Abstract
Objective
To analyze the survival rates of patients with COVID-19 supported with extracorporeal membrane oxygenation (ECMO) and compare the survival rates of patients with COVID-19 supported with ECMO to patients with influenza supported with ECMO.
Design
A systematic review and meta-analysis to assess the impact of ECMO as supportive therapy of COVID-19.
Setting
The authors performed a search through the Cochrane, EMBASE, and MEDLINE/PubMed databases from inception to February 19, 2021, for studies reporting hospitalized patients with COVID-19 managed with ECMO.
Participants
A total of 134 studies were selected, including 6 eligible for the comparative meta-analysis of COVID-19 versus influenza.
Interventions
The authors pooled the risk ratio and random effects model.
Measurements and Main Results
The primary endpoint was the overall mortality of patients with COVID-19 receiving ECMO. Of the total number of 58,472 patients with COVID-19 reported, ECMO was used in 4,044 patients. The analysis suggested an overall in-hospital mortality of 39% (95% CI 0.34-0.43). In the comparative analysis, patients with COVID-19 on ECMO had a higher risk ratio (RR) for mortality when compared to influenza patients on ECMO: 72/164 (44%) v 71/186 (38%) RR 1.34; 95% CI 1.05-1.71; p = 0.03.
Conclusions
ECMO could be beneficial in patients with COVID-19, according to the authors’ meta-analysis. The reported mortality rate was 39%. This systematic analysis can provide clinical advice in the current era and ongoing pandemic.
Keywords: COVID-19, ECMO, critical care, mortality, intensive care, ARDS
THE SARS-Cov-2 Coronavirus pandemic continues to threaten global health, causing economic burden and social disruption. Extracorporeal membrane oxygenation (ECMO) was used largely in patients with COVID-19 with acute respiratory distress syndrome (ARDS), inducing health systems to modulate infrastructures and reallocate devices and personnel with significant financial commitments.
Venovenous (VV) ECMO is an invasive technique that oxygenates the blood and removes CO2 while the failing lung is rested and is given time to recover. The management of patients on ECMO generally is performed in tertiary care referral centers, as it requires expertise in the treatment of refractory respiratory failure and severe ARDS.1 , 2
Although reports on the use of ECMO from previous epidemics exist,1 , 3, 4, 5, 6, 7, 8, 9, 10 dedicated guidelines were produced during the COVID-19 pandemic to help triage patients in the face of reduced resources.11
Initial ECMO guidelines for COVID-19–related ARDS were based on pre–COVID-19 trials,1 , 4 and ECMO was started in patients <71 years old with severe initial presentation and a short duration of mechanical ventilation (MV) before ECMO (ie, <7 or <11 days).12 , 13
Data on ECMO efficacy in COVID-19 related ARDS are limited and come mainly from case reports or experiences of single centers. This systematic review and meta-analysis aimed to summarize evidence from all available studies to assess the mortality of patients with COVID-19 treated with VV ECMO.
Materials and Methods
This research was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines14 (Supplemental Figure 1).
The authors searched the Cochrane, EMBASE, and MEDLINE/PubMed databases from inception to February 19, 2021. For Pubmed, they used the following search string: (ECMO [tiab] or Extracorporeal membrane oxygenation [tiab] or veno-venous [tiab] or veno arterial [tiab] or extracorporeal cardiopulmonary resuscitation [tiab] or ECPR [tiab] or venovenus [tiab] or venoarterial [tiab]) and (covid-19 [tiab] or coron* [tiab] or covid [tia b] or nCov [tiab] or sars [tiab]) not (animal [mh] or animal* [mh] or pig [ti] or pig* [ti] or rat [ti] or rat* [ti] or horse [ti] or horse* [ti] or preclinical [mh] or pre-clinical [mh]).
The current meta-analysis included studies that met the Population, Interventions, Comparison, and Outcomes criteria (Table 1 ). Studies were included if reporting patients with COVID-19 treated with ECMO. The primary analyzed endpoint was the overall mortality of patients with COVID-19 receiving ECMO. Secondary outcomes and associated variables were: the percentage of patients receiving ECMO among the COVID-19 investigated cohorts, male versus female, ECMO duration, and time on MV before ECMO implantation.
Table 1.
Participants, Intervention, Comparison, and Outcomes (PICO) of the Present Meta-Analysis
| PICO | Description |
|---|---|
| Population | Patients diagnosed with COVID-19 who developed ARDS and were put on VV ECMO |
| Intervention | VV ECMO |
| Comparison | None (in a secondary analyses we performed a comparison with influenza patients who were put on ECMO) |
| Outcome | Mortality |
Abbreviations: ARDS, acute respiratory distress syndrome; PICO, participants, intervention, comparison, and outcomes; VV ECMO: veno venous extracorporeal membrane oxygenation.
Studies reporting the comparison between ECMO therapy in COVID-19 and influenza-related ARDS were also searched, and the findings meta-analyzed.
The study protocol is available at https://www.crd.york.ac.uk/PROSPERO/ under registration number CRD42021229145.
Data Extraction
Two authors separately reviewed all potentially eligible manuscripts (title and abstract level first, full text thereafter). Disagreements were reviewed by a third reviewer, who had a deciding vote.
Risk of Bias
The quality of the included studies was assessed using the Newcastle-Ottawa scale (NOS)15 and its modification for case series and reports, according to Murad et al.16 The NOS was designed to judge a study by 3 perspectives: the study's selection, the comparability, and the determination of the outcomes. A favorable judgment was made by awarding a star. Nine stars indicated the highest strength, and 6 or more stars signified elevated quality. The modified NOS scale by Murad et al was used for case series and/or reports16; a score of 8 marks is considered the maximum as items related to comparability and adjustment (which are not relevant to non comparative studies) and retained items that focused on selection, representativeness of cases, and the ascertainment of outcomes and exposure were removed.
The methodologic quality of eligible studies was independently assessed by 2 reviewers, with disagreements resolved through discussion with a third reviewer.
Data Synthesis and Analysis
A meta-analysis of single proportions summarizing data using the inverse variance method (95% CI)17 was conducted to examine the rate of the primary and secondary outcomes in VV ECMO treatment for COVID-19. A meta-analysis of continuous variables was performed for some of the secondary outcomes: mean ratio (MR) with 95% CI was calculated, and a pooled estimate, meta-MR, was computed weighting MRs according to the variance and the number of participants in the study.18
In the comparative meta-analysis, the study authors’ primary measure of association was the risk ratio (RR) of mortality between groups. Secondary variables included the mean difference (MD) of ECMO duration, the duration of MV before ECMO placement, peak serum creatinine concentration, and the RR of renal replacement therapy.
Given the diversity of studies and populations, the authors did not assume a common effect size and expected considerable heterogeneity. Therefore, a priori use of the random-effects model19 was decided using the meta, metafor, and dmetar packages for R and R-Studio Version 1.3 for macOS. This model reduces the probability of type II errors. For each study, the effects estimates are presented as squares, and proportions with their 95% CI are presented as horizontal lines. The chi-square test and the I2 were used to assess heterogeneity among the studies. Heterogeneity was classified as low (25%), moderate (50%), or high (75%).20 The estimation of the mean and standard deviation in studies reporting only median values and interquartile ranges was accomplished using the methodology described by Hozo et al.21 Results were summarized using effect estimates and their associated 95% CI. Publication bias was examined by visual inspection of the funnel plot and tested by Egger's test.22 A p value below 0.05 was considered suspicious of publication bias.
Results
Three prospective investigations, 82 retrospective observational analyses, and 49 case reports and/or series of patients with COVID-19 on ECMO, for a total 134 studies (references to the 134 studies in Supplemental Appendix 1), were selected for the systematic review and meta-analysis of single proportions or single means, and 6 23, 24, 25, 26, 27, 28 of them were eligible for the comparative meta-analysis of patients with COVID-19 on ECMO versus patients with influenza on ECMO. According to the NOS,15 the quality scores of the included studies ranged from 6 to 9 for retrospective studies, indicating elevated quality as listed in Supplemental Table 1. Supplemental Table 2 shows the score modified for case series and reports.16
A total of 4,044 out of 58,472 (6.9%) patients with COVID-19 received VV ECMO (Table 2 ). In the 77 studies reporting sex, males were 2,606 out of 3,455 (71%) patients (Table 2), and the mean age was 51 years.
Table 2.
Pooled Data of Mortality of COVID-19 Patients on VV-ECMO and of Other Categorical and Continuous Variables
| Categorical Variables | ||||||
|---|---|---|---|---|---|---|
| Variable | No of Studies | Patients, n/N | Proportion | 95% CI | Heterogeneity | Risk of Bias (Egger's test) |
| Mortality | 102 | 1,508/3,793 | 0.39 | 0.34-0.43 | I2 53%; p < 0.01 | - |
| ECMO/COVID-19 cases | 65 | 4,044/58,472 | 0.07 | 0.05-0.09 | I2 97%; p < 0.01 | + |
| Male sex | 77 | 2,606/3,455 | 0.71 | 0.67-0.74 | I2 23%; p = 0.04 | + |
| Continuous variables | ||||||
| Variable | No of Studies | Patients | Mean, d | 95% CI | Heterogeneity | Risk of Bias |
| ECMO Duration | 31 | 3,176 | 15 | 13.34-27.62 | I2 98%; p < 0.01 | + |
| MV prior ECMO | 26 | 1,747 | 4.25 | 3.32-5.18 | I2 99%; p < 0.01 | + |
NOTE. Data are presented as categorical and continuous variables. Categorical variables are expressed as proportions and 95% CI according to the random effect model single outcome meta-analysis; continuous variables are indicated as means and 95% CI.
Abbreviations: ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation.
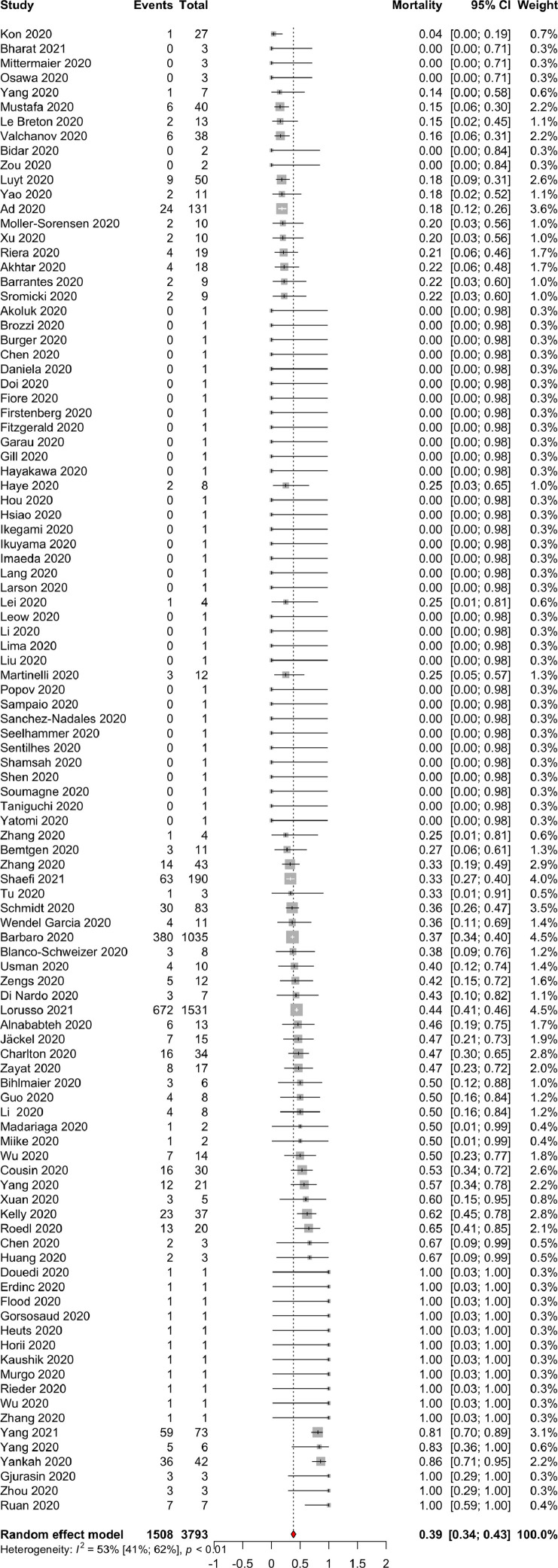
In a meta-analysis of single proportions to calculate an overall proportion from studies reporting a single variable, random-effect pooled estimates of 102 studies analyzing 3,793 patients suggested an overall in-hospital mortality of patients with COVID-19 on ECMO of 39% (1508/3793) (95% CI 34-43; I2 = 53%; P of heterogeneity < 0.01) (Fig 1 ; Table 2) with low risk of publication bias (Supplemental Tables 1 and 2). Patients with COVID-19 had been 4.25 (3.32-5.18) days on invasive MV before receiving ECMO, and ECMO lasted 14.25 (13.34-27.62) days (Table 2).
Fig 1.
A forest plot displaying the random-effect pooled estimates of 102 studies analyzing 3,793 patients suggested an overall in-hospital mortality proportion of 0.39 (95% CI [0.34-0.43]; I2 = 53%; P of heterogeneity < 0.01) ordered by treatment effects.
The overall study quality was acceptable (Supplemental Tables 1 and 2).
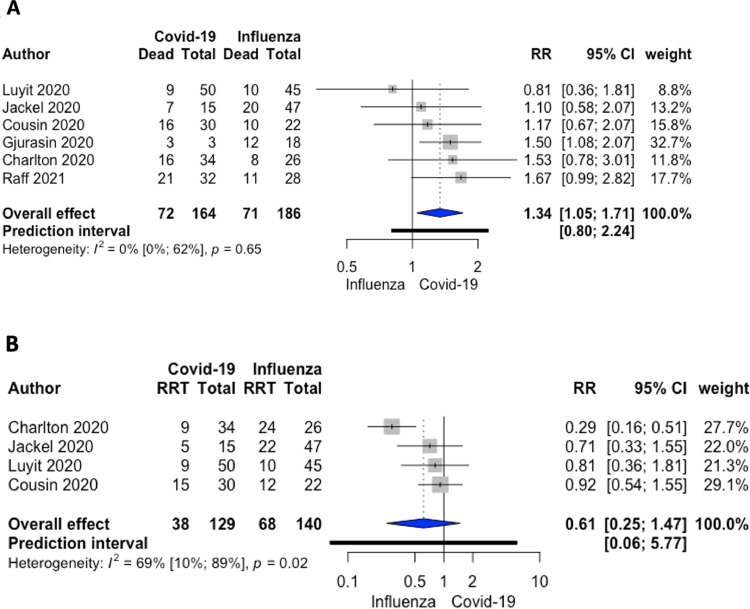
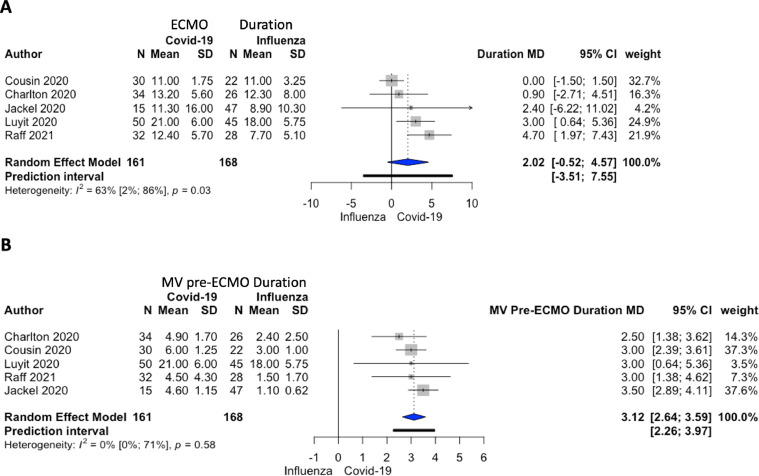
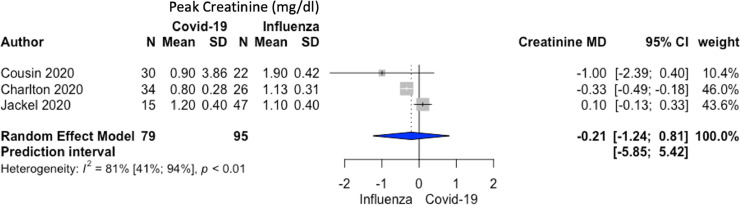
The comparative meta-analysis restricted to 6 studies (350 overall patients) showed that patients with COVID-19 on ECMO had a higher RR of mortality when compared to patients with influenza on ECMO: 72/164 (44%) v 71/186 (38%) RR 1.34; 95% CI 1.05-1.71; p = 0.03; I2 = 0%; P of heterogeneity = 0.65 (Fig 2A). The authors also found a longer MV duration before ECMO initiation in patients with COVID-19 compared to influenza patients without a differences in renal replacement therapy, ECMO duration, and peak serum creatinine concentration (Fig. 2B, 3 , and 4 ).
Fig 2.
(A) A forest plot depicting mortality in patients treated with ECMO in COVID-19 and influenza. Relative risk 1.34 (95% CI [1.05-1.71]; I2 = 0%; P of heterogeneity = 0.65) ordered by treatment effects. (B) A forest plot showing renal replacement therapy in patients treated with ECMO in COVID-19 and influenza. Relative risk 0.61 (95% CI [0.25-1.47]; I2 = 69%; P of heterogeneity = 0.02) ordered by treatment effects. ECMO, extracorporeal membrane oxygenation; RR, relative risk, RRT, renal replacement therapy.
Fig 3.
(A) A forest plot visualizing extracorporeal membrane oxygenation duration in patients with COVID-19 and influenza. Mean difference 2.02 days (95% CI [–0.52 to 4.57]; I2 = 63%; P of heterogeneity = 0.03) ordered by treatment effects. (B) A forest plot of mechanical ventilation duration pre-extracorporeal membrane oxygenation in patients with COVID-19 versus influenza. Mean difference 3.12 days (95% CI [2.64-3.59]; I2 = 0%; P of heterogeneity = 0.58) ordered by treatment effects. ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; MD, mean difference.
Fig 4.
A forest plot of peak serum creatinine concentration in patients with COVID-19 versus influenza. Mean difference –0.21 mg/dL (95% CI [–1.24 to 0.81]; I2 = 81%; P of heterogeneity < 0.01) ordered by treatment effects. MD, mean difference.
Discussion
Key Findings and Relationship to Previous Studies
Mortality
In this extensive systematic review, the authors first investigated mortality in a meta-analysis of single proportions to better understand the survival rates of patients with COVID-19 supported with ECMO. They identified 134 studies, and 102 reported an overall mortality rate of 39% (1,508/3,793 patients).
This mortality rate was consistent with experience from EOLIA (37%) and CESAR (37%) randomized controlled trials, performed in the pre–COVID-19 era,29 and were much lower than the initial data of patients with COVID-19 requiring ECMO reported from China in early 2020.30, 31, 32, 33
According to this latter evidence, it seems that mortality is improving over time, and this is consistent with the Extracorporeal Life Support Organization (ELSO) Registry, suggesting that criteria for placing patients with COVID-19 on ECMO became more stringent over time, favoring survivability.
However, in this comparison meta-analysis, the authors found 6 studies comparing ECMO in COVID-19 to ECMO in influenza. According to the 350 patients analyzed, mortality in patients with COVID-19 on ECMO was higher than in patients with influenza on ECMO (RR 1.34; 95% CI 1.05-1.71; p = 0.03), as previously reported.34 Still, this data are expected to vary across time as newer COVID-19 to influenza comparisons will be reported.
Male Sex
Interestingly, 71% of patients who underwent ECMO were male. This had already been noticed and referred not to the general population of infected people but to the severe clinical presentations as the ones requiring VV ECMO.35 The causes behind this sex-based unbalance remain unclear. Social, psychological, and genetic factors could all be contributing to this gender skew. Men, who are recognized in research and practice to be more impacted by cardiovascular illnesses, diabetes, chronic pulmonary disease, hypertension, and cancer, have a high incidence of disease in most situations.36 All of these factors have been connected to a high COVID-19 fatality rate.37
ECMO Duration
Even if several reports included in this meta-analysis were still incomplete as patients were still on ECMO at the moment of the publication, according to the authors' analysis, the mean ECMO duration in patients with COVID-19 was 15 days (longer than the 9 days reported in the CESAR trial38). This probably was ascribed to a different pathophysiologic representation in COVID-19 compared to pneumonia and ARDS of other etiologies and involves angiogenesis, pulmonary vasculitis, and thrombosis.39 Also, the higher ECMO duration compared to influenza might indicate more considerable pathogenicity, leading to respiratory complications and to higher mortality.40
MV
An MV duration before ECMO initiation of 4 days is longer than pre-2020 investigations, and this might underline a lack of uniformity in intubation protocols and late calls for ECMO referral centers (overwhelmed during the pandemic surge). It also may be displaying the tendency to wait longer before placing a patient on ECMO, tolerating lower PaO2/FIO2 ratios compared to the standard ARDS care.41 This comparison meta-analysis further confirmed this data: MV pre-ECMO duration in COVID-19 was increased by 3 days (95% CI 2.64-3.59; p < 0.001) versus MV pre-ECMO duration in influenza.
Limitations of the Study
The authors’ systematic review had limitations. First of all, by pooling observational studies, this review could not overcome the limitations of its primary studies included, which were relatively small numbers, and, still, none was based on a randomized allocation. Meta-analysis of observational studies is notoriously challenging due to heterogeneity (in subjects, outcome definitions, study design, etc), incomplete data, and bias. Also, secondary outcome measurements were missing in many studies because the focus often was mortality. However, the present systematic review and meta-analysis are relevant and may guide current practice helping clinicians to consider patients for ECMO therapy according to the current ELSO guidelines,11 even if only by emphasizing the limitations of the available clinical evidence. It is worth noting that the present analysis reported the highest number of patients with COVID-19 treated with ECMO to date.
Third, duplicate publication bias might have occurred as it is challenging to detect double ECMO runs reports, especially in large cohorts extracted from international databases.
Finally, the reduced mortality from ECMO duration compared to the ELSO registry could have been the representation of publication bias, as authors tend to publish favorable outcomes with shorter runs in fewer sick patients and, thus, overestimate survivability.
Conclusions
To this day, this systematic review included the highest number of patients with COVID-19 with ECMO outcomes. The results suggested that ECMO could be advantageous for patients with COVID-19 with ARDS. The mortality rate for patients with ARDS due to COVID who received ECMO support was 39% (95% CI 34-43). In the authors' view this systematic analysis of the literature can be of benefit and provide clinical advice in the current era and ongoing pandemic.
Conflict of Interest
None.
Footnotes
Financial support: None (the study was supported by departmental funds only).
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.jvca.2021.11.006.
Appendix. Supplementary materials
Appendix 1. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1053/j.jvca.2016. 07.016
References
- 1.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Pieri M, Landoni G, et al. Venovenous ECMO treatment, outcomes, and complications in adults according to large case series: A systematic review. Int J Artif Organs. 2021;44:481–488. doi: 10.1177/0391398820975408. [DOI] [PubMed] [Google Scholar]
- 3.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: A review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Clemens F, Elbourne D, et al. CESAR: Conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. doi: 10.1186/1472-6963-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 6.Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 8.Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: A systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monaco F, Belletti A, Bove T, et al. Extracorporeal membrane oxygenation: Beyond cardiac surgery and intensive care unit: Unconventional uses and future perspectives. J Cardiothorac Vasc Anesth. 2018;32:1955–1970. doi: 10.1053/j.jvca.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 11.Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopal K, Keller SP, Akkanti B, et al. Advanced pulmonary and cardiac support of COVID-19 patients: Emerging recommendations from ASAIO-a Living working document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLaren G, Combes A, Brodie D. Saying no until the moment is right: initiating ECMO in the EOLIA era. Intensive Care Med. 2020;46:1894–1896. doi: 10.1007/s00134-020-06185-1. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed.
- 16.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich JO, Adhikari NKJ, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol. 2011;64:556–564. doi: 10.1016/j.jclinepi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Green S. Heterogeneity, Cochrane Handbook for Systematic Reviews of Interventions version 6.1 . Updated September 2020. Available at: http://www.training.cochrane.org/handbook. Accessed .
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousin N, Bourel C, Carpentier D, et al. SARS-CoV-2 versus influenza associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. ASAIO J. 2021;67:125–131. doi: 10.1097/MAT.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlton M, Dashey S, Stubbs A, et al. Comparing SARS-CoV-2 and influenza A(H1N1)pdm09-infected patients requiring ECMO - A single-centre, retrospective observational cohort experience. J Infect. 2021;82:84–123. doi: 10.1016/j.jinf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjurašin B, Santini M, Krajinović V, et al. A retrospective comparison between influenza and COVID-19-associated ARDS in a Croatian tertiary care center. Wien Klin Wochenschr. 2021;133:406–411. doi: 10.1007/s00508-020-01759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jäckel M, Rilinger J, Lang CN, et al. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza - a single-center registry study. Artif Organs. 2021;45:593–601. doi: 10.1111/aor.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luyt C-E, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann Intensive Care. 2020;10:158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raff LA, Reid TD, Johnson D, et al. Comparative outcomes between COVID-19 and influenza patients placed on veno-venous extracorporeal membrane oxygenation for severe ARDS. Am J Surg. 2021 doi: 10.1016/j.amjsurg.2021.04.004. S0002-9610(21)00233-6. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Combes A, Peek GJ, Hajage D, et al. ECMO for severe ARDS: Systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46:2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnino MW, Moskowitz A, Thompson GS, et al. Comparison between patients hospitalized with influenza and COVID-19 at a tertiary care center. J Gen Intern Med. 2021;36:1689–1695. doi: 10.1007/s11606-021-06647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baiardo Redaelli M, Landoni G, Di Napoli D, et al. Novel coronavirus disease (COVID-19) in Italian patients: Gender differences in presentation and severity. Saudi J Med Med Sci. 2021;9:59–62. doi: 10.4103/sjmms.sjmms_542_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID-19 pandemic: Are men vulnerable and women protected? JACC Case Rep. 2020;2:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 38.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 39.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piroth L, Cottenet J, Mariet A-S, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1053/j.jvca.2016. 07.016