Abstract
COVID-19 pandemic continues to be a global threat, affecting more than 200 countries/territories at both human and economic level. This necessitates the rapid development of highly reliable diagnostic methods in order to effectively and accurately diagnose the pathology to prevent the spread of COVID-19. Currently, RT-PCR is the most widely used method worldwide for SARS-CoV-2 detection. Serological assays are being used for sero-surveys of SARS-CoV-2 antibody prevalence in the community. Radiology imaging has been useful in the clinical diagnosis of COVID-19. These methods have their own limitations and there are continued efforts to develop easier, economic, highly sensitive and specific, point-of-care methods. Reverse transcription-loop mediated isothermal amplification (RT-LAMP), nucleic acid sequence-based amplification (NASBA), CRISPR-Cas-based detection, and digital PCR are such techniques being employed in research laboratories, with many awaiting diagnostic approval from competent authorities. This review highlights the rapidly expanding array of existing and in-development diagnostic tests/strategies that may be used to diagnose SARS-CoV-2 infection in both clinical and research settings.
Keywords: COVID-19, Diagnosis, Nucleic acids, CRISPR-Cas, Nanobodies, Biosensors, Serology
1. Introduction
COVID-19 (Coronavirus disease 2019) was first reported in the month of December 2019 in Wuhan city of China and was designated as pandemic by World Health Organisation (WHO) on March 11, 2020. A novel coronavirus, named as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) by the International Committee on Taxonomy of Viruses (ICTV), is responsible for COVID-19 [1], [2]. Coronaviruses, first isolated by Tyrrell and Bynoe [3] from common cold patients, belong to the order Nidovirales, family Coronaviridae and subfamily, Coronavirinae. Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus are the four genera of subfamily Coronavirinae [4]. Seven subtypes of coronavirus are known to infect humans whereby alphacoronaviruses cause mild or asymptomatic while betacoronaviruses cause severe infections and fatalities. SARS-CoV-2 is a betacoronavirus with 96.2% sequence similarity (whole genome level) to a bat coronavirus (RatG13) and 79.5% to SARS-CoV, which indicate its origin from bats in China and transmission from animals to humans but intermediated host and exact route of transmission are still not known [5], [6].
COVID-19 has spread to over 200 countries/territories with more than 246 million cases and 5 million deaths worldwide as on October 31, 2021 and thousands dying daily. United States is the most affected country with about 46 million cases and over 0.74 million deaths followed by India with 34.3 million cases and 0.45 million deaths [7]. Several COVID-19 vaccines (Covishield, Pfizer-BioNTech mRNA, Moderna mRNA-1273, Sputnik V, AZD1222 Vaxzevria, COVAXIN, Covovax) have been approved for human administration, while many are in various stages of development [8]. Different countries are experiencing multiple waves due to mutating nature of SARS-CoV-2. As COVID-19 is a major global health threat, it has piqued the interest of research community globally in areas such as origin, pathogenesis, diagnosis and treatment.
It is crucial to diagnose COVID-19 at an early stage in order to control its spread. Although molecular detection by RT-PCR is the gold standard method used currently, but its shortcomings such as costly equipment, need for trained personnel, false positive/ negative results and longer time taken curtail the effort to contain this outbreak [9]. COVID-19 pandemic has driven the scientists to develop highly reliable diagnostic techniques at a fast pace in order to detect the pathology and thereby prevent infection transmission. Despite the fact that the SARS CoV-2's chemical and structural characteristics were previously unknown, government and private research institutions and biomedical business set-ups have swiftly developed methods beneficial for accurate diagnosis of COVID-19 [10].
A good diagnostic test must take into account the period of suspected infection, patient's medical history, symptoms and the overall clinical picture. Furthermore, accuracy of a diagnostic test is greatly reliant on the time when it is performed. When the virus is still in its incubation stage and there aren't enough viral RNA copies in circulation, neither antibodies nor viral antigens are detectable, making both serological and molecular testing ineffective during the first week of infection [11]. So highly sensitive methods are required for early detection of virus. This review outlines various methods available for the sensitive and specific detection of SARS-CoV-2 virus.
2. SARS-CoV-2: structure
SARS-CoV-2 belongs to the family of enveloped coronaviruses containing non-segmented RNA genome (positive single strand) of size 29.9 kb and 5’-ORF1a-1b-S-3a-3b-E-M-6-7a-8a-N-3’-polyA tail. About 70% genome is covered by overlapping ORF1a and ORF1b, which further translates into polyproteins forming 16 non-structural proteins (nsp1‐nsp16). Nsp 12 possesses RdRp (RNA-dependent RNA polymerase) activity responsible for virus replication and transcription [5], [12]. Phosphorylated N protein and genomic RNA together form the nucleocapsid which is enveloped by phospholipid bilayer that possesses membrane (M) protein, spike (S) protein and envelope (E) protein (Fig. 1 ). M is the transmembrane protein responsible for nutrient transport, release of bud and envelope formation. E protein is necessary for the virus assembly and release, possesses ion channel activity and is reported to have role in pathogenesis of virus [13]. N is the only nucleocapsid protein and is composed of two domains ‐both required for RNA binding. N also binds to M protein and replicase complex. S protein, a trimer, forms the spike structure on the surface of virus and attachment to host cell receptor occurs via S1 and S2 subunits (S1 determines host cell range and S2 mediates membrane fusion). In addition, there are replicases and other accessory proteins (3a, 6, 7a, 7b, 8, and 10) with unknown functions [14], [15].
Fig. 1.
SARS-CoV-2 virion structure (S: spike protein, N: nucleocapsid protein; E: envelope protein and M: membrane protein).
3. Diagnostic methods
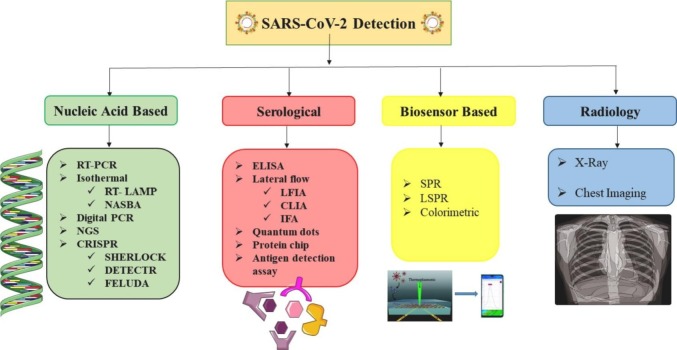
Different SARS-CoV-2 detection approaches (Fig. 2 ) include swift antigen or antibody testing, immunological enzymatic serological testing and nucleic acid based molecular tests, as the most often used procedures. Other techniques, such as isothermal nucleic acid amplification, clusters of regularly interspaced short palindromic repeats/Cas (CRISPR/Cas)-based approaches, digital PCR and chemiluminescence, are already being used in research settings or are awaiting diagnostic approval.
Fig. 2.
Methods for SARS-CoV-2 detection.
3.1. Nucleic acid based detection
3.1.1. Real time reverse transcription polymerase chain reaction (rRT-PCR)
FDA has authorized 235 molecular tests for the fast detection of SARS-CoV-2, while the EMA (European Medicine Agency) has authorized 192 PCR based procedures. RT-PCR based molecular tests are the gold standard for obtaining a verified diagnosis of COVID-19. It works by reverse transcription of SARS-CoV-2 RNA into cDNA followed by measuring viral load by the cycle threshold (Ct) (Fig. 3A). High viral load in the sample is indicated by lower Ct values [16]. SARS-CoV-2 genome regions coding for the RdRp, spike, nucleocapsid and enveloped proteins are utilized to construct specialized primers and probes. RT-PCR has flaws and critiques that might cause false-positive or false-negative findings, compromising the pandemic's proper management [17], [18]. In addition, impurities and interferers in the sample or added by the operator hinder the reaction. Other constraints include the analysis execution time, which in non-automated systems might take up to 24 h to provide a conclusion that can be conveyed to the patient. During the early stages of pandemic, when diagnostic techniques had not yet been optimized and standardized, a significant fraction of COVID-19 positive patients were identified as false-negative due to the low sensitivity of the primers and probes used, or the inaccuracy of the entire RT-PCR procedure. This assay cannot be used to determine if a person had SARS-CoV-2 infection in past and has recovered [19]. Allplex 2019-nCoV assay developed by Seegene Inc., South Korea detects SARS-CoV-2 by direct rRT-PCR without needing RNA extraction if sample is transported in UTM (universal transport medium) or molecular water [20]. Also, various companies have developed modified RT-PCR assays such as Cobas® Liat® (Roche Molecular 145 Systems, USA), Xpert® Xpress SARS-CoV-2 (Cepheid, USA) and ID NOW™ (Abbott, USA) which have been approved by United States FDA (USFDA) under emergency use authorization.
Fig. 3.
Diagrammatical representation of (A) RT-PCR and (B) RT-LAMP based SARS-CoV-2 detection.
3.1.2. Isothermal detection
Isothermal amplification techniques are being developed to detect viral RNAs as alternative to RT-PCR. Isothermal detection methods can prepare samples and reagents quickly and can be coupled to a variety of readouts, making these more user-friendly and accessible [21]. Isothermal amplification, when integrated with portable devices, enhances the nucleic acid based on-site tests' accuracy and sensitivity. Integrated microfluidic devices have been used for single-cell and single-molecule investigations. Loop-mediated isothermal amplification (LAMP), transcription-mediated amplification (TMA), nucleic acid sequence-based amplification (NASBA), recombinase polymerase amplification (RPA) and rolling circle amplification (RCA) are examples of traditional isothermal-based tests.
3.1.2.1. Reverse transcription-loop mediated isothermal amplification (RT-LAMP)
At a constant temperature of 60–65 °C, LAMP is a highly accurate, sensitive, accessible, point of care (POC) device and fast amplification method that amplifies DNA/RNA by employing 4–6 primers that bind to six distinct regions of the target genome [22]. The method begins with acquiring sample from a COVID-19 patient and includes a step-by-step procedure for amplifying viral RNA. The results can be analysed by turbidity of PCR tubes, change of colour or fluorescence, making it a simple technique (Fig. 3B). It has higher specificity than RT-PCR due to the use of multiple primers. Recently, Lu et al. [23] developed an RT-LAMP test targeting the S (one primer set), RdRp (one primer set) genes and N (four primer sets), with RdRp primer set exhibiting the best specific detection of SARS-CoV-2 RNA with a sensitivity of three copies per million.
In India, Sree Chitra Tirunal Institute for Medical Sciences and Technology Thiruvananthapuram has developed Chitra GeneLAMP-N diagnostic test kit for SARS-CoV-2 detection. This kit is regarded as one of the world's first confirmatory diagnostic tests for the SARS-CoV-2 N-gene and provides result within 2 h [24].
3.1.2.2. Nucleic acid sequence-based amplification (NASBA)
It employs a two-stage isothermal amplification assay capable of producing a large copy number within 1.5–2 h and requires fewer cycles than RT-PCR and LAMP, resulting in reduced incubation time and a lower overall frequency of error. It is robust, sensitive and specific for single stranded RNA (ssRNA) detection and holds immense potential for use at POC in low-resource settings [25]. In previous epidemics, NASBA was utilized to detect SARS-CoV. Fluorochromes are also used in the reaction for real-time monitoring. Multiplex RT-NASBA is the modified version that can aid in detection of multiple viral infections at the same time. Xing et al. [26] analysed 614 clinical samples from patients with respiratory tract infections, 201 preclinical, 25 clinically diagnosed and 14 COVID-19 positive samples using the RTisochip™-W system that has the capacity of analysing 16 samples in single run in 90 min with minimal limit of detection equal or less than 50 copies/μL.
Transcription-mediated amplification (TMA) is a single tube-based isothermal amplification-test that employs RNA polymerase and reverse transcriptase to produce billions of copies of RNA in short time. This method can target both RNA and DNA using two primers, and the amplicons are ssRNA [27]. This method's operating temperature range of 37–41 °C eliminates the requirement for expensive thermocyclers. TMA has been used to identify a wide range of viral and bacterial infections.
3.1.3. Next generation sequencing (NGS)
Apart from electron microscopy and other techniques, NGS has been critical in the discovery of SARS-CoV-2 and its variants, as well as in the development of existing molecular diagnostic tools. It was feasible to comprehensively describe the whole genome of SARS-CoV-2 using NGS, confirming that it belonged to the β-coronavirus genus [28]. NGS is being employed in molecular epidemiology and identification of new molecular variations, rather than for diagnostic reasons. Because of high cost illumine and the need for highly skilled molecular and bioinformatics staff, its diagnostic use is restricted. A majority of commercial kits rely on NGS techniques paired with hybrid capture methods. These platforms are made up of biotinylated RNA probes that hybridize to SARS-CoV-2 RNA fragments. The biotinylated probes are then amplified in PCR using streptavidin-coated beads [29].
Newer NGS methods have been devised for detecting changes in the SARS-CoV-2 genome, allowing for the identification of newly emerging variants that are relevant epidemiologically and for the development of new vaccines. Amplicon-based metagenomic sequencing is the most potent way for quickly identifying and fully characterizing SARS-CoV-2 and other pathogens [30]. Patients' normal microbiomes can be identified using metagenomic sequencing, whereas SARS-CoV-2 RNA may be amplified and sequenced using amplicon-based sequencing. Combining amplicon-based and metagenomic sequencing allows researchers to diagnose COVID-19 infection, as well as detect secondary infections caused by other organisms that aggravate the disease. Whole-genome sequencing is the most effective approach for molecular characterization of SARS-CoV-2, identification of novel variants during genomic surveillance screening, and designing genome-based therapeutic strategies [31].
3.1.4. Clustered regularly interspaced short palindromic repeats (CRISPR)
CRISPR modules are the acquired immune systems that operate against invading genetic elements such as viruses and are encoded by prokaryotic organisms, particularly bacteria. CRISPR-associated endonuclease's collateral cleavage activity is employed in CRISPR-based nucleic acid detection methods, as well as in almost all CRISPR-based SARS-CoV-2 detection approaches. Cas9, Cas12, and Cas13 are CRISPR-associated endonucleases that cut the SARS-CoV-2 RNA sequence [32]. Several CRISPR-based assays viz. SHERLOCK, DETECTR and FELUDA (Fig. 4 ) for diagnosing COVID-19 have been reported, each employing a distinct isothermal amplification methodology.
Fig. 4.
CRISPR-Cas technologies for SARS-CoV-2 detection: (A) SHERLOCK; (B) DETECTR; (C) FELUDA.
3.1.4.1. SHERLOCK (specific high sensitivity enzymatic reporter unlocking)
SHERLOCK employs Cas13a as an effector enzyme (Fig. 4A) and was targeted to the S and ORF1ab genes of SARS-CoV-2. Cas12a and Cas13a enzymes exhibit a collateral cleavage activity which can be explained as cleavage of an additional RNA non-specifically following the cleavage of target RNA [33], [34]. Cas13 remains inactive if two or more mismatches are found in target RNA and efficiently distinguishes between SARS-CoV-2 and other identical viruses [35]. A quenched fluorescent ssRNA reporter was utilized in SHERLOCK technology. Its sensitivity has been enhanced by using recombinase polymerase amplification (RPA) or reverse transcriptase-RPA (RT-RPA) to amplify specific DNA or RNA prior to the commencement of the reaction [36]. The test may be performed using RNA extracted from patient samples, as in qRT-PCR, and read out with a dipstick in less than an hour without the need for any additional equipment. As a consequence, SHERLOCK for SARS-CoV-2 detection is considerably faster than qRT-PCR and showed 93.1% sensitivity. Joung et al. [37] further modified SHERLOCK to develop STOP (SHERLOCK Testing in One Pot) for POC diagnosis of COVID-19 and named it STOPCovid. Ding et al. [38] reported AIOD-CRISPR (All-In-One Dual CRISPR-Cas12a), a POC assay for the detection of SARS-CoV-2 and HIV (Human immunodeficiency virus).
3.1.4.2. DETECTR (DNA endonuclease-targeted CRISPR trans reporter)
An RT-LAMP-based DETECTR assay was designed to detect SARS-CoV-2. The Cas12 protein from Lachnospiraceae sp. (LbCas12a) or other organisms was designed to recognize and detect SARS-CoV-2 gRNA (Fig. 4B). This approach involves recognition of amplified DNA targets by crRNA-Cas12a combination [39], which when binds to target DNA cleaves non-target FQ-DNA reporters indiscriminately. This assay may detect as little as 10 copies/μl with a total sample assay time of around 45 min and provides visual detection through LFA (lateral flow assay) and employs a reporter molecule tagged with fluorescence dye (fluorescein amidite) which produces colour when cleaved [40].
3.1.4.3. FELUDA (FnCas9 editor linked uniform detection assay)
The Indian Council of Medical Research (ICMR) has approved CRISPR/Cas9 based assay-FELUDA for the detection of SARS-CoV-2 in clinical samples. This technique was devised by the CSIR (Council of Scientific and Industrial Research)—India's Institute of Genomics and Integrative Biology to provide a platform for the development of POC diagnostic assay for COVID-19 that can detect single nucleotide variations. Cas9 from Francisella novicida (FNCas9), which has a high mismatch sensitivity even by single mismatch, was used in this methodology (Fig. 4C). FELUDA is a potential technique for differentiating viruses that have nearly identical genomes (e.g., SARS-CoV and SARS-CoV-2). Inactivation of the virus, extraction of the RNA, reverse transcription, isothermal amplification, and detection are typical process steps. This is a rapid, easy to use, affordable and cost-effective SARS-CoV-2 detection test having high sensitivity (100%) and specificity (97%), comparable to qPCR, SHERLOCK and DETECTR tests [41].
3.1.5. Digital PCR
Digital PCR (dPCR) is a technical advancement over the widely used RT-PCR procedures, and is further classified into cdPCR (chip digital PCR) and ddPCR (droplet digital PCR), with latter being most commonly used. ddPCR is based on the principles of sample micro-partitioning and DNA ultra-dilutions via the reaction mix's water-oil emulsion. In ddPCR, a master mix is prepared which includes probes, primers, non-target and target genes, and other reagents. This master mix is partitioned into thousands of replicate droplets, each droplet performing individual PCR reaction detected by fluorescence [42] . Applications of digital PCR includes identification of low expressed targets, microbiological investigations, low copy number (≤3 copies) genes, low viral load detection, analysis of liquid biopsy samples, single-cell analysis and mutational analysis [43]. Falzone et al. [44] demonstrated that the Probe and EvaGreen systems have high sensitivity and accuracy for detecting N gene of SARS-CoV-2 in lower quantities. In comparison to other molecular techniques, ddPCR imparts improved stability, sensitivity, specificity and repeatability [45] and can accurately detect very low viral load, allowing clinicians to diagnose COVID-19 infection in asymptomatic or paucisymptomatic patients [46]. These claims were also supported by a recent study in which 63 of the qRT-PCR negative samples were found positive for SARS-CoV-2 using ddPCR, with 55% exhibiting COVID-19 symptoms [47]. Its higher precision corresponds to its best performance as LoD that showed a limit of detection of 2.1 and 1.8 copies/reaction for ORF1ab and N gene of SARS-CoV-2, respectively, compared to RT-PCR, which had a LoD of 873.2 copies/reaction for N gene and 1039 copies/reaction for ORF1ab in an experimental setting. It has a 500-fold higher sensitivity than RT-PCR [48]. High sensitivity of cdPCR was also demonstrated for detecting SARS-CoV-2 N gene in false RT-PCR negative COVID-19 patients, thus enhancing the sensitivity of molecular testing [49]. The ability of ddPCR to detect viral loads in sputum samples rather than nasal swabs revealed that using sputum samples improves detection rate [50]. Thus, the sensitive ddPCR outperforms qRT-PCR in clinical diagnosis of COVID-19 by reducing false negative reports.
3.2. Serological assays
As SARS-CoV-2 infection causes acute immunological reaction in patients, serological assays are good options for COVID-19 diagnosis but these have only been approved for areas where nucleic acid testing is not feasible and for monitoring the extent of the outbreak. A variety of serological assays were recommended by WHO such as chemiluminescence assay (CLIA), enzyme-linked immunosorbent assay (ELISA), western blotting (WB), immune-fluorescence assay (IFA) and protein microarray. CLIA and ELISA were the first lines of screening for earlier pandemics caused by SARS-CoV and MERS-CoV. These assays are based on detection of IgA, IgM and IgG-antibodies for COVID-19 in blood samples. Zhang et al. [51] used ELISA for detection of IgG and IgM in COVID-19 patients' sera. A peptide-based luminescent immunoassay has been proposed by Cai et al. [52] to detect IgG and IgM for COVID-19. When combined with RT-PCR detection, this test may help to improve the accuracy of SARS-CoV-2 infection diagnosis.
The SARS-CoV-2 spike protein's receptor-binding domain (RBD) was synthesised in human cells and was used to develop a series of chemical luminescence assays for the detection of RBD-specific IgM, IgA, and IgG. The RBD-specific IgG, IgM and IgA kits had diagnostic sensitivities of 96.8, 96.8, and 98.8%, respectively, with respective specificities of 98.1, 92.3 and 99.8% [53].
Li et al. [54] developed lateral flow immunoassay (LFIA) for the detection of IgM and IgG simultaneously. Many antibody-based kits such as DZ-Lite SARS-CoV-2 CLIA IgM and IgG kits (Diazyme, USA), Platelia SARS-CoV-2 Total Ab (Bio-Rad, USA), Wondfo SARS-CoV-2 Antibody Test (Guangzhou Wondfo Biotech, China), FREND™ COVID-19 IgG/IgM Duo (NanoEntek, South Korea) and ScheBo SARS-CoV-2 Quick (ScheBo Biotech, Germany) have been approved for emergency use, with the advantage of being applied at the POC. However, serological detection of COVID-19 has limitations due to the time-dependent development of antibodies during infection. The rates of IgM and IgG antibodies against S and N protein were studied, and it was observed that S-IgM and N-IgM peaked during the second week of infection, while S-IgG and N-IgG peaked during the third week [55]. So, during the initial phase of infection, nucleic acid assays are highly recommended, whereas sero assays are confined to surveillance of community infection [56].
3.2.1. Lateral flow immuno assay (LFIA)
With the rise in the number of people suspected of having COVID-19, it became imperative to develop quick and low-cost testing procedures in order to conduct widespread monitoring programmes. To deal with this scenario, many rapid tests have been developed to detect viral antigens or anti-SARS-CoV-2 human antibodies in nasal, salivary, oropharyngeal swabs and blood samples [57]. Rapid antigenic and antibody tests are faster than RT-PCR based procedures, with execution times ranging from 10 to 30 min (Table 1 ), lower costs, and a simpler approach that does not necessitate the presence of highly skilled individuals. A majority of these tests use LFIA devices for rapid detection of viral proteins or human antibodies against SARS-CoV-2 antigens. Rapid antigen assays allow the identification of COVID-19 positive patients by detecting SARS-CoV-2 N or S proteins or RBDs (viral antigens) in swabs obtained from the subject's upper airways [58].
Table 1.
Lateral flow assay kits commercially available for COVID-19 diagnosis.
| Company | Kit name | Target | Features | Reference |
|---|---|---|---|---|
| MyLab Discovery Solutions | CoviSelf™ | Spike protein | Mobile app synchronized Time: 15 min |
[63] |
| Cellex Inc. | qSARS-CoV-2 IgG/IgM rapid test | IgG and IgM | Time: 15–20 min Sensitivity: 93.8% Specificity: 96% |
[64] |
| Luminostics, Inc. | Clip COVID rapid antigen test | Nucleocapsid protein | Time: 30 min | [65] |
| Pharmact AG | BELTEST-IT COV-2 | IgG and IgM | Sensitivity: IgM 98.1% and IgG 98.2% Specificity IgG 99.7% IgM 99.5% Time: 20 min |
[66] |
| Pharmact AG | BELTEST-IT AGS COV-2 | Not reported | Time: 15 min Sensitivity: 92.2% Specificity: 100% |
[67] |
| ChemBio Diagnostic System, Inc. | DPP COVID-19 IgM/IgG system | Nucleocapsid protein | Sensitivity: 93.5% Specificity: 94.4% Time: 15 min |
[68] |
| Celltrion | Celltrion DiaTrust™ | Nucleocapsid and receptor binding domains | Time: 15 min Specificity: 99.03% Sensitivity: 93.33% |
[69] |
| Acon | Flowflex™ SARS-CoV-2 | Nucleocapsid protein | Time: 15 min Sensitivity: 97.1% Specificity: 99.6% |
[70] |
| Xtrava Health | SPERA™ COVID-19 Ag test | Nucleocapsid protein | Time: 15 min Sensitivity: 92% Specificity: 97% |
[71] |
| ANP Technologies, Inc. | NIDS® COVID-19 antigen rapid test | Nucleocapsid protein | Time: 15 min | [72] |
| GenBody Inc. | GenBody COVID-19 Ag | Nucleocapsid protein | Time: 10–20 min Sensitivity: 96.8% Specificity: 98.9% |
[73] |
| InBios International, Inc. | SCoV-2 Ag Detect™ | Nucleocapsid protein | Time: 20 min Sensitivity: 86.67% Specificity: 100% |
[74] |
| Access Bio, Inc. | CareStart™ COVID-19 antigen home test | Nucleocapsid protein | Time: 10 min Sensitivity 93.8% Specificity 99.3% |
[75] |
| Becton, Dickinson and Company (BD) | BD Veritor™ at-home COVID-19 test | Nucleocapsid protein | Time: 15 min | [76] |
Fast antigen and rapid antibody tests produce results that are visible with the naked eye in a short period of time and can be performed at POC without the need for any additional instruments or sample processing [59]. Being more cost-effective, easy-to-use, and fast diagnostic technologies, these are ideal for global pandemic management.
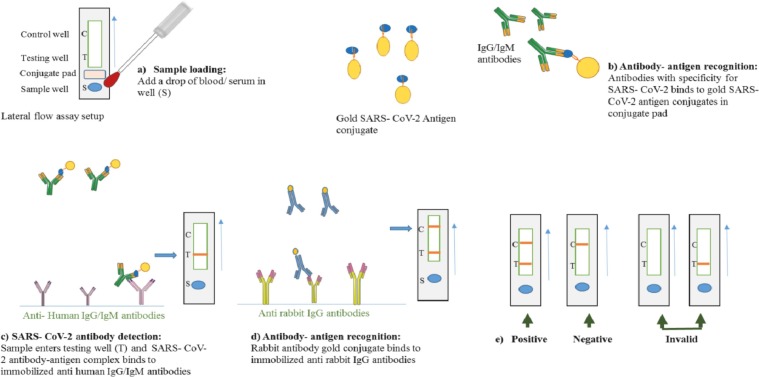
LFIA, commonly known as immune chromatographic assays or strip tests, use single axis of operation. LFIA strips comprise of a sample pad, a conjugate pad, a nitrocellulose membrane and an absorbent pad. The liquid sample applied to the sample pad migrates through the conjugate pad that conjugate pad contains the analyte specific antibodies conjugated to fluorescent dyes or coloured colloidal gold nanoparticles. The sample along with conjugated antibody bound to analyte then reaches the detection zone which is a porous membrane having specific biological molecules immobilized in lines. These biological molecules are mostly antigens or antibodies and these react with the analyte bound to conjugated antibody. A suitable response on the test line results from the recognition of the analyte while a response on control line indicates that the sample has properly run through the strip. The end of the strip consists of an absorbent pad that prevents the backflow of liquid (Fig. 5). Absorbent pad helps in increasing the sensitivity of assay by allowing use of large sample volume [58], [59], [60].
Fig. 5.
Schematic overview of lateral flow assay setup for SARS-CoV-2 detection.
Cross reactivity due to similarities between antigens of SARS-CoV-2 and other related coronaviruses like SARS-CoV and MERS-CoV is the biggest challenge faced by serological assays. S1 subunit of S protein has emerged as a better antigen for use in serological tests, because the S2 subunit is conserved among coronaviruses and therefore exhibits cross-reactivity. This has contributed to decreased number of false positive results [61], [62].
3.3. Biosensors
Advances in bioelectronics have led to the development of analytical assays and devices for accurate and rapid detection of pathogens. Biosensors detect pathogens by biochemical reactions (enzyme based) or specific binding to analyte. For the diagnosis of COVID-19, a variety of biosensor platforms are now available. Colorimetric, fluorescence-based, localized surface plasmon resonance (LSPR), electrochemical surface-enhanced Raman scattering (SERS) and other platforms for detecting SARS-CoV-2 particles are among them. SARS-CoV-2 proteins and RNA may both be detected with electrochemical biosensors [77]. Zhu et al. [78] combined nanoparticles with RT-LAMP to develop biosensor for SARS-CoV-2 detection. Field effect transistor biosensor has been developed by conjugating graphene sheet with S protein antibody for detection of SARS-CoV-2 without prior isolation of virus RNA [79]. Biosensors based on surface plasmon resonance (SPR) allow for real-time diagnostics of analytes, as well as real-time interaction and binding affinity at the atomic level. A photothermal biosensor based on a prism-based SPR method was recently reported to detect SARS-CoV-2 coronavirus. This biosensing method employs the plasmonic photothermal effect and targeted surface plasmon resonance to provide a promising and accurate clinical diagnosis of COVID-19. The sensing mechanism is based on functionalization of gold nano islands with complementary DNA receptors and heat-activated nucleic acid hybridization to detect the RNA SARS-CoV-2 coronavirus sequence [80]. SARS-CoV-2 nucleocapsid antibodies were also detected using a portable SPR sensor equipment. The nanomolar anti-SARS-CoV-2 antibodies could be detected in undiluted human serum in under 15 min after the SPR sensor was coated with a peptide monolayer and functionalized with recombinant protein [81].
Qiu et al. [82] demonstrated dual-function plasmonic biosensor based on the concept of nucleic acid hybridization for detection of RdRp, ORF1ab and E gene of SARS-CoV-2. Most of these devices are in development stages and are yet to be approved for diagnosis of COVID-19 patients. Plasmonic biosensors based on nanotechnology are portable platforms for detecting layer-by-layer biomolecules, proteins and viral particles that are easy, simple and easily demonstrative. AuNPs particle-based detection has recently received a lot of interest for colorimetric biosensing applications. The thiol-modified functionalized AuNPs used in this colorimetric test were used to capture thiol-modified antisense oligonucleotides (ASOs) specific for the SARS CoV-2 N-gene. The sensing concept relies on RNA-DNA hybridization and colour variation of agglomerated AuNPs to achieve a detection limit of 0.18 ng/μl of SARS-CoV-2 RNA. After RNA separation, this methodology offers the benefits of reliability, repeatability, and speed of response, taking just 10 min to identify positive COVID-19 patient samples [83].
3.4. Nanobodies assays
Nanobodies or single variable domain (VHH) of camelid heavy-chain-only antibodies have become popular and highly effective antibody fragment type for SARS-CoV-2 diagnosis and potential therapy. Nanobodies are small (12–15 kDa), stable, and cheap to make in bacteria and yeast. Because of their small size, they are well suited for accessing hidden epitopes such as cracks in target proteins [84]. They can be used as imaging agents, diagnostics and structural biology techniques instead of traditional antibodies. Nanobodies are being researched for their potential to treat cancer and inflammatory disorders directly. There is a discovery and characterization of a high-affinity nanobody (H11-H4) against SARS-CoV-2 spike protein, which prevents spike attachment to ACE2 (Angiotensin-converting enzyme 2) in vitro. According to the structural analysis, nanobodies in association with either the full-length spike or RBD from SARS-CoV-2 target an epitope that is immediately adjacent to and slightly overlaps the ACE2 binding area. SPR revealed that H11-H4 binds RBD, with a KD (dissociation constant) of 5 nM. RBD and spike binding to ACE2 was inhibited by this nanobody [85]. This suggests that the nanobody epitope on the RBD of spike coincides with the ACE2 binding site. The superposition of the RBD–ACE2 complex on the H11-H4–RBD complex demonstrates that H11-H4 would block ACE2 binding to RBD [86]. This is due to Van der Waals collisions, primarily between H11-H4 areas that are not in touch with the RBD and ACE2 region.
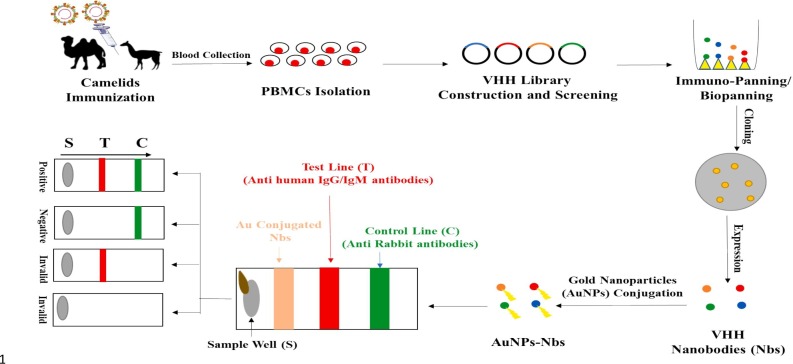
Four camels immunized with the SARS-CoV-2 RBD domain were used to create nanobody phage display libraries. 381 nanobodies were found to capable of recognising SARS-CoV-2 RBD while seven nanobodies blocked the interaction between RBD and human ACE2. Among these nanobodies, Nb11–59 had the highest anti-SARS-CoV-2 activity having higher affinity (KD = 21.6–106 nM) and with greater than 99% purity in Pichia pastoris [87]. Nanobody based assays are relatively new for detecting SARS-CoV-2 antigens, but are potentially beneficial for the development of futuristic POC assays. PBMCs (peripheral blood mononuclear cells) obtained from the blood of SARS-CoV-2 immunized camelids are utilized for library construction and VHH screening. These are then cloned and expressed in a suitable vector followed by conjugation with gold nanoparticles (AuNPs). These AuNPs attached nanobodies can be utilized to build subsequent lateral flow-based as well as ELISA-based assays for SARS-CoV-2 detection (Fig. 6 ).
Fig. 6.
Schematic representation of nanobodies based detection of SARS-CoV-2.
3.5. Radiology
COVID-19 was clinically diagnosed in its early stages of the disease, when the causative agent was unknown, by examining the patient's respiratory and extra-respiratory symptoms as well as using radiological imaging modalities. Symptoms were typically modest in 80–90% of COVID-19 positive persons, although a small proportion of patients had severe symptoms that required hospitalization [88]. Interstitial pneumonia, which is linked with cytokine storm, respiratory failure and multiorgan failure, affects around 16% of patients and can result in death.
As a result, a comprehensive clinical examination of all these symptoms, as well as radiological and laboratory data, aids doctors in accurately diagnosing COVID-19 infection and resulting in initiating successful therapy procedures on time. Chest X-ray (CXR) and computed tomography (CT) are the most powerful radiological imaging tools for diagnosing COVID-19 related pneumonia. Due to false-negative results reported from RT-PCR in some cases, CT scan has proven to be more sensitive for COVID-19 diagnosis than RT-PCR [89], [90]. As CT scan is commonly used to better visualize lung abnormalities, which are typically characterized by bilateral interstitial ground-glass opacities [91]. The CT scan, in particular, has a high-resolution power and a sensitivity of 95–100%, but its major limitation is its limited specificity that does not allow for the differentiation of pulmonary abnormalities associated with various etiological agents other than SARS-CoV-2 and the risk of cumulative radiation exposure. CXR is often used in patients with moderate to severe symptomatology who have interstitial opacities (71.7%) or alveolar opacities (60.5%) in both lungs and are suspected of having COVID-19 infection (64.5%) [92]. Despite the inexpensive cost and quick radiological results achieved by CRX, some lung abnormalities are not clearly apparent by this method. Furthermore, the need for highly skilled radiologists and expensive equipment associated with radio imaging methods limits their usage in COVID-19 diagnosis.
3.6. Machine learning, block chain and artificial intelligence (AI)
There is very limited evidence for use of AI in clinical diagnosis. However, it can be coupled with POC assays and radiological imaging to hasten the testing procedure and aiding self-testing. IKONOS is one such tool developed by Gomes et al. [93] which collaborates chest X-ray with machine learning to differentiate COVID-19 infection from other pneumonia infections.
Shiri et al. [94] employed deep learning algorithms to create an ultra-low dose CT examination procedure that predicted full dosage CT images, resulting in an 89% reduction in CT radiation. However, it had a disadvantage that correct lesion structure or density could not be recovered for many patients.
Similarly, ResNet-101, a deep learning system employed in conjunction with CT imaging, demonstrated 99.51% accuracy in distinguishing COVID-19 from other pneumonia illnesses. So, deep learning, block chain, and AI-based technologies may be utilized as adjuvants to conventional methods, reducing the workload on technicians and radiologists while improving detection accuracy [95].
4. Conclusion
SARS-CoV-2 diagnosis is of paramount importance in curing and containing the disease spread and currently relies on molecular methods, specifically RT-PCR, which have limitations such as the need for expensive equipment, trained personnel, and low accuracy and sensitivity. Several serological, rapid antigen and biosensor based tests have been approved for SARS-CoV-2 detection but their application is currently limited to community surveillance of pandemic. Technologies for accurate, specific and sensitive POC detection of SARS-CoV-2 are being developed and include improved nucleic acid based methods like NASBA and RT-LAMP, CRISPR-Cas and its variations, nanobodies based LFA, SPR assays, paper assays, semiconductors based binding assays, use of aptamers functionalized with quantum dots and employing functionalized nanostructures in order to improve the sensitivity of PCR based methods. Combination of state-of-the-art molecular tests with artificial intelligence and LFAs will allow POC diagnosis to become more common in future, especially in the case of global pandemics like COVID-19.
Declaration of competing interest
None.
References
- 1.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/J.ENVRES.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fathizadeh H., Afshar S., Masoudi M.R., Gholizadeh P., Asgharzadeh M., Ganbarov K., Köse Ş., Yousefi M., Kafil H.S. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: a review. Int. J. Biol. Macromol. 2021;188:740–750. doi: 10.1016/J.IJBIOMAC.2021.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyrrell D.A., Bynoe M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1966;1:76–77. doi: 10.1016/S0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4016–4026. doi: 10.26355/EURREV_202004_20871. [DOI] [PubMed] [Google Scholar]
- 5.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Coronavirus (COVID-19) Dashboard 2021. https://covid19.who.int/
- 8.WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. 2021. https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
- 9.Bulilete O., Lorente P., Leiva A., Carandell E., Oliver A., Rojo E., Pericas P., Llobera J. PanbioTM rapid antigen test for SARS-CoV-2 has acceptable accuracy in symptomatic patients in primary health care. J. Infect. 2021;82:391–398. doi: 10.1016/J.JINF.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmi G. Rapid coronavirus tests: a guide for the perplexed. Nature. 2021;590:202–205. doi: 10.1038/D41586-021-00332-4. [DOI] [PubMed] [Google Scholar]
- 11.Krajewski P.K., Matusiak Ł., Szepietowski J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35:e632–e634. doi: 10.1111/JDV.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baral P.K., Yin J., James M.N.G. Treatment and prevention strategies for the COVID 19 pandemic: a review of immunotherapeutic approaches for neutralizing SARS-CoV-2. Int. J. Biol. Macromol. 2021;186:490–500. doi: 10.1016/J.IJBIOMAC.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur M., Sharma A., Kumar S., Singh G., Barnwal R.P. SARS-CoV-2: insights into its structural intricacies and functional aspects for drug and vaccine development. Int. J. Biol. Macromol. 2021;179:45–60. doi: 10.1016/J.IJBIOMAC.2021.02.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.Sen, Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- an update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kralik P., Ricchi M. A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front. Microbiol. 2017;8:108. doi: 10.3389/FMICB.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varlamov D.A., Blagodatskikh K.A., Smirnova E.V., Kramarov V.M., Ignatov K.B. Combinations of PCR and isothermal amplification techniques are suitable for fast and sensitive detection of SARS-CoV-2 viral RNA. Front. Bioeng. Biotechnol. 2020;8:1294. doi: 10.3389/FBIOE.2020.604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., Scarlata S., Agrò F.E. COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 2020;288:192–206. doi: 10.1111/JOIM.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yüce M., Filiztekin E., Özkaya K.G. COVID-19 diagnosis - a review of current methods. Biosens. Bioelectron. 2021;172 doi: 10.1016/J.BIOS.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merindol N., Pépin G., Marchand C., Rheault M., Peterson C., Poirier A., Houle C., Germain H., Danylo A. SARS-CoV-2 detection by direct rRT-PCR without RNA extraction. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Rev. Med. Virol. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaouch M. Loop-mediated isothermal amplification (LAMP): an effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2. Rev. Med. Virol. 2021 doi: 10.1002/RMV.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu R., Wu X., Wan Z., Li Y., Zuo L., Qin J., Jin X., Zhang C. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virol. Sin. 2020;35:344–347. doi: 10.1007/S12250-020-00218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sree Chitra Tirunal Institute joins hands with Tata Sons to augment production of COVID-19 testing Kits | Department Of Science & Technology. 2020. https://dst.gov.in/sree-chitra-tirunal-institute-joins-hands-tata-sons-augment-production-covid-19-testing-kits
- 25.Floriano I., Silvinato A., Bernardo W.M., Reis J.C., Soledade G. Accuracy of the polymerase chain reaction (PCR) test in the diagnosis of acute respiratory syndrome due to coronavirus: a systematic review and meta-analysis. Rev. Assoc. Med. Bras. 2020;66:880–888. doi: 10.1590/1806-9282.66.7.880. [DOI] [PubMed] [Google Scholar]
- 26.Xing W., Liu Y., Wang H., Li S., Lin Y., Chen L., Zhao Y., Chao S., Huang X., Ge S., Deng T., Zhao T., Li B., Wang H., Wang L., Song Y., Jin R., He J., Zhao X., Liu P., Li W., Cheng J. A high-throughput, multi-index isothermal amplification platform for rapid detection of 19 types of common respiratory viruses including SARS-CoV-2. Engineering. 2020;6:1130–1140. doi: 10.1016/J.ENG.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabir M.A., Ahmed R., Iqbal S.M.A., Chowdhury R., Paulmurugan R., Demirci U., Asghar W. Diagnosis for COVID-19: current status and future prospects. expert revMol. Diagn. 2021;21:269–288. doi: 10.1080/14737159.2021.1894930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brun A.L., Gence-Breney A., Trichereau J., Ballester M.C., Vasse M., Chabi M.L., Mellot F., Grenier P.A. COVID-19 pneumonia: high diagnostic accuracy of chest CT in patients with intermediate clinical probability. Eur. Radiol. 2020;31:1969–1977. doi: 10.1007/S00330-020-07346-Y. 2020 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M., Fu A., Hu B., Tong Y., Liu R., Liu Z., Gu J., Xiang B., Liu J., Jiang W., Shen G., Zhao W., Men D., Deng Z., Yu L., Wei W., Li Y., Liu T. Nanopore targeted sequencing for the accurate and comprehensive detection of SARS-CoV-2 and other respiratory viruses. Small. 2020;16:2002169. doi: 10.1002/SMLL.202002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giallonardo F., Duchene S., Puglia I., Curini V., Profeta F., Cammà C., Marcacci M., Calistri P., Holmes E.C., Lorusso A. Genomic epidemiology of the first wave of SARS-CoV-2 in Italy. Viruses. 2020;12:1438. doi: 10.3390/V12121438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kames J., Holcomb D.D., Kimchi O., DiCuccio M., Hamasaki-Katagiri N., Wang T., Komar A.A., Alexaki A., Kimchi-Sarfaty C. Sequence analysis of SARS-CoV-2 genome reveals features important for vaccine design. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-72533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganbaatar U., Liu C. CRISPR-based COVID-19 testing: toward next-generation point-of-care diagnostics. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/FCIMB.2021.663949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F., Abudayyeh O.O., Gootenberg J.S., Sciences C., Mathers L. A protocol for detection of COVID-19 using CRISPR diagnostics. Bioarchive. 2020;8:1–8. [Google Scholar]
- 34.Kellner M.J., Koob J.G., Gootenberg J.S., Abudayyeh O.O., Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019;14:2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taleghani N., Taghipour F. Diagnosis of COVID-19 for controlling the pandemic: a review of the state-of-the-art. Biosens. Bioelectron. 2021;174 doi: 10.1016/J.BIOS.2020.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P., Malik Y.S., Ganesh B., Rahangdale S., Saurabh S., Natesan S., Srivastava A., Sharun K., Yatoo M.I., Tiwari R., Singh R.K., Dhama K. CRISPR-cas system: an approach with potentials for COVID-19 diagnosis and therapeutics. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/FCIMB.2020.576875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joung J., Ladha A., Saito M., Kim N.-G., Woolley A.E., Segel M., Barretto R.P.J., Ranu A., Macrae R.K., Faure G., Ioannidi E.I., Krajeski R.N., Bruneau R., Huang M.-L.W., Yu X.G., Li J.Z., Walker B.D., Hung D.T., Greninger A.L., Jerome K.R., Gootenberg J.S., Abudayyeh O.O., Zhang F. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med. 2020;383:1492–1494. doi: 10.1056/NEJMC2026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding X., Yin K., Li Z., Lalla R.V., Ballesteros E., Sfeir M.M., Liu C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020;11:4711. doi: 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J.S., Ma E., Harrington L.B., Da Costa M., Tian X., Palefsky J.M., Doudna J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/SCIENCE.AAR6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azhar M., Phutela R., Kumar M., Ansari A.H., Rauthan R., Gulati S., Sharma N., Sinha D., Sharma S., Singh S., Acharya S., Sarkar S., Paul D., Kathpalia P., Aich M., Sehgal P., Ranjan G., Bhoyar R.C., Singhal K., Lad H., Patra P.K., Makharia G., Chandak G.R., Pesala B., Chakraborty D., Maiti S. Rapid and accurate nucleobase detection using FnCas9 and its application in COVID-19 diagnosis. Biosens. Bioelectron. 2021;183 doi: 10.1016/J.BIOS.2021.113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei S., Chen S., Zhong Q. Digital PCR for accurate quantification of pathogens: principles, applications, challenges and future prospects. Int. J. Biol. Macromol. 2021;184:750–759. doi: 10.1016/J.IJBIOMAC.2021.06.132. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y., Ding C., Chen Q., Xie J., Yu J., Shi Y., Jiang C., Zhang Z., He H., Ge Y., Li W., He J., Gao Y. Digital PCR assay for the effective detection of COVID-19 patients with SARS-CoV-2 low viral load. J. Virol. Methods. 2021;295 doi: 10.1016/J.JVIROMET.2021.114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falzone L., Musso N., Gattuso G., Bongiorno D., Palermo C.I., Scalia G., Libra M., Stefani S. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int. J. Mol. Med. 2020;46:957–964. doi: 10.3892/IJMM.2020.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., Zhao Y., Zhang X., Zhu T., Peng T., Xie J., Gao Y., Wang D., Dai X., Fang X. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224 doi: 10.1016/J.TALANTA.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ternovoi V.A., Lutkovsky A.V., Ponomareva R.Yu., Gladysheva E.P., Chub E.V., Tupota N.L., Smirnova A.M., Nazarenko A.A., Loktev V.B., Gavrilova E.V., Agafonov A.P., Maksyutov R.A. Detection of SARS-CoV-2 RNA in nasopharyngeal swabs from COVID-19 patients and asymptomatic cases of infection by real-time and digital PCR. Klin. Lab. Diagn. 2020;65:785–792. doi: 10.18821/0869-2084-2020-65-12-785-792. [DOI] [PubMed] [Google Scholar]
- 47.Kim K.B., Choi H., Lee G.D., Lee J., Lee S., Kim Y., Cho S.-Y., Lee D.-G., Kim M. Analytical and clinical performance of droplet digital PCR in the detection and quantification of SARS-CoV-2. Mol. Diagn. Ther. 2021;25:617–628. doi: 10.1007/S40291-021-00547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suo T., Liu X., Feng J., Guo M., Hu W., Guo D., Ullah H., Yang Y., Zhang Q., Wang X., Sajid M., Huang Z., Deng L., Chen T., Liu F., Xu K., Liu Y., Zhang Q., Liu Y., Xiong Y., Chen G., Lan K., Chen Y. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg.Microbes Infect. 2020;9:1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poggio P., Songia P., Vavassori C., Ricci V., Banfi C., Barbieri S.S., Garoffolo G., Myasoedova V.A., Piacentini L., Raucci A., Scopece A., Sommariva E., Vinci M.C., Carcione D., Biondi M.L., Mancini M.E., Formenti A., Andreini D., Assanelli E.M., Agostoni P., Camera M., Colombo G.I., Pesce M. Digital PCR for high sensitivity viral detection in false-negative SARS-CoV-2 patients. Sci. Rep. 2021;11:4310. doi: 10.1038/s41598-021-83723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu F., Yan L., Wang N., Yang S., Wang L., Tang Y., Gao G., Wang S., Ma C., Xie R., Wang F., Tan C., Zhu L., Guo Y., Zhang F. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020;71:793–798. doi: 10.1093/CID/CIAA345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W., Du R.H., Li B., Zheng X.S., Lou Yang X., Hu B., Wang Y.Y., Xiao G.F., Yan B., Shi Z.L., Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai X., Chen J., Long Q., Deng H., Liu P., Fan K., Liao P., Liu B., Wu G., Chen Y., Li Z., Wang K., Zhang X., Tian W., Xiang J., Du H., Wang J., Hu Y., Tang N., Lin Y., Ren J., Huang L., Wei J., Gan C., Chen Y., Gao Q., Chen A., He C., Wang D.-X., Hu P., Zhou F.-C., Huang A., Wang D., Hu J.- li. A peptide-based magnetic chemiluminescence enzyme immunoassay for serological diagnosis of coronavirus disease 2019. J. Infect. Dis. 2020;222:189–193. doi: 10.1093/INFDIS/JIAA243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H., Zeng W., He H., Zhao D., Jiang D., Zhou P., Cheng L., Li Y., Ma X., Jin T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020;17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis COVID-19, fingerstick blood, lateral flow immunoassay, point-of-care testing, rapid IgM-IgG combined test, SARS-CoV-2 virus infection. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H., Zhang F., Luo W., Niu X., Hu P., Wang L., Peng H., Huang Z., Feng L., Li F., Zhang F., Li F., Zhong N., Chen L. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg.Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C.Y., Fomsgaard A., Krogfelt K.A., Jørgensen C.S. 2020. Evaluation of Nine Commercial SARS-CoV-2 Immunoassays. [DOI] [Google Scholar]
- 57.Wu J.L., Tseng W.P., Lin C.H., Lee T.F., Chung M.Y., Huang C.H., Chen S.Y., Hsueh P.R., Chen S.C. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J. Infect. 2020;81:435–442. doi: 10.1016/J.JINF.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J.H., Choi M., Jung Y., Lee S.K., Lee C.S., Kim J., Kim J., Kim N.H., Kim B.T., Kim H.G. A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2) Biosens. Bioelectron. 2021;171 doi: 10.1016/J.BIOS.2020.112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., Chan R.C., Tsang D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129 doi: 10.1016/J.JCV.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montesinos I., Gruson D., Kabamba B., Dahma H., Van den Wijngaert S., Reza S., Carbone V., Vandenberg O., Gulbis B., Wolff F., Rodriguez-Villalobos H. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020;128 doi: 10.1016/J.JCV.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loeffelholz M.J., Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg.Microbes Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okba N.M.A., Widjaja I., Li W., GeurtsvanKessel C.H., Farag E.A.B.A., Al-Hajri M., Park W.B., Oh M., Reusken C.B.E.M., Koopmans M.P.G., Bosch B.-J., Haagmans B.L. Serologic detection of Middle East respiratory syndrome coronavirus functional antibodies. Emerg. Infect. Dis. 2020;26:1024–1027. doi: 10.3201/EID2605.190921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coviself By Mylab - India’s First Rapid Antigen Self Test Kit 2021. https://coviself.com/
- 64.qSARS-CoV-2 IgG/IgM Rapid Test 2021. https://cellex.com/
- 65.2020. COVID-19 - Clip Health.https://cliphealth.com/products/covid-19/ [Google Scholar]
- 66.BELTEST-IT COV-2 https://pharmact.de/en/beltest-it-cov-2/
- 67.BELTEST-IT AGS COV-2 - Pharmact GmbH 2021. https://pharmact.de/en/beltest-it-ags-cov-2/
- 68.<collab>DPP® SARS-CoV-2 IgM/IgG System Europe – Chembio Diagnostics Inccollab. 2021. https://chembio.com/products/dpp-sars-cov-2-igm-igg-system-europe/
- 69.DiaTrustTM COVID-19 Ag Rapid Test 2021. https://www.celltrion.com/en-us/kit/DiatrustAg
- 70.SARS-CoV-2 Antigen Rapid Test - ACON LABS INC 2021. https://www.aconlabs.com/sars-cov-2-antigen-rapid-test/
- 71.SPERATM COVID-19 Ag TEST | Xtrava Health 2021. https://www.xtravahealth.com/spera-covid19-antigen-test
- 72.COVID-19 Antigen Rapid Test | anptech 2021. https://www.anptinc.com/covid-19-test
- 73.GenBody COVID-19 Ag 2021. http://genbody.co.kr/bbs/board.php?bo_table=human01&wr_id=22
- 74.<collab>SCoV-2 Ag DetectTM Rapid Test – InBios International Inccollab. 2021. https://inbios.com/scov-2-ag-detecttm-rapid-test/
- 75.CareStartTM COVID-19 Antigen Home Test – Access Bio 2020. https://accessbio.net/products/covid-19-detection-kits/carestart-covid-19-antigen-home-test
- 76.COVID-19 Rapid Antigen Test | BD VeritorTM Plus System 2021. https://bdveritor.bd.com/en-us/rapid-antigen-testing/covid-19
- 77.Abid S.A., Ahmed Muneer A., Al-Kadmy I.M.S., Sattar A.A., Beshbishy A.M., Batiha G.E.S., Hetta H.F. Biosensors as a future diagnostic approach for COVID-19. Life Sci. 2021;273 doi: 10.1016/J.LFS.2021.119117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu X., Wang X., Han L., Chen T., Wang L., Li H., Li S., He L., Fu X., Chen S., Xing M., Chen H., Wang Y. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 2020;166 doi: 10.1016/J.BIOS.2020.112437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Kim S.Il. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 80.Basso C.R., Malossi C.D., Haisi A., Barbosa A.N., Grotto R.T., Junior J.P.A., Pedrosa V.de A. Fast and reliable detection of SARS-CoV-2 antibodies based on surface plasmon resonance. Anal. Methods. 2021;13:3297–3306. doi: 10.1039/D1AY00737H. [DOI] [PubMed] [Google Scholar]
- 81.Maddali H., Miles C.E., Kohn J., O’Carroll D.M. Optical biosensors for virus detection: prospects for SARS-CoV-2/COVID-19. ChemBioChem. 2021;22:1176–1189. doi: 10.1002/CBIC.202000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 83.Moitra P., Alafeef M., Dighe K., Frieman M.B., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14:7617–7627. doi: 10.1021/ACSNANO.0C03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pymm P., Adair A., Chan L.-J., Cooney J.P., Mordant F.L., Allison C.C., Lopez E., Haycroft E.R., O’Neill M.T., Tan L.L., Dietrich M.H., Drew D., Doerflinger M., Dengler M.A., Scott N.E., Wheatley A.K., Gherardin N.A., Venugopal H., Cromer D., Davenport M.P., Pickering R., Godfrey D.I., Purcell D.F.J., Kent S.J., Chung A.W., Subbarao K., Pellegrini M., Glukhova A., Tham W.-H. Nanobody cocktails potently neutralize SARS-CoV-2 D614G N501Y variant and protect mice. Proc. Natl. Acad. Sci. 2021;118 doi: 10.1073/PNAS.2101918118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., Tan T.K., Rijal P., Dumoux M., Ward P.N., Ren J., Zhou D., Harrison P.J., Weckener M., Clare D.K., Vogirala V.K., Radecke J., Moynié L., Zhao Y., Gilbert-Jaramillo J., Knight M.L., Tree J.A., Buttigieg K.R., Coombes N., Elmore M.J., Carroll M.W., Carrique L., Shah P.N.M., James W., Townsend A.R., Stuart D.I., Owens R.J., Naismith J.H. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020;27:846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- 86.Ye G., Gallant J.P., Massey C., Shi K., Tai W., Zheng J., Odle A.E., Vickers M.A., Shang J., Wan Y., Drelich A., Kempaiah K.R., Tat V., Perlman S., Du L., Tseng C.-T., Aihara H., LeBeau A.M., Li F. BioRxiv; 2020. The Development of a Novel Nanobody Therapeutic for SARS-CoV-2. 2020.11.17.386532. [DOI] [Google Scholar]
- 87.Gai J., Ma L., Li G., Zhu M., Qiao P., Li X., Zhang H., Zhang Y., Chen Y., Gong R., Wan Y. A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. MedComm. 2021;2:101–113. doi: 10.1101/2020.08.09.242867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H., Ai L., Lu H., Li H. Clinical and imaging features of COVID-19. Radiol.Infect. Dis. 2020;7:43–50. doi: 10.1016/J.JRID.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Qahtani M., AlAli S., AbdulRahman A.K., Salman Alsayyad A., Otoom S., Atkin S.L. The prevalence of asymptomatic and symptomatic COVID-19 in a cohort of quarantined subjects. Int. J. Infect. Dis. 2021;102:285–288. doi: 10.1016/J.IJID.2020.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Long C., Xu H., Shen Q., Zhang X., Fan B., Wang C., Zeng B., Li Z., Li X., Li H. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020;126 doi: 10.1016/J.EJRAD.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdi A., Jalilian M., Sarbarzeh P.A., Vlaisavljevic Z. Diabetes and COVID-19: a systematic review on the current evidences. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/J.DIABRES.2020.108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ippolito D., Maino C., Pecorelli A., Allegranza P., Cangiotti C., Capodaglio C., Mariani I., Giandola T., Gandola D., Bianco I., Ragusi M., Franzesi C.T., Corso R., Sironi S. Chest X-ray features of SARS-CoV-2 in the emergency department: a multicenter experience from northern italian hospitals. Respir. Med. 2020;170 doi: 10.1016/J.RMED.2020.106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomes J.C., Santana M.A., Bandeira J., Valença M.J.S., de Souza R.E., Ismael A.M., dos Santos W.P., Barbosa V.A.de F. IKONOS: an intelligent tool to support diagnosis of COVID-19 by texture analysis of X-ray images. Res. Biomed. Eng. 2020;2020:1–14. [Google Scholar]
- 94.Shiri I., Akhavanallaf A., Sanaat A., Salimi Y., Askari D., Mansouri Z., Shayesteh S.P., Hasanian M., Rezaei-Kalantari K., Salahshour A., Sandoughdaran S., Abdollahi H., Arabi H., Zaidi H. Ultra-low-dose chest CT imaging of COVID-19 patients using a deep residual neural network. Eur. Radiol. 2020;31:1420–1431. doi: 10.1007/S00330-020-07225-6. 2020 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ardakani A.A., Kanafi A.R., Acharya U.R., Khadem N., Mohammadi A. Application of deep learning technique to manage COVID-19 in routine clinical practice using CT images: results of 10 convolutional neural networks. Comput. Biol. Med. 2020;121 doi: 10.1016/J.COMPBIOMED.2020.103795. [DOI] [PMC free article] [PubMed] [Google Scholar]