Graphical abstract
Abbreviations: APDs, Antipsychotic drugs; C. elegans, Caenorhabditis elegans; SDA, Serotonin Dopamine Antagonist; E. coli, Escherichia coli BOD-Biochemical Oxygen Demand; Min., Minutes; 5-HT, 5-hydroxytryptamine; D2, Dopamine Receptor 2; NSM, Neurosecretory Motor Neuron; ADF, Amphid Neuron; HSN, Hermaphrodite Specific Neuron; GPR, G coupled Protein Receptor; C, Control Group; E, Exposed Group; SD, Standard Deviation; C-0h, Control Group at 0 h; C-2h, Control Group at 2 h; C-4h, Control Group at 4 h; C-6h, Control Group at 6 h; C-8h, Control Group at 8 h; C-10h, Control Group at 10 h; C-12h, Control Group at 12 h. E-2h, Exposure Group at 2 h; E-4h, Exposure Group at 4 h; E-6h, Exposure Group at 6 h; E-8h, Exposure Group at 8 h; E-10h, Exposure Group at ten; E-12h, Exposure Group at 12 h
Keywords: Antipsychotic drugs, Risperidone, Behavioral alteration, C. elegans, N2 Wild type, omega bends, Reversals, Turn counts, Peristaltic speed, Pharyngeal pumping, 5-H2T, D2
Highlights
-
•
Risperidone Drug induced alteration in Caenorhabditis elegans.
-
•
Liquid Culture Assay requires lower drug concentration.
-
•
Feeding and Locomotion behavior are vital parameters for onset of toxicity.
-
•
Alteration in feeding and locomotion reflects time-dependent toxicity.
-
•
Higher duration of exposures show impregnated alteration in behavior.
Abstract
Antipsychotic drugs (APDs) are prescribed for the treatment of psychiatric illness. However, these drugs can also contribute to several developmental and behavioral disorders. Contemporary studies to evaluate the toxic effects of numerous atypical antipsychotics are reported to cause behavioral alteration at variable doses in mammals and nematodes. Risperidone, the second most prescribed drug in India, requires more exploration of its adverse effects on humans. Here, we explore effects on feeding behavior and locomotion patterns due to risperidone exposure in C. elegans model. The study targets to work out the toxic effects of risperidone exposure on feeding and locomotion behavior in addition to the expected pharmacological effects.
N2 wild type strain was exposed in liquid culture assay for 2, 4, 6, 8, 10, and 12 hours with fixed 50 µM concentration. Feeding behavior was depleted due to inhibition in pharyngeal pumping varying from 11.05% - 45.67% in a time-dependent manner. Results of locomotion assay also show time-varying increase in reversals (4.9%–34.03%) and omega bends (26.23%–62.17%) with reduction in turn counts (29.07%- 42.2%) and peristaltic speed (31.38%–42.22%) amongst exposed groups as to control. The present work shows behavioral alterations due to risperidone exposure (50 µM) in C. elegans is in a time-dependent manner. The study concludes that risperidone exposure in C. elegans produces toxic effects with time, possibly caused by antagonizing other receptors apart from serotonin (5-H2T) and dopamine (D2) adding to its expected pharmacological effects.
Introduction
Antipsychotic drugs (APDs) are increasingly being prescribed to adolescents, children, and even pregnant women. These drug types include tranquilizers and neuroleptics. They are mainly administered to help tackle mental illness such as schizophrenia, bipolar disorder and psychotic depression (Findling et al., 1998, Lanczik et al., 1998, Yaeger et al., 2006, Zito et al., 2000). APDs bind to and block serotonin & dopamine receptors, thereby producing neurodevelopmental disorders (Donohoe et al., 2006). Also, studies show that they cause several developmental defects such as convulsions (Stoner et al., 1997), floppy infant syndrome (Lanczik et al., 1998) and lower development scores (Yoshida et al., 1998). Visual distress has been reported in monkeys due to the exposure of APDs (Ullberg et al., 1970) while the exposure of APDs in rodents has been reported to cause behavioral disruptions (Hironaka and Umemura, 1988, Robertson et al., 1980). Previous studies on classes of APDs have identified novel molecular targets and revealed adverse effects on development over a range of concentrations (Donohoe et al., 2006). Altered neuronal development by APDs on axonal outgrowth & neuronal migration have been critically evaluated in C. elegans (Donohoe et al., 2008). Action mechanisms of APDs with clozapine for development and pharyngeal pumping alterations is another investigative study carried out in C. elegans (Hao and Buttner, 2014).
A recent study with risperidone and aripiprazole revealed serotonin and dopamine-dependent behavioral alterations in pharyngeal pumping mechanism and gentle touch response wherein C. elegans N2 strain was exposed to 150 μM and 300 μM concentrations respectively (Osuna-Luque et al., 2018).
Risperidone, an atypical antipsychotic, is effective in treating autism, bipolar disorder, and schizophrenia. This research relied on risperidone over other antipsychotics because of its extensive use as a prescription drug, it is the second most prescribed drug in about 26.7 % of cases. (Grover et al., 2014). Risperidone is a serotonin dopamine antagonist (SDA) found to present adverse effects like extrapyramidal syndrome, hyperprolactinemia, neuroleptic malignant syndrome, and several other behavioral and metabolic disorders in humans (Horacek et al., 2006). Previous studies related to the class of antipsychotics in terms of their adverse effects have been examined in this research. After the examination, it is evident that interventions concerning risperidone are scanty, requiring it to be investigated as it is a commonly prescribed drug for psychiatric illness. C. elegans being a self-fertilizing, hermaphrodite and due to its ease of culture, smaller life cycle (3–5 days at 22° C) is an emerging animal model of choice amongst toxicologists and geneticists.(Brenner, 1973, Byerly et al., 1976, Weinhouse et al., 2018). It has 60–80% of homology to the human genome (Kaletta and Hengartner, 2006); at least 38 % protein-coding genes and 40% genes associated with human diseases facilitate extrapolating findings to humans (Apfeld and Alper, 2018).
Pharyngeal pumping is the rhythmic feeding mechanism in C. elegans, which is autonomous. Pharyngeal pumping is controlled by a neuronal network composed of several neurons (approximately 19–20). The neurons have fourteen sub-types. Pharyngeal pumping rate in C elegans depends on feeding history and the type of food. (Hobson et al., 2006, Shtonda and Avery, 2006, Song et al., 2013).
Nonetheless, environmental factors such as exposure to drugs or xenobiotics may alter the pharyngeal pumping rate. The pharynx in C. elegans is a muscular organ, which autonomously and constantly pumps the food inside the body. It comprises neurons, accessory cells, and muscle cells: each is 20 in number wrapped by a basement membrane (Avery and You, 2018). Locomotion is a neuromuscular activity regulated by AVA, AVB, AVD, and PVC, which are the four bilaterally symmetric interneuron pairs. AVD and AVA are responsible for pairing A-type motor neurons. Their combination forms a neural circuit responsible for backward movement. In contrast, AVB and PVC are responsible for pairing the B-type motor neurons, forming a neural circuit responsible for the forward movement in C. elegans (Chalfie et al., 1985, White et al., 1976). Since pharyngeal pumping rate and locomotion behavior are primary observations encountered in the onset of adverse effects (due to any drug or xenobiotic), this research aims at exploring effects on behavioral characteristics of C. elegans after risperidone exposure. This work targets to study the time dependent pattern of alteration observed by risperidone drug exposure in C. elegans. The main limitation of this study is the impossibility of using human subjects for testing any possible alteration due to the pharmacology of the risperidone drug. Also, extrapolating research data from other subjects for human application does not always yield perfect results.
Materials and methods
Chemicals and glassware
Risperidone powder (purity ≥ 98%) and Sodium Hypochlorite bleach solution were procured from Sigma-Aldrich (USA). Sodium chloride, Peptone, Agar, Cholesterol, Calcium Chloride, Magnesium Sulphate, Dihydrogen Potassium Phosphate were of analytical grade (∼99% purity) and were purchased from SRL (Mumbai, India). Milli-Q water was obtained from the LABINDIA Water Purification System. All calibrated glasswares used were of research-grade from Borosil.
Culture, maintenance and exposure
Wild type N2 strain of Caenorhabditis elegans was used for the experiments by exposing them to a liquid culture assay. The strain was obtained as a gift from Dr. Amir Nazir (CSIR-CDRI Lucknow). Animal culture was carried out using nematode growth medium plates. The plates were seeded with E. coli (OP50 strain) and were maintained at 22 °C in Biochemical Oxygen Demand (BOD) incubator (Thermo Fisher Scientific, Model-Precision) (Brenner, 1973). An age synchronized population was obtained for exposure by bleaching gravid animals using the modified method to help avoid variation due to age differences among the worms (Mishra, Gaur, and Agarwal, 2020). After age synchronization, animals were allowed to incubate at 22 °C, for 36–40 h to obtain L4 worms. For the liquid culture assay, 1 mL M9 buffer was used to obtain worm wash. Afterward, centrifugation was done at 200 rpm for 1 min to allow the worms to be free from adhered E. coli (Willhite and Mirkes, 2014) and were pipetted out in an Eppendorf tube using a cut tip for well exposure.
Drug preparation and exposure
The risperidone drug stock solution of 3 mM concentration was prepared as per the described method (Donohoe et al., 2006). The exposure was carried out with 50 µM final concentration as with lower concentrations than this showed unnoticeable effects on the exposed group. A volume of 20 µL (∼15–25 worms) of synchronized L4 worm wash was transferred into each well. L4 stage worms were exposed following age synchronization, in a sterile 96 well plate with a total volume of 200 µL in triplicate. The groups were classified in terms of exposure duration, i.e., 2, 4, 6, 8, 10 & 12 h. The time interval of two hours was selected considering short life span of C. elegans in order to observe pattern of alteration due to variable exposure durations.
Feeding behavior
C. elegans has a pharynx with similar pumping action to the mammalian heart (Hao and Buttner, 2014). It is autonomous and is responsible for pumping food inside the body. Pharyngeal pumping is a measure of feeding behavior and the amount of food taken by a worm. Age synchronized worms were used for pharyngeal pumping assay, Four drops each of 20 µL worm solution (∼10–15 worms) from control and exposed wells were pipetted out using cut tip. They were transferred into quadrants prepared by markings on a freshly seeded plate. Ten animals from each well with a total of 30 animals were used for counting. Pharyngeal pumping was counted for 1 min, and observations were recorded.
Locomotion
A Compound microscope with Phase Contrast (Micros Austria MCX51) was used to make video clips for locomotion assay. The WormLab (Version 3.0.0) helped perform the analysis.
Omega bends
It involves a worm curling back so that its head touches the tail & produces an omega shape during motion (Pierce-Shimomura et al., 1999). Four drops each of 20 µL containing approximately 10–15 worms were placed in a freshly non-seeded NGM plate to provide room for drying. The worms were allowed to move on the bed for 30sec-1 min. 3 min video was recorded after the worms started moving freely.
Peristaltic speed
It is defined as the speed with which a worm covers the peristaltic track length during its forward motion (Chao, 2016). 20 µL of the solution containing worms from each well were taken onto a fresh non-seeded NGM plate. A total of thirty animals were recorded from each set of control and exposed wells for 3 min.
Reversals
It is defined as the body waveforms in the anterior direction when a worm moves for several seconds and changes its movement backward (Tsalik and Hobert, 2003). 20 µL volume of solution was taken from the well and spotted in a fresh non-seeded NGM plate and kept for air drying. The worms were then unrestricted to move on the bed for 30 sec-1 min. In total, 30 animals were recorded for 3 min duration from each set of control and exposed wells.
Turn counts
It is defined as a count of perpendicular bending with respect to the pharynx during movement (Pierce-Shimomura et al., 1999). 20 µL of solution was pipetted and transferred into a fresh non-seeded NGM plate. Afterward, 30 animals were recorded from each set of control and exposed wells for 3 min.
The Control groups were C-0 h, C-2 h, C-4 h, C-6 h, C-8 h, C-10 h, and C-12 h for 2, 4, 6, 8, 10, and 12 h respectively. The exposed groups were E-2 h, E-4 h, E-6 h, E-8 h, E-10 h, and E-12 h, for 2, 4, 6, 8, 10, and 12 h. The time was within two hours as toxicity trends are expected to be affected by exposure time and keeping C. elegans life span into consideration.
Statistical analysis
The information from each duration is shown as Mean ± Standard Deviation (n = 30). The Graph Pad Prism software version 8.4.3 helped plot graphs. Two-way Analysis of Variance (ANOVA) with multiple comparison test was performed for data analysis between exposed and control to a sub-lethal dose of risperidone for various intervals. 'ns' denotes non-significant differences, P < 0.05 represented as *, P < 0.01 as ** and P < 0.001, represented as ***, means a high level of significance.
Results
Risperidone prescribed in the treatment of psychiatric disorders is found to show various toxic effects in terms of feeding and locomotion behavior of C. elegans.
Effects of risperidone on feeding behavior
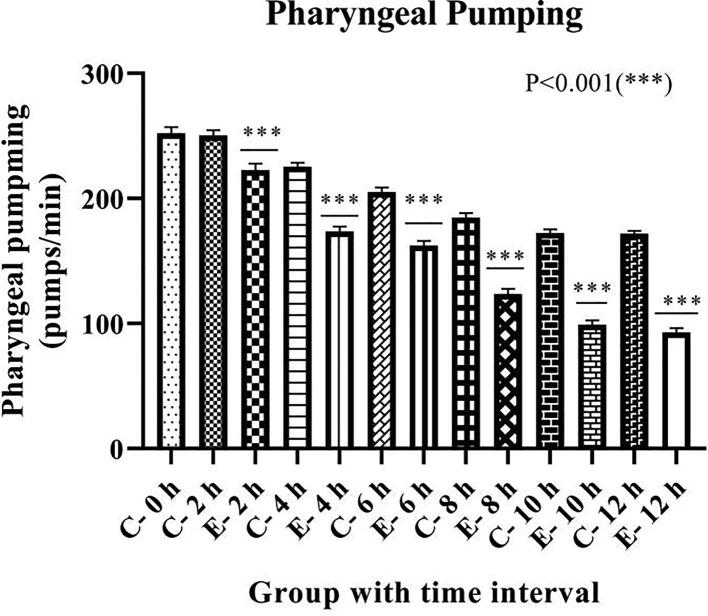
Feeding behavior is evaluated by pharyngeal pumping count. Fig. 1 the pharyngeal pumping rate expressed as pumps per minute in each group. Results show inhibitory effects of risperidone on pharyngeal pumping, thereby causing suppression in feeding behavior amongst the exposed group in contrast to respective control groups. The suppression in feeding behavior is in a time-dependent manner, i.e., 11.05% in 2 h, 22.07% in 4 h, 20.8% in 6 h, 33.07% in 8 h, 42.5% in 10 h, and 45.67% in 12 h in pharyngeal pumping when compared to its respective control.
Fig. 1.
Effect of risperidone exposure of 50 µM concentration at 2, 4, 6, 8, 10 and 12 hours of duration at 22 °C on pharyngeal pumping in C. elegans. Results are presented as mean ± SD. Asterisk (***) represented significant difference between control and exposed group (Two-way ANOVA, P < 0.001).
Effects of risperidone on locomotion
This is carried out in terms of vital markers of locomotion behavior, i.e., omega bends, peristaltic speed, reversals, and turn counts.
Omega bends
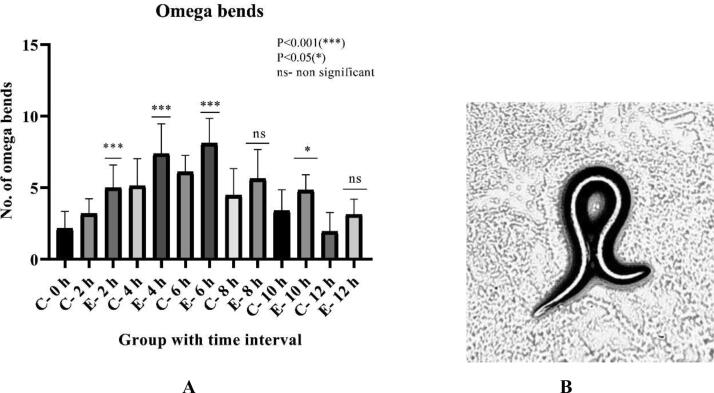
Fig. 2A depicts variation in omega bends among exposed and the control group of worms. There is a 56.25% increase after 2 h of exposure, 43.4% increase in 4 h, 32.78% in 6 h, 26.23% in 8 h, 42.05% in 10 h, and 62.17% in 12 h, which was found to be significant.
Fig. 2A.
Effect of risperidone exposure of 50 µM concentration at 2, 4, 6, 8, 10, and 12 hours of duration on omega bend in C. elegans. Outcomes demonstrated as mean ± SD. Asterisk (***) stood for a large difference between the exposed and control group in Fig. 2B omega bend in C. elegans at 10X magnification. An increase in omega turns (serotonergic effect) due to risperidone’s exposure effect.
Peristaltic speed
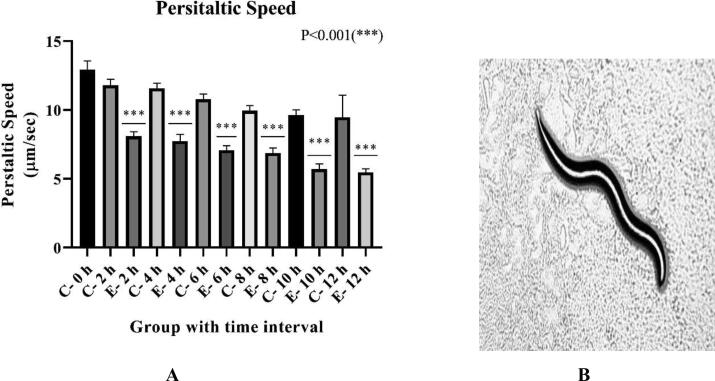
In Fig. 3A, the peristaltic speed of exposed worms shows a 31.38% drop in the speed after 2 h of exposure, 32.4% in 4 h, 34.32% in 6 h, 30.98% in 8 h, 40.7% in 10 h, and 42.22% in 12 h, which was significant in comparison to respective control groups.
Table 1.
Data showing values of behavioral parameters against exposure duration.
| Time (hours) | n | Behavioral Parameter Analyzed |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locomotion |
Feeding |
||||||||||
| Reversals |
Turn counts |
Omega Bends |
Peristaltic Speed |
Pharyngeal Pumping |
|||||||
| C | E | C | E | C | E | C | E | C | E | ||
| 0 | 30 | 318.23 ± 6.31 | – | 67.43 ± 4.37 | – | 2.16 ± 1.17 | – | 12.91 ± 0.65 | – | 252 ± 5.14 | – |
| 2 | 30 | 285.43 ± 4.17 | 299.4± 3.49 | 66.36 ± 3.69 | 43.233 ± 3.430 | 3.2 ± 1.03 | 5 ± 1.59 | 11.79 ± 0.43 | 8.09 ± 0.33 | 250.33 ± 4.30 | 222.66 ± 5.28 |
| 4 | 30 | 263.30 ± 5.18 | 280.73 ± 6.30 | 54.23 ± 3.51 | 34.133 ± 2.515 | 5.13 ± 1.90 | 7.36 ± 2.09 | 11.57 ± 0.38 | 7.74 ± 0.47 | 225.26 ± 3.46 | 173.53 ± 3.98 |
| 6 | 30 | 242.23 ± 4.88 | 263.33 ± 5.20 | 47.23 ± 4.90 | 31.466 ± 4.554 | 6.1 ± 1.15 | 8.1 ± 1.74 | 10.78 ± 0.36 | 7.08 ± 0.32 | 205.2 ± 3.58 | 162.4 ± 3.61 |
| 8 | 30 | 223.46 ± 10.88 | 252.16 ± 4.80 | 44.86 ± 3.33 | 25.9 ± 4.641 | 4.46 ± 1.87 | 5.63 ± 2.04 | 9.94 ± 0.38 | 6.86 ± 0.37 | 184.73 ± 3.61 | 123.63 ± 4.31 |
| 10 | 30 | 187.9 ± 6.321 | 230.6 ± 7.98 | 17.96 ± 2.83 | 15.96 ± 2.00 | 3.4 ± 1.45 | 4.83 ± 1.08 | 9.62 ± 0.39 | 5.7 ± 0.38 | 172.4 ± 3.035 | 99.13 ± 3.58 |
| 12 | 30 | 137.133 ± 3.848 | 183.80 ± 3.44 | 17.433 ± 1.454 | 15.1 ± 1.90 | 1.93 ± 1.33 | 3.13 ± 1.07 | 9.46 ± 1.60 | 5.46 ± 0.26 | 171.966 ± 2.173 | 92.96 ± 3.44 |
Table 1 represents data for feeding and locomotion behavioral pattern (reversals, omega bends, turn counts and peristaltic speed) for control and exposed groups against time interval from 2, 4, 6, 8, 10 and 12 hours. The data is represented as Mean ± SD (n = 30) for each group.
Fig. 3A.
Effect of risperidone exposure of 50 µM concentration at 2, 4, 6, 8, 10, and 12 hours of duration on peristaltic speed in C. elegans. Outcomes demonstrated as mean ± SD. Asterisk (***) stood for a large difference between the exposed and control group Fig. 3B Peristaltic movement shown by C. elegans at 10X magnification. An indication of slower movement (dopaminergic and serotonergic effects) due to the effects of risperidone exposure.
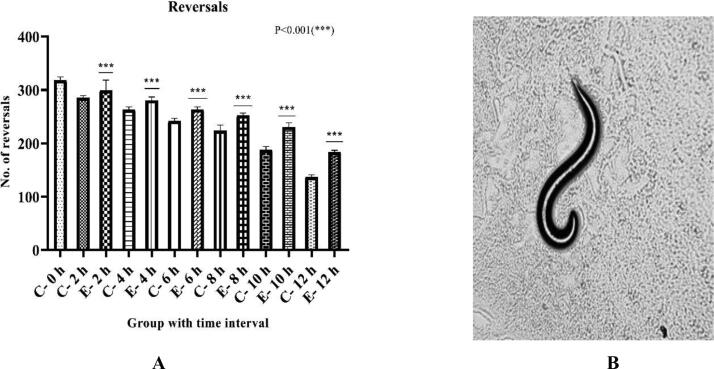
Reversals
Fig. 4A shows significant variation in reversals with the enhancement of 4.9% in 2 h, 6.61% in 4 h, 8.71% in 6 h, 12.84% in 8 h, 22.7% in 10 h, and 34.03% in 12 h in the exposed group compared to their respective control group.
Fig. 4A.
Effect of risperidone exposure of 50 µM concentration at 2, 4, 6, 8, 10, and 12 hours of duration on reversals in C. elegans. Outcomes are demonstrated as mean ± SD. Asterisk (***) stood for a large difference between the exposed and control group in Figure 4B. The reversal was shown by C. elegans at 10X magnification. An indication of slower movement (dopaminergic and serotonergic effects) due to the effects of risperidone exposure.
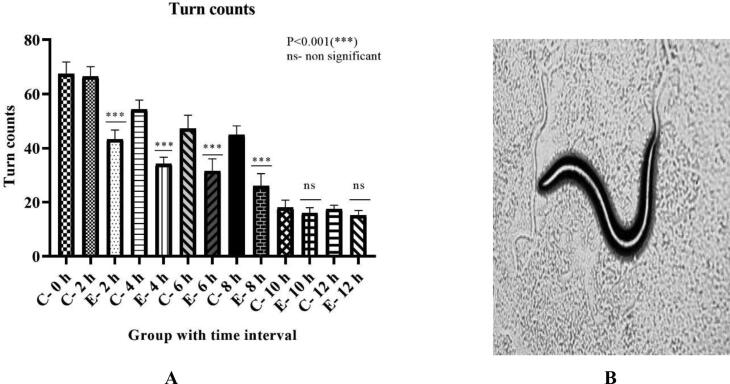
Turn counts
In Fig. 5A, turn counts are significantly reduced with 34.85% in 2 h of exposure, 37.06% in 4 h, 29.07% in 6 h, and 42.2% in 8 h in exposed groups compared to control groups. As the exposure duration exceeds 8 h, no significant difference was obtained.
Fig. 5A.
Effect of risperidone exposure of 50 µM concentration at 2, 4, 6, 8, 10, and 12 hours of duration on turn counts in C. elegans. Outcomes are demonstrated as mean ± SD. Asterisk (***) stood for a large difference between the exposed and control group in Figure 5B Turn counts shown by C. elegans at 10X magnification. An indication of a slower turn (dopaminergic and serotonergic effects) due to the effects of risperidone exposure.
Discussion
Risperidone is prescribed due to its therapeutic properties. However, several pieces of researches have shown that they have several developmental, behavioral, and physiological aftereffects (Horacek et al., 2006). Thus, this is a potential drug to investigate its toxic effects in detail. According to the research findings made by (Cope et al., 2009) on the intake of risperidone in rats, feeding and locomotion are the two useful concepts that can be used to evaluate onset of adverse effects of APDs. Also, (Donohoe et al., 2006), in their research, discovered that the egg-laying and growth were moderately affected due to risperidone in C. elegans. Moreover (Donohoe et al., 2009), discovered behavioral alterations due to clozapine, fluphenazine, olanzapine, and trifluoperazine in a class of APDs involving the plate assay method in their research.
We have adopted Liquid culture assay for this study as it allows variety of experimental procedures which require high throughput to be carried out conveniently (Scanlan et al., 2018), moreover nematodes encounter less mechanical stress in liquid culture assay than those in plate assay (Win et al., 2013).
Pharyngeal Pumping involves intake of bacteria through peristaltic movement is controlled by serotonin, octapine and tyramine (Horvitz et al., 1982, Rex et al., 2004). Serotonin (5-HT) produced by NSM, ADF and HSN neurons modulates feeding behavior by activating NSM. It stimulates pharyngeal pumping responding to food availability.(Avery and Horvitz, 1990, Avery and Thomas, 1997, Sawin et al., 2000)
The data show pharyngeal pumping inhibition amongst exposed groups in comparison to control, thereby reflecting altered feeding behavior. The pattern of alterations observed shows a significant reduction with exposure durations perhaps by antagonizing octapine, tyramine receptors along with serotonin (5-HT2) and dopamine (D2) receptors. This denotes possible alteration due to the pharmacology in addition to the off target effects due to risperidone drug in exposed animals compared to control in time dependent manner. As the exposure duration goes beyond 8 h, exposed groups show similar patterns potentially due to biological half-life of risperidone regulating further increase in toxicity due to its breakdown by xenobiotic-metabolizing enzyme.
Feeding behavior was reported to be inhibited in C. elegans supporting current study due to the effects of a class of APDs however risperidone was not evaluated for pharyngeal pumping assay in this study (Donohoe et al., 2006). Our results show significant inhibitory effects in pharyngeal pumping mechanism compared to modest effects due to Risperidone (150 μM) and aripiprazole (300 μM) treatment observed in C. elegans (Osuna-Luque et al., 2018). This could be explained due to variation in exposure duration and use of liquid culture assay for drug exposure which requires lesser concentration by increasing bioavailability (Zheng et al., 2013). Behavioral alterations have also been reported by (Donohoe et al., 2009) for class of APDs involving plate assay method where higher drug concentrations were used. Developmental delay and pharyngeal pumping reduction have already been studied extensively for variable concentrations of Clozapine in C. elegans model (Hao and Buttner, 2014). Our study suggests risperidone exposure tends to antagonize the above mentioned receptors thereby inhibiting pharyngeal pumping mechanism in time varying manner.
The findings of the study reveal that locomotion patterns (omega bends, peristaltic speed, reversal, and turn count) which are essential markers of toxicological assessment, show alteration amongst exposed groups in a time-sensitive manner compared to the respective control groups. Locomotion, which is a critical marker for the assessment of onset of toxic effects (Hahm et al., 2015, Peliti et al., 2013, Shtonda and Avery, 2006) being a neuromuscular activity, is affected due to several factors (Lüersen et al., 2016). Locomotion in C. elegans, whether forward or backward, comprises various patterns. AVA, AVB, AVD, and PVC are four bilaterally symmetric interneuron pairs responsible for locomotion in C. elegans. A-type motor neurons formed by the combination of AVA and AVD bring about backward movement while AVB and PVC provide input to the B-type motor neurons responsible for the forward movement in C. elegans (Chalfie et al., 1985, White et al., 1976). Reversals, omega bends, turn counts and peristaltic speed are significant markers of locomotion behavior in C. elegans (Li et al., 2013).
Omega Bend and Reversal patterns show an increased number in the exposed group, while turn counts show an increased number in the control group. Peristaltic speed shows a decrease amongst the exposed groups in comparison to respective controls. However, the pattern shows impregnation of toxicity around 8 h of exposure durations. Increased number of omega bends and reversals show the alteration set due to the pharmacology, a motor response developed in C. elegans. Omega bends and reversals are behavior shown by the worm to prevent itself from the toxicant (Huang et al., 2006). It is a measure of the neurological state of an animal with respect to its environment (Zhao et al., 2003). The findings show similarity with haloperidol (an atypical APD) induced reduction of locomotor activity in mice (Marx et al., 2006).
In our observations notable reduction in peristaltic speed and turn counts shows relatable patterns where less activity count in mice due to increased duration of risperidone treatment was reported by (Cope et al., 2009). Another study by (Lee Stubbeman et al., 2017) showing similar results wherein early-life exposure of risperidone reduced locomotor activity and amphetamine was used to induce hyperactivity in rats. Locomotion in Caenorhabditis elegans is regulated by serotonin as neurotransmitter (Chase and Koelle, 2007, Horvitz et al., 1982). SER-1 mediates a 5-HT induced decrease in motility along with MOD-1 to control locomotive function in C. elegans (Hobson et al., 2006). The alteration observed here is probably due to risperidone antagonizing other receptors along with D2 and 5-HT2A.
C. elegans have GPRs viz. SER-4 (5-HT1ce), SER-1 (5-HT2ce) and SER-7 (5-HT7ce) sharing similar characteristics to 5-HT1, 5-HT2, and 5-HT7 receptors as in mammals (Hamdan et al., 1999, Hobson et al., 2003, Olde and McCombie, 1997). Detailed study of mechanism of action has its scope and can be utilized in its extrapolation to humans.
The alteration observed amongst control groups with respect to duration may be due to factors responsible for the changes that may occur in the natural environment and lab culture conditions in C. elegans (Lant and Storey, 2010). Overcrowding, limited food, and less oxygen availability can also be responsible for it to occur (Sobkowiak et al., 2011). (Fujiwara et al., 2017) report of variation amongst control groups with variable exposure duration in rats also supports our observations.
Conclusion
Risperidone, an atypical antipsychotic prescribed for treatment of psychiatric disorder is found to produce altered locomotion and feeding behavior in C. elegans in a time-dependent manner. This study demonstrates a critical observation of inhibitory feeding and locomotion behavior using a liquid culture assay, which showed its effects on behavioral parameters at lower concentration and posing lesser mechanical stress in worms than that of plate assay. Risperidone antagonizes serotonin (5-HT) and dopamine (D2) receptors along with additional receptors thereby producing inhibitory effects on feeding and locomotion. The study sets the stage for exploring damage to other vital parameters due to the pharmacology of risperidone drug with detailed study of mechanism of its action. This also provides scope of probing the role of antioxidants/antidotes/herbal products in minimizing these effects when provided in combination with the drug.
Funding
Mr. Aaditya Vikram Gaur is grateful to National Forensic Sciences University (Erstwhile Gujarat Forensic Sciences University) for providing GFSU Research Fellowship in First Year of the Doctoral Studies. He is also thankful to University Grant Commission (UGC), New Delhi, India for providing the Junior Research Fellowship (Grant No. 190520237933) from second year onwards.
CRediT authorship contribution statement
Aaditya Vikram Gaur: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing – original draft, Data curation. Rakhi Agarwal: Conceptualization, Methodology, Validation, Resources, Investigation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Authors express their deep gratitude to Vice Chancellor and Campus Director, National Forensic Sciences University for permitting and providing facilities and instruments for research study. The study was approved by University Research Committee vide No. PhD/FS/RA/007. This work is a part of Ph.D. Thesis of Mr. Aaditya Vikram Gaur.
References
- Apfeld J., Alper S. “What Can We Learn About Human Disease from the Nematode C. Elegans?”. Meth. Mol. Biol. (Clifton N.J.) 2018;1706:53–75. doi: 10.1007/978-1-4939-7471-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L., Horvitz H.R. Effects of Starvation and Neuroactive Drugs on Feeding in Caenorhabditis Elegans. J. Exp. Zool. 1990;253(3):263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Avery Leon, Thomas James H. 1997. Feeding and Defecation. [PubMed] [Google Scholar]
- Avery, Leon, and Young-Jai You. 2018. “C. Elegans Feeding.” WormBook: The Online Review of C. Elegans Biology [Internet]. [DOI] [PMC free article] [PubMed]
- Brenner S. The Genetics of Behaviour. Br. Med. Bull. 1973;29(3):269–271. doi: 10.1093/oxfordjournals.bmb.a071019. [DOI] [PubMed] [Google Scholar]
- Byerly L., Cassada R.C., Russell R.L. The Life Cycle of the Nematode Caenorhabditis Elegans. I. Wild-Type Growth and Reproduction. Dev. Biol. 1976;51(1):23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J.E., White J.G., Southgate E., Thomson J.N., Brenner S. The Neural Circuit for Touch Sensitivity in Caenorhabditis Elegans. J. Neurosci. 1985;5(4):956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, Elizabeth. 2016. “Optimizing Pharmacological Lifespan Extension : Testing Chemical Compounds for Additive Effects on Longevity.”
- Chase D.L., Koelle M.R. Biogenic Amine Neurotransmitters in C. Elegans. WormBook : Online Rev. C. Elegans Biol. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M.B., Li X., Jumbo-Lucioni P., DiCostanzo C.A., Jamison W.G., Kesterson R.A., Allison D.B., Nagy T.R. Risperidone Alters Food Intake, Core Body Temperature, and Locomotor Activity in Mice. Physiol. Behav. 2009;96(3):457–463. doi: 10.1016/j.physbeh.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Aamodt E.J., Osborn E., Dwyer D.S. Antipsychotic Drugs Disrupt Normal Development in Caenorhabditis Elegans via Additional Mechanisms besides Dopamine and Serotonin Receptors. Pharmacol. Res. 2006;54(5):361–372. doi: 10.1016/j.phrs.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Jarvis R.A., Weeks K., Aamodt E.J., Dwyer D.S. Behavioral Adaptation in C. Elegans Produced by Antipsychotic Drugs Requires Serotonin and Is Associated with Calcium Signaling and Calcineurin Inhibition. Neurosci. Res. 2009;64(3):280–289. doi: 10.1016/j.neures.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Weeks K., Aamodt E.J., Dwyer D.S. Antipsychotic Drugs Alter Neuronal Development Including ALM Neuroblast Migration and PLM Axonal Outgrowth in Caenorhabditis Elegans. Int. J. Dev. Neurosci. 2008;26(3-4):371–380. doi: 10.1016/j.ijdevneu.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling R.L., Schulz S.C., Reed M.D., Blumer J.L. The Antipsychotics. A Pediatric Perspective. Pediatr. Clin. North Am. 1998;45(5):1205–1232. doi: 10.1016/s0031-3955(05)70070-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Ando H., Ushijima K., Horiguchi M., Yamashita C., Fujimura A. Dosing-Time-Dependent Effect of Rivaroxaban on Coagulation Activity in Rats. J. Pharmacol. Sci. 2017;134(4):234–238. doi: 10.1016/j.jphs.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Grover S., Avasthi A., Sinha V., Lakdawala B., Bathla M., Sethi S., Mathur D.M., Kathuria P., Shah S., Sai Baalasubramanian D., Agarwal V., Deka K. Indian Psychiatric Society Multicentric Study: Prescription Patterns of Psychotropics in India. Indian J. Psychiatry. 2014;56(3):253. doi: 10.4103/0019-5545.140632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm Jeong-Hoon, Kim Sunhee, DiLoreto Race, Shi Cheng, Lee Seung-Jae V., Murphy Coleen T., Nam Hong Gil. C. Elegans Maximum Velocity Correlates with Healthspan and Is Maintained in Worms with an Insulin Receptor Mutation. Nat. Commun. 2015;(6):8919. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan F.F., Ungrin M.D., Abramovitz M., Ribeiro P. Characterization of a Novel Serotonin Receptor from Caenorhabditis Elegans: Cloning and Expression of Two Splice Variants. J. Neurochem. 1999;72(4):1372–1383. doi: 10.1046/j.1471-4159.1999.721372.x. [DOI] [PubMed] [Google Scholar]
- Hao L., Buttner E.A. Methods for Studying the Mechanisms of Action of Antipsychotic Drugs in Caenorhabditis Elegans. J. Visualized Exp. 2014;84:1–8. doi: 10.3791/50864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka N., Umemura T. Influence of Maternal Chlorpromazine on Discrimination Learning in Rat Offspring. Neurotoxicol. Teratol. 1988;10(3):199–205. doi: 10.1016/0892-0362(88)90018-9. [DOI] [PubMed] [Google Scholar]
- Hobson R.J., Geng J., Gray A.D., Komuniecki R.W. SER-7b, a Constitutively Active Gαs Coupled 5-HT 7-like Receptor Expressed in the Caenorhabditis Elegans M4 Pharyngeal Motorneuron. J. Neurochem. 2003;87(1):22–29. doi: 10.1046/j.1471-4159.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- Hobson R.J., Hapiak V.M., Xiao H., Buehrer K.L., Komuniecki P.R., Komuniecki R.W. SER-7, a Caenorhabditis Elegans 5-HT7-like Receptor, Is Essential for the 5-HT Stimulation of Pharyngeal Pumping and Egg Laying. Genetics. 2006;172(1):159–169. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horacek J., Bubenikova-Valesova V., Kopecek M., Palenicek T., Dockery C., Mohr P., H??schl C. Mechanism of Action of Atypical Antipsychotic Drugs and the Neurobiology of Schizophrenia. CNS Drugs. 2006;20(5):389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Horvitz H., Chalfie M., Trent C., Sulston J., Evans P. Serotonin and Octopamine in the Nematode Caenorhabditis Elegans. Science. 1982;216(4549):1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Huang K.-M., Cosman P., Schafer W.R. Machine Vision Based Detection of Omega Bends and Reversals in C. Elegans. J. Neurosci. Methods. 2006;158(2):323–336. doi: 10.1016/j.jneumeth.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Kaletta T., Hengartner M.O. Finding Function in Novel Targets: C. Elegans as a Model Organism. Nat. Rev. Drug Discovery. 2006;5(5):387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Lanczik M., Knoche M., Fritze J. Psychopharmacotherapy during pregnancy and lactation. 1: Pregnancy. Der. Nervenarzt. 1998;69(1):1–9. doi: 10.1007/s001150050232. [DOI] [PubMed] [Google Scholar]
- Lant B., Storey K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis Elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010;6(1):9–50. doi: 10.7150/ijbs.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Stubbeman Bobbie, Brown Clifford J., Yates Justin R., Bardgett Mark E. Early-Life Risperidone Enhances Locomotor Responses to Amphetamine during Adulthood. Eur. J. Pharmacol. 2017;812:256–263. doi: 10.1016/j.ejphar.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yu., Gao Shan, Jing Haiming, Qi Lijuan, Ning Junyu, Tan Zhuangsheng, Yang Kexin, Zhao Chaoying, Ma Ling, Li Guojun. Correlation of Chemical Acute Toxicity between the Nematode and the Rodent. Toxicol. Res. 2013;2(6):403–412. [Google Scholar]
- Lüersen Kai, Gottschling Dieter Christian, Döring Frank. Complex Locomotion Behavior Changes Are Induced in Caenorhabditis Elegans by the Lack of the Regulatory Leak K+ Channel TWK-7. Genetics. 2016;204(2):683–701. doi: 10.1534/genetics.116.188896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx Christine E., Shampine Lawrence J., Duncan Gary E., VanDoren Margaret J., Chistina Grobin A., Massing Mark W., Madison Roger D., Bradford Daniel W., Butterfield Marian I., Lieberman Jeffrey A., Leslie Morrow A. Clozapine Markedly Elevates Pregnenolone in Rat Hippocampus, Cerebral Cortex, and Serum: Candidate Mechanism for Superior Efficacy? Pharmacol. Biochem. Behav. 2006;84(4):598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Mishra Sarita, Gaur Aaditya Vikram, Agarwal Rakhi. “Standardization of Synchronization Procedure to Collect the Similar Aged C. Elegans”. 2020;268–71 [Google Scholar]
- Olde Bjorn, Richard McCombie W. Molecular Cloning and Functional Expression of a Serotonin Receptor from Caenorhabditis Elegans. J. Mol. Neurosci. 1997;8(1):53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- Osuna-Luque Jaime, Rodríguez-Ramos Ángel, del Mar Gámez-del-Estal María, Ruiz-Rubio Manuel. Behavioral Mechanisms That Depend on Dopamine and Serotonin in Caenorhabditis Elegans Interact With the Antipsychotics Risperidone and Aripiprazole. J. Exp. Neurosci. 2018;12 doi: 10.1177/1179069518798628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peliti Margherita, Chuang John S., Shaham Shai, Bettinger Jill C. Directional Locomotion of C. Elegans in the Absence of External Stimuli. PLoS One. 2013;8(11):e78535. doi: 10.1371/journal.pone.0078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura Jonathan T., Morse Thomas M., Lockery Shawn R. The Fundamental Role of Pirouettes in Caenorhabditis Elegans Chemotaxis. J. Neurosci. 1999;19(21):9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex Elizabeth, Molitor Scott C., Hapiak Vera, Xiao Hong, Henderson Megan, Komuniecki Richard. Tyramine Receptor (SER-2) Isoforms Are Involved in the Regulation of Pharyngeal Pumping and Foraging Behavior in Caenorhabditis Elegans. J. Neurochem. 2004;91(5):1104–1115. doi: 10.1111/j.1471-4159.2004.02787.x. [DOI] [PubMed] [Google Scholar]
- Robertson R.T., Majka J.A., Peter C.P., Bokelman D.L. Effects of Prenatal Exposure to Chlorpromazine on Postnatal Development and Behavior of Rats. Toxicol. Appl. Pharmacol. 1980;53(3):541–549. doi: 10.1016/0041-008x(80)90367-1. [DOI] [PubMed] [Google Scholar]
- Sawin Elizabeth R, Ranganathan Rajesh, Robert Horvitz H. C. Elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron. 2000;26(3):619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Scanlan Leona D., Lund Steven P., Coskun Sanem Hosbas, Hanna Shannon K., Johnson Monique E., Sims Christopher M., Brignoni Karina, Lapasset Patricia, Petersen Elijah J., Elliott John T., Nelson Bryant C. Counting Caenorhabditis Elegans: Protocol Optimization and Applications for Population Growth and Toxicity Studies in Liquid Medium. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-19187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtonda Boris Borisovich, Avery Leon. Dietary Choice Behavior in Caenorhabditis Elegans. J. Exp. Biol. 2006;209(Pt 1):89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowiak Robert, Kowalski Mateusz, Lesicki Andrzej. Concentration- and Time-Dependent Behavioral Changes in Caenorhabditis Elegans after Exposure to Nicotine. Pharmacol. Biochem. Behav. 2011;99(3):365–370. doi: 10.1016/j.pbb.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Song Bo-Mi, Faumont Serge, Lockery Shawn, Avery Leon. Recognition of Familiar Food Activates Feeding via an Endocrine Serotonin Signal in Caenorhabditis Elegans. eLife. 2013;2:e00329. doi: 10.7554/eLife.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner Steven C., Sommi Roger W., Marken Patricia A., Anya Innocent, Vaughn James. Clozapine Use in Two Full-Term Pregnancies. J. Clin. Psychiatry. 1997;58(8):364–365. doi: 10.4088/jcp.v58n0806f. [DOI] [PubMed] [Google Scholar]
- Tsalik Ephraim L., Hobert Oliver. Functional Mapping of Neurons That Control Locomotory Behavior in Caenorhabditis Elegans. J. Neurobiol. 2003;56(2):178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Ullberg S., Lindquist N.G., Sjöstrand S.E. Accumulation of Chorio-Retinotoxic Drugs in the Foetal Eye. Nature. 1970;227(5264):1257–1258. doi: 10.1038/2271257a0. [DOI] [PubMed] [Google Scholar]
- Weinhouse Caren, Truong Lisa, Meyer Joel N., Allard Patrick. Caenorhabditis Elegans as an Emerging Model System in Environmental Epigenetics. Environ. Mol. Mutagen. 2018;59(7):560–575. doi: 10.1002/em.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. “The Structure of the Ventral Nerve Cord of Caenorhabditis Elegans”. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1976;275(938):327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- Willhite C.C., Mirkes P.E. In: Encyclopedia of Toxicology (Third Edition) Wexler P., editor. Academic Press; Oxford: 2014. Developmental Toxicology; pp. 14–44. [Google Scholar]
- Win Myat Thu, Thu Yasuhiko Yamamoto, Munesue Seiichi, Han Dong, Harada Shin-Ichi, Yamamoto Hiroshi. Validated Liquid Culture Monitoring System for Lifespan Extension of Caenorhabditis Elegans through Genetic and Dietary Manipulations. Aging Dis. 2013;4(4):178–185. [PMC free article] [PubMed] [Google Scholar]
- Yaeger Deborah, Smith Healy G., Altshuler Lori L. Atypical Antipsychotics in the Treatment of Schizophrenia during Pregnancy and the Postpartum. Am. J. Psychiatry. 2006;163(12):2064–2070. doi: 10.1176/ajp.2006.163.12.2064. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Smith B., Craggs M., Kumar R. Neuroleptic Drugs in Breast-Milk: A Study of Pharmacokinetics and of Possible Adverse Effects in Breast-Fed Infants. Psychol. Med. 1998;28(1):81–91. doi: 10.1017/s0033291797005965. [DOI] [PubMed] [Google Scholar]
- Zheng Shan-Qing, Ding Ai-Jun, Li Guo-Ping, Wu Gui-Sheng, Luo Huai-Rong, Wicker-Thomas Claude. Drug Absorption Efficiency in Caenorhbditis Elegans Delivered by Different Methods. PLoS One. 2013;8(2):e56877. doi: 10.1371/journal.pone.0056877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito J.M., Safer D.J., dosReis S., Gardner J.F., Boles M., Lynch F. Trends in the Prescribing of Psychotropic Medications to Preschoolers. JAMA. 2000;283(8):1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]