM. Mapping progressive brain structural changes in early Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2008;46:1597–1612. doi: 10.1016/j.neuropsychologia.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98:653–665. doi: 10.1016/B978-0-444-52006-7.00041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarci K. Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer's disease. Front Aging Neurosci. 2014;6:316. doi: 10.3389/fnagi.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study) Arch Gerontol Geriatr. 2008;47:302–310. doi: 10.1016/j.archger.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 21.Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14:401–407. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 23.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 24.Bullich S, Villemagne VL, Catafau AM, Jovalekic A, Koglin N, Rowe CC, et al. Optimal reference region to measure longitudinal amyloid-β change with 18F-Florbetaben PET. J Nucl Med. 2017;58:1300–1306. doi: 10.2967/jnumed.116.187351. [DOI] [PubMed] [Google Scholar]
- 25.Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulkava S, Muggalla P, Sulkava R, Ollila HM, Peuralinna T, Myllykangas L, et al. Melatonin receptor type 1A gene linked to Alzheimer's disease in old age. Sleep (Basel) 2018;41:zsy103. doi: 10.1093/sleep/zsy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep (Basel) 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Veasey SC, Wood MA, Leng LZ, Kaminski C, Leight S, et al. Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer's disease with injury to pedunculopontine cholinergic neurons. Am J Pathol. 2005;167:1361–1369. doi: 10.1016/S0002-9440(10)61223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 31.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sämann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, et al. Development of the brain's default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21:2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 33.Manuel DG, Garner R, Finès P, Bancej C, Flanagan W, Tu K, et al. Alzheimer's and other dementias in Canada, 2011 to 2031: a microsimulation Population Health Modeling (POHEM) study of projected prevalence, health burden, health services, and caregiving use. Popul Health Metr. 2016;14:37. doi: 10.1186/s12963-016-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]