Abstract
Background
The COVID-19 pandemic has negatively impacted the psychological well-being of individuals and society. Previous studies conducted on coronavirus outbreaks including Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome pandemic found that posttraumatic stress disorder (PTSD), depression, and anxiety were the most common mental health problems and long-term consequences of these outbreaks. Currently, comprehensive and integrated information on the global prevalence of PTSD due to the COVID-19 pandemic is lacking.
Objective
In the present meta-analysis, we examined the global prevalence and associated risk factors of PTSD in patients/survivors of COVID-19, health professionals, and the population at large.
Design
Meta-analysis.
Data Source
Cochrane, CINAHL, Embase, MEDLINE, PubMed, Scopus, Web of Science, and manual search up to June 2021.
Methods
We included studies evaluating the prevalence of PTSD during the COVID-19 pandemic in either patients/survivors, health professionals, and the population at large. The data were analyzed using logit transformation with the random-effects model. Risk of bias assessment was conducted using Hoy and colleagues.
Results
A total of 63 studies (n = 124,952) from 24 different countries were involved. The overall pooled estimate of PTSD prevalence was 17.52% (95% CI 13.89 to 21.86), with no evidence of publication bias (t=-0.22, p-value=0.83). This study found a high prevalence of PTSD among patients with COVID-19 (15.45%; 95% CI 10.59 to 21.99), health professionals (17.23%; 95% CI 11.78 to 24.50), and the population at large (17.34%; 95% CI 12.21 to 24.03). Subgroup analyses showed that those working in COVID-19 units (30.98%; 95% CI, 16.85 to 49.86), nurses (28.22%; 95% CI, 15.83 to 45.10), those living in European countries (25.05%; 95% CI 19.14 to 32.06), and studies that used Clinician-Administered PTSD Scale for DSM-5 (30.18%, 95% CI 25.78 to 34.98) demonstrated to have the highest PTSD prevalence compared to other subgroups. Meta-regression analyses revealed that the elderly (above age 65) had lower PTSD prevalence (-1.75, 95% CI -3.16 to -0.34) than the adult population.
Conclusion and Implications
Substantial PTSD prevalence was found in patients with COVID-19, health professionals, and the population at large. Moderator analysis revealed that age, unit of work, health profession, continent, and assessment tools as significant moderators. Mental health services are needed for everyone, especially adults under the age of 65, those who work in COVID-19 units, nurses, and people in the European continent.
Registration
The study protocol was registered with the International database of prospective registered systematic reviews (PROSPERO): CRD42020218762.
Tweetable abstract: The pooled PTSD prevalence during COVID-19 pandemic for patients with COVID-19, health professionals, and the population at large was 17.52%.
Keywords: PTSD, COVID-19, Meta-analysis, Prevalence, Risk factors
What is already known
-
•
Previous meta-analyses focused more on the prevalence of psychological effects without further analysis on associated PTSD's risk factors.
-
•
PTSD prevalence rates in previous meta-analyses were retrieved from a small number of studies.
What the paper adds
-
•
Patients/survivors of COVID-19, health professionals, and the population at large were found to have substantial rates of PTSD, with the overall pooled estimate of PTSD prevalence being 17.52% (95% CI 13.89–21.86) during the COVID-19 pandemic.
-
•
Adults under the age of 65, those who work in COVID-19 units, nurses, and people from the European continent had a higher risk for developing PTSD during the COVID-19 pandemic.
1. Introduction
The infection of severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2) or what is now widely known as COVID-19 is the latest coronavirus outbreak after Severe Acute Respiratory Syndrome in 2003 and Middle East Respiratory Syndrome in 2012. Since the start of January 2020, over 180 million COVID-19 cases were reported with 3 million confirmed deaths worldwide (WHO, 2020). According to the WHO, the reproductive number is estimated to be around 2–4 for COVID-19, which is higher than influenza.
Quarantine and lockdown restrictions have been placed on populations worldwide in an attempt to stop the spread of COVID-19 (WHO, 2020). However, these social interaction restrictions, along with the high numbers of infection and deaths have negatively impacted the psychological well-being of individual and society (Asim et al., 2020). Thus, long-term psychological consequences of COVID-19 among vulnerable populations should be considered a major problem (Chirico and Ferrari, 2021). Studies conducted after the previous coronavirus outbreaks found that posttraumatic stress disorder (PTSD) (Fan et al., 2021), depression, anxiety (Rogers et al., 2020), and burnout (Magnavita et al., 2021) were the most common mental health problems and long-term consequences of these outbreaks. During Severe Acute Respiratory Syndrome pandemic, the prevalence of PTSD ranged between 5% to 18% (Salehi et al., 2021; Wu et al., 2005, 2009) while the prevalence of Middle East Respiratory Syndrome was higher ranging between 36% to 42.9% (Park et al., 2020; Salehi et al., 2021).
The “Population Exposure Model” developed by Deborah DeWolfe for the Department of Health and Human Services espouses that different segments of the population may be more or less affected based on exposure to the traumatic event (DeWolfe, 2004). The model considers a community perspective as well as individual psychological effects. It is believed that the individuals who are most personally, physically, and psychologically exposed to a traumatic event are likely to be affected the most. This model further observed the macro-view of the entire community and the gradation of trauma effect across population groups (U.S Department of Health and Human Services, 2004). Based on the population exposure model, we proposed that three population groups should be analysed to assess the PTSD associated effects of the COVID-19 pandemic into those directly exposed or affected (patients/survivors), those who witness the suffering of those affected (health professionals), and everyone else not in the previous categories as the population at large.
People infected by COVID-19 may experience feelings of trauma due to the hospitalization and the disease itself and also stigmatization from family and friends after recovery or release from quarantine due to the viral nature of the outbreak. Health professionals, such as doctors, nurses, and paramedics, who work on the frontline, are also seen as a vulnerable group during the pandemic (Javed et al., 2020). Fear, work overload, shortage of self-protection gear and medication, deaths of colleagues, and isolation from family and friends can increase the risk for mental health problems in this population (Marshall, 2020). Furthermore, demographic characteristics and different numbers of COVID-19 cases in each country could also have different effects on mental health problems globally. Children, adolescents, older adults, and people with disabilities are considered to be vulnerable populations during the COVID-19 pandemic. Being away from school, friends, and colleagues, distance from family, staying at home for an extended period, lack of knowledge about the disease, and having a weaker immune system could result in more negative outcomes among these groups (Javed et al., 2020; Vahia et al., 2020). Thus, more nuanced analyses with these differentiated population groups could provide better information to improve the management and treatment of mental health problems.
Diagnostic and Statistical Manual of Mental Disorders 5th edition defines PTSD as “exposure to death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence” (American Psychiatric Association, 2013). Current research shows that PTSD can be measured using either interview or self-report assessment tools, although the Clinician Administered PTSD Scale is recognized as the gold standard in PTSD assessment (Weathers et al., 2013). Current evidence shows that numerous instruments have also been developed to measure the diagnosis and symptoms severity of PTSD in the clinical setting. Although meta-analyses on the prevalence of PTSD during the COVID-19 outbreak have been conducted either specifically in this period (Arora et al., 2020) or in comparisons with PTSD prevalence during Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome pandemic (Park et al., 2020; Salehi et al., 2021; Vos, 2020), the number of studies included in these meta-analyses were limited. Thus, a more updated and comprehensive meta-analysis on the most current prevalence of PTSD for those directly exposed and those who indirectly witnessed COVID-19 and their associated factors is needed to provide a more detailed perspective on the impact that COVID-19 has had on people. Therefore, this study aimed to examine the prevalence of PTSD during the COVID-19 pandemic among patients/survivors of COVID-19, health professionals, and the population at large, along with the associated risk factors as valuable information for developing better interventions and management of PTSD for the COVID-19 pandemic.
2. Methods
2.1. Search strategy and eligibility criteria
This study was registered to the international database of prospective registered systematic reviews (PROSPERO) with registration number: CRD42020218762. Reporting of our study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (Moher et al., 2015). A comprehensive literature search without language restrictions was conducted in seven databases, including Cochrane library, CINAHL, Embase, Medline-Ovid, PubMed, Scopus, and Web of Science up to June 2021. Manual search in the references list of previously published meta-analyses or systematic reviews was also done, and identified potential studies were searched in Google scholar to find more eligible studies. The search was conducted using combination keywords ‘prevalence’ OR 'incident' OR ‘incidence’ OR 'rate’ OR 'number' OR 'proportion' OR 'probability' AND ‘posttraumatic stress disorder’ OR 'post-traumatic stress disorder' OR 'PTSD' AND 'Covid-19′ OR 'Covid 19′ OR 'coronavirus 19′ OR "SARS Cov 19′ OR 'SARS-COV-2 (Supplementary Table 1).
This study focused on PTSD prevalence measured during the COVID-19 pandemic. More specifically, we only included studies that (1) measured PTSD prevalence as the outcome, (2) all participants diagnosed with COVID-19, either patients/survivors, health professionals (including auxiliary workers) and population at large, (3) PTSD condition can be diagnosed either using a standardized mental health diagnostic manual (DSM-III, DSM III-R, DSM-IV, DSM-IV-R, DSM-5, ICD-10) or those using validated PTSD assessment tools based on the recognized threshold. Articles were excluded if they were (1) not relevant to the topic, (2) PTSD not related to COVID-19 pandemic, (3) irrelevant study designs, (4) study protocol, (5) meta-analysis/systematic reviews, (6) studies that did not provide sufficient data and (7) studies published in different articles with duplicate participants. Regarding the study design, this study included only observational studies, either cohort or cross-sectional. Cohort study is an approach to follow study participants over a period of time after being exposed to certain risk factors (Barrett and Noble, 2019). While cross-sectional refers to a study that measures the outcome as well as the exposures in study participants at the same time (Setia, 2016).
2.2. Data extraction and quality assessment
All databases were comprehensively searched, and articles were screened using EndNote version 9.3 software. After removal of duplicates, articles were screened by title and abstract and then eligible studies were screened by full text. All the data from the eligible studies in the analysis were extracted using standard pre-designed tables with study and participant characteristics. In order to evaluate the study quality, the risk of bias assessment tool developed by Hoy and colleagues, which determines the internal and external validity for prevalence studies was used (Hoy et al., 2012). This is a 10-item assessment tool with each item rated as 1 for low risk and 0 for high risk and the overall scores ranging from 0 to 10 with the assessment conducted by two independent raters. The overall quality of the included eligible studies was categorised based on the risk of bias rated as low (9–10), moderate (7–8), and high (0–6) risk of bias. Two reviewers independently appraised the included studies. The two reviewers met to discuss their results and come to a consensus for each item on the checklist for each study. A third reviewer was consulted if there was a discrepancy in data extraction between the two primary reviewers and a consensus regarding the information was needed.
2.3. Data analysis
Data analyses were conducted using the metaprop module in R software package version 4.0.2. The data was analyzed using logit transformation random-effects model to account for the variability and heterogeneity of prevalence rates among the included studies (Lin and Xu, 2020). The prevalence of PTSD was pooled for the overall population and then divided into three groups according to the population exposure model: patients/survivors, health professionals, and the population at large. The main outcomes were presented in proportion format with corresponding 95% confidence interval (95%CI) and 95% prediction interval (95%PrI) along with statistical heterogeneity results (Tau2, I2, Q-statistic, and p-value). I2 value of ≤25% indicated low heterogeneity, ≥25% to ≤75% indicated moderate heterogeneity, and ≥75% indicated high heterogeneity (Higgins et al., 2003).
When high statistical heterogeneity is observed among the included studies, moderator analysis with sub-group and meta-regression were used to find moderator variables that can help explain the observed heterogeneity. The following pre-specified participants’ characteristics (gender, age group, marital status, education level, unit of work, profession specifically for health professionals, and population type) and study-related groups (country, continent, gross domestic product, total COVID-19 case, and total death number) were used in the moderator analysis. A p-value less than 0.05 indicates a significant moderator effect of the categorical or continuous variables.
In order to assess for potential publication bias among the included studies, the Peter's method was used for this study (Peters et al., 2006). This method is based on weighted linear regression on the inverse of total sample single proportion where a p-value of less than 0.1 indicates the existence of publication bias. Furthermore, sensitivity analysis was conducted to evaluate the robustness of the study findings. First, we excluded studies with moderate and high risk of bias based on the study quality. Second, we excluded studies using non-recommended assessment tools according to PTSD guidelines by the American Psychological Association (American Psychological Association, 2020). There are 11 assessment tools recommended by the American Psychological Association in their PTSD guidelines including the Clinician-Administered PTSD Scale for DSM-5, PTSD symptom scale interview (PSS-I and PSS-I-5), Structured Clinical Interview; PTSD module (SCID PTSD module), Structured Interview for PTSD (SIP or SI-PTSD), Treatment-outcome Posttraumatic stress disorder scale, Davidson Trauma Scale, Impact of Event Scale, Mississippi Scale for Combat-Related PTSD (MISS or M-PTSD), Modified PTSD Symptom Scale, PTSD Checklist for DSM-5, PTSD Symptom Scale self-report version, and Short PTSD Rating Interview (American Psychological Association, 2020).
3. Results
3.1. Selection of studies
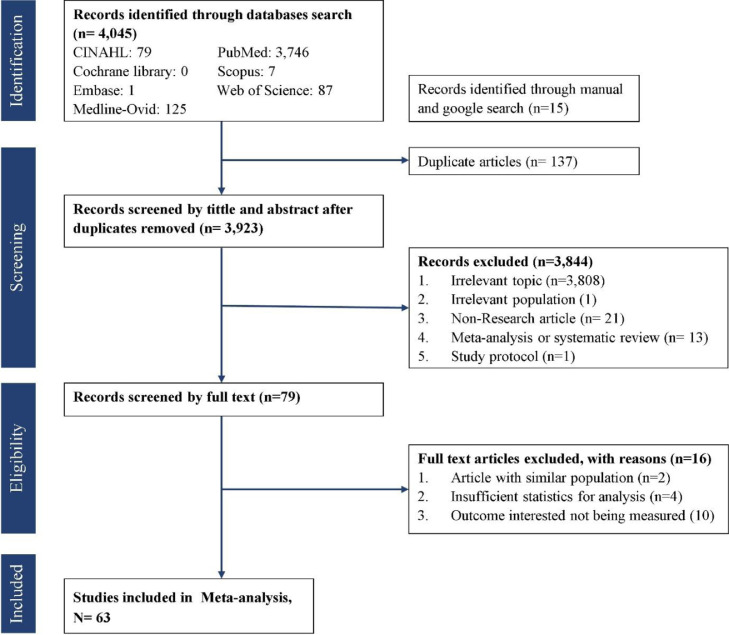
A total of 4045 studies were retrieved from the databases of Cochrane library, CINAHL, Embase, Medline-Ovid, PubMed, Scopus, and Web of Science. Fifteen articles were found through manual search in Google scholar and previous meta-analyses (Arora et al., 2020; Li et al., 2021; Yuan et al., 2021) up to June 2021. About 79 full-text articles were retrieved for further consideration. Finally, 63 studies were included in the final analysis (Fig. 1 )
Fig 1.
PRISMA flow diagram.
A total of 72 proportion estimates from 63 studies were used with 124,952 participants in various population and countries. About 11 (15.3%) proportion estimates PTSD prevalence among COVID-19 patients/survivors, 24 (33.3%) health workers (medical nurse, nurse, clinical psychologist, physiotherapist, medical assistances, administration staff), 36 (50%) population at large (pregnant women, college students, generally healthy population, psychiatric patients, young people, cancer patients, rheumatoid arthritis patients, multiple sclerosis patients, academic staff, workers), and 1 (1.4%) mixed population were included in the analysis. Studies were conducted in 24 different countries with most of the studies conducted in mainland China (47%), around February to April (75%), all published in 2020, and a majority used PTSD checklist as diagnostic assessment tools (44.4%) (Table 1 ).
Table 1.
Data extraction of included studies of PTSD prevalence during COVID-19 pandemic.
| No | Author (year) | Study setting | Study design | Diagnostic criteria | Study population | Sample size | PTSD prevalence | Study population characteristics | Time of study | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alshehri et al., 2020 | Saudi Arabia | Cross-sectional | PCL-S | Population at large | 1374 | 22.63% | Mean age: NARange age: 18->55GenderMale: 674 (49.05%)Female: 700 (50.95%) | June 2020 | 9 – L |
| 2 | Berthelot et al., 2020 | Canada | Cohort | PCL-5 | Population at large | 1258 | 1.19% | Mean age: 29.27Range age: 18–46GenderMale: -Female: 1258(100%) | April 2020 | 9 – L |
| 3 | Blekas et al., 2020 | Greece | Cross-sectional | PTSD-8 | Health professionals | 270 | 16.67% | Mean age: 37.61Range age: NAGenderMale: 71 (22.9%)Female: 199 (77.1%) | April 2020 | 9 – L |
| 4 | Cai et al., 2020 | China | Cross-sectional | PTSD-SS | Patients/survivor | 126 | 30.95% | Mean age:45.7Range age: 11–72GenderMale: 60 (47.6%)Female: 66 (52.4%) | February-March 2020 | 8 – M |
| 5 | Caillet et al., 2020 | France | Cross-sectional | IES-R | Health professionals | 208 | 25% | Mean age: 35Range age: NAGenderMale: 52 (25%)Female: 156 (75%) | April 2020 | 7 – M |
| 6 | Castelli et al., 2020 | Italy | Cross-sectional | PCL-5 | Population at large | 1321 | 20% | Mean age: 35.1Range age: NAGenderMale: 399 (31%)Female: 922(69%) | March-April 2020 | 8 – M |
| 7 | Chang et al., 2020 | South Korea | Cross-sectional | PCL-5 | Patients/survivor | 64 | 20.31% | Mean age:54.7Range age: NAGenderMale: 28 (43.7%)Female: 36 (56.3%) | February-April 2020 | 8 – M |
| 8 | Chew et al., 2020 (a) | Singapore | Cross-sectional | IES-R | Health professionals | 277 | 12.27% | Mean age: 35Range age: NAGenderMale: 84 (30.3%)Female: 193 (69.7%) | April-June 2020 | 9 – L |
| Chew et al., 2020 (b) | India | Cross-sectional | IES-R | Health professionals | 384 | 2.08 | Mean age: 27.7Range age: NAGenderMale: 133 (34.5%)Female: 251 (65.4%) | April-June 2020 | 9 – L | |
| Chew et al., 2020 (c) | Malaysia | Cross-sectional | IES-R | Health professionals | 175 | 6.29% | Mean age: 32.4Range age: NAGenderMale: 57 (32.6%)Female: 118 (67.4%) | April-June 2020 | 9 – L | |
| Chew et al., 2020 (d) | Vietnam | Cross-sectional | IES-R | Health professionals | 60 | 15% | Mean age: 34.7Range age: NAGenderMale: 16 (26.7%)Female: 44 (73.3%) | April-June 2020 | 9 – L | |
| Chew et al., 2020 (e) | Indonesia | Cross-sectional | IES-R | Health professionals | 250 | 11.60% | Mean age: 33.2Range age: NAGenderMale: 110 (44%)Female: 140 (56%) | April-June 2020 | 9 – L | |
| 9 | Chew, Nicolas et al., 2020 (a) | Singapore | Cross-sectional | IES-R | Health professionals | 480 | 7.5% | Mean age: 29Range age: 25–35GenderMale: NAFemale: NA | February-April 2020 | 9 – L |
| Chew, Nicolas et al., 2020 (b) | India | Cross-sectional | IES-R | Health professionals | 426 | 7.28% | Mean age: 29Range age: 25–35GenderMale: NAFemale: NA | February-April 2020 | 9 – L | |
| 10 | Chi et al., 2020 | China | Cross-sectional | PCL | Population at large | 2038 | 30.81% | Mean age: 20.56Range age: NAGenderMale: 755 (37%)Female: 1283 (63%) | February 2020 | 9 – L |
| 11 | Czeisler et al., 2020 | United States | Cross-sectional | IES-6 | Population at large | 5470 | 4.59% | Mean age: NARange age: 18–44GenderMale: 2676 (48.9%)Female: 2784 (50.9%)Others: 10 (0.2%) | April-June 2020 | 9 – L |
| 12 | DiCrosta et al., 2020 | Italy | Cross-sectional | IES-R | Population at large | 1253 | 35.59% | Mean age: 39.48Range age: 18–65GenderMale: 445 (35.5%)Female: 808 (64.5%) | April 2020 | 9 – L |
| 13 | Einvik et al., 2021(a) | Norway | Cross sectional | PCL-5 | Patients/survivor (hospitalised) | 125 | 9.5% | Mean age: NARange age: NAGenderMale: NAFemale: NA | June 2020 | 7 – M |
| Einvik et al., 2021(b) | Norway | Cross sectional | PCL-5 | Patients/survivor (non-hospitalised) | 458 | 7.0% | Mean age: NARange age: NAGenderMale: NAFemale: NA | June 2020 | 7 – M | |
| 14 | Fekih-Romdhane et al., 2020 | Tunisia | Cross sectional | IES-R | Population at large | 603 | 33.0% | Mean age: 29.2Range age: >18GenderMale: 157 (26%)Female: 446 (74%) | April 2020 | 8 – M |
| 15 | Forte et al., 2020 | Italy | Cross sectional | IES-R | Population at large | 2291 | 27.72% | Mean age: 30.0Range age: 18–89GenderMale: 580 (25.2%)Female: 1708 (74.6%)Other: 3 (0.2%) | March 2020 | 9 – L |
| 16 | Giusti et al., 2020 | Italy | Cross sectional | IES-6 | Health professionals | 330 | 36.67% | Mean age: 44.6Range age: 18–89GenderMale: 124 (37.4%)Female: 206 (62.6%) | May 2020 | 8 – M |
| 17 | Gonzaler-Sanguino et al., 2020 | Spain | Cross sectional | PCL-C-2 | Population at large | 3480 | 13.97% | Mean age: 37.92 Range age: 18–80GenderMale: 870 (25%)Female: 2610 (75%) | March 2020 | 9 – L |
| 18 | Goularte et al., 2021 | Brazil | Cross-sectional | IES-R | Population at large | 1996 | 34.22% | Mean age: 34.22Range age: NAGenderMale: 320 (15.5%)Female: 1676 (84.5%) | May-July 2020 | 9 – L |
| 19 | Gu et al., 2020 | China | Cross-sectional | IES-R | Covid-19 patients | 461 | 24.95% | Mean age: NARange age: 18->50GenderMale:162 (35.1%)Female: 299 (64.9%) | February 2020 | 8 – M |
| 20 | Guo, Qian et al., 2020 | China | Cross-sectional | PCL-5 | Patients/survivors | 103 | 7.8% | Mean age: 42.50Range age: 18–75GenderMale:59 (57.3%)Female: 44 (42.7%) | February 2020 | 9 – L |
| 21 | Guo, Jing et al., 2020 | China | Cross-sectional | PCL-5 | Mixed population | 2441 | 72.6% | Mean age: NARange age: 18->51GenderMale:1172 (48%)Female: 1296 (52%) | February 2020 | 9 – L |
| 22 | Hao et al., 2020 (a) | China | Cross-sectional | IES-R | Population at large | 76 | 31.58% | Mean age: 32.8Range age: NAGenderMale: 25 (32.9%)Female: 51 (37.1%) | February 2020 | 8 – M |
| Hao et al., 2020 (b) | China | Cross-sectional | IES-R | Population at large | 109 | 13.76% | Mean age: 33.1Range age: NAGenderMale: 41 (37.6%)Female: 68 (62.4%) | February 2020 | 8 – M | |
| 23 | Huang, et al., 2020 | China | Cross-sectional | PTSD-SS | Health professionals | 230 | 27.39% | Mean age: 32.6Range age: -GenderMale: 43 (18.7%)Female: 187 (61.3%) | February 2020 | 8 – M |
| 24 | Janiri et al., 2021 | Italy | Cross-sectional | CAPS-5 | Patients/survivors | 381 | 30.2% | Mean age: 53.1Range age: NAGenderMale: 51 (44.3%)Female: 64 (55.7% | April-October 2020 | 8 – M |
| 25 | Johnson et al., 2020 | Norway | Cross-sectional | PCL-5 | Health professionals & public service providers | 1773 | 11.68% | Mean age: NARange age: 18->60GenderMale: 166 (15.3%)Female: 1507 (84.7%) | March-April 2020 | 10 – L |
| 26 | Joseph et al., 2020 | Saudi Arabia | Cross-sectional | IES-6 | Population at large | 584 | 59.93 | Mean age: NARange age: 15–44GenderMale: 361 (61.8%)Female: 223 (38.2%) | April-May 2020 | 9 – L |
| 27 | Karatzias et al., 2020 | Ireland | Cross-sectional | ITQ | Population at large | 1041 | 17.68% | Mean age: NARange age: 15->65GenderMale: 505 (48.5%)Female: 536 (51.5%) | March 2020 | 9 – L |
| 28 | Lahav, 2020 | Israel | Cross-sectional | PCL-5 | Population at large | 976 | 5.53% | Mean age: 33.1Range age: NAGenderMale: 180 (18.4%)Female: 796 (81.6%) | April 2020 | 9 – L |
| 29 | Leng et al., 2020 | China | Cross-sectional | PLC—C | Health professionals | 90 | 5.6% | Mean age: NARange age: 20–40GenderMale: 25 (17.8%)Female: 65 (72.2%) | March 2020 | 9 – L |
| 30 | Liang et al., 2020 (a) | China | Cross-sectional | PCL-C | Population at large | 570 | 12.81% | Mean age: NARange age: 14–35GenderMale: 205 (36%)Female: 365 (64%) | January 2020 | 9 – L |
| 31 | Liang et al., 2020 (b) | China | Cross-sectional | PCL-C | Population at large | 584 | 14.38% | Mean age: NARange age: 14–35GenderMale: 223 (38.2%)Female: 361 (61.8%) | January 2020 | 9 – L |
| 32 | Li, Q, 2020 | China | Cross-sectional | IES-R | Population at large | 1109 | 67.09% | Mean age: NARange age: 18->60GenderMale: 622 (56.1%)Female: 487 (43.9%) | March 2020 | 9 – L |
| 33 | Li-Xuenyuan et al., 2020 | China | Cross-sectional | IES-R | Health professionals | 225 | 31.56% | Mean age: NARange age: 21–60GenderMale: 63 (28%)Female: 162 (72%) | January-March 2020 | 8 – M |
| 34 | Li Xiuchuan et al., 2020 | China | Cohort | PCL-5 | Health professionals | 356 | 61.80% | Mean age: 31.3Range age: NAGenderMale: 49 (13.8%)Female: 307 (86.2%) | January-March 2020 | 8 – M |
| 35 | Liu CH et al., 2020 | United States | Cross-sectional | PCL-C | Population at large | 898 | 4.34% | Mean age: 24.5Range age: 18–30GenderMale: 127 (14.1%)Female: 730 (81.3%)Other: 41 (4.6%) | April-May 2020 | 9 – L |
| 36 | Liu, Dong et al., 2020 | China | Cross-sectional | PCL-5 | Patients/ survivors | 675 | 12.44% | Mean age: 53.58Range age: NAGenderMale: 317 (47%)Female: 358 (53%) | April 2020 | 8 – M |
| 37 | Liu, Nianqi et al., 2020 | China | Cross-sectional | PCL-5 | Population at large | 285 | 7% | Mean age: NARange age: NAGenderMale: 130 (45.6%)Female: 155 (54.4%) | January 2020 | 9 – L |
| 38 | Luceno-Moreno et al., 2020 | Spain | Cross-sectional | IES-R | Health professionals | 1422 | 56.6 | Mean age: 43.88Range age: 19–68GenderMale: 194 (13.6%)Female: 1228 (86.4%) | April 2020 | 9 – L |
| 39 | Mazza et al., 2020 | Italy | Cross-sectional | PCL-5 | Patients/survivors | 402 | 28% | Mean age: 57.8Range age: 18–87GenderMale: 256 (63.7%)Female: 146 (36.3%) | April-June 2020 | 9 – L |
| 40 | Qi et al., 2020 | China | Cross-sectional | PCL-C | Covid-19 patients | 43 | 12.20% | Mean age: 40.01Range age: NAGenderMale: 18 (41.9%)Female: 25 (58.1%) | February 2020 | 7 – M |
| 41 | Ramirez et al., 2020 | Mexico | Cross-sectional | IES-R | Population at large | 3932 | 27.21% | Mean age: 33Range age: 18–77GenderMale: 1004 (25.5%)Female: 2928 (74.5%) | March-April 2020 | 9 – L |
| 42 | Ren et al., 2020 | China | Cross-sectional | PCL-5 | Population at large | 1172 | 6.99% | Mean age: 22Range age: NAGenderMale: 360 (30.7%)Female: 812 (69.3%) | March 2020 | 9 – L |
| 43 | Romito et al., 2020 | Italy | Cross-sectional | IES-R | Population at large | 77 | 36.36% | Mean age: 56.6Range age: 22–85GenderMale: 39 (50.6%)Female: 38 (49.4%) | April 2020 | 9 – L |
| 44 | Rossi et al., 2020(a) | Italy | Cross-sectional | GPS-PTSS | Population at large | 1379 | 49.38% | Mean age: 39Range age: NAGenderMale: 315 (22.8%)Female: 1064 (77.2%) | March 2020 | 9 – L |
| Rossi et al., 2020(b) | Italy | Cross-sectional | GPS-PTSS | Health professionals | 18,147 | 36.73% | Mean age: 38Range age: NAGenderMale: 3700 (20.4%)Female: 14,447 (79.6%) | March 2020 | 9 – L | |
| 45 | Seyahi et al., 2020 (a) | Turkey | Cross-sectional | IES-R | Health professionals | 535 | 40.93% | Mean age: 31Range age: 19–58GenderMale: 181 (33.8%)Female: 354 (66.2%) | April 2020 | 9 – L |
| Seyahi et al., 2020 (b) | Turkey | Cross-sectional | IES-R | Population at large | 1688 | 26.18% | Mean age: 38.2Range age: 16–81GenderMale: 503 (29.8%)Female: 1185 (70.2%) | April 2020 | 9 – L | |
| 46 | Shevlin et al., 2020 | United Kingdom | Cross-sectional | ITQ | Population at large | 2025 | 16.79% | Mean age: 45.44Range age: 18–83GenderMale: 978 (48.3%)Female: 1047(51.7%) | March 2020 | 9 – L |
| 47 | Si et al., 2020 | China | Cross-sectional | IES-6 | Health professionals | 863 | 40.21% | Mean age: NARange age: NAGenderMale: 253 (29.3%)Female: 610 (70.7%) | February-March 2020 | 9 – L |
| 48 | Song et al., 2020 | China | Cross-sectional | PCL-5 | Health professionals | 14,825 | 9.13% | Mean age: 34Range age: 18->40GenderMale: 5289 (35.7%)Female: 9536 (64.3%) | February-March 2020 | 9 – L |
| 49 | Sun Luna et al., 2020 | China | Cross-sectional | PCL-5 | Population at large | 2091 | 4.6% | Mean age: NARange age: 18->60GenderMale: 819 (39.2%)Female: 1272 (60.8%) | January-February 2020 | 9 – L |
| 50 | Sun Shufang et al., 2020 | China | Cross-sectional | IES | Population at large | 1912 | 67.05% | Mean age: 20.28Range age: 18–49GenderMale: 578 (30.23%)Female: 1334 (69.77%) | March-April 2020 | 9 - L |
| 51 | Tan et al., 2020 | China | Cross-sectional | IES-R | Population at large | 673 | 10.85% | Mean age: 30.8Range age: 18–83GenderMale: 501 (74.4%)Female: 172 (25.6%) | February 2020 | 8 – M |
| 52 | Tang et al., 2020 | China | Cross-sectional | PCL-C | Population at large | 2485 | 2.70% | Mean age: 19.81Range age: 16–27GenderMale: 960 (39.2%)Female: 1525 (60.8%) | February 2020 | 8 – M |
| 53 | Tarsitani et al., 2021 | Italy | Cohort | PCL-5 | Patients/survivors | 115 | 10.4% | Mean age: 58Range age: 48–67GenderMale: 2 (17%)Female: 10 (83%) | April 2020 | 9 – L |
| 54 | Tomaszek et al., 2020 | Poland | Cross-sectional | IES-R | Population at large | 184 | 69.57% | Mean age: 21.92Range age: 18–48GenderMale: 29 (15.8%)Female: 155 (84.2%) | March-April 2020 | 9 – L |
| 55 | Wang, Ya-Xi et al., 2020 | China | Cross-sectional | PCL-C | Health professionals | 202 | 16.83% | Mean age: 32Range age: 29–40GenderMale: 25 (12.4%)Female: 177 (87.6%) | February-March 2020 | 8 – M |
| 56 | Wang Ying et al., 2020 | China | Cross-sectional | IES-R | Health professionals | 1897 | 9.75% | Mean age: 34Range age: 18->40GenderMale: 332 (17.5%)Female: 1565 (82.5%) | January-February 2020 | 9 – L |
| 57 | Wang-yuan et al., 2020 | China | Cross-sectional | IES-R | Population at large | 6213 | 9.30% | Mean age: 50.57Range age: NAGenderMale: 3278 (52.8%)Female: 2935 (47.2%) | April 2020 | 8 – M |
| 58 | Wathelet et al., 2021 | France | Cross-sectional | PCL-5 | Population at large | 22,883 | 19.5% | Mean age: 21.2Range age: NAGenderMale: 925 (20.8%)Female: 3408 (76.5%)Others: 123 (2.8%) | June-July 2020 | 9 – L |
| 59 | Yin et al., 2020 | China | Cross-sectional | PCL-5 | Health professionals | 371 | 3.8% | Mean age: 35.3Range age: 18–60GenderMale: 143 (38.5%)Female: 228 (61.5%) | February 2020 | 9 – L |
| 60 | Zanghi et al., 2020 | Italia | Cross-sectional | SSS-DSM-IV | Population at large | 432 | 31.71% | Mean age: 40.4Range age: NAGenderMale: 155 (35.9%)Female: 277 (64.1%) | May 2020 | 8 – M |
| 61 | Zhang et al., 2020 | China | Cross-sectional | PCL-C | Health professionals | 642 | 20.87% | Mean age: NARange age: NAGenderMale: 96 (14.95%)Female: 546 (85.05%) | June 2020 | 8 – M |
| 62 | Zhao et al., 2020 | China | Cross-sectional | PCL-5 | Population at large | 515 | 5.63% | Mean age: NARange age: NAGenderMale: 173 (33.6%)Female: 342 (66.4%) | January-February 2020 | 9 – L |
| 63 | Zhou et al., 2020 | China | Cross-sectional | PCL-5 | Population at large | 859 | 2.68% | Mean age: 32.68Range age: 20–47GenderMale: 0Female: 859 (100%) | February-March 2020 | 8 – M |
Abbreviations: Post-traumatic stress disorder checklist-survey (PCL-S); Post-traumatic stress disorder checklist-based DSM 5(PCL-5); Clinical-administered PTSD Scale for DSM-5 (CAPS-5); Posttraumatic stress disorder-8 inventory (PTSD-8); Post-traumatic stress disorder self-rating scale (PTSD-SS); Impact Event Scale-Revision (IES-R); The abbreviated PTSD checklist (PCL); Six items Impact Event Scale (IES-6); Post-traumatic stress disorder checklist-reduced version (PCL-C-2); International Trauma Questionnaire (ITQ); The PTSD checklist-civilian (PCL-C); The global psychotrauma screen, post-traumatic stress symptoms subscale (GPS-PTSS); Impact Event Scale (IES); The short screening scale Diagnostic and Statistical Manual for Mental Disorders 4th Edition (SSS-DSM-IV); Not available (NA).
3.2. PTSD prevalence
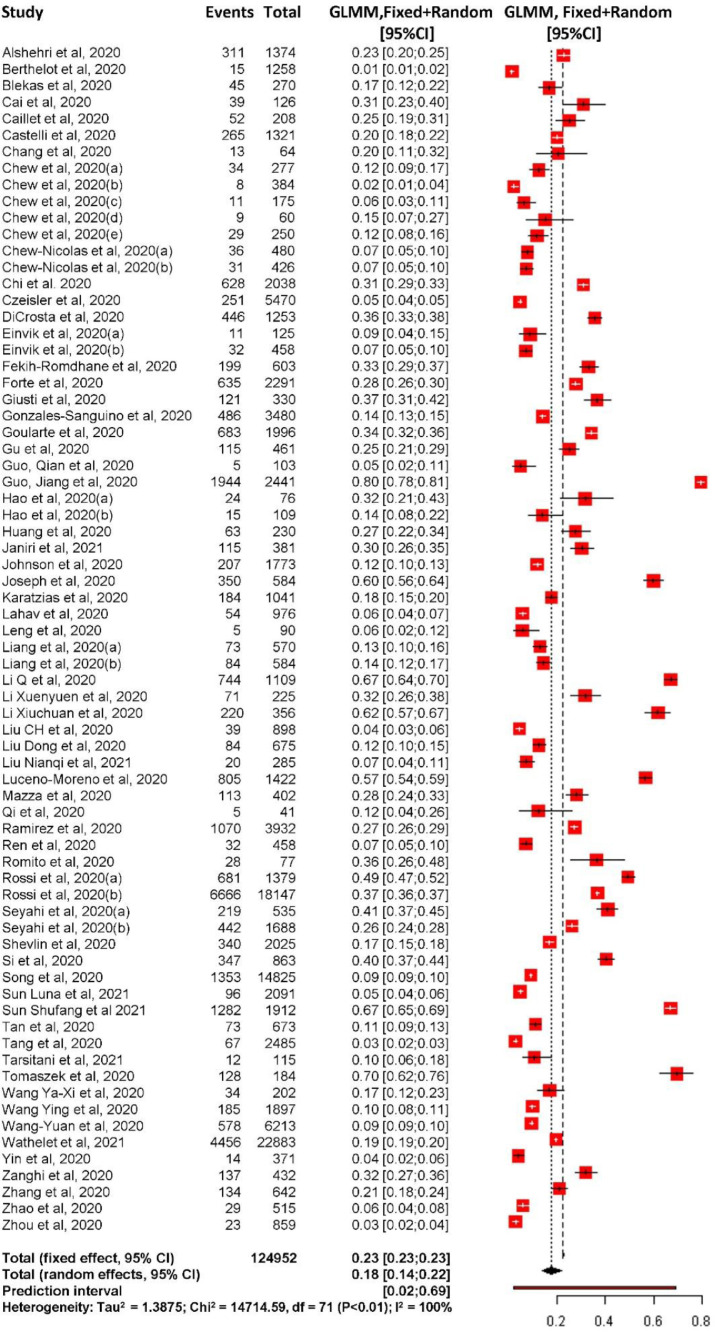
The results showed that the overall prevalence of PTSD during the COVID-19 pandemic was 17.52% (95% CI 13.89% to 21.86%) with high heterogeneity: I2=99.7% and τ2=1.39. The prediction interval showed that the proportion of PTSD in future similar studies would range between 1.96% to 69.36% (Fig. 2 ). Regarding publication bias, the regression test indicated no evidence of publication bias with t = 0.22, p-value=0.83 (Supplementary Figure 1).
Fig 2.
Forest plot overall PTSD prevalence during COVID-19 pandemic.
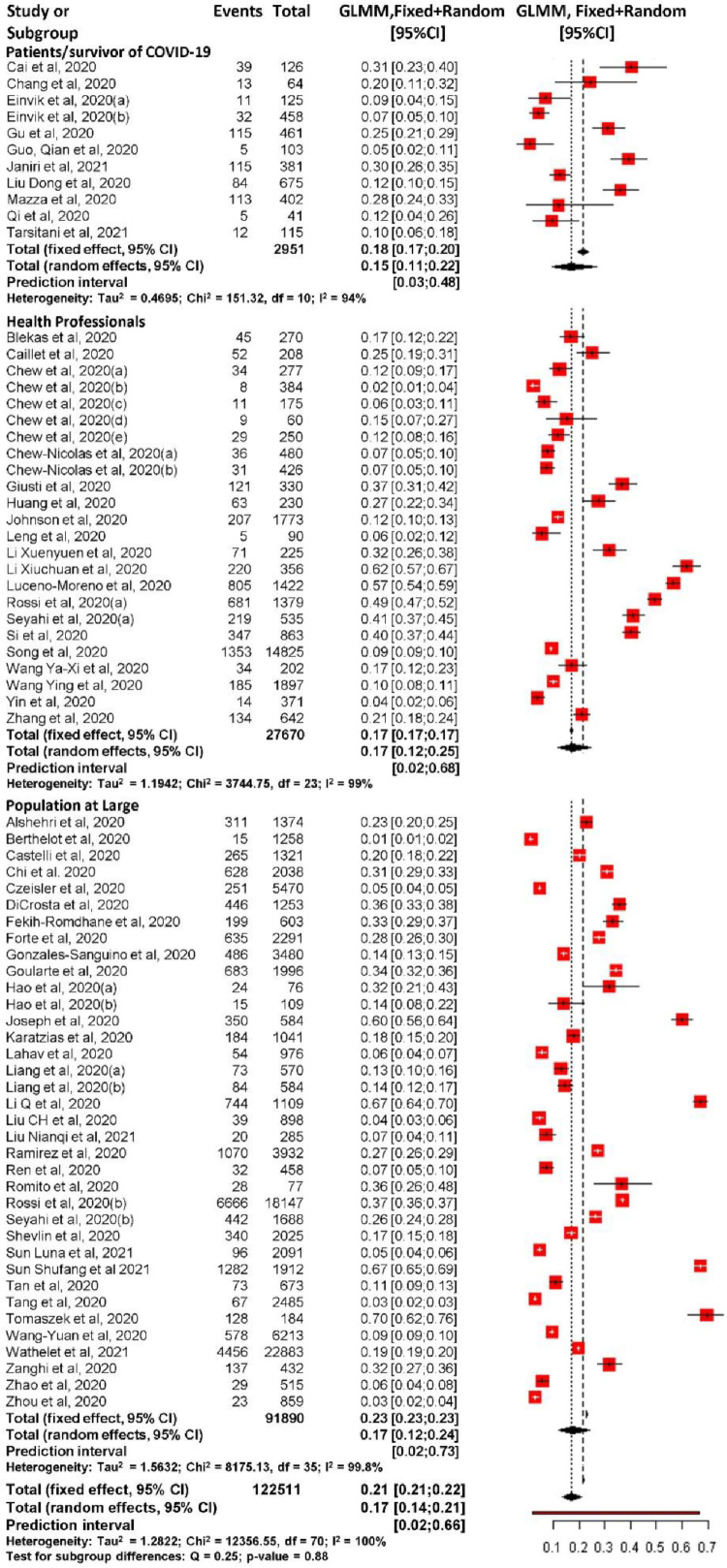
Among the three different population groups according to the population exposure model (patients/survivors of COVID-19, health professionals, and the population at large), there were no statistically significant difference in PTSD prevalence. Those with direct exposure, patients/survivors of COVID-19, had the lowest proportion of PTSD at 15.45% (95% CI 10.59 to 21.99; 95% PrI 3.46% to 48.23%) with heterogeneity: I2=94.3%, τ2=0.47. Among the witness to exposure group, or health professionals, the PTSD prevalence rate was 17.23% (95% CI 11.78 to 24.50; 95% PrI 2.02% to 67.81%) with heterogeneity: I2=99.3%, and τ2=1.19. The population at large or general population not directly exposed and not part of the health professionals had prevalence rate of 17.34% (95% CI 12.21 to 24.03; 95% PrI 1.57% to 73.40%) with heterogeneity: I2=99.8% and τ2=1.56 (Fig. 3 ).
Fig 3.
Forest plot of PTSD prevalence during COVID-19 pandemic in three different populations.
3.3. Moderator analysis
Subgroup analyses and meta-regression were conducted based on participants’ characteristics (gender, age, marital status, educational level), health professionals’ characteristics (unit of work and profession), and studies’ characteristics (countries’ continent, gross domestic product, total case, and total death case).
Regarding participants’ characteristics, subgroup analyses found age, gender, marital status, and educational level were not statistically significant moderators. While meta-regression analysis found age as the only statistically significant moderator with those in the elderly group (>65 years old) had lower PTSD prevalence (−1.75, 95% CI −3.16 to −0.34) during the COVID-19 pandemic compared to adults (18–65 years old) (Table 2 ).
Table 2.
Moderator analysis of PTSD prevalence during COVID-19 pandemic.
| Subgroup analysis |
Meta-regression analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | n of study (event sample size) | Pooled estimate% (95% CI) | I2(%) | p-value | Pooled estimate (95% CI) | p-value |
| Participants’ characteristics | ||||||
| Mean Age | 17 | – | – | – | ref | |
| −0.02 (−0.07 to 0.02) | 0.269 | |||||
| Age | ||||||
| Adult (18–65 years old) | 12 (35,799) | 25.67 (17.12 to 36.60) | 99.6 | 0.089 | ref | |
| Elderly (>65 years old) | 5 (544) | 5.68 (0.85 to 29.72) | 89.7 | −1.75 (−3.16 to −0.34) | 0.015 | |
| Gender | ||||||
| Male | 22 (12,264) | 21.86 (13.41 to 33.58) | 99.2 | 0.519 | ref. | |
| Female | 22 (30,193) | 26.21 (18.91 to 35.11) | 99.4 | 0.23 (−0.49 to 0.95) | 0.535 | |
| Marital Status | ||||||
| Single/not married | 11 (3277) | 22.90 (12.86 to 37.40) | 98.4 | 0.423 | ref | |
| Married | 11 (6455) | 30.86 (17.99 to 47.60) | 99.2 | 0.41 (−0.59 to 1.41) | 0.421 | |
| Education level | ||||||
| High school and below | 8 (2210) | 37.37 (21.59 to 56.40) | 98.2 | 0.889 | ref | |
| Bachelor and over | 8 (4238) | 35.73 (22.77 to 51.17) | 99.3 | −0.07 (−1.07 to 0.93) | 0.888 | |
| Health worker | ||||||
| Unit of work | ||||||
| Not work in Covid-19 unit | 3 (1670) | 13.16 (6.79 to 23.96) | 93.8 | 0.049 | ref | |
| Work in Covid-19 unit | 4 (1420) | 30.98 (16.85 to 49.86) | 97.3 | 1.08 (−0.05 to 2.20) | 0.060 | |
| Health profession | ||||||
| Medical doctor | 4 (830) | 10.80 (6.12 to 18.38) | 84.3 | 0.003 | ref | |
| Nurse | 5 (2422) | 28.22 (15.83 to 45.10) | 97.8 | 1.18 (0.21 to 2.15) | 0.017 | |
| Others | 1 (65) | 7.69 (4.42 to 12.19) | – | −0.39 (−2.04 to 1.25) | 0.637 | |
| Study Characteristics | ||||||
| Countries’ continent | ||||||
| Asia | 44 (50,798) | 15.50 (11.29 to 20.92) | 99.4 | 0.017 | ref. | |
| Europe | 22 (13, 554) | 25.05 (19.14 to 32.06) | 99.6 | 0.59 (0.01 to 1.17) | 0.046 | |
| America | 5 (59,997) | 8.08 (2.47 to 23.37) | 99.8 | −0.73 (−1.78 to 0.32) | 0.173 | |
| Countries GDP | ||||||
| Low income | 4 (1473) | 9.88 (3.37 to 25.61) | 96.9 | 0.342 | ref. | |
| Upper middle income | 37 (51,851) | 17.05 (12.15 to 23.41) | 99.6 | 0.63 (−0.60 to 1.86) | 0.313 | |
| High income | 31 (71,378) | 19.35 (13.93 to 26.23) | 99.7 | 0.78 (−0.46 to 2.03) | 0.217 | |
| Countries’ total case | ||||||
| Non top 10 country | 49 (57,409) | 15.73 (11.62 to 20.94) | 99.5 | 0.142 | ref. | |
| Top 10 country | 23 (67,159) | 21.84 (15.65 to 29.61) | 99.8 | 0.09 (−0.13 to 0.28) | 0.356 | |
| Countries’ total death | ||||||
| Non top 10 country | 50 (55,316) | 16.07 (11.92 to 21.31) | 99.4 | 0.223 | ref. | |
| Top 10 country | 22 (69,252) | 21.18 (15.00 to 29.03) | 99.8 | 0.22 (−0.08 to 0.51) | 0.158 | |
| Assessment tools | ||||||
| CAPS-5 | 1 (381) | 30.18 (25.78 to 34.98) | 0.00 | <0.0001 | ref | |
| PCL (5/S/C/C2) | 21 (64,758) | 10.60 (6.39 to 17.09) | 99.7 | −1.29 (−3.60 to 1.03) | 0.276 | |
| IES (R/6) | 26 (36,163) | 21.68 (15.49 to 29.47) | 99.4 | −0.43 (−2.75 to 1.86) | 0.705 | |
Abbreviations: Study size (n); Confidence Interval (CI); Gross Domestic Product (GDP); Reference (ref); Clinical-Administered PTSD Scale for DSM-5 (CAPS-5); Post-Traumatic Stress Disorder Checklist-5/Survey/Civilian/Reduced version (PCL-5/S/C/C2); Impact Event Scale-Revision/6 (IES-R/6).
Note: Significancy level <0.05.
According to health professionals’ characteristics, subgroup analyses found the unit of work and health profession as significant moderators. Health professionals who worked in COVID-19 units showed higher PTSD prevalence (30.98%, 95% CI 16.85 to 49.86) compared to those who did not work in COVID-19 units (13.16%, 95% CI 6.79 to 23.96). Among health professionals, nurses were found to have the highest PTSD prevalence with (28.22%, 95%CI 15.83 to 45.10), followed by medical doctors (10.80%, 95% CI 6.12 to18.38), and others (physiotherapists, care assistants, and admission staff) (7.69%, 95% CI 4.42 to 12.19). Meta-regression analysis also showed that nurses had a higher PTSD prevalence (1.18, 95% CI 0.21 to 2.15) compared to medical doctors (Table 2).
According to studies’ characteristics, subgroup analyses found the study's continent and assessment tools as significant moderators while countries’ GDP, total case, and death case were not. Regarding the continents, Europe showed the highest prevalence of PTSD (25.05%, 95% CI 19.14 to 32.06) compared to Asia (15.50%, 95% CI 11.29 to 20.92) and America (8.08%, 95% CI 2.47 to 23.37). Furthermore, assessment tools were also found as a significant moderator. Studies that used Clinician-Administered PTSD Scale for DSM-5 showed the highest PTSD prevalence (30.18%, 95% CI 25.78 to 34.98) compared to PTSD Checklist/for DSM-5/S/C/C2 (10.60%, 95% CI 6.39 to 17.09), and Impact of Event Scale/Revised/6 (21.68%, 95% CI 15.49 to 29.47). Meta-regression analysis showed that people who lived in Europe had higher PTSD prevalence (0.59, 95% CI 0.01 to 1.17) than those who lived in Asia (Table 2).
3.4. Quality assessment
All included studies were evaluated using the ten items risk of bias tool developed specifically for prevalence meta-analysis by Hoy and colleagues. Two independent raters conducted the evaluation, and there was no disagreement between raters for each article included in this study. Overall results showed 41 (65.1%) and 22 (34.49%) studies were classified as low and moderate risk of bias.
3.5. Sensitivity analysis
Sensitivity analyses was conducted according to the studies’ quality and assessment tools used. According to study quality, 22 studies with moderate risk of bias were excluded, the results were not significantly different with prevalence of PTSD 16.93% (95% CI 12.46 to 22.60; 95% PrI 1.55 to 72.53). Based on instruments used in studies included, after four studies measuring PTSD using posttraumatic stress disorder-8 inventory, The global psychotrauma screen, post-traumatic stress symptoms subscale and The short screening scale Diagnostic and Statistical Manual for Mental Disorders 4th Edition were excluded, studies were categorized into Clinician-administered PTSD Scale for DSM-5, PTSD checklist/for DSM-5/S/C/C2, and Impact of event scale/Revised/6. The result showed no significant difference in prevalence of PTSD, 16.83% (95% CI 13.20 to 21.21; 95%PrI1.82 to 68.82).
4. Discussion
To our knowledge, this is the first meta-analysis to include the prevalence of PTSD in different segments of the population according to the population exposure model with higher rates of PTSD during COVID-19 in a comprehensive review using 63 studies with 124,952 participants. Although almost half of the studies included were conducted in mainland China, our study includes data from 23 other countries representing three continents: Asia, America, and Europe. Substantial prevalence rates were found across groups of patients/survivors of COVID-19, health professionals, and the population at large. This study also determined age, working unit, health profession, continent, and PTSD assessment tools as significant moderators for PTSD prevalence during the COVID-19 pandemic.
4.1. Main findings
This study found that the COVID-19 pandemic has affected all populations who were either directly or indirectly exposed to the disease. This study's overall pooled PTSD prevalence was higher than the prevalence rate found during the Severe Acute Respiratory Syndrome pandemic. A study from China in 2009 found the prevalence rate of PTSD of 131 Severe Acute Respiratory Syndrome survivors to be about 4% and 5% at one and three months after discharge, respectively (Wu et al., 2005). However, the higher PTSD prevalence during the COVID-19 pandemic might be due to its high reproductive number. Although the mortality rate of COVID-19 (13%) (Abdelghany et al., 2021) might be less than Severe Acute Respiratory Syndrome (15%) (Chan-Yeung and Xu, 2003) and Middle East Respiratory Syndrome (35%) (WHO, 2019), yet the reproductive number of COVID-19 is relatively high (1.8–3.6) (WHO, 2019) when compared to Severe Acute Respiratory Syndrome (1.7–1.9) and Middle East Respiratory Syndrome (<1) (Petrosillo et al., 2020). This higher reproductive number can be seen in the higher number of COVID-19 cases. According to the WHO, the duration of COVID-19 is currently longer than previous coronavirus outbreaks, whereas the Severe Acute Respiratory Syndrome outbreak ended eight months after the first case was reported (WHO, 2015), while for COVID-19, the pandemic is still spreading more than a year since the first reported case.
4.1.1. Prevalence rate according to population exposure
The current study results indicate that there are similar and considerable rates of PTSD for both those who are directly or indirectly exposed to COVID-19. For those directly exposed to COVID −19, patients could experience trauma from procedures such as respiratory failure and tracheotomy. This result is supported by previous studies, which showed that either direct or indirect exposure to trauma could lead to PTSD (Lee et al., 2017; May and Wisco, 2016; Szogi and Sullivan, 2018). The more severe physical symptoms and a longer hospitalization period may also lead to more trauma for patients diagnosed with COVID-19. Thus, mental health support is crucial for this population.
The findings of the current meta-analysis revealed that the prevalence of PTSD among health professionals was higher than patients/survivors. The current study findings demonstrate a higher prevalence of PTSD of 15.5% among health professionals compared to a previous study with 11.9% (Chirico et al., 2021). In addition, other studies have shown that health professionals faced higher number of traumatic incidences compared to other professionals in the social and trading sectors (Magnavita et al., 2021). Health professionals play an essential role in the pandemic as the frontline responders. Hospitals and clinics being the service centers for patients affected by the COVID-19 virus and overcrowding when the case counts are high leaves health professionals without enough time for rest and relaxation. Initially, uncertainty about COVID-19 and the lack of guidelines for taking care of the patients resulted in feelings of frustration and anger among health professionals. This may have generated moral injury that could be considered as a serious threat to mental stability (Chirico et al., 2020). However, as frontline service providers with experience in health care services, some professionals may have a higher ability to process the trauma from COVID-19 and have positive results such as post-traumatic growth. A previous survey study reports that the rate of posttraumatic growth in nurses during COVID-19 was 39.3% (Chen et al., 2020).
The prevalence of PTSD among the population at large or those not directly exposed was also quite high compared to the average global prevalence pre-COVID-19 (Kessler et al., 2017). Although this population was not exposed to COVID-19 directly, stressful situations such as lockdowns, economic instability, social isolation, and media reporting of information during the pandemic most likely had a negative effect on psychological well-being of the population at large. Providing information on essential elements of the COVID-19 pandemic to the population at large to reduce stress for trauma including increasing the sense of safety, staying connected, promoting calm and sense of self, collective efficacy, and remaining hopeful could also be effective methods in reducing PTSD.
4.2. Subgroup and moderator analyses
This study indicates that although the elderly was considered as a vulnerable population, it was found that they were more likely to have less negative health outcomes than other age groups. A study by Ditlevsen and Elklit (2010) found that the prevalence of PTSD among adults tends to be higher than the elderly while a study by Robert et al. (2012) found that PTSD prevalence among older adults was 4.5%, which was lower than reported rates of younger age. The possible explanation could be that the elderly has cumulated life experiences offering them a higher resilience to post-traumatic events including COVID-19 pandemic compared to younger age groups. Resiliency is the ability to adapt and being flexible and persistently toward hard situations and as well as ability to tolerate negative emotions and failures. It has been recognized as a protective factor against the experienced negative life events (Oginska-Bulik and Kobylarczyk, 2016). Further, from a biological perspective, because people's prefrontal cortex is not fully mature until the age of 20, they have difficulty coping with traumas after they experience them (Johnson et al., 2009).
In terms of health professionals' characteristics, our study found those who worked in COVID-19 units showed five times greater PTSD prevalence than those who did not work in COVID-19 units. Being exposed to highly stressful situations such as witnessing death, trauma, and working overtime, and overcrowded settings could be a major reason for the psychosomatic problems seen in health professionals working in the COVID-19 units during the pandemic. The general director of WHO, Tedros Adhanom Ghebreyesus, notes that “many (health care workers) have themselves become infected, and while reporting is scant, we estimate that at least 115,000 health care workers have paid the ultimate price in the service of others” (Euronews, 2021). The current study findings also indicate that nurses were at high risk of having PTSD than other health professionals. Similarly, nurses who work in COVID-19 units have shown to have a 16.31 higher risk of developing PTSD (Moon et al., 2021). As part of the frontline health workers, nurses are facing high stress in taking care of people with COVID-19. At the start of the pandemic, armed only with limited information about COVID-19 and basic training of universal precautions, nurses tended to have more direct contact with patients and work more than eight hours every day. The shortage of nurses and personal protective equipment could have led to an increased number of health workers being overworked, becoming infected, and dying from COVID-19. Nurses experience fear of their own deaths or the deaths of loved ones that could result in the development of PTSD (Marshall, 2020). Therefore, providing full support for health professionals to ensure positive outcomes from witnessing exposure to COVID-19 should be encouraged. In addition, in-person or virtual in-service training on essential elements related to COVID-19 and treatments should be available and accessible to health professionals and health care institutions should ensure periodic comprehensive screening and occupational health surveillance of PTSD symptoms to ensure healthcare professionals’ well-being and prompt treatment is provided (Chirico and Magnavita, 2020). Furthermore, it is essential to develop adequate psychological support to help health professionals through the challenges of the COVID-19 pandemic. We suggest that the psychological intervention for PTSD for health professionals should consist of two pillars: (1) providing sufficient information related to COVID-19, training, and personal protective equipment; and (2) providing psychological support for health workers to improve their ability to cope with mental problems.
Regarding study characteristics, subgroup analysis and meta-regression also revealed that there were significant differences in PTSD prevalence rates among the continents. Europe showed the highest number of PTSD compared to Asia and America. As the largest contributor to new COVID-19 cases and death (Smith-Spark et al., 2020), people who live in European countries have higher risk to develop pandemic-related PTSD. Furthermore, the social restriction caused businesses struggle to survive, unemployment due to COVID-19 pandemic has demonstrated to cause significant health loss in high-income countries and these situations have collectively impacted on people's mental health condition including development of PTSD (WHO, 2020). Since the outbreak of COVID-19 in Europe, the level of stress and anxiety have risen significantly (United Nation, 2020). However, this finding should be interpreted with caution, as only three studies reported the prevalence rates of PTSD in low-income countries. Thus, more studies are needed to further explore the prevalence of PTSD in more countries and continents to have a comprehensive view and better understanding of the global pandemic-related PTSD.
Subgroup analyses found that the PTSD assessment tool among the included studies was a significant moderator. Studies that used Clinician-Administered PTSD Scale for DSM-5 and PTSD Checklist/for DSM-5/S/C/C2 as instruments to measure PTSD showed the highest and lowest prevalence, respectively. Of all the instruments, the Clinician-Administered PTSD Scale for DSM-5, PTSD Checklist/for DSM-5/S/C/C2, and Impact of Event Scale/Revised/6 showed high validity and reliability (Blevins et al., 2015; Creamer et al., 2003; Weathers et al., 2018). Clinician-Administered PTSD Scale for DSM-5 is the gold standard for PTSD assessment (Weathers et al., 2013) which is an interview-based instrument, while PTSD Checklist/for DSM-5/S/C/C2 and Impact of Event Scale/Revised/6 are self-reported ones. Different thresholds used in several studies might have also influenced the pooled PTSD prevalence in studies that used PTSD Checklist/for DSM-5/S/C/C2 as the assessment tool. A score of 34 has been suggested as the cut-off PTSD Checklist/for DSM-5/S/C/C2 (Murphy et al., 2017). Murphy et al. (2017) found positive agreement between PTSD Checklist/for DSM-5/S/C/C2, Impact of Event Scale/Revised and Clinician-Administered PTSD Scale for DSM-5 in identifying PTSD, different results found in this study might be related to low score of positive predictive value of PTSD Checklist/for DSM-5/S/C/C2 (45.8%) while the negative prediction value was (89.3%) (Verhey et al., 2018) to determine the existence of PTSD. Furthermore, different rates of PTSD among three different populations might also be related to the instrument used. About 72.7% of studies that measured PTSD among patients/survivors of COVID-19 used PTSD Checklist/for DSM-5/S/C/C2. In addition, studies that measured PTSD on health professionals and the population at large dominantly use Impact of Event Scale/Revised/6 More comprehensive assessments using interview and self-report-based instruments are needed instead of relying on one specific type of assessment tool only.
4.3. Strengths and limitations
This meta-analysis has numerous strengths. Firstly, this meta-analysis included more studies than the previous meta-analyses and provided PTSD prevalence among three different groups (patients/survivors of COVID-19, health professionals, and the population at large) as well as exploring moderator factors to help explain the identified statistical heterogeneity. Secondly, a comprehensive literature search without language restrictions was conducted with independent screening, careful data extraction, and rigorous quality assessment. Finally, sensitivity analyses were also conducted and revealed the robustness of the current study findings. Despite the numerous strengths of the current study, some limitations should be considered when interpreting the results. Most of the studies included in the analysis were conducted between one to eight months after the outbreak. However, PTSD is usually diagnosed at least six months after exposure to trauma. Furthermore, not all studies provide demographic characteristics of those with PTSD or information prior to the pandemic such as previous mental disorder diagnosis that could be associated with PTSD; subgroup analyses were measured based on available data only. Therefore, future studies meeting the diagnostic criteria of PTSD and better reporting of demographic and study characteristics for more accurate measurement of prevalence are needed. As the pandemic is not yet over, more studies are needed to explore the long-term impact of the COVID-19 pandemic on PTSD.
4.4. Conclusions and implications
To our knowledge, this is the first meta-analysis to examine the incidence of PTSD in the COVID-19 pandemic in the overall global population and by comparison groups in terms of exposure and moderator factors. Substantial PTSD prevalence rates was found in patients/survivors diagnosed with COVID-19, health professionals, and the population at large. Moderator analysis revealed age, unit of work, health profession, continent, and PTSD assessment tool as significant moderators.
As WHO recommends improving mental health service, findings from this study can be used to develop programs needed to offer support for people who are at high risk for developing PTSD, especially in adults under the age of 65, health professionals who work in the COVID-19 units, nurses, and those who live in the European countries. Further psychological support as part of health services for those who suffer from PTSD due to COVID-19 is needed.
CRediT authorship contribution statement
Ninik Yunitri: Data curation, Formal analysis, Software, Visualization, Writing – original draft. Hsin Chu: Software, Validation. Xiao Linda Kang: Validation, Writing – review & editing. Hsiu-Ju Jen: Software, Validation. Li-Chung Pien: Software, Validation. Hsiu-Ting Tsai: Software, Validation. Abdu Rahim Kamil: Software, Validation. Kuei-Ru Chou: Conceptualization, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
No conflict interest to be declared.
Acknowledgments
Funding
None.
Data Availability
As this study is a meta-analysis of previous data, no new data were generated in support of this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijnurstu.2021.104136.
Appendix. Supplementary materials
References
- Abdelghany T.M., Ganash M., Bakri M.M., Qanash H., Al-Rajhi A.M.H., Elhussieny N.I. SARS-CoV-2, the other face to SARS-CoV and MERS-CoV: future predictions. Biomed. J. 2021;44(1):86–93. doi: 10.1016/j.bj.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychological Association . PTSD guideline. 2020. PTSD assessment instruments. [Google Scholar]
- Arora T., Grey I., Ostlundh L., Lam K.B.H., Omar O.M., Arnone D. The prevalence of psychological consequences of COVID-19: a systematic review and meta-analysis of observational studies. J. Health Psychol. 2020 doi: 10.1177/1359105320966639. [DOI] [PubMed] [Google Scholar]
- Arora T., Grey I., Ostlundh L., Lam K.B.H., Omar O.M., Arnone D. The prevalence of psychological consequences of COVID-19: a systematic review and meta-analysis of observational studies. J. Health Psychol. 2020 doi: 10.1177/1359105320966639. [DOI] [PubMed] [Google Scholar]
- Asim M., van Teijlingen E., Sathian B. Coronavirus Disease (COVID-19) and the risk of post-traumatic stress disorder: a mental health concern in Nepal. Nepal J. Epidemiol. 2020;10(2):841–844. doi: 10.3126/nje.v10i2.29761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D., Noble H. What are cohort studies? Evid. Based Nurs. 2019;22(4):95–96. doi: 10.1136/ebnurs-2019-103183. [DOI] [PubMed] [Google Scholar]
- Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.K. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M., Xu R.-.H. SARS: epidemiology. Respirology. 2003;8(supplement (supplement 1)):9–14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Sun C., Chen J.-j., Jen H.-j., Kang X.L., Kao C.-c., Chou K.-R. A large scale survey on trauma, burnout, and posttraumatic growth among nurse during the COVID-19 pandemic. Int. J. Ment. Health Nurs. 2020:1–15. doi: 10.1111/inm.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico F., Ferrari G. Role of the workplace in implementing mental health interventions for high-risk groups among the working age population after the COVID-19 pancemic. J. Health Soc. Sci. 2021;6(2):145–150. [Google Scholar]
- Chirico F., Ferrari G., Nucera G., Szarpak L., Crescenzo P., Ilesanmi O. Prevalence of Anxiety, depression, burnout syndrome, and mental health disorders among healthcare workers during the COVID_19 pandemic: a rapid umbrella review of systematic reviews. J. Health Soc. Sci. 2021;6(2):209–220. [Google Scholar]
- Chirico F., Magnavita N. The crucial role of occupational health surveilance for health-care workers during the COVID-19 pandemic. Workplace Health Saf. 2020 doi: 10.1177/2165079920950161. [DOI] [PubMed] [Google Scholar]
- Chirico F., Nucera G., Magnavita N. Protecting the mental ehalth of healthcare workers during the COVID-19 emergency. Br. J. Psychiatry. 2020;18(1):1–2. [Google Scholar]
- Creamer M., Bell R., Failla S. Paychometric properties of the impact of event scale-revised. Behav. Res. Ther. 2003;4(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- DeWolfe D.J. American Psychological Association; Arlington: 2004. Mental Health Response to Mass Violence and Terrorism: A Training Manual: (532512006–001) [Google Scholar]
- Ditlevsen D.N., Elklit A. The combined effect of gender and age on posttraumatic stress disorder: do men and women show differences in the lifespan distribution of the disorder? Ann. Gener. Psychiatry. 2010;9(32):1–12. doi: 10.1186/1744-859X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euronews . 2021. COVID-19: Estimated 115,000 Healthcare Workers have Died from Disease, says WHO. [Google Scholar]
- Fan F.C., Zhang S.Y., Cheng Y. Incidence of psychological illness after coronavirus outbreak: a meta-analysis study. J. Epidemiol. Community Health. 2021 doi: 10.1136/jech-2020-215927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analysis. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Javed B., Sarwer A., Soto E.B., Mashwani Z.U. The coronavirus (COVID-19) pandemic’s impact on mental health. Int. J. Health. Plann. Manage. 2020;35(5):993–996. doi: 10.1002/hpm.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Blum R.W., Giedd J.N. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J. Adolesc. Health. 2009;45(3):216–221. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G., Degenhardt L., de Girolamo G., Dinolova R.V., Ferry F., Florescu S., Gureje O., Haro J.M., Huang Y., Karam E.G., Kawakami N., Lee S., Lepine J.P., Levinson D., Navarro-Mateu F., Pennell B.E., Piazza M., Posada-Villa J., Scott K.M., Stein D.J., Ten Have M., Torres Y., Viana M.C., Petukhova M.V., Sampson N.A., Zaslavsky A.M., Koenen K.C. Trauma and PTSD in the WHO World Mental Health Surveys. Eur. J. Psychotraumatol. 2017;8(sup5) doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Furnham A., Merritt C. Effect of directness of exposure and trauma type on mental health literacy of PTSD. J. Ment. health. 2017:1–7. doi: 10.1080/09638237.2016.1276531. [DOI] [PubMed] [Google Scholar]
- Li Y., Scherer N., Felix L., Kuper H. Prevalence of depression, anxiety and post-traumatic stress disorder in health care workers during the COVID-19 pandemic: a systematic review and meta-analysis. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0246454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci. Rep. 2020;3(3):e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnavita N., Capitanelli I., Arnesano G., Iuliano A., Mauro I., Suraci F., Chirico F. Common occupational trauma: is there a relationship with workers mental halth? Trauma Care. 2021;1:66–74. [Google Scholar]
- Magnavita N., Chirico F., Garbarino S., Bragazzi N.L., Santacroce E., Zaffina S. SARS/MERS/SARS-CoV-2 Outbreaks and Burnout Syndrome among Healthcare Workers. An umbrella systematic review. Int. J. Environ. Res. Public Health. 2021;18(8) doi: 10.3390/ijerph18084361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. Impact of COVID-19 on Nurses’ Mental Health. Issues Ment. Health Nurs. 2020;41(10):853–854. doi: 10.1080/01612840.2020.1819083. [DOI] [PubMed] [Google Scholar]
- May C.L., Wisco B.E. Defining trauma: how level of exposure and proximity affect risk for posttraumatic stress disorder. Psycho. Trauma. 2016;8(2):233–240. doi: 10.1037/tra0000077. [DOI] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart P., group, P.P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. System. Rev. 2015;4(1) doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon D.J., Han M.A., Park J., Ryu S.Y. Post-traumatic stress and related factors among hospital nurses during the COVID-19 outbreak in Korea. Psychi. Quart. 2021;92(4):1381–1391. doi: 10.1007/s11126-021-09915-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Ross J., Ashwick R., Armour C., Busuttil W. Exploring optimum cut-off scores to screen for probable posttraumatic stress disorder within a sample of UK treatment-seeking veterans. Eur. J. Psychotraumatol. 2017;8(1) doi: 10.1080/20008198.2017.1398001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nation., 2020. Concerns are raised over the threat of COVID-19 to mental health in Europe.
- Oginska-Bulik N., Kobylarczyk M. Association between resiliency and posttraumatic growth in firefighters: the role of stress appraisal. Int. J. Occup. Saf. Ergon. 2016;22(1):40–48. doi: 10.1080/10803548.2015.1109372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.Y., Park W.B., Lee S.H., Kim J.L., Lee J.J., Lee H., Shin H.S. Posttraumatic stress disorder and depression of survivors 12 months after the outbreak of Middle East respiratory syndrome in South Korea. BMC Public Health. 2020;20(1):605. doi: 10.1186/s12889-020-08726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Ruston L. Comparison of two methods to detect publication bias in meta-analysis. JAMA Network. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert H.P., Goldstein R.B., Southwick S.M., Grant B.F. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: results from wave 2 of the national epidemiologic survey on alcohol and related conditions. Am. J. Geriatr. Psychiatry. 2012;20(5):380–390. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M., Amanat M., Mohammadi M., Salmanian M., Rezaei N., Saghazadeh A., Garakani A. The prevalence of post-traumatic stress disorder related symptoms in Coronavirus outbreaks: a systematic-review and meta-analysis. J. Affect. Disord. 2021;282:527–538. doi: 10.1016/j.jad.2020.12.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia M.S. Methodology series module 3: cross-sectional studies. Indian J. Dermatol. 2016;61(3):261–264. doi: 10.4103/0019-5154.182410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Spark L., Braithwaite S., Frater J. CNN; 2020. Europe Was Largest Contributor to New Covid-19 Cases and Death in the Past Week, WHO Says. [Google Scholar]
- Szogi E.G., Sullivan K.A. Malingered posttraumatic stress disorder (PTSD) and the effect of direct versus indirect trauma exposure on symptom profiles and detectability. Psychol. Inj. Law. 2018;11:351–361. [Google Scholar]
- U.S Department of Health and Human Services . Center for Mental Health Services, Substance Abuse and Mental Health Service Administration; Rockville, Maryland: 2004. Mental Health Response to Mass Violence and Terrorism: A Training Manual; p. 20857. [Google Scholar]
- Vahia I.V., Jeste D.V., Reynolds C.F., 3rd Older adults and the mental health effects of COVID-19. JAMA. 2020;324(22):2253–2254. doi: 10.1001/jama.2020.21753. [DOI] [PubMed] [Google Scholar]
- Verhey R., Chibanda D., Gibson L., Brakarsh J., Seedat S. Validation of the posttraumatic stress disorder checklist - 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18(1):109. doi: 10.1186/s12888-018-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, J., 2020. Prevalence and predictors of the early psychological impact of the COVID-19 pandemic compared with SARS and MERS: a systematic review and meta-analysis.
- Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., M K.T. 2013. The Clinician Administered PTSD Scale for DMS-5 (CAPS-5) [Google Scholar]
- Weathers F.W., Bovin M.J., Lee D.J., Sloan D.M., Schnurr P.P., Kaloupek D.G., Keane T.M., Marx B.P. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30(3):383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19) Pandemic. [Google Scholar]
- WHO . 2020. Facing Mental Health Fallout from the Coronavirus Pandemic. [Google Scholar]
- WHO . Key Facts. World Health Organization; 2019. Middle East respiratory syndrome coronavirus (MERS-CoV) [Google Scholar]
- WHO . 2019. Report of the WHO-China joint mission on Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- WHO . WHO international; 2015. Summary Probable SARS Case With Onset of Illness from 1 November 2002 to 31 July 2003. [Google Scholar]
- WHO . Brussel; 2020. WHO Warning On Lockdown Mental Health. [Google Scholar]
- Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress after SARS. Emerg. Infect. Dis. 2005;11(8):1297–1300. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Fang Y., Guan Z., Fan B., Kong J., Yao Z., Liu X., Fuller C.J., Susser E., Lu J., Hoven C.W. The psychological impact of SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Canad. J. Psychiatry. 2009;54(5):302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Gong Y.M., Liu L., Sun Y.K., Tian S.S., Wang Y.J., Zhong Y., Zhang A.Y., Su S.Z., Liu X.X., Zhang Y.X., Lin X., Shi L., Yan W., Fazel S., Vitiello M.V., Bryant R.A., Zhou X.Y., Ran M.S., Bao Y.P., Shi J., Lu L. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this study is a meta-analysis of previous data, no new data were generated in support of this research.