Abstract
Purpose:
To examine the effectiveness of the recombinant zoster vaccine for preventing herpes zoster ophthalmicus in the general US population.
Design:
Retrospective, observational cohort study.
Participants:
Individuals enrolled in the OptumLabs® Data Warehouse (OLDW) (OptumLabs, Cambridge, MA) who were age-eligible for herpes zoster vaccination (≥50 years old) from 2018 through 2019. OLDW is a longitudinal, de-identified administrative claims and electronic health record database of patients in the United States with commercial insurance, Medicare Part D or Medicare Advantage
Methods:
Patients were required to have ≥365 days of continuous enrollment to be eligible. Those with a diagnosis code of herpes zoster or an immunocompromising condition within one year prior to study inclusion were excluded. Vaccination with the recombinant zoster vaccine was ascertained by CPT codes, and herpes zoster ophthalmicus was ascertained by ICD-10 codes. Cox proportional hazards regression models were used to estimate the hazard ratio of HZO associated with RZV, and inverse-probability weighting was used to control for confounding. Vaccine effectiveness was calculated from hazard ratios.
Main Outcome(s) and Measure(s):
Incidence of herpes zoster ophthalmicus in vaccinated vs. unvaccinated person-time and vaccine effectiveness were assessed.
Results:
From January 1, 2018 to December 31, 2019 a total of 4 842 579 individuals were included in this study. 177 289 (3.7%) received two valid doses of RZV. The incidence rate of herpes zoster ophthalmicus was 25.5 (95% CI: 17.4, 35.8) cases per 100 000 person-years in the vaccinated group compared to 76.7 (95% CI: 74.7, 78.7) in the unvaccinated group. The overall adjusted effectiveness of RZV against herpes zoster ophthalmicus was 89.1% (95% CI: 82.9, 93.0).
Conclusions:
The effectiveness of RZV against herpes zoster ophthalmicus in individuals ages 50 years old and above is high in a real-world setting. However, the low vaccination rate in this study highlights the public health need to increase herpes zoster vaccination. Ophthalmologists can play an important role in recommending vaccination to eligible patients.
INTRODUCTION
Herpes zoster (HZ; shingles) is a viral infection which occurs with the reactivation of the varicella zoster virus (VZV).1,2 One in three Americans will develop HZ during their lifetime.3,4 Herpes zoster ophthalmicus (HZO), a form of HZ presenting in the ophthalmic division of the trigeminal nerve, makes up 10–20% of HZ cases.5,6 There are many complications associated with HZO. Approximately 20% of HZO patients develop postherpetic neuralgia (PHN), which can result in chronic debilitating pain.7 50% or more of patients can develop ocular complications, such as corneal scarring, glaucoma, cataract formation, and permanent vision loss.8–11,5 Patients with HZO can develop a chronic or recurrent course and require ongoing treatment.10 Due to the significant morbidity and decreased quality of life associated with HZO, prevention of HZO is crucial.
The Food and Drug Administration approved two vaccines against HZ. Zoster Vaccine Live (ZVL/Zostavax; Merck & Co, Inc, Whitehouse Station, NJ) was approved for immunocompetent adults ages ≥60 in 2006 and ages ≥50 in 2011. Recombinant Zoster Vaccine (RZV/Shingrix; GlaxoSmithKline, Philadelphia, PA) was approved in late 2017 for immunocompetent adults ≥50 years old. Clinical trials and real-world studies show that ZVL reduces the incidence of HZ by approximately 50% with effectiveness waning by an average of about 10% each year.12–19 RZV was approved after clinical trials demonstrated ≥90% vaccine efficacy in individuals ≥50 years old, though long-term effectiveness is unknown given its recent entry into the marketplace.20,21 RZV has also demonstrated high effectiveness against HZ in a real-world setting.22 Due to RZV’s greater efficacy, RZV is preferred and ZVL is no longer being marketed in the US.23
Although both vaccines have proven efficacy and effectiveness in clinical trials and real-world settings, the impact of the HZ vaccines on HZO is not well understood.12–15,20,21,24 No findings related to HZO were reported in the ZVL clinical trials.12–15 There are also limited studies studying the effectiveness of the ZVL vaccine in real-world populations, and only one addressed HZO specifically.13,16,18,19 Kovac et al. performed a pooled post-hoc analysis of the RZV clinical trials which suggested that RZV reduces all HZ-related complications, including ophthalmic disease, defined as HZ affecting any eye structure as per investigator’s judgement.24 However, it is unclear if this definition captures all types of HZO cases, including ones that affect only the skin or forehead.
Given the serious complications associated with HZO, understanding the effectiveness of RZV on HZO in the real-world is crucial. Common barriers to vaccination include lack of physician knowledge and lack of strong clinical recommendations from physicians.25,26 The purpose of this study was to determine the effectiveness of RZV for preventing HZO in real-world practice through a large-scale cohort study using administrative claims data. By focusing on the ocular benefits of vaccination, ophthalmologists could play a bigger role in the public health sphere by championing vaccination efforts to prevent a debilitating eye disease.
METHODS
Database
OptumLabs® Data Warehouse (OLDW; OptumLabs, Eden Prairie, MN) provides retrospective de-identified healthcare claims for HIPAA-compliant research. OLDW contains administrative claims and electronic health records of over 200 million patients in the United States enrolled in commercial insurance, Medicare Advantage, or Medicare Part D plans. OLDW individuals represent all regions of the US, with a greater proportion from the Central and Southern regions.
Cohort selection
The retrospective study cohort consisted of individuals from January 1, 2018, through December 31, 2019, who met two inclusion criteria: (1) have reached the age of 50 by 2018 or 2019, which meant becoming age eligible for RZV based on ACIP recommendations and (2) have continuously enrolled in OLDW for at least 365 days. The index date was defined as the date that a patient met both criteria. Patient ages were estimated by birth years, since exact birth dates were concealed to maintain patient confidentiality. From January 1, 2018 to December 31, 2018, all patients ≥50 years old with at least a year of continuous enrollment were included in the cohort. From January 1, 2019 to December 31, 2019, only patients who turned 50 years-old during the year and who had at least a year of continuous enrollment were included in the study. This was done to exclude individuals that may have received RZV vaccination prior to joining OLDW. This exclusion does not apply to 2018 as the vaccine was made available starting October 2017.
Figure 1 provides cohort selection details. Individuals were excluded if they were diagnosed with HZ/HZO or classified as immunocompromised within 1 year prior to the index date. Cases of HZ and HZO were identified by using codes in the International Classification of Disease (ICD) 10th Edition (HZ as B02.xx; HZO as B02.3x). Diagnoses of HZ and HZO from physician claims and facility claims from any clinical setting (inpatient hospital, long-term care, emergency department, outpatient hospital, office visits, other unclassified as defined by OLDW) were included. All cases of HZ, not just HZO, were excluded because HZO may be coded as HZ and because a recent case of HZ offers an immunity boost which is protective against a future occurrence. Immunocompromised status was determined with an ICD-9/10 code for human immunodeficiency virus, acquired immunodeficiency syndrome, leukemia, or lymphoma (eBox1), or an immunosuppressive medication prescription (eBox2).16 Those who received only a single dose of RZV or a dose of RZV prior to January 1, 2018 were excluded. Only 143 individuals (0.08% of total vaccinated cohort) received the first dose of RZV prior to January 1, 2018. Patients developing HZ/HZO during the time between the two RZV doses or ≤ 30 days after the second dose of RZV were excluded to provide adequate time for an immune response to develop following vaccination.
Figure 1.
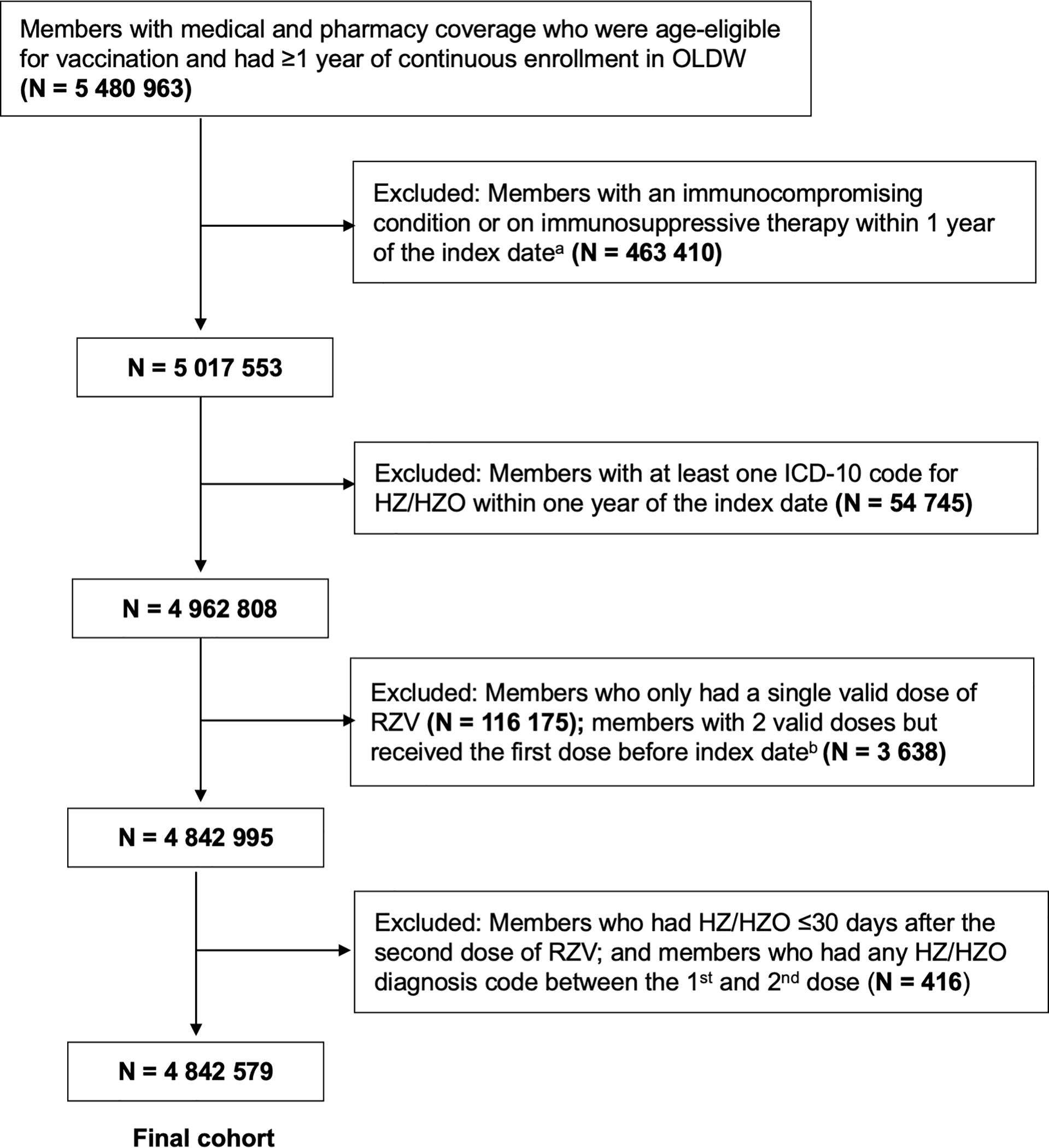
Flow diagram of inclusion and exclusion criteria for study cohort
OLDW = OptumLabs Data Warehouse; ICD = International Classification of Disease; HZ = Herpes zoster; HZO = Herpes Zoster Ophthalmicus; RZV = Recombinant Zoster Vaccine
a Index date was defined as the date at which an individual was eligible for study inclusion.
b Two valid doses of recombinant zoster vaccine were defined as receiving the second dose between 30 and 210 days after the first dose.
Exposure and outcome
The exposure of interest was receipt of two valid doses of RZV. RZV vaccination was determined by a Current Procedural Terminology code for RZV (90750) or through pharmacy claims by the brand name for RZV. An individual was categorized as vaccinated after receiving a valid two-dose RZV regimen. The second dose was considered valid only if it occurred 30–210 days after the first dose, which follows the vaccination timing recommendation set by the ACIP.27 Those receiving a second dose of RZV outside of this time frame were excluded. The outcome of interest was the first code for HZO (ICD-9 053.2x, ICD-10 B02.3x).
Covariates and follow-up
Confounders were designated as time-fixed or time-varying covariates for the inverse probability (IP)-weighted Cox proportional-hazards regression analysis. Time-fixed covariates included sex (female, male, unknown), race (Asian, Black, Hispanic, White, unknown), region (Midwest, Northeast, South, West, unknown), insurance type (commercial, Medicare Advantage) and ZVL vaccination within 1 year prior to the index date. Additional details regarding race and region are found in eMethods 1.
Time-varying covariates were age, healthcare utilization (measured as inpatient stay, long-term care, emergency department visits, outpatient hospital visits and office visits), age-adjusted Charlson Comorbidity Index, and systemic antiviral use. The antiviral medications that were included for analysis included valacyclovir (Valtrex), acyclovir (Zovirax), and famciclovir (Famvir). Time-varying covariates were updated every 6-months.28,29 Since the number of events for inpatient stay, long-term care, emergency department visits, and antiviral use among this cohort was low, these variables were treated as binary variables. Outpatient hospital visits and office visits were kept as continuous variables. Individuals contributed to unvaccinated person-time until they received the second valid dose of RZV, after which they contributed to vaccinated person-time. If an individual completed the second RZV dose within a 6-month interval, the 6-month period was split into an unvaccinated and vaccinated portion. Individuals were followed in this study from their index date until one of the following occurrences: an HZO diagnosis, the development of an immunocompromised status (eBox1), ZVL vaccination, disenrollment from the insurance plan, or the end of the study period (December 31, 2019).
Statistical analysis
HZO incidence rates were calculated as the number of HZ cases per 100 000 person-years, with 95% confidence intervals assuming HZO occurrences followed a Poisson distribution. Hazard ratios associated with RZV were estimated using Cox proportional-hazards regression models.
Cox regression models were stratified by birth year with calendar time as the timescale. Person-time was coarsened into 6-month periods for analysis due to time-varying covariates. Inverse probability (IP)-weighting was used to adjust for measured confounding so that (1) RZV vaccination was not associated with covariates and (2) censoring was not associated with covariates or RZV vaccination. IP-weights were time-updated by using the treatment and censoring logistic regression models that included fixed and time-varying covariates.22 Adjusted hazard ratios were estimated through weighting both the IP-weighted product of treatment and IP-weighted product of censoring in the Cox models. A robust sandwich-type variance estimator, a conservative approach for IP-weighted variance estimation, was used to calculate the 95% confidence intervals.30 Estimated vaccine effectiveness was calculated using 1- hazard ratio x 100%.
Subgroup analyses of HZO incidence rate and vaccine effectiveness were performed for age groups, sex, race, region and those with no prior history of ZVL. Treatment status was used as a multiplicative interaction term in the Cox regression models to assess the difference in vaccine effectiveness among subgroups. Note that small cell counts <11 could not be reported in order to protect patient privacy. OptumLabs requires cell counts <11 to be reported as <11 rather than reporting true values. In order to prevent back calculation, the value in a corresponding cell of the same subgroup is lowered and reported with a greater than sign to ensure that the total count within the subgroup stays the same. Therefore, the HZO incidence rate in each affected subgroup was reported with greater than and less than signs.
Analyses were preformed using R Software version 3.6.3 (The R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org). Informed consent was not required for this study since only de-identifiable data were available for analyses. The study was granted approval by the Institutional Review Board of the University of California, San Francisco, and it has adhered to the Declaration of Helsinki.
RESULTS
This study included a total of 4 842 579 individuals with 7 491 570 person-years from January 1, 2018 to December 31, 2019. Table 1 provides the demographic characteristics for individuals on their index dates. 177 289 (3.7%) received a valid two-dose regimen of RZV. Median follow-up time for the vaccinated cohort was 730 days (IQR: 730 – 730) and median follow-up time post-vaccination was 211 days (IQR: 86 – 391). Median duration of follow-up in the unvaccinated cohort was 730 days (IQR: 365 – 730). Median age at the index date was 72 years-old (IQR 69–77) for those vaccinated and was 64 years-old (IQR 56–73) for the unvaccinated group. Demographics for gender, race/ethnicity, and region were similar between vaccinated and unvaccinated individuals. Healthcare utilization was also similar. Females, whites, and individuals from the South comprised the majority of both groups. Only 2.4% and 1.4% of individuals from the vaccinated and unvaccinated cohorts, respectively, received a ZVL vaccination within 1 year prior to the index date.
Table 1.
Characteristics of the study population at the index datea by vaccination status
| Characteristicb | Unvaccinated (n = 4 665 290) | Vaccinated (n = 177 289) | Overall (n = 4 842 579) |
|---|---|---|---|
| Age, median (IQR), y | 64 (56 – 73) | 72 (69 – 77) | 65 (56 – 73) |
| Sex | |||
| Female | 2 424 614 (51.9) | 103 144 (58.2) | 2 526 758 (52.2) |
| Male | 2 239 176 (48.0) | 73 643 (41.5) | 2 312 819 (47.8) |
| Unknown | 2 500 (0.1) | 502 (0.3) | 3002 (0.1) |
| Race/Ethnicity | |||
| Asian | 146 406 (3.1) | 6 257 (3.5) | 152 663 (3.2) |
| Black | 517 054 (11.1) | 12 991 (7.3) | 530 045 (10.9) |
| Hispanic | 470 660 (10.1) | 8 558 (4.8) | 479 218 (9.9) |
| White | 3 051 395 (65.4) | 131 738 (74.3) | 3 183 133 (65.7) |
| Unknownc | 479 775 (10.3) | 17 745 (10.0) | 497 520 (10.3) |
| Insurance Type | |||
| Commercial | 2 516 462 (53.9) | 20 479 (11.6) | 2 536 941 (52.4) |
| Medicare Advantage | 2 148 828 (46.1) | 156 810 (88.4) | 2 305 638 (47.6) |
| Region | |||
| Midwest | 1 185 708 (25.4) | 57 866 (32.6) | 1 254 574 (25.7) |
| Northeast | 603 123 (12.9) | 19 109 (10.8) | 622 232 (12.8) |
| South | 2 157 968 (46.3) | 77 712 (43.8) | 2 235 680 (46.2) |
| West | 646 878 (13.9) | 22 579 (12.7) | 669 457 (13.8) |
| Unknownd | 71 613 (1.5) | 23 (0.0) | 71 636 (1.5) |
| Inpatient Stay e | |||
| ≥ 1 visits | 405 000 (8. 7) | 16 284 (9.2) | 421 284 (8.7) |
| No visits | 4 260 290 (91.3) | 161 005 (90.8) | 4 421 295 (91.3) |
| Long-term Care e | |||
| ≥ 1 visits | 94 857 (2.0) | 2 490 (1.4) | 97 347 (2.0) |
| No visits | 4 570 433 (98.0) | 174 799 (98.6) | 4 745 232 (98.0) |
| Emergency Room Visit e | |||
| ≥ 1 visits | 967 397 (20.7) | 34 911 (19.7) | 1 002 308 (20.7) |
| No visits | 3 697 893 (79.3) | 142 378 (80.3) | 3 849 271 (79.3) |
| Outpatient Visit e | |||
| Median (IQR) | 1.0 (0.0–3.0) | 2.0 (1.0–5.0) | 1.0 (0.0–3.0) |
| Office Visit | |||
| Median (IQR) | 5.0 (2.0–11) | 9.0 (5.0–16) | 6.0 (2.0–11) |
| Charlson Comorbidity Index f | |||
| Median (IQR) | 2.0 (1.0–4.0) | 3.0 (3.0–4.0) | 2.0 (1.0–4.0) |
| ZVL Vaccination within 1 Year Prior to Index Date | |||
| No | 4 599 704 (98.6) | 173 011 (97.6) | 4 772 715 (98.6) |
| Yes | 65 586 (1.4) | 4 278 (2.4) | 69 864 (1.4) |
IQR = Interquartile range.
The index date was defined as the date at which an individual was eligible for study inclusion.
Values are reported as No. (%) unless otherwise indicated.
The unknown race category includes individuals with either unknown or missing race. As defined in OLDW, unknown race is for individuals who are not in one of the pre-defined categories (Asian, white, black, Hispanic) or who have mixed race.
The unknown region category includes individuals in either unknown or other regions. In OLDW, the pre-defined region categories are (Midwest, Northeast, South, West, Other, Unknown). The Other and Unknown categories were combined given the low prevalence of herpes zoster in each individual group.
Healthcare utilization was assessed in the 1 year prior to the index date.
Charlson comorbidity index was assessed in the 1 year prior to the index date.
Table 2 reports the number of HZO cases per age group and gender. Overall, the vaccinated cohort had 30 HZO cases with a total follow-up time of 117 517 person-years. The unvaccinated cohort had 5 654 HZO cases with a total follow-up time of 7 374 053 person-years. The incidence rate of HZO was 25.5 (95% CI: 17.4,35.8) cases per 100 000 person-years in the vaccinated group, while the rate was 76.7 (95% CI: 74.7,78.7) cases per 100 000 person-years in the unvaccinated group.
Table 2.
Incidence of herpes zoster per 100 000 person-years by demographic characteristics and recombinant zoster vaccination status from 2018 to 2019
| Unvaccinated | Vaccinated | |||||
|---|---|---|---|---|---|---|
| Number of Cases | Number of Person-Years | Incidence Rate (95% CI) | Number of Cases | Number of Person-Years | Incidence Rate (95% CI) | |
| Overall | ||||||
| 5 654 | 7 374 053 | 76.7 (74.7, 78.7) | 30 | 117 517 | 25.5 (17.4, 35.8) | |
| Age group, y a | ||||||
| 50–59 | 1 226 | 2 343 779 | 52.3 (49.4, 55.3) | 0 | 2 142 | 0.0 |
| 60–79b | 3 282 | 4 051 347 | 81.0 (78.3, 83.8) | > 19 | 90 069 | > 21.1 (13.0, 32.1) |
| 80+ | 1 146 | 978 927 | 117.1 (110.4, 124.0) | <11 | 25 306 | <43.5 (22.6,74.4) |
| Sex | ||||||
| Male | > 2254 | 3 510 499 | > 64.2 (61.6, 66.9) | > 19 | 49 190 | > 38.6 (23.8, 58.7) |
| Female | 3 389 | 3 858 797 | 87.8 (84.9, 90.8) | <11 | 67 947 | < 16.2 (8.4, 27.7) |
| Unknownc | < 11 | 4 757 | < 231.2 (120.1, 395.9) | 0 | 380 | 0.0 |
OptumLabs requires cell counts <11 to be reported as <11 rather than reporting true values in order to protect patient privacy. In order to prevent back calculation, the value in a corresponding cell of the same subgroup is lowered and reported with a greater than sign to ensure that the total case count within the subgroup stays the same. Each affected subgroup’s HZO incidence rate was reported with greater than and less than signs. Numbers are not reported for race/ethnicity subgroups due to the cell suppression policy.
Incidence rates for age group 60–69 and 70–79 have been combined together to protect patient privacy due to low cell counts.
The unknown sex category includes individuals with either unknown or missing sex.
Figure 2 summarizes the unadjusted and adjusted effectiveness of RZV. Covariate balance improved with IP-weighting, demonstrated through smaller standardized mean differences (estimates of average causal effects) in the weighted strata compared to the unweighted strata.31 The overall unadjusted effectiveness of RZV was 73.3% (95% CI: 61.7, 81.4). The overall adjusted effectiveness of RZV was 89.1% (95% CI: 82.9, 93.0). Vaccine effectiveness in patients who did not receive ZVL vaccination within 1 year prior to the index date was similar to the overall unadjusted and adjusted effectiveness. Vaccine effectiveness was 100% for ages 50–59, Hispanic ethnicity, and those who received ZVL vaccination within 1 year prior to the index date; however, the confidence intervals could not be calculated for these subgroups because there were no cases of HZO in the vaccinated cohort. Unadjusted RZV effectiveness was 74.4% (95% CI: 38.2, 89.4) for ages 60–69, 73% (95% CI: 56.5, 83.3) for ages 70–79, and 72.1% (95% CI: 43.9, 86.1) for ages ≥80. Adjusted RZV effectiveness was 87.6% (95% CI: 59.9, 96.2) for ages 60–69 and 88.9% (95% CI: 80.6, 93.7) for ages 70–79. Adjusted RZV effectiveness remained high at 88.8% (95% CI: 77.5, 94.5) for ages ≥80. Females had higher RZV adjusted effectiveness, 95.3% (95% CI: 91.2, 97.5), compared to males, 79.0% (95% CI: 63.1, 88.0). Adjusted effectiveness by reported races were similar.
Figure 2.
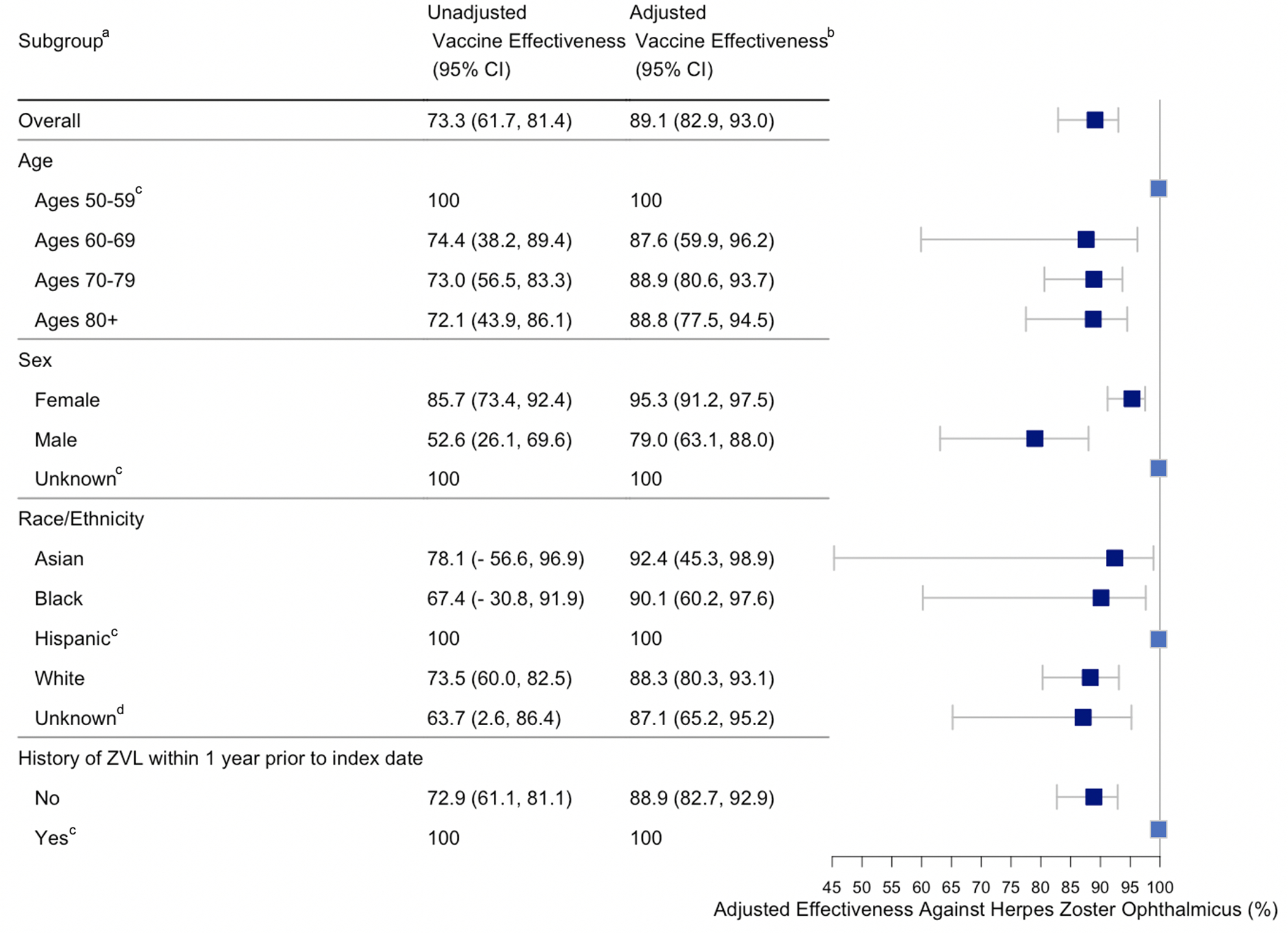
Recombinant zoster vaccine effectiveness against HZO by age, sex, race/ethnicity and history of ZVL from 2018 to 2019
CI = Confidence interval.
ZVL = Zoster vaccine live.
a Values are reported as %.
b Vaccine effectiveness was adjusted using inverse probability weighting for age, sex, race, region, prior zoster vaccine live, antiviral use, healthcare utilization, insurance type, and Charlson comorbidity index.
c Vaccine effectiveness was 100% in the subgroup, but the confidence interval could not be calculated because there were no cases of HZO in the vaccinated cohort.
d The unknown race/ethnicity category includes individuals with either unknown or missing race/ethnicity.
DISCUSSION
This study provides novel information supporting the effectiveness of RZV in preventing HZO, with an overall adjusted vaccine effectiveness of 89.1% (95% CI: 82.9, 93.0). There are two randomized, placebo-controlled trials that evaluated the efficacy of the RZV for preventing HZ, ZOE-50 and ZOE-70. ZOE-50 enrolled adults ≥50 years old. RZV had an overall efficacy between 96.6% and 97.9% for all age groups.20 ZOE-70 enrolled adults ≥70 years old and had an RZV efficacy of 89.8%.21 In a pooled analysis from the two trials, there was 1 HZ-related ophthalmic complication among the 32 HZ cases that occurred in the 13 881 who received RZV and there were 7 HZ-related ophthalmic complications among the 477 HZ cases that occurred in the 14 035 individuals receiving the placebo. Vaccine efficacy against HZ-related complications excluding PHN was 93.7% in the pooled analysis. However, the efficacy against HZO was unclear since the paper only commented on HZ cases with ophthalmologic disease complications, which they defined as “HZ affecting any eye structure as per investigator’s judgment.” This definition most likely does not capture all HZO cases, such as those only involving the skin or the forehead. Our study included any HZO diagnosis code, which would encompass HZO cases only involving the skin or forehead. Our estimate of vaccine effectiveness for preventing HZO is comparable to that found in the clinical trials leading to approval of RZV for prevention of HZ. The adjusted vaccine effectiveness was greater in females than males. Effectiveness was similar among age groups and races.
The CDC has recognized the importance of studies that assess vaccine effectiveness outside of clinical trial settings, so a real-world study like this one provides important information on the merits of vaccination from an ocular perspective. Real-world studies generally include patients with a wider range of health compared to clinical trials. Our study also included patients with a history of prior ZVL vaccination, which was an exclusion in the primary trials. 20,21
Understanding the effectiveness of RZV on HZO is important given the large disease burden. It is estimated that there are around 1.2 million new cases of HZ in the United States annually with 10–20% of them being HZO, and it is estimated that 6.6% of HZ cases involving the eye result in large healthcare expenditures and permanent vision decrement.8,32 In addition, the incidence of HZO continues to rise, with an estimated relative increase in the incidence of 3.6% per year from 1994 to 2018.33 HZO has a wide variety of complications that can be acute, chronic or recurrent.6,9 The most common complication is PHN and the most common ocular manifestations are keratitis, uveitis, and conjunctivitis.8,9 More rarely, HZO can involve the optic nerve, retina or CNS.6
Given the significant burden of this disease, the low vaccination rates are particularly alarming.34,35 In our study, 3.7% of eligible patients received two valid doses of RZV. Historically, low vaccination coverage was an issue with ZVL as well. Almost a decade after introduction of ZVL, only 30.6% of adults 60 years and older had received ZVL as of 2015.35,36 Barriers to ZVL vaccination in the United States included high cost, insurance coverage, and the absence of strong recommendations from general practioners.25,26 Even with increased HZ education and coverage of the vaccine in insured patients over the age of 50, primary care physicians did not prioritize the HZ vaccine as much as the influenza and pneumococcal vaccines.34
RZV shows greater efficacy compared to ZVL, so improving vaccination coverage is an important public health issue.12,13 Lack of physician support for vaccination is known to be an important barrier to vaccination.38 This study provides evidence to support RZV vaccination from an ocular perspective, which may improve physician attitudes towards RZV. Secondly, interventions targeted at increasing the number of physicians recommending RZV can also promote vaccination. Jung et al. highlighted how ophthalmologists can increase vaccination rates by recommending the vaccine to their patients.39 The American Academy of Ophthalmology (AAO) published a policy statement encouraging ophthalmologists to strongly recommend RZV vaccination in those aged 50 and older without contraindications 40 The vaccinated group in our study was older than the unvaccinated group, highlighting the need to improve vaccination efforts in eligible younger patients. According to a survey on general ophthalmologists and cornea specialists, all respondents have seen at least one HZO case and 87% have treated recurrent or chronic cases of HZO within the past year.41 With their training and clinical experience around HZO, ophthalmologists have the knowledge to educate patients about the high disease burden associated with HZO and the benefits of RZV vaccination. Data from this study showing the effectiveness of RZV on preventing HZO may both strengthen RZV recommendations from ophthalmologists and increase the total number of physicians promoting RZV vaccination.
This study has limitations. The retrospective nature of this study is inherently limited by the quality of ICD coding. Physicians may code for HZO when trying to rule out the diagnosis and individuals may not seek care when presenting with mild forms of HZO. Furthermore, generalizability is limited as only patients with commercial insurance, Medicare Part D, and Medicare Advantage were included; uninsured patients or those with other insurance forms were not included in this database study. Health status could be different in study participants compared to the general American population. However, the database was very large and geographically representative of the United States population. Since there was no randomization in this study, the unvaccinated and vaccinated cohorts were different in certain characteristics. Vaccinated individuals were more likely to be older and sicker, resulting in higher risk of developing zoster. Without adjusting for those confounders, the vaccine effectiveness would be biased towards the null. The robust method that we utilized to adjust for time-fixed and time-varying covariates allowed for a fair comparison of vaccine effectiveness between unvaccinated and vaccinated individuals.
The study only excluded patients with a diagnosis of HZ or HZO up to a year prior to the index date. A person with a more remote history of HZ or HZO may have unintentionally been included in this study. However, a study on the effectiveness of RZV on HZ published by our research group illustrated that vaccine effectiveness results did not change with a one year vs. five-year look-back time period.22 Another limitation is that since HZO is a rare outcome and our study period is limited to two years, some subgroups have larger confidence intervals or couldn’t estimate confidence intervals. This study also could not assess waning of RZV effectiveness, since RZV was only introduced in late 2017 and individuals had relatively short post-vaccination follow-up time. A future study with a longer study period is needed to confirm long-term effectiveness. Lastly, effectiveness of the vaccine in individuals with a history of HZO cannot be determined since they were excluded from the study population. There is a published case report of HZO reactivation following RZV vaccination, but the reactivation occurred three weeks post vaccination and is not sufficient to establish a definite association.42 Future research studying the safety and effectiveness of RZV vaccination in those with a prior history of HZO would be beneficial, as well as reporting of adverse events to the Vaccine Adverse Event Reporting System.
In conclusion, RZV demonstrates higher efficacy and effectiveness compared to ZVL. The results of this study should encourage clinicians to provide strong recommendations for RZV, especially given previous low HZ vaccine coverage, the rising incidence of HZ and HZO, and the significant morbidity associated with HZO. Ophthalmologists should counsel patients on the potential benefits of RZV vaccination with the understanding that future studies are needed to investigate long-term outcomes and effectiveness of this vaccine.
Supplementary Material
Acknowledgments
Financial Support: National Institutes of Health (NIH, National Eye Institute and Office of Research on Women’s Health), Bethesda, Maryland; NIH Grant R01 EY028739 to NRA); OptumLabs Warehouse research credit through OptumLabs®, Cambridge, Massachussetts; The Department of Ophthalmology at the University of California San Francisco is supported by an unrestricted grant from the Research to Prevent Blindness Foundation, a core grant from the National Eye Institute at the National Institutes of Health (EY06190) and the That Man May See Foundation.
Abbreviations and Acronyms:
- ACIP
Advisory Committee on Immunization Practices
- AAO
American Academy of Ophthalmology
- CDC
Centers for Disease Control
- CPT
Current Procedural Terminology
- FDA
Food and Drug Administration
- HZ
herpes zoster
- HZO
herpes zoster ophthalmicus
- ICD
International Classification of Disease
- OLDW
Optum Labs Data Warehouse
- PHN
postherpetic neuralgia
- RZV
recombinant zoster vaccine
- US
United States
- ZVL
zoster vaccine live
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
REFERENCES
- 1.Hope-Simpson RE. The Nature of Herpes Zoster: A Long-term Study and a New Hypothesis. Proc R Soc Med. 1965;58(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI. Herpes Zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(3 Suppl):21–26. doi: 10.1586/erv.10.30 [DOI] [PubMed] [Google Scholar]
- 4.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of Herpes Zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recomm Rep. 2008;57(5):1–30. [PubMed] [Google Scholar]
- 5.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–12. doi: 10.1016/j.ophtha.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Borkar DS, Tham VM, Esterberg E, et al. Incidence of Herpes Zoster Ophthalmicus: Results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120(3):451–456. doi: 10.1016/j.ophtha.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yawn BP, Wollan PC, Sauver JLS, Butterfield LC. Herpes Zoster Eye Complications: Rates and Trends. Mayo Clin Proc. 2013;88(6):562–570. doi: 10.1016/j.mayocp.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen EJ, Kessler J. Persistent dilemmas in zoster eye disease. Br J Ophthalmol. 2016;100(1):56–61. doi: 10.1136/bjophthalmol-2015-306700 [DOI] [PubMed] [Google Scholar]
- 10.Tran KD, Falcone MM, Choi DS, et al. Epidemiology of Herpes Zoster Ophthalmicus: Recurrence and Chronicity. Ophthalmology. 2016;123(7):1469–1475. doi: 10.1016/j.ophtha.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szeto SKH, Chan TCY, Wong RLM, Ng ALK, Li EYM, Jhanji V. Prevalence of Ocular Manifestations and Visual Outcomes in Patients With Herpes Zoster Ophthalmicus. Cornea. 2017;36(3):338–342. doi: 10.1097/ICO.0000000000001046 [DOI] [PubMed] [Google Scholar]
- 12.Oxman MN, Levin MJ, Johnson GR, et al. A Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older Adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- 13.Schmader KE, Levin MJ, Gnann JW, et al. Efficacy, Safety, and Tolerability of Herpes Zoster Vaccine in Persons Aged 50–59 Years. Clin Infect Dis. 2012;54(7):922–928. doi: 10.1093/cid/cir970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the Efficacy of Zoster Vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55(10):1320–1328. doi: 10.1093/cid/cis638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison VA, Johnson GR, Schmader KE, et al. Long-term Persistence of Zoster Vaccine Efficacy. Clin Infect Dis. 2015;60(6):900–909. doi: 10.1093/cid/ciu918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes Zoster Vaccine in Older Adults and the Risk of Subsequent Herpes Zoster Disease. JAMA. 2011;305(2):160–166. doi: 10.1001/jama.2010.1983 [DOI] [PubMed] [Google Scholar]
- 17.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes Zoster Vaccine Effectiveness against Incident Herpes Zoster and Post-herpetic Neuralgia in an Older US Population: A Cohort Study. PLoS Med. 2013;10(4). doi: 10.1371/journal.pmed.1001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng HF, Harpaz R, Luo Y, et al. Declining Effectiveness of Herpes Zoster Vaccine in Adults Aged ≥60 Years. J Infect Dis. 2016;213(12):1872–1875. doi: 10.1093/infdis/jiw047 [DOI] [PubMed] [Google Scholar]
- 19.Baxter R, Bartlett J, Fireman B, et al. Long-Term Effectiveness of the Live Zoster Vaccine in Preventing Shingles: A Cohort Study. Am J Epidemiol. 2018;187(1):161–169. doi: 10.1093/aje/kwx245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. 10.1056/NEJMoa1501184. doi: 10.1056/NEJMoa1501184 [DOI] [PubMed]
- 21.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Kim E, Kong CL, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021;(ciab121). doi: 10.1093/cid/ciab121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merck. PRODUCT DISCONTINUATION NOTICE: ZOSTAVAX® (Zoster Vaccine Live); 2020. Accessed October 8, 2020. https://www.merckvaccines.com/wp-content/uploads/sites/8/2020/06/US-CIN-00033.pdf
- 24.Kovac M, Lal H, Cunningham AL, et al. Complications of herpes zoster in immunocompetent older adults: Incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine. 2018;36(12):1537–1541. doi: 10.1016/j.vaccine.2018.02.029 [DOI] [PubMed] [Google Scholar]
- 25.Hurley LP, Allison MA, Dooling KL, et al. Primary care physicians’ experience with zoster vaccine live (ZVL) and awareness and attitudes regarding the new recombinant zoster vaccine (RZV). Vaccine. 2018;36(48):7408–7414. doi: 10.1016/j.vaccine.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkin Z, Cohen EJ, Goldberg JD, et al. Studying Physician Knowledge, Attitudes, and Practices Regarding the Herpes Zoster Vaccine to Address Perceived Barriers to Vaccination. Cornea. 2013;32(7):976–981. doi: 10.1097/ICO.0b013e318283453a [DOI] [PubMed] [Google Scholar]
- 27.Dooling KL. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67. doi: 10.15585/mmwr.mm6703a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Li B, Couris CM, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. doi: 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suaya JA, Chen S-Y, Li Q, Burstin SJ, Levin MJ. Incidence of Herpes Zoster and Persistent Post-Zoster Pain in Adults With or Without Diabetes in the United States. Open Forum Infect Dis. 2014;1(2). doi: 10.1093/ofid/ofu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence Rate of Herpes Zoster Ophthalmicus: A Retrospective Cohort Study from 1994 through 2018. Ophthalmology. 2019;0(0). doi: 10.1016/j.ophtha.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aris E, Montourcy M, Esterberg E, Kurosky SK, Poston S, Hogea C. The adult vaccination landscape in the United States during the Affordable Care Act era: Results from a large retrospective database analysis. Vaccine. 2020;38(14):2984–2994. doi: 10.1016/j.vaccine.2020.02.057 [DOI] [PubMed] [Google Scholar]
- 35.Williams WW, Lu P-J, O’Halloran A, et al. Surveillance of Vaccination Coverage among Adult Populations - United States, 2015. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2017;66(11):1–28. doi: 10.15585/mmwr.ss6611a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2016. Published March 14, 2019. Accessed July 6, 2020. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html
- 37.Elkin ZP, Cohen EJ, Goldberg JD, et al. Improving Adherence to National Recommendations for Zoster Vaccination Through Simple Interventions. Eye Contact Lens. 2014;40(4):225–231. doi: 10.1097/ICL.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabana MD, Rand CS, Powe NR, et al. Why Don’t Physicians Follow Clinical Practice Guidelines?: A Framework for Improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458 [DOI] [PubMed] [Google Scholar]
- 39.Jung JJ, Elkin ZP, Li X, et al. Increasing Use of the Vaccine Against Zoster Through Recommendation and Administration by Ophthalmologists at a City Hospital. Am J Ophthalmol. 2013;155(5):787–795.e2. doi: 10.1016/j.ajo.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 40.Policy Statement: Recommendations for Herpes Zoster Vaccine for Patients 50 Years of Age and Older. Ophthalmology. 2018;125(11):1813–1816. doi: 10.1016/j.ophtha.2018.07.003 [DOI] [Google Scholar]
- 41.Sy A, McLeod SD, Cohen EJ, et al. Practice Patterns and Opinions in the Management of Recurrent or Chronic Herpes Zoster Ophthalmicus. Cornea. 2012;31(7):786–790. doi: 10.1097/ICO.0b013e31823cbe6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann A, Matoba A. Reactivation of Herpes Zoster Stromal Keratitis After HZ/su Adjuvanted Herpes Zoster Subunit Vaccine. Ophthalmology. 2018;125(11):1682. doi: 10.1016/j.ophtha.2018.08.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


