To the Editor:
Convalescent plasma (CP) therapy uses neutralizing antibodies harvested from recovered patients to treat viral infections, including severe acute respiratory syndrome coronavirus (SARS-CoV) and influenza A.1 The emerging data from randomized controlled trials and observational studies suggest—consistent with historical precedent—that CP therapy has limited efficacy in severely ill patients with coronavirus disease 2019 (COVID-19) treated late in the disease course. However, early treatment with high-titer CP exhibits signs of efficacy.2 Additionally, CP use in immunocompromised hosts unable to generate endogenous antibodies suggests a mortality benefit and rapid clinical improvement.3 , 4 One group of patients who benefit from CP therapy are those with primary or secondary B-cell deficiencies with a high risk of severe COVID-19 due to their reduced ability to produce neutralizing antibodies.5 , 6 These patients can also have prolonged refractory COVID-19 that can last many months and include the generation of novel viral variants. In this context, Vax-plasma is CP from patients who have recovered from natural infection and have been subsequently vaccinated. It can have 10 to 100 times higher antibody titers than does standard high-titer CP with a broad coverage of known COVID-19 variants.7 Herein, we present our first experience using Vax-plasma in an immunocompromised patient with refractory COVID-19.
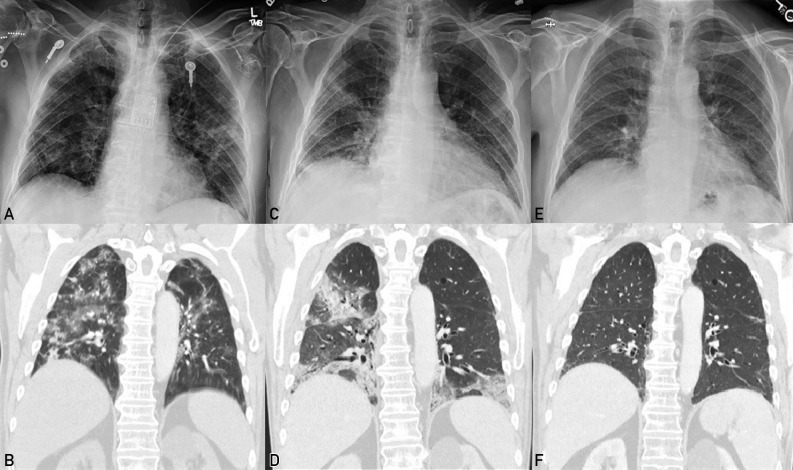
In the fall of 2020, a 68-year-old patient with a history of mantle cell lymphoma and recently diagnosed with COVID-19 presented in the emergency department with shortness of breath, cough, and fever. His medical history was remarkable for metastatic mantle cell lymphoma treated with 6 cycles of rituximab and bendamustine, followed by an autologous stem cell transplant 2 years before this admission. He received maintenance therapy with rituximab every 2 months with the last infusion 2 months before this presentation. His examination was remarkable for nonlabored breathing and coarse crackles in both bases. His white blood cell count was 3800 cells/μL; lymphocyte count, 140 cells/μL; C-reactive protein level, 141 mg/L; ferritin level, 849 μg/L; and D-dimer level, 19,418 ng/mL. Chest radiography revealed airspace opacities bilaterally, and computed tomography of the lungs revealed numerous ground-glass opacities consistent with COVID-19 pneumonia (Figure 1 A and B). The patient received remdesivir for 5 days but developed hypoxia, with repeated lung imaging revealing worsening patchy infiltrates. The patient next received broad-spectrum antibiotics along with dexamethasone and remdesivir for 5 additional days. His infectious disease work-up was negative for superimposed infection, so the broad-spectrum antibiotics were discontinued. In addition, 2 U of high-titer CP was administered on consecutive days. His fever and hypoxia resolved, his C-reactive protein level diminished to 37 mg/L, and the patient was dismissed after being hospitalized for 2 weeks.
Figure 1.
Imaging during the patient’s illness. Panels A and B correspond to his first hospitalization. Panels C and D correspond to his last hospitalization. Panel E and F correspond to his follow-up visit after receiving Vax-plasma. A, Chest radiography revealing diffuse airspace opacities in both lungs. B, Computed tomography of the chest revealing multiple bilateral consolidations and ground-glass opacities. C, Chest radiography revealing patchy opacities in both lungs with consolidative changes. D, Computed tomography of the chest revealing airspace disease with consolidations. E, Chest radiography revealing near complete interval resolution of patchy bilateral infiltrates. F, Computed tomography of the chest revealing interval resolution of airspace opacities in both lungs.
Unfortunately, the patient had recurrent episodes of fever, shortness of breath, and hypoxia, requiring intermittent admissions to the intensive care unit and receiving multiple courses of remdesivir, dexamethasone, and high-titer CP, requiring 9 rehospitalizations. He was also diagnosed with pulmonary embolism and cryptogenic organizing pneumonia, receiving anticoagulation and high doses of corticosteroids with initial improvement, but was then rehospitalized with the same symptomatology. He had a persistently positive SARS-CoV-2 polymerase chain reaction result and was diagnosed with refractory COVID-19 (Figure 2 ).
Figure 2.
| Fever | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) | (−) | (+) | (−) | |
| Hypoxia | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | |
| Lymphocyte absolute count (×109/L) | 0.14 | 0.19 | 0.28 | 0.12 | 0.31 | 0.09 | 0.22 | 0.27 | 0.16 | 0.15 | ND | |
| C-reactive protein level (mg/L) | 141 | 87 | 118 | ND | 162 | 82 | 124 | 106 | 70 | 84 | <3 | |
| Ferritin level (µg/L) | 849 | 1154 | ND | 7670 | 4242 | 4345 | 3100 | 1070 | 791 | 791 | ND | |
| Remdesivir | 10 d | (−) | 5 d | 5 d | (−) | 5 d | 5 d | 5 d | (−) | 5 d | (−) | |
| Corticosteroidsc | 5 d | 10 d | 10 d | 10 d | PDN Taper | + + + | 10 d | PDN taper + | PDN taper + | PDN taper + | + | |
| CP | 2 U | (−) | 2U | (−) | (−) | 2 U | 2 U | (−) | (−) | 2 U | (−) | |
| Vax-plasma | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | 2 U | 1 U |
| Day 0 | Day 8-21 hospitalization | Day 28-38 hospitalization | Day 40-48 hospitalization | Day 78-90 hospitalization | Day 97-109 hospitalizationd | Day 195-200 hospitalization | Day 226-235 hospitalizatione | Day 247-254 hospitalization | Day 262-264 hospitalization | Day 269-276 hospitalization | Day 305 follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 PCR |
(+) | ND | ND | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) |
| SARS-CoV-2 nudeocapsid antibody | ND | ND | ND | ND | (−) | (−/+)f | (−/+)f | (+) | (+) | (−) | ND | |
| SARS-CoV-2 spike antibody (U/mL) | ND | ND | ND | ND | ND | ND | 1.9 | ND | ND | 2.9–4.1g/>250>250h | >250 | |
| SARS-CoV-2 neutralizing antibody | ND | ND | ND | ND | ND | ND | ND | ND | ND | (−)i/286j | ND |
COP, cryptogenic organizing pneumonia; COVID-19, coronavirus disease 2019; CP, convalescent plasma; ND, no data; PCR, polymerase chain reaction; PDN, prednisone; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; U, units.
SARS-CoV-2 PCR, Cobas SARS-CoV-2 assay (Roche Molecular Systems, Inc.); SARS-CoV-2 nucleocapsid antibody, Roche Elecsys Anti-SARS-CoV-2 Reagent assay (Roche Diagnostics); SARS-CoV-2 spike antibody, SARS-CoV-2 spike glycoprotein antibody (Roche Elecsys Anti-SARS-CoV-2 S Reagent assay from Roche Diagnostics); SARS-CoV-2 neutralizing antibody, IMMUNO-COV.
Patients received dexamethasone initially between 5 and 10 d and then PDN at different doses after the diagnosis of COP.
Admission was complicated by aspiration pneumonia and COP. PDN 20 mg per day was initiated and then tapered down slowly.
The patient required mechanical ventilation, and nasopharynx and bronchoalveolar lavage samples resulted positive for SARS-CoV-2.
SARS-CoV-2 nucleocapsid antibody test results were negative before receiving CP and then turned positive after receiving two units of high-titer CP.
SARS-CoV-2 spike protein antibody test results after receiving the first and second unit of high-titer CP.
SARS-CoV-2 spike protein antibody test results after receiving the first and second unit of super high-titer CP (Vax-plasma).
SARS-CoV-2 neutralizing antibodies after receiving two units of high-titer CP.
SARS-CoV-2 neutralizing antibodies after receiving two units of super high-titer CP (Vax-plasma).
During his last hospitalization in the summer of 2021, the patient presented again with fever, hypoxia, and persistent pulmonary infiltrates (Figure 1 C and D). He received remdesivir, corticosteroids, and 2 U of high-titer CP. The semiquantitative detection of total antibodies against the SARS-CoV-2 spike protein (Roche Elecsys Anti-SARS-CoV-2 S assay, Roche Diagnostics) resulted 2.9 U/mL after the first infusion of CPT and 4.1 U/mL after the second unit. The IMMUNO-COV SARS-CoV-2 neutralizing antibody test result was negative. As he continued to have hypoxia, Vax-plasma was administered. After the first infusion, the semiquantitative detection of total antibodies against SARS-CoV-2 spike antibody was repeated and resulted greater than 250 U/mL (Roche Elecsys Anti-SARS-CoV-2 S assay). After the second dose of Vax-plasma the following day, the semiquantitative detection of total antibodies resulted greater than 250 U/mL and IMMUNO-COV SARS-CoV-2 neutralizing antibody test resulted positive (level was 286). No adverse effects were reported. The patient improved progressively, and on dismissal 7 days later, he was afebrile and not hypoxic. At follow-up, he remained afebrile, with normal oximetry results, and imaging revealed almost complete resolution of pulmonary infiltrates (Figure 1 E and F). He also had a negative SARS-CoV-2 RNA test result 1 month after his last hospitalization and 305 days after his first positive test result. He has subsequently received monthly outpatient infusions of Vax-plasma, and after 10 weeks of follow-up, the patient has not been admitted to the hospital.
To our knowledge, this is the first report of Vax-plasma treatment in a patient with COVID-19 unable to mount normal antibody responses to the disease. Notably, the serum neutralizing antibody response from individuals who have been infected is enhanced after receiving a messenger RNA vaccine and could effectively neutralize an array of COVID-19 variants.7 , 8 In conclusion, the use of Vax-plasma is a promising therapy that can be included in the treatment and prevention of COVID-19 in immunocompromised patients.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Mair-Jenkins J., Saavedra-Campos M., Kenneth Baillie J., et al. Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klassen S.A., Senefeld J.W., Johnson P.W., et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senefeld J.W., Klassen S.A., Ford S.K., et al. Use of convalescent plasma in COVID-19 patients with immunosuppression. Transfusion. 2021;61(8):2503–2511. doi: 10.1111/trf.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchini M., Corsini F., Focosi D., Cruciani M. Safety and efficacy of convalescent plasma in COVID-19: an overview of systematic reviews. Diagnostics (Basel) 2021;11(9):1663. doi: 10.3390/diagnostics11091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hueso T., Pouderoux C., Péré H., et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tepasse P.R., Hafezi W., Lutz M., et al. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190(2):185–188. doi: 10.1111/bjh.16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt F, Weisblum Y, Rutkowska M, et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape [published online ahead of print September 20, 2021]. Nature. https://doi.org/10.1038/s41586-021-04005-0. [DOI] [PMC free article] [PubMed]
- 8.Stamatatos L, Czartoski J, Wan YH, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection [published online ahead of print March 25, 2021]. Science. 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed]