Abstract
Background
The Covid-19 pandemic has had dramatic consequences on the progression of numerous pathologies, especially neoplastic ones. The orientation of hospital activities toward the care of patients with SARS-Cov2 infection has caused significant delays in the diagnosis and therapy of many other pathologies. What about severe hypercalcemia? The aim of this work was to determine the clinical and biological presentation, etiologies, mortality, and the impact of the Covid-19 pandemic on severe hypercalcemia.
Material and methods
we conducted a retrospective study for 84 months (September 2014 to September 2021) at the Nephrology Unit in University Hospital Mohammed VI, Oujda, Morocco. Included were all adult patients diagnosed with severe hypercalcemia (defined as corrected total serum calcium of >3.5 mmol/l or > 14.0 mg/dl) and who had benefited from one or more hemodialysis sessions.
Results
66 episodes of severe hypercalcemia occurred in 64 patients. The mean age was 57 ± 15 years and 57.6% were female. The mean corrected serum calcium at admission was 16.9 ± 2.1 mg/dl and 33.3% had more than 18.0 mg/dl. Malignancies represented 80.4% of all etiologies. Acute kidney injury was observed in 69.7%. The delta drop in serum calcium 48 h after initiation of medical treatment was 4.64 ± 1.63 mg /dl. Mortality was noted in 14% of all cases. Electrocardiographic abnormalities were observed in 58.3%, 87.5% and 85.7%, respectively, in group 1 (14.0–16.0 mg/dl), group 2 (16.1–18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p = 0.04). The mean serum potassium value was 5.1 ± 1.3, 4.0 ± 1.0, and 3.7 ± 0.7 respectively, in group 1 (14.0–16.0 mg/dl), group 2 (16.1–18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p < 0.001). Newly diagnosed neoplasia, severe hypercalcemia (> 16.0 mg/dl), and mortality have been observed in 15.4% vs. 23.7% (p = 0.31), 25% vs. 50% (p = 0.03), and 35.7% vs. 52.6% (p = 0.13) respectively, in patients before and during the Covid-19 pandemic.
Conclusions
The Covid-19 pandemic caused an increase in both the incidence and severity of hypercalcemia and the hemodialysis practiced in this context remains efficient and safe.
Keywords: Severe hypercalcemia, Covid-19 pandemic, Neoplasia, Mortality, Acute kidney injury, Acute hemodialysis
1. Introduction
Severe hypercalcemia, also called hypercalcemic crisis or malignant hypercalcemia, is serious electrolyte disturbance and life-threatening condition. Its incidence remains low and does not exceed 1% of all patients admitted to emergency departments [1]. Habitually, we divide hypercalcemia into mild hypercalcemia with serum calcium levels of <12.0 mg/dl, moderate hypercalcemia with serum calcium levels between 12.0 mg/dl and 14.0 mg/dl, and severe hypercalcemia with serum calcium levels above 14.0 mg/dl [2]. In adults, malignancies seem to be the main etiologies of severe hypercalcemia, with primary hyperparathyroidism and other types of endocrinopathy as the second cause, followed by less frequent causes, such as iatrogenic etiologies and granulomatous diseases [3]. The most serious complications of severe hypercalcemia that can be life-threatening to patients are cardiovascular, neurological, and kidney complications. Acute kidney injury (AKI) is a frequent complication of severe hypercalcemia and is usually functional, secondary to hypovolemia, itself linked to polyuria caused by hypercalcemia and hypercalciuria, or acute tubular necrosis caused by a nephrotoxic drug, an iodinated contrast agent, associated sepsis or direct tubular toxicity as is the case with light chains in multiple myeloma. The use of renal replacement therapy (RRT) is often necessary to immediately and effectively correct the hypercalcemia, uremia, and electrolyte disturbances related to AKI. In the current setting of the Covid-19 pandemic, we can assume that the incidence of severe hypercalcemia has increased. Although, since the start of the pandemic, neoplasia from all causes has been a high priority alongside Covid-19 patients, dramatic consequences have occurred.
Indeed, severe hypercalcemia, due to the unavailability of injectable calcitonin in many countries, is increasingly an indication for acute hemodialysis, and its incidence probably increased during the period of the Covid-19 pandemic. The discontinuation of anticancer treatment, the delay in the management of patients with cancer disease, the delay in diagnosis of cancer disease, the fear of patients of being infected by attending hospital structures, the orientation of hospital activities toward Covid-19 activities, and the difficulty of moving patients between regions and cities could explain the increase in cases of severe hypercalcemia during this pandemic. The aggravation of the epidemiological situation during the occurrence of several Covid-19 epidemiological waves in Morocco has contributed to the emergence of severe hypercalcemia. Very few studies have been published on severe hypercalcemia and no studies have been published on the impact of the pandemic on severe hypercalcemia [[3], [4], [5]]. The aim of this study was to determine the clinical and biological presentation, etiologies, organ complications, kidney function, therapeutic methods, mortality, and the impact of the Covid-19 pandemic on the incidence of severe hypercalcemia.
2. Materials and methods
We conducted a retrospective study for 84 months (September 2014 to September 2021) at the Nephrology, Dialysis, and Kidney transplantation Unit in University Hospital Mohammed VI, Oujda, in eastern Morocco, North Africa. Included were all adult patients (> 16 years) diagnosed with severe hypercalcemia that was defined as corrected total serum calcium of ≥ 3.5 mmol/l (≥14.0 mg/dl) and who had benefited from one or more hemodialysis sessions in the acute dialysis unit, whatever the level of kidney function. The total seum calcium was corrected according to the following formula: calcemia measured mg/l + (40 - Blood level of serum albumin g/l). Acute kidney injury (AKI) was defined using the KDIGO (Kidney Disease Improving Global Outcomes) criteria according to the level of increase in blood creatinine levels and urine output, and all patients were classified stage 3 “Failure” because they all underwent a hemodialysis session regardless of the serum creatinine value [6]. Ethics Committee approval and informed consent were not needed because the study was observational and retrospective and the need for written informed consent was waived due to the retrospective nature of this research. The study was performed with absolute respect for international ethical rules, anonymity, and data protection.
3. Case presentation
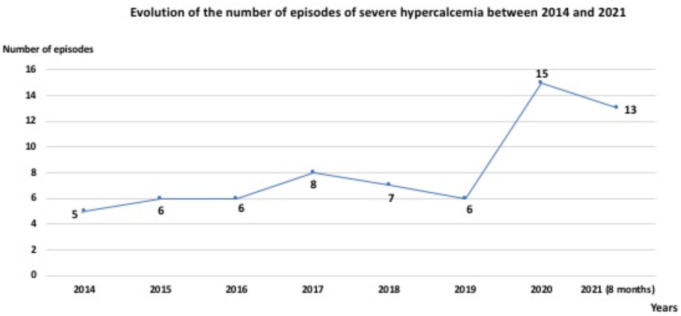
During the study period, 197 patients were admitted to emergency and various medical units for hypercalcemia with corrected total serum calcium greater than 3 mmol/L (12.0 mg/dl) and among them, 66 (33.5%) episodes of severe hypercalcemia occurred in 64 patients. 62.2% of severe hypercalcemia cases were recorded during the last four years (2018–2021) vs. 37.8% recorded during the first four years (2014–2017) of the study and 42.4% (28 cases) of the 66 cases collected were recorded during the two years of Covid-19 pandemic (2020,2021). Fig. 1 shows the evolution of the number of cases of severe hypercalcemia between 2014 and 2021.
Fig. 1.
Evolution of the number of episodes of severe hypercalcemia between 2014 and 2021.
The 66 severe hypercalcemia episodes occurred in 64 patients. The mean age of patients was 57 ± 15 years with extremes of 16 and 88 years and 57.6% were female. The mean of corrected total serum calcium was 16.9 ± 2.1 mg/dl with extremes of 14.0 and 2.32 mg/dl. 56% of patients had hypokalemia (serum potassium <3.5 mmol/L).
Malignancies represented 80.4% of all etiologies. Hematologic diseases such as multiple myeloma, lymphoma, and leukemia occupied the first position (41%) followed by solid organ cancer, including breast, stomach, brain, lung, and pancreatic cancers (39.4%). Multiple myeloma alone represented 36.4% of all cases. Primary hyperparathyroidism was observed in 6 cases, two of whom had multiple endocrine neoplasias. Hard-water syndrome was observed in three cases of patients who were on chronic hemodialysis. All patients received intravenous rehydration (except patients under chronic hemodialysis) according to their volume and heart condition, intravenous glucocorticoids, and all of them received bisphosphonates. The bisphosphonates (zoledronic acid) were used in a full single dose in patients with estimated glomerular filtration rate (GFR) > 30 ml/min/1.73 m2 and reduced by half in patients who presented acute kidney injury or chronic kidney disease with an estimated GFR < 30 ml/min/1.73m2 or under chronic hemodialysis. No side effects have been observed with the use of reduced doses of zoledronate in patients with kidney impairment. No patient received salmon calcitonin subcutaneously because it was not available in our country. Oral calcimimetic drugs were not used in our emergency setting. Intravenous rehydration is immediately started in all patients as soon as hypercalcemia is diagnosed. The use of renal replacement therapy (RRT) with low calcium dialysate (1.25 mmol/l) was performed in all cases. Each patient had at least two hemodialysis sessions. The first hemodialysis session was performed within six hours after the patient's admission. The second hemodialysis session was performed between 12 and 24 h later. No death or major incident was observed during the hemodialysis sessions, notably no heart rhythm disturbances, cardiac arrest, disturbances of consciousness, or severe hypotension occurred. Intravenous rehydration was maintained during hemodialysis sessions. The delta drop in serum calcium observed 48–72 h after initiation of medical treatment was 4.71 ± 1.63 mg /dl. No patient had a kidney biopsy during hospitalization. 67.5% of patients with acute kidney failure had fully recovered normal kidney function within 72 h of admission. In-hospital mortality was observed in 14% of all cases. Death occurred after a significant drop in serum calcium and was not directly related to hypercalcemia. Clinical and biological parameters, etiologies, management, and mortality of all episodes of severe hypercalcemia are reported in Table 1 .
Table 1.
Clinical and biological parameters, etiologies, management, and mortality of patients admitted for severe hypercalcemia
| Parameters (n = 66) | N (%) |
|---|---|
| Age, years #⁎ | 57 ± 15 |
| Age, groups | |
| < 45 years | 10 (15.2) |
| 45–65 years | 30 (45.4) |
| > 65 years | 26 (39.4) |
| Female gender | 38 (57.6) |
| Medical history | |
| Type 2 diabetes | 12 (18.2) |
| Arterial hypertension | 11 (16.7) |
| End-stage renal disease under dialysis | 08 (12.1) |
| Neoplasia | 20 (30.3) |
| Clinical presentation | |
| Neurologic symptoms (disorientation, confusion, coma..) | 25 (37.9) |
| Digestive symptoms (nausea, vomiting, abdominal pain..) | 52 (78.8) |
| Bone pain | 40 (60.6) |
| Urinary stones | 15 (22.7) |
| Oligoanuria (< 400 ml/ 24 h) | 24 (36.4) |
| Arterial hypotension (systolic blood pressure < 100 mmHg) | 38 (57.6) |
| Electrocardiographic abnormalities (shortening of the QT interval, heart block..) | 49 (74.2) |
| Acute kidney injury (chronic kidney disease not included) | 46 (69.7) |
| Serum creatinine, mg/dl⁎ (chronic kidney disease under dialysis not included) | 2.2 [1.6, 6.9] |
| Serum creatinine (mmol/l)⁎ (chronic kidney disease under dialysis not included) | 194 [140, 607] |
| Serum albumin, g/l # | 31.4 ± 5.5 |
| Corrected total serum calcium, mg/dl # | 16.9 ± 2.1 |
| Corrected total serum calcium, mmol/l # | 4.22 ± 0.52 |
| Corrected total calcium groups, mg/dl | |
| 14.0–16.0 | 26 (39.4) |
| 16.1–18.0 | 18 (27.3) |
| 18.1–19.9 | 16 (24.2) |
| ≥ 20.0 | 06 (9.1) |
| Serum hemoglobin, g/dl # | 9.5 ± 2.6 |
| Serum potassium, mmol/l # | 4.3 ± 1.2 |
| Serum potassium <3.5 mmol/L | 25 (37.8) |
| Serum bicarbonates, mmol/l # | 17.2 ± 3.5 |
| Etiology of severe hypercalcemia | |
| Humoral hypercalcemia of malignancy | 27 (41.0) |
| Multiple bone metastases and solid cancer (breast, uterus, pancreas, kidney, lungs,..) | 26 (39.4) |
| Primary hyperparathyroidism | 06 (9.1) |
| Hard water syndrome | 03 (4.5) |
| Others (tuberculosis, acute toxicity…) | 04 (6.0) |
| Duration of the first dialysis session, minutes | 151 ± 27 |
| Corrected total serum calcium after 48 h of treatment, mg/dl # | 12.7 ± 2.5 |
| Delta drop in corrected total serum calcium, mg/dl | 4.71 ± 1.63 |
| In-hospital mortality | 9 (14) |
variables expressed by median, interquartiles.
variable expressed by mean and Standard type.
The mean age was respectively 65.3 ± 9.6 vs 55 ± 15.0 years in deceased and non-deceased patients (p = 0.02). Mortality was observed in 30.8% vs. 13.2% respectively, in groups of elderly patients (≥ 65 years) vs. non-elderly patients (<65 years) (p = 0.08). Neoplasia was observed in 100% vs. 76.5% respectively, in groups of deceased and non-deceased patients (p = 0.04). The mean value of corrected total serum calcium was respectively, 17.8 ± 2.9 vs 16.6 ± 1.8 mg/dl in deceased and non-deceased patients (p = 0.07).
Electrocardiographic abnormalities (shortening of the QT interval, heart block…) were observed in 58.3%, 87.5%, and 85.7%, respectively, in group 1 (14.0–16.0 mg/dl), group 2 (16.1–18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p = 0.04). Mortality was observed in 19.2%, 11.8%, and 28.6%, respectively, in groups 1 (14.0–16.0 mg/dl), 2 (16.1–18.0 mg/dl), and 3 (> 18.0 mg/dl) (p = 0.04). The mean serum potassium value was 5.1 ± 1.3, 4.0 ± 1.0 and 3.7 ± 0.7 respectively, in group 1 (14.0–16.0 mg/dl), group 2 (16.1–18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p < 0.001).
Newly diagnosed neoplasia, severe hypercalcemia (> 16.0 mg/dl), and mortality have been observed in 15.4% vs. 23.7% (p = 0.31), 25% vs. 50% (p = 0.03), and 35.7% vs. 52.6% (p = 0.13) respectively, in patients before and during the Covid-19 pandemic.
4. Discussion
Despite its relative frequency, its severity, the difficulty of its management, the absence of codified treatment, and the great interest of the subject, very few series have been published on severe hypercalcemia since the 1970s. Moreover, most of the published series include all stages of hypercalcemia and frequently use the 12.0 mg/dl cut-off as their only inclusion criterion.
Ours is the largest series, 66 cases, published in the literature on severe hypercalcemia, with strict respect for the definition of severe hypercalcemia, and thus provides current and relevant data on the subject. That the incidence of severe hypercalcemia is significantly increasing in our unit is clear, since 62.2% of cases were collected during the last four years of the study vs. 37.8% during the first four years of the study [7]. When we analyze the results closely, we observe that 42.4% (28 cases) of severe hypercalcemia occurred during the year from March 2020 to March 2021 whereas, before 2020, the number of cases had varied from 5 to 8 per year. The number of cases tripled in 2020 compared to previous years. This very significant increase in cases coincides with the current context of the Covid-19 pandemic which has deeply disrupted, throughout the world and in our country, the various hospital activities of nephrology including kidney transplantation, acute dialysis, and clinical nephrology [[8], [9]]. Moreover, even if the majority of services taking care of patients with neoplastic disease have tried to maintain their activities, these have been deeply impacted with significant delays recorded in the diagnostic and therapeutic management of these patients.
Very few articles have been published on severe hypercalcemia during the pandemic. A recent literature review addressed the management of severe hypercalcemia associated with primary hyperparathyroidism while raising the role of cinacalcet [10].
In the French series of Mousseaux et al., published in 2019, the authors identified, in ten years, 131 cases of hypercalcemia in an intensive care milieu, defined as hypercalcemia exceeding 12.0 mg/dl [3]. In this series of 131 cases, the prevalence of AKI was 82.4% with recourse to renal replacement therapy in 23.1% of cases. Notably, 25% of the patients had a corrected calcemia under 14 mg/dl which partly explains the low use of renal replacement therapy. Neoplasia represented 58.2% of etiologies, and intra-hospital mortality occurred in 21.3% of cases in the study of Mousseaux et al. This mortality was greater in the neoplasia group vs. the no neoplasia group (62% vs. 13.4%). Patients with hypercalcemia and neoplasia tend to have a limited survival of several months, and it is not clear if this poor prognosis is related to the advanced stage of malignancy, severe hypercalcemia, malnutrition, inflammation, associated comorbidities, or all of these conditions [4]. Primary hyperparathyroidism is the second leading cause of severe hypercalcemia. Other rarer etiologies may be encountered such as hard water syndrome or vitamin D toxicity. The hard water syndrome was first described in 1967, when failure of a dialysis unit water softener led to 12 patients being dialyzed against hard water, causing severe symptomatic hypercalcemia and hypermagnesemia [11]. It is important to evoke this syndrome in the context of hypercalcemia in a chronic hemodialysis patient. In the series of Camus et al., published in 1996, 33 cases of severe hypercalcemia were collected in 10 years and all patients had a hemodialysis session with free calcium dialysate [12]. In this study of 33 cases, the hemodynamic tolerance was not good, with a non-negligible incidence of arterial hypotension and cardiac rhythm disorders, but the observed effect on the lowering of calcemia was interesting. At the same time, the bisphosphonates that have revolutionized the treatment of hypercalcemia were not available during the 1980s and 1990s. Currently, dialysis with free calcium dialysate is not recommended. First-line treatment of a hypercalcemic crisis includes hydration, administration of furosemide after rehydration, intravenous glucosteroids, salmon calcitonin, and intravenous bisphosphonates.
Nevertheless, this arsenal of measures, except salmon calcitonin, which is not available in all countries and medical centers, cannot deeply and rapidly lower the serum calcium; even combined, the time of action of these treatments is approximately 48 h. Rehydration, diuretics, and corticosteroids have a less powerful hypocalcemic effect but their association with other treatments results in effective and prolonged action. The onset of action of calcitonin is 2 to 6 h and should be repeated every 6 to 8 h without exceeding 48 h due to the development of tachyphylaxis, itself linked to downregulation of calcitonin receptors. For bisphosphonates, the onset of action is 48 h and lasts up to 30 days. The use of dialysis with low calcium dialysate (1.25 mmol/l) makes it possible to lower serum calcium, restore kidney function, improve clinical symptoms and reduce morbimortality in a few hours after initiation [[13], [14]]. In our study, the delta drop in serum calcium 48 h after initiation of medical treatment, including hemodialysis sessions, was 4.71 ± 1.63 mg /dl and reflects the effectiveness of the treatment initiated. In our series, mortality was not directly related to hypercalcemia and death occurred after correction of hypercalcemia. In this context, mortality remains linked to other complications, particularly infectious, cardiovascular, and neoplastic.
5. Conclusions
Our study shows a significant increase in cases of severe hypercalcemia as well as in the average corrected total serum calcium level during the Covid-19 pandemic. This is linked to the progression of pre-existing neoplastic disease and the delays in diagnosing new neoplastic diseases resulting from the major impact of the Covid-19 pandemic on the overall organization of hospital activities. Hemodialysis with low calcium dialysate should be performed within hours of the diagnosis, particularly in the presence of neurological symptoms and/or electrocardiographic abnormalities, whatever the kidney function, because cardiac arrhythmia may rapidly progress into complete heart block and cardiac arrest. One to three hemodialysis sessions are usually sufficient to significantly lower the calcium value and correct any associated electrolyte disturbances, thus contributing to an improvement in the overall morbidity and mortality of these patients. Today, hospital activities have resumed their usual rhythms guaranteeing better management of neoplastic disease.
Funding
This work has not received any financing or sponsorship.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.Guimard C., Batard E., Lavainne F., Trewick D. Is severe hypercalcemia immediately life-threatening? Eur J Emerg Med Off J Eur Soc Emerg Med. avr 2018;25(2):110–113. doi: 10.1097/MEJ.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 2.Mirrakhimov A.E. Hypercalcemia of malignancy: an update on pathogenesis and management. North Am J Med Sci. nov 2015;7(11):483–493. doi: 10.4103/1947-2714.170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mousseaux C., Dupont A., Rafat C., Ekpe K., Ghrenassia E., Kerhuel L., et al. Epidemiology, clinical features, and management of severe hypercalcemia in critically ill patients. Ann Intensive Care. 27 nov 2019;9(1):133. doi: 10.1186/s13613-019-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralston S.H., Gallacher S.J., Patel U., Campbell J., Boyle I.T. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1 avr 1990;112(7):499–504. doi: 10.7326/0003-4819-112-7-499. [DOI] [PubMed] [Google Scholar]
- 5.Krolewicz K., Stec Z., Niemczyk S. Hypercalcemia in the nephrology department patients - incidence, etiology and impact on renal function. Pol Merkur Lek Organ Pol Tow Lek. 24 févr 2021;49(289):9–12. [PubMed] [Google Scholar]
- 6.Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- 7.Bentata Y., El Maghraoui H., Benabdelhak M., Haddiya I. Management of hypercalcaemic crisis in adults: current role of renal replacement therapy. Am J Emerg Med. juin 2018;36(6):1053–1056. doi: 10.1016/j.ajem.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Bentata Y. Impact of the COVID-19 pandemic on the nephrology activity of hemodialysis started in an emergency context: a view from Morocco. J Nephrol. févr 2021;34(1):15–17. doi: 10.1007/s40620-020-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentata Y. Organ shortage: major or minor impact of the COVID-19 pandemic? Exp Clin Transp Off J Middle East Soc Organ Transp. 2021 Jun;19(6):624–626. doi: 10.6002/ect.2020.0371. [DOI] [PubMed] [Google Scholar]
- 10.Alfadhli E.M. Management of primary hyperparathyroidism with severe hypercalcemia during the COVID-19 pandemic. Clin Ther. 2021 Apr;43(4):711–719. doi: 10.1016/j.clinthera.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard H., Pile T. Hard water syndrome: a case series of 30 patients from a London haemodialysis unit. Clin Kidney J. févr 2020;13(1):111–112. doi: 10.1093/ckj/sfz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camus C., Charasse C., Jouannic-Montier I., Seguin P., Tulzo Y.L., Bouget J., et al. Calcium free hemodialysis: experience in the treatment of 33 patients with severe hypercalcemia. Intensive Care Med. févr 1996;22(2):116–121. doi: 10.1007/BF01720717. [DOI] [PubMed] [Google Scholar]
- 13.Wang C.-C., Chen Y.-C., Shiang J.-C., Lin S.-H., Chu P., Wu C.-C. Hypercalcemic crisis successfully treated with prompt calcium-free hemodialysis. Am J Emerg Med. nov 2009;27(9) doi: 10.1016/j.ajem.2009.01.026. 1174.e1–3. [DOI] [PubMed] [Google Scholar]
- 14.Trabulus S., Oruc M., Ozgun E., Altiparmak M.R., Seyahi N. The use of low-calcium hemodialysis in the treatment of hypercalcemic crisis. Nephron. 2018;139(4):319–331. doi: 10.1159/000488502. [DOI] [PubMed] [Google Scholar]