Abstract
Background
It is important to identify further predictors of outcome after successful transcatheter mitral valve repair (TMVR), as optimal patient selection remains difficult.
Objective
The study investigates the prognostic benefit of the mean arterial pressure (MAP) to right atrial pressure (RAP) ratio (MAP/RAP ratio) after successful TMVR in patients with congestive heart failure (CHF) and severe mitral regurgitation (MR).
Method
Patients with CHF and severe MR were enrolled after successful TMVR (MR ≤ 2+ at discharge). The primary endpoint was a composite of all-cause mortality or hospitalisation for heart failure. The median follow-up time was 16 ± 9 months. Receiver Operating Characteristic (ROC) analysis was applied to assess the discriminatory power of the MAP/RAP ratio. The predictive value of the MAP/RAP ratio threshold was investigated using a Kaplan-Meier analysis. Multivariable logistic regression analysis was conducted to evaluate independent risk factors for the combined primary endpoint.
Results
145 patients (median age 76 [69–80 years], 60.3% male) were included. ROC curve analysis showed that MAP/RAP ratio was associated with an area under the curve of 0.62 (95% confidence interval (CI) 0.53–0.71; p = 0.01). A MAP/RAP ratio threshold of 7.13 was associated with 67.4% sensitivity and 57.0% specificity for the combined primary endpoint. Event-free survival was significantly lower in the MAP/RAP ratio < 7.13 group compared to those with MAP/RAP ratio ≥ 7.13 (62.2% versus 39.4%; log-rank p = 0.022). In logistic regression analysis MAP/RAP ratio was an independent predictor for the combined primary endpoint (odds ratio 0.75; 95% CI 0.62–0.90; p = 0.002).
Conclusions
The MAP/RAP ratio is associated with an unfavorable outcome in patients undergoing successful TMVR. Therefore, this new index could improve prognostic assessment of TMVR candidates.
Keywords: Mitral regurgitation, Chronic heart failure, Transcatheter mitral valve repair, MAP/RAP ratio
Abbreviations: CHF, congestive heart failure; LV-EF, left ventricular ejection fraction; MAP, Mean arterial pressure; NYHA, New York Heart Association; RAP, Mean right atrial pressure; TAPSE, tricuspid annular plane systolic excursion; TMVR, transcatheter mitral valve repair
1. Introduction
Severe functional mitral regurgitation (FMR) due to left ventricular remodeling occurs in up to 25% of patients with congestive heart failure (CHF) and is associated with an unfavorable prognosis [1]. Transcatheter mitral valve repair (TMVR) with the MitraClip system (Abbott Vascular, Abbott Park, Illinois) offers a valuable treatment option for these patients [2]. Early data from non-randomised observational studies, mostly including heart failure patients with FMR, show that TMVR is safe, effectively reduces FMR and can improve patients' functional status [3], [4], [5], [6], [7], [8], [9], [10]. Nevertheless, numerous predictors of adverse outcome after TMVR have been identified, e.g. procedural failure, New York Heart Association (NYHA) functional class IV before TMVR, high NT-proBNP levels, severely reduced left ventricular ejection fraction (LV-EF) and concomitant right ventricular (RV) impairment [6], [9], [11], [12], [13], [14]. Evidence to date suggests that different subgroups of CHF patients do not benefit equally from TMVR. Indeed, two simultaneously published randomized clinical trials have obtained contradictory results [15], [16]. Both trials compared MitraClip plus optimal medical therapy (OMT) with OMT alone in patients with CHF and FMR. These seemingly contradictory results can be at least partially explained by variations in patient selection. Hence, an appropriate patient selection for TMVR is still difficult. It is therefore crucial to identify further risk factors of poorer prognosis to better define the most appropriate patients in whom TMVR has significant benefit. Although specific clinical and echocardiographic predictors of worse prognosis have already been identified, the utility of invasively derived haemodynamic measurements is not well established. Recently, pulmonary artery pulsatility index (PAPi), defined as the ratio of pulmonary artery pulse pressure to right atrial pressure, was the first haemodynamic index to show an association with outcome in patients with CHF and FMR after TMVR [17]. The present study analyses a new index, the ratio of mean arterial pressure (MAP) to right atrial pressure (RAP), which describes the interaction between systemic and pulmonary circulation. This new index takes into account both left and right ventricular factors in congestive heart failure and may serve as an additional marker of biventricular congestion. Thus, MAP/RAP ratio may be more able to predict outcome in patients with CHF undergoing TMVR than variables describing systemic or pulmonary circulation alone. Therefore, the aim of the present study is to investigate the utility of the MAP/RAP ratio in predicting outcome in CHF patients undergoing TMVR.
2. Methods
2.1. Patient cohort
The study retrospectively analyses data from an ongoing single-center registry of the Bremen Institute for Cardiovascular Research (BIHKF) at the Bremen Heart Centre. The following inclusion criteria were defined for study enrolment: successful TMVR (MR ≤ 2 at discharge) of severe FMR, CHF regardless of underlying heart disease with a reduced LV-EF ≤ 50%, NYHA functional class III and IV prior to TMVR, established optimal heart failure drug and device therapy (if indicated) at least 3 months prior to TMVR in accordance with the currently valid recommendations [18].
TMVR was performed based on the Heart Team's decision if the mitral valve was anatomically suitable for TMVR, the patient was deemed inoperable or the surgical risk was considered unacceptably high. The logistic European System for Cardiac Surgical Risk Evaluation (logistic EuroSCORE) was used as a decision aid. All study participants were fully informed about the procedure and signed a written informed consent form. The study is in accordance with the Declaration of Helsinki. The locally appointed ethics committee has approved the research protocol.
2.2. Echocardiographic and haemodynamic evaluation
Transthoracic and transoesophageal echocardiographic examinations were recorded at baseline. The severity grade of MR was graded based on current guidelines [19], [20]. Transthoracic echocardiographic evaluations were done before hospital discharge, 30 days after TMVR and, if feasible, at follow-up. The severity of MR after TMVR was assessed using the technique described by Foster et al. [21].
All included study participants underwent a baseline invasive haemodynamic examination during the screening period while conscious and without sedation. Right heart catheter measurements were performed under stable chronic heart failure conditions, defined as optimal drug therapy for heart failure with no changes in medication in the previous 3 months. Right heart catheterisation was done with a single lumen, balloon-tipped, flow-guided Swan-Ganz catheter (Arrow International, Inc, Reading, Pennsylvania, USA) to achieve the following parameters: systemic arterial systolic and diastolic pressure (RR syst., RR diast.) and MAP, pulmonary capillary wedge pressure (PCWP), pulmonary artery systolic, mean and diastolic pressure (PASP, PAP mean, PAP diast.) and RAP. Cardiac output (CO) was analysed using the Fick method. Stroke volume (SV) was computed as CO / heart rate and the stroke volume index (SVi) as SV / body surface area (BSA). BSA was assessed based on the Du Bois formula. Systemic and pulmonary vascular resistance (SVR, PVR) were calculated as the ratio of the pressure drop across the vascular bed and CO and expressed in metric units (dyn*s*cm−5). All values are given at end-expiration. PAPi was computed as (PASP – PAP diast.) / RAP. Transpulmonary gradient was defined as PAP mean – PCWP and MAP/RAP ratio as MAP/RAP.
2.3. TMVR and clinical outcome (study endpoints)
All interventions were carried out under general anaesthesia with fluoroscopic and (3D) transoesophageal echocardiographic guidance. The MitraClip procedure was done as previously mentioned [3], [22]. Primary endpoint was a composite of all-cause mortality or heart failure hospitalisation. The single endpoints all-cause mortality and hospitalisation for heart failure were also analysed. Serious in-hospital adverse events were documented (intervention-related death, disabling stroke, non-fatal myocardial infarction, pericardial tamponade, urgent cardiovascular surgery due to adverse events, acute kidney failure requiring dialysis, in-hospital bleeding and vascular access complications according to Mitral Valve Academic Research Council [MVARC] criteria) [23].
Standardised follow-up of the study participants was undertaken by the Bremen Institute for Cardiovascular Research (BIHKF) after 30 days and after a mean follow-up time of 16 ± 9 months. If a study participant was unable to attend the follow-up visit, a telephone interview was held with either the patient themselves, their relatives or their general practitioners. During long-term follow-up, major adverse cardiac and cerebrovascular events (MACCE) were documented (disabling stroke, non-fatal myocardial infarction, death from all causes, necessary interventional or surgical re-intervention). Health-related quality of life was assessed using a standardised questionnaire (EQ-5D) and changes in NYHA functional class.
2.4. Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median (interquartile range) and were tested with the unpaired Student s t-test for normally distributed variables or the Mann-Whitney U test for skewed data. Distribution of data was assessed by Kolmogorov-Smirnov test and distribution analysis. Categorical variables are displayed as numbers and proportions and were compared with the Fisher's exact test or chi-square test. Receiver operating characteristic (ROC) curves were applied to estimate the discriminatory ability of the MAP/RAP ratio and to define a corresponding cut-off score for the combined primary endpoint. This cut-off score was used to dichotomize the study cohort into two groups (high and low ratio group). Pearson s and Spearman Rho correlation function were applied to determine the relationship between MAP/RAP ratio and haemodynamic variables (CI, PCWP, PVR, SVR, PASP) and between MAP/RAP ratio and echocardiographic parameters of LV and RV systolic function (LV-EF, tricuspid annular plane systolic excursion [TAPSE] and doppler tissue imaging S [DTI-S ]). Multicollinearity was studied by using the variance inflation factor and a value ≥ 4 was defined as an index of multicollinearity. The Kaplan-Meier method was used for survival analysis. The log-rank test was used to estimate the event-free survival of combined primary and individual secondary endpoints. Multivariable binary logistic regression analysis using stepwise backward elimination was used to determine the relationship between MAP/RAP ratio and combined primary endpoint adjusting for any associated covariates previously reported, including TAPSE, LV-EF, creatinine levels, NT-proBNP levels, NYHA functional class IV°, severe tricuspid valve regurgitation (TR III°) and for those variables that differ significantly between the groups (see Appendix 1). Goodness-of-fit was assessed using the Hosmer-Lemeshow-Test. A multivariable Cox proportional hazards regression analysis with stepwise backward elimination was conducted to investigate the impact of relevant variables on all-cause mortality. Individual variables were deleted in a stepwise backward selection until a model with a significance level of 0.05 in all variables was achieved. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 27 (SPSS, Inc, Chicago, Illinois).
3. Results
3.1. Clinical characteristics
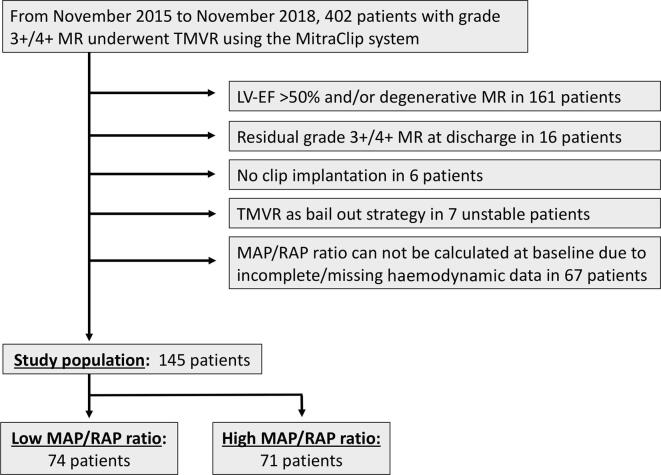
The Bremen MitraClip registry contains records of 402 patients in the time period between November 2015 and November 2018. Of these, 145 patients were eligible for at least 12 months follow-up, had complete invasive haemodynamics and fulfilled the aforementioned inclusion criteria (Fig. 1). Clinical follow-up was achieved in all included study participants. Mean follow-up time was 16 ± 9 months. Median age of the study population was 76 (69–79.5) years (67.6% male). Median MAP/RAP ratio of the study population was 7.07 (5.20–10.58).
Fig. 1.
Study flow chart. LV-EF: left ventricular ejection fraction; MAP: mean arterial pressure; MR: mitral regurgitation; RAP: right atrial pressure; TMVR: transcatheter mitral valve repair;
3.2. Validation of discriminative capacity and optimal cut-off value for MAP/RAP ratio
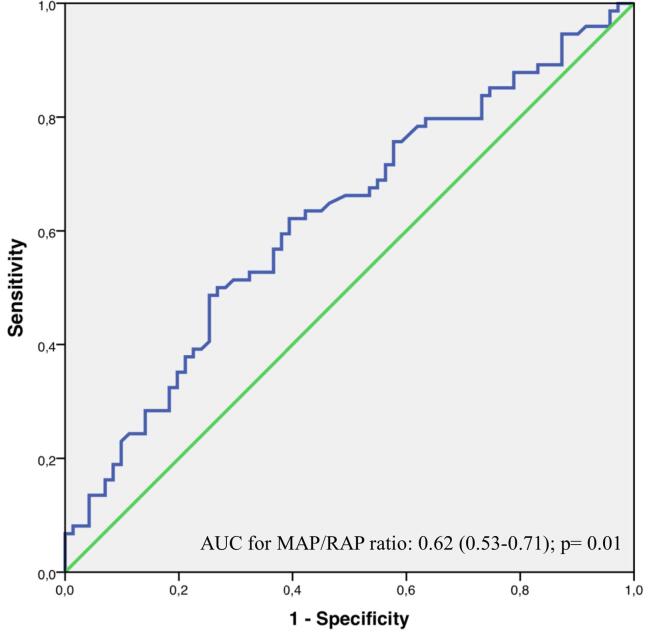
MAP/RAP ratio showed discriminative capacity for combined primary endpoint (area under the curve (AUC) 0.62 [0.53–0.71]; p = 0.01; Fig. 2). ROC curve analysis showed that optimal sensitivity and specificity for combined primary endpoint was achieved using a simple nearest-to-median-threshold of 7.13 (sensitivity 67.4%, specificity 57.0%; Fig. 2).
Fig. 2.
Receiver operating characteristic curve analysis for MAP/RAP ratio. AUC: area under the curve; MAP: mean arterial pressure; RAP: right atrial pressure.
This cut-off value for MAP/RAP ratio was used to dichotomize the study population into a low ratio (<7.13, n = 74; 51%) and a high ratio (≥7.13, n = 71; 49%) group.
3.3. Comparison of baseline variables
The proportion of male patients was significantly higher in the low ratio group (Table 1). The vast majority of patients had several comorbidities with hypertension and coronary artery disease being the most common. Of patients included, 37.9% had a history of myocardial infarction. Patients in the low ratio group presented a higher body mass index and trended to show higher levels of creatinine, respectively. Patients in the low ratio group were more likely to have hypertension and received more often an implantable cardioverter/ defibrillator (ICD). The proportion of patients who were in NYHA functional class IV before TMVR was similar in both groups. The study population received maximal tolerated medical heart failure treatment with no differences in discharge medication between groups except the higher proportion of loop diuretics administered in the low ratio group (Table 1).
Table 1.
Demographic baseline characteristics according to MAP/RAP ratio before the MitraClip procedure.
| All Patients n = 145 |
MAP/RAP < 7.13 n = 74 |
MAP/RAP ≥ 7.13 n = 71 |
p-value | |
|---|---|---|---|---|
| Age years median (IQR) |
76 (69–79.5) | 74 (68–79) | 77 (71–80) | 0.12 |
| Male gender n (%) |
98 (67.6) | 58 (78.4) | 40 (56.3) | 0.007 |
| Body mass index median kg/m2 IQR |
25.7 (23.0–29.1) | 27.4 (24.6–30.0) | 24.0 (22.4–27.1) | <0.001 |
| NYHA functional class IV n (%) |
25 (17.2) | 14 (18.9) | 11 (15.5) | 0.86 |
| Coronary artery disease n (%) |
88 (60.7) | 45 (60.8) | 43 (60.6) | 0.17 |
| Previous myocardial infarction n (%) |
55 (37.9) | 30 (40.6) | 25 (35.2) | 0.61 |
| Previous CABG n (%) |
41 (28.3) | 27 (36.6) | 14 (19.8) | 0.31 |
| Chronic atrial fibrillation n (%) |
70 (48.3) | 39 (52.7) | 31 (43.7) | 0.55 |
| ICD n (%) |
40 (27.6) | 26 (35.2) | 14 (19.7) | 0.04 |
| ICD-CRT n (%) |
19 (13.1) | 9 (12.2) | 10 (14.1) | |
| PM-CRT n (%) |
2 (1.4) | 1 (1.4) | 1 (1.4) | |
| Hypertension n (%) |
106 (73.1) | 61 (82.4) | 45 (63.4) | 0.01 |
| Chronic obstructive pulmonary disease, n (%) | 21 (14.5) | 14 (18.9) | 7 (9.9) | 0.22 |
| Diabetes mellitus n (%) |
30 (20.7) | 20 (27.1) | 10 (14.0) | 0.15 |
| Logistic EuroSCORE median % (IQR) |
17.4 (12.0–29.3) | 17.0 (12.8–30.0) | 18.5 (11.0–28.9) | 0.92 |
| NT-proBNP median ng/l (IQR) |
4324 (2147–9575) | 5240 (2434–12957) | 3918 (2049–7954) | 0.13 |
| Creatinine median mg/dl (IQR) |
1.3 (1.0–1.75) | 1.4 (1.1–2.0) | 1.3 (1.0–1.6) | 0.07 |
| Renal replacement therapy n (%) |
4 (2.8%) | 0 | 4 (5.6) | 0.05 |
| No of Clips implanted n (%) |
||||
| 1 | 73 (50.3) | 35 (47.3) | 38 (53.5) | |
| 2 | 70 (48.3) | 39 (52.7) | 31 (43.7) | 0.23 |
| 3 | 2 (1.4) | 0 (0) | 2 (2.8) | |
| Discharge medication | ||||
| Beta-blocker n (%) |
131 (90.3) | 68 (91.9) | 63 (88.7) | 0.36 |
| ACE/R inhibitors n (%) |
118 (83.1) | 62 (83.8) | 56 (78.9) | 0.72 |
| Loop diuretics n (%) |
131 (90.3) | 70 (94.6) | 61 (85.9) | 0.03 |
| Aldosteron-antagonists n (%) |
71 (49.0) | 37 (50.0) | 34 (47.9) | 0.87 |
| angiotensin receptor-neprilysin inhibitor, n (%) | 3 (2.1) | 1 (1.4) | 2 (2.8) | 0.57 |
ACE/R: Angiotensin converting enzym/ receptor blockers; CABG: coronary artery bypass graft; CRT: cardiac resynchronization therapy; ICD: implantable cardioverter/ defibrillator; IQR: interquartile range; NT-proBNP: Brain natriuretic peptid; NYHA: New York Heart Association; PM: pacemaker.
Variables of systemic blood pressure were comparable between groups (Table 2), whereas PASP, PAP mean, PCWP, PAP diast. and RAP were significantly higher in the low ratio group. CI and PAPi were significantly lower in the low ratio group. SVR and PVR did not differ significantly between groups (Table 2).
Table 2.
Baseline invasive haemodynamic parameters according to MAP/RAP ratio before the MitraClip procedure.
| All Patients n = 145 |
MAP/RAP < 7.13 n = 74 |
MAP/RAP ≥ 7.13 n = 71 |
p-value | |
|---|---|---|---|---|
| MAP/RAP Ratio IQR |
7.07 (5.20–10.58) | 5.25 (4.29–5.94) | 10.71 (8.60–16.83) | <0.001 |
| HR mean beats/min ± SD |
77 ± 14.77 | 77 ± 13.1 | 77 ± 16.4 | 0.86 |
| RR syst. mean mmHg ± SD |
127 ± 26.0 | 124 ± 25.7 | 130 ± 26.1 | 0.18 |
| RR diast. mean mmHg ± SD |
71 ± 12.77 | 71 ± 12.6 | 70 ± 13.1 | 0.99 |
| RR mean median mmHg (IQR) |
88 (81–101) | 88 (82–97) | 89 (81–103) | 0.14 |
| PASP mean mmHg ± SD |
60 ± 16.3 | 64.9 ± 15.4 | 54 ± 15.4 | <0.001 |
| PAP mean mean mmHg ± SD |
39 ± 10.6 | 44 ± 10.2 | 34 ± 8.8 | <0.001 |
| PCWP mean mmHg ± SD |
29 ± 10.6 | 34 ± 9.9 | 23 ± 8.7 | <0.001 |
| V-wave mean mmHg ± SD |
42 ± 15.7 | 49 ± 13.1 | 35 ± 15.1 | <0.001 |
| PAP diast. mean mmHg ± SD |
25 ± 9.3 | 29 ± 9.2 | 21 ± 7.5 | <0.001 |
| RAP mean mean mmHg ± SD |
13 ± 6.7 | 18 ± 5.1 | 8.0 ± 3.4 | <0.001 |
| CO median l/min (IQR) |
3.5 (2.9–4.2) | 3.4 (2.7–4.1) | 3.5 (3.0–4.3) | 0.24 |
| CI mean l/min/m2 ± SD |
2.0 ± 0.60 | 1.8 ± 0.42 | 2.1 ± 0.71 | 0.007 |
| PVR mean dyn*s*cm−5 ± SD |
279 ± 201 | 290 ± 216 | 267 ± 184 | 0.5 |
| TVR mean dyn*s*cm−5 ± SD |
1786 ± 617 | 1713 ± 604 | 1849 ± 625 | 0.17 |
| CFP mean mmHg ± SD |
0.48 ± 0.24 | 0.58 ± 0.24 | 0.37 ± 0.18 | <0.001 |
| PAPi median (IQR) |
2.69 (1.9–4.0) | 2.0 (1.5–2.6) | 4.0 (3.0–5.9) | <0.001 |
| LVSWi mean g/m−1/m2 ± SD |
22.0 ± 10.8 | 18.0 ± 7.6 | 26.2 ± 12.1 | <0.001 |
| RVSWi mean g/m−1/m2 ± SD |
8.9 ± 4.2 | 8.1 ± 3.3 | 9.8 ± 4.8 | 0.01 |
| TPG median mmHg (IQR) |
11 (6–15) | 11 (6–15) | 11 (6–15) | 0.75 |
| PA compliance median ml/mmHg (IQR) |
1.4 (1.0–1.9) | 1.3 (0.9–1.7) | 1.6 (1.1–2.2) | 0.02 |
| SV median ml/beat (IQR) |
46 (36–58) | 45 (35–57) | 47 (38–63) | 0.25 |
| SVi median ml/m2/beat (IQR) |
24.9 (19.6–29.8) | 23.5 (18.2–28.5) | 25.5 (21.4–33.5) | 0.04 |
| LVEDV median ml (IQR) |
127 (101–186) | 138 (106–183) | 122 (100–187) | 0.41 |
CFP: cardiac filling pressures; CI: cardiac index; CO: cardiac output; DPG: diastolic pulmonary gradient; HR: heart rate; IQR: interquartile range; LVEDV: left ventricular enddiastolic diameter; LVSWi: left ventricular stroke work index; PA: pulmonary artery; PAPdiast.: pulmonary artery diastolic pressure; PAPi: pulmonary artery pulsatility index; PAP mean: mean pulmonary artery pressure; PASP: pulmonary artery systolic pressure; PCWP: postcapillary wedge pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RR diast.: systemic artery diastolic pressure; RR mean: systemic artery mean pressure; RR syst.: systemic artery systolic pressure; RVSWi: right ventricular stroke work index; SV: stroke volume; SVi: stroke volume index; TPG: transpulmonary gradient.
The degree of MR severity was similar between groups but patients in the low ratio group show significantly lower LV and RV systolic function as assessed by LV-EF, TAPSE and DTI-S‘ (Table 3). Concomitant TR III° was more frequent in the group with low ratio. Patients in the high ratio group trend to show higher systolic pulmonary pressure (Table 3).
Table 3.
Baseline echocardiographic parameters according to MAP/RAP ratio before the MitraClip procedure.
| All Patients n = 145 |
MAP/RAP < 7.13 n = 74 |
MAP/RAP ≥ 7.13 n = 71 |
p-value | |
|---|---|---|---|---|
| Vena contracta width mean mm ± SD |
8 ± 1.3 | 8 ± 1.2 | 8 ± 1.4 | 0.98 |
| EROA median cm2 (IQR) |
0.31 (0.24–0.42) | 0.30 (0.23–0.43) | 0.31 (0.25–0.42) | 0.73 |
| Regurgitant volume median ml (IQR) |
48 (37–64) | 45 (33–63) | 50 (38–67) | 0.18 |
| LV-EF mean % ± SD |
35 ± 9.2 | 34 ± 8.5 | 37 ± 9.7 | 0.02 |
| LVEDD mean mm ± SD |
59 ± 9.4 | 59 ± 8.4 | 59 ± 10.4 | 0.66 |
| LVESD mean mm ± SD |
50 ± 11.2 | 52 ± 11.3 | 48 ± 11 | 0.12 |
| RVEDD mean mm ± SD |
39 ± 7.9 | 41 ± 8.6 | 36 ± 6.3 | 0.002 |
| TAPSE mean mm ± SD |
18 ± 4.3 | 17 ± 4.4 | 19 ± 3.9 | 0.008 |
| DTI-S‘ median cm/s (IQR) |
11.0 (9.0–12.0) | 9.0 (7.0–11.75) | 11.0 (9.0–12.5) | 0.048 |
| TR severity grade III° n (%) |
20 (14.2) | 16 (22.2) | 4 (5.8) | 0.001 |
| PaPsyst. mean mmHg ± SD |
53 ± 13.6 | 51 ± 13.1 | 55 ± 14.0 | 0.09 |
DTI-S‘: doppler tissue imaging S‘; EROA: effective regurgitant orifice area; IQR: interquartile range; LVEDD: left ventricular enddiastolic diameter; LV-EF: left ventricular ejection fraction; LVESD: left ventricular endsystolic diameter; PaPsyst.: pulmonary artery systolic pressure; RVEDD: right ventricular enddiastolic diameter; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation.
3.4. Relationship of MAP/RAP ratio to echocardiographic and haemodynamic parameters
A weak to modest positive correlation between MAP/RAP ratio and echocardiographic parameters of LV and RV function was observed (LV-EF: r = 0.288, p < 0.001; TAPSE: r = 0.262, p = 0.001; DTI-S‘: r = 0.259, p = 0.028). No correlation between MAP/RAP ratio and haemodynamic variables of vascular resistance was found (SVR: r = 0.106, p = 0.19; PVR: r = -0.004, p = 0.96). There was a strong inverse correlation between MAP/RAP ratio and PCWP and a modest inverse correlation between MAP/RAP ratio and PASP (PCWP: r = -0.556, p < 0.001; PASP: r = -0.391, p < 0.001), whereas MAP/RAP ratio and CI were positively correlated (r = 0.337, p 〈0 0 1). MAP/RAP ratio and PAPi show a strong positive correlation (r = 0.788, p < 0.001).
3.5. Short and long-term outcome
The rate of in-hospital mortality was 1.4% (n = 2). Both deceased patients were in the low ratio group. No procedure related death occurred. In-hospital bleeding and vascular access complications according to MVARC criteria were reported in 6 patients (4.1%, 3 in each group). In four of these cases, the hemorrhage was associated with the site of vascular access. In two cases, the reason was due to gastrointestinal bleeding. Two patients (1.4%, one in each group) presented with pericardial tamponade and required pericardiocentesis. All-cause mortality at 30 days after TMVR was 3.4% (n = 5). Patients with low MAP/RAP ratio showed a higher mortality rate 30 days postprocedural as compared with high MAP/RAP ratio, although statistically not significant (5.4%, n = 4 vs. 1.4%, n = 1; p = 0.36).
The rate of heart failure hospitalisation at 30 days postprocedural was 9.7% (n = 14) and trend to be higher in the low ratio group (13.5%, n = 10 vs. 5.6%, n = 4; p = 0.09). At 30 days after the MitraClip procedure, 20.3% of patients in group A (n = 15) and 4.2% in group B (n = 3) continued to show residual high-grade tricuspid regurgitation despite successful TMVR (p = 0.025).
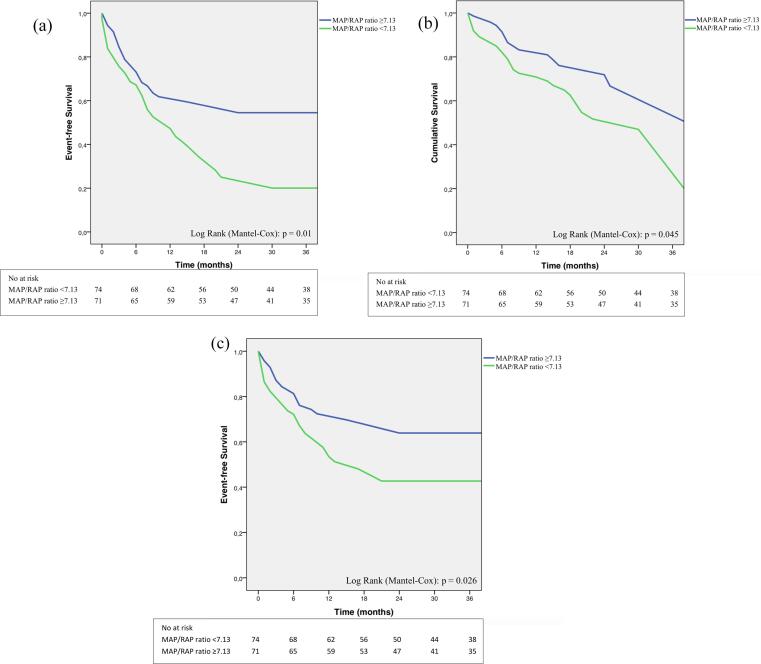
The combined primary endpoint at long-term follow-up occurred in 74 patients (51.0%). Median time to first event was 5 (2–9) months. Kaplan-Meier analysis for combined primary endpoint revealed a significantly lower event-free survival in patients with low MAP/RAP ratio as compared with high MAP/RAP ratio (62.2%, n = 46 vs. 39.4%, n = 28; log-rank p = 0.01; Fig. 3a). The secondary endpoint of all-cause mortality occurred in 47 patients (32.4%). Median time to all-cause mortality was 7 (3–15) months. Patients with low MAP/RAP ratio showed a significantly higher mortality rate as compared with high MAP/RAP ratio (40.5%, n = 30 vs. 23.9%, n = 17; log-rank p = 0.043, Fig. 3b).
Fig. 3.
a) Kaplan-Meier estimate of combined primary endpoint (composite of all-cause mortality and heart failure hospitalisation). Kaplan-Meier analysis showed a significantly lower event-free survival in the low MAP/RAP ratio group (<7.13) compared to the high MAP/RAP ratio group (≥7.13). b) Kaplan-Meier estimate of all-cause mortality. Kaplan-Meier analysis showed a significantly lower survival in the low MAP/RAP ratio group (<7.13) compared to the high MAP/RAP ratio group (≥7.13). c) Kaplan-Meier estimate of heart failure hospitalisation. Kaplan-Meier analysis showed a significantly lower hospitalisation rate for heart failure in the low MAP/RAP ratio group (<7.13) compared to the high MAP/RAP ratio group (≥7.13). MAP: mean arterial pressure; RAP: right atrial pressure.
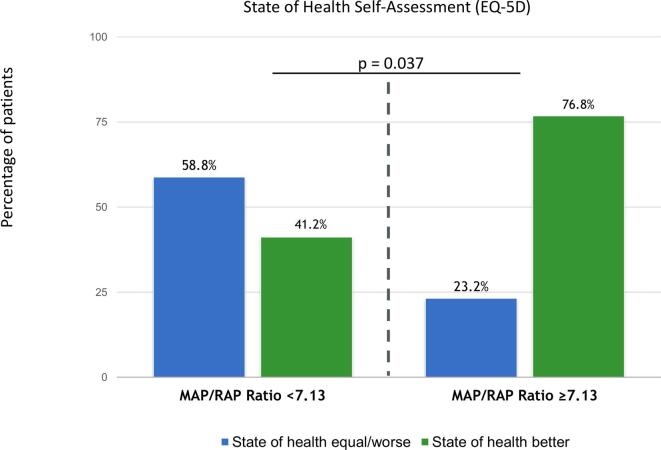
During long-term follow-up, a total of 53 patients (36.6%) were hospitalised for congestive heart failure (secondary endpoint). Median time to first hospitalisation for heart failure was 9 (5–17) months. Study participants in the low MAP/RAP ratio group demonstrated a significantly higher hospitalisation rate for heart failure compared to the high ratio group (44.6%, n = 33 vs. 28.2%, n = 20; log-rank p = 0.026, Fig. 3c). At long-term follow-up, 14.9% of patients in the low ratio group stayed in NYHA functional class IV and no one in the high ratio group (p = 0.002). In addition, patients with low MAP/RAP ratio were more likely to report that they did not benefit from TMVR (Fig. 4).
Fig. 4.
Health-realted quality of life self-assessment at long-term follow-up according to the MAP/RAP ratio group. MAP: mean arterial pressure; RAP: right atrial pressure.
According to the multivariable binary logistic regression analysis MAP/RAP ratio, PAPi, PaPsyst. and NYHA functional class IV prior to intervention were identified as the only independent predictors for combined primary endpoint (Table 4). Goodness-of-fit was assessed using the Hosmer-Lemeshow-Test, indicating a good model fit, p = 0.18. Furthermore, MAP/RAP ratio, PAPi and NT-proBNP levels remained the strongest independent variables for all-cause mortality in Cox regression analysis also after adjusting for other known haemodynamic, echocardiographic and clinical parameters (Table 4). There were no signs of multicollinearity in the regression models (all variance inflation factors below 2.5).
Table 4.
Multivariable Analysis for the combined primary endpoint and all-cause mortality at long-term follow-up.
| Combined Primary Endpoint |
All-Cause Mortality |
|||
|---|---|---|---|---|
| OR (CI) | P Value | HR (CI) | P Value | |
| Multivariable analysis | ||||
| MAP/RAP Ratio | 0.75 (0.62–0.90) | 0.002 | 0.82 (0.70–0.95) | 0.009 |
| PaP syst. | 1.5 (1.05–2.17) | 0.025 | 1.3 (0.98–1.72) | 0.067 |
| PAPi | 0.96 (0.92–1.0) | 0.047 | 0.96 (0.93–0.99) | 0.01 |
| NYHA IV | 9.3 (2.2–38.8) | 0.002 | 2.23 (0.98–5.08) | 0.055 |
| TR severity grad III° | 3.0 (0.80–11.7) | 0.10 | – | – |
| NT-pro BNP (per ng/l) | – | – | 1.21 (1.12–1.30) | 0.005 |
CI: confidence interval; HR: hazard ratio; MAP/RAP ratio: Mean arterial pressure to mean right atrial pressure ratio; NT-proBNP: Brain natriuretic peptid; NYHA: New York Heart Association functional class; OR: odds ratio; PAPi: Pulmonary artery pulsatility index; PaPsyst.: pulmonary artery systolic pressure; TR: tricuspid regurgitation.
4. Discussion
The current study is the first to demonstrate that a low MAP/RAP ratio in candidates for TMVR was associated with a higher rate of all-cause mortality and hospitalisation for heart failure at long-term follow-up. Furthermore, the study identifies MAP/RAP ratio as an independent predictor and defines a cut-off value of 7.13 as predictive for worse clinical outcome. The predictive value of this newly developed index is not influenced by other covariates.
In the presented study, we have tried to introduce a new index that takes into account both left and right ventricular factors in congestive heart failure. MAP is a surrogate parameter for systolic and diastolic blood pressure and also for systemic vascular resistance. In addition, MAP affects left ventricular end-diastolic pressure, especially in patients with additionally compromised diastolic function that is superimposed on heart failure with reduced ejection fraction [24]. On the contrary, RAP reflects right ventricular preload. Higher levels of RAP indicate elevated right-sided filling pressures which predict poor prognosis in advanced heart failure [25], [26]. Therefore, the MAP/RAP ratio includes the functionality of both ventricles in a single index that provides a more comprehensive look at the mutual interplay between the systemic and pulmonary circulation. Consequently, MAP/RAP ratio may serve as an additional marker of biventricular congestion. In this study the discriminatory capacity of MAP/RAP ratio remains modest despite a strong association with outcome. MAP/RAP ratio should therefore not be used in isolation for patient selection. Due to its limited sensitivity and specificity thresholds it seems to be obvious, that there is not an important threshold effect of MAP/RAP ratio. Therefore, MAP/RAP ratio should be interpreted as a continuous rather than a categorial variable. MAP/RAP ratio and PAPi are of added value in risk stratification especially when they are interpreted together with other clinical and echocardiographic variables that have already demonstrated their utility in patient selection [3], [4], [5], [6], [7], [8], [9], [10]. In the present study, MAP/RAP ratio and PAPi were found to be the only haemodynamic correlates of outcome because traditional haemodynamic parameters, i. e. blood pressure, PCWP, CI and PCWP, did not have a positive impact on prognostic assessment.
A weak correlation between MAP/RAP ratio and echocardiographic variables of LV- and RV-systolic function was observed. This is consistent with previous findings that invasively derived indices of cardiac function have been shown to correlate poorly with echocardiographic markers of left and right ventricular function [27]. The data from the current study underscore the hypothesis that calculation of MAP/RAP ratio beyond the determination of echocardiographic variables could provide additional and clinically meaningful prognostic information when selecting optimal candidates for TMVR. Unfortunately, some CHF patients who underwent TMVR for severe MR do not report improvement in quality of life despite successful MR reduction [6], [9], [28], [11], [12], [13], [14]. In this study, nearly 60% of study participants in the low ratio group declared in a quality of life self-assessment that they didn‘t benefit from successful TMVR. On the other hand, nearly 80% of patients in the high ratio group reported that their functional status improved after TMVR. These data further suggest that MAP/RAP ratio may help to identify potential non-responder patients after successful TMVR. Two lately published randomised controlled trials which investigated the outcome of MitraClip therapy in heart failure patients with severe MR found apparently conflicting results and exacerbate the issue of optimal patient selection [15], [16]. The MITRA-FR trial failed to demonstrate a positive effect of TMVR in addition to optimal drug therapy on the primary composite endpoint of all-cause death and unplanned rehospitalisation for heart failure at 12 months. Conversely, the COAPT study demonstrated that MitraClip significantly reduced the primary endpoint of hospitalisations for heart failure and also mortality after 24 months. One possible explanation for these contradictory results could be related to substantial differences in patient selection. For example, identifying additional risk factors for poorer prognosis, such as the MAP/RAP ratio, could significantly simplify the selection of suitable patients for TMVR.
Further studies are needed to obtain more information to optimise patient selection. The upcoming RESHAPE-HF-2 trial (RESHAPE-HF-2, ClinicalTrials.gov Identifier: NCT01772108 Ref.) has the potential to offer additional insights into this topic.
4.1. Study limitations
A limitation of the study is the small sample size and the lack of a conservative control group. Nevertheless, it can be emphasized as a strength of the study that the influence of the MAP/RAP ratio on prognosis could be investigated in a patient cohort reflecting everyday treatment without the common exclusion criteria for randomised studies.
4.2. Conclusions
This is the first study to show an strong association between MAP/RAP ratio and outcome in CHF patients undergoing TMVR for severe MR. A MAP/RAP ratio of <7.13 predicts a poorer outcome regardless of other known risk factors. Therefore, MAP/RAP ratio may be used in risk stratification to identify optimal candidates for TMVR.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Rico Osteresch received lecture honoraria from Abbott Vascular. Christian Frerker received lecture honoraria and travel support from Abbott Vascular. The other authors have no relationships with industry to declare].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100903.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Rossi A., Dini F.L., Faggiano P., Agricola E., Cicoira M., Frattini S., Simioniuc A., Gullace M., Ghio S., Enriquez-Sarano M., Temporelli P.L. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97(20):1675–1680. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P.J., Iung B., Lancellotti P., Lansac E., Rodriguez Munoz D., Rosenhek R., Sjogren J., Tornos Mas P., Vahanian A., Walther T., Wendler O., Windecker S., Zamorano J.L., Group ESCSD ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Franzen O., Baldus S., Rudolph V., Meyer S., Knap M., Koschyk D., Treede H., Barmeyer A., Schofer J., Costard-Jackle A., Schluter M., Reichenspurner H., Meinertz T. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: Emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur. Heart J. 2010;31(11):1373–1381. doi: 10.1093/eurheartj/ehq050. [DOI] [PubMed] [Google Scholar]
- 4.Whitlow P.L., Feldman T., Pedersen W.R., Lim D.S., Kipperman R., Smalling R., Bajwa T., Herrmann H.C., Lasala J., Maddux J.T., Tuzcu M., Kapadia S., Trento A., Siegel R.J., Foster E., Glower D., Mauri L., Kar S. Acute and 12-month results with catheter-based mitral valve leaflet repair: The EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J. Am. Coll. Cardiol. 2012;59(2):130–139. doi: 10.1016/j.jacc.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 5.Nickenig G., Estevez-Loureiro R., Franzen O., Tamburino C., Vanderheyden M., Lüscher T.F., Moat N., Price S., Dall’Ara G., Winter R., Corti R., Grasso C., Snow T.M., Jeger R., Blankenberg S., Settergren M., Tiroch K., Balzer J., Petronio A.S., Büttner H.-J., Ettori F., Sievert H., Fiorino M.G., Claeys M., Ussia G.P., Baumgartner H., Scandura S., Alamgir F., Keshavarzi F., Colombo A., Maisano F., Ebelt H., Aruta P., Lubos E., Plicht B., Schueler R., Pighi M., Di Mario C. Percutaneous mitral valve edge-to-edge Repair: In-hospital results and 1-year follow-up of 628 patients of the 2011–2012 pilot European Sentinel Registry. J. Am. Coll. Cardiol. 2014;64(9):875–884. doi: 10.1016/j.jacc.2014.06.1166. [DOI] [PubMed] [Google Scholar]
- 6.Puls M., Lubos E., Boekstegers P., von Bardeleben R.S., Ouarrak T., Butter C., Zuern C.S., Bekeredjian R., Sievert H., Nickenig G., Eggebrecht H., Senges J., Schillinger W. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: Results from the German transcatheter mitral valve interventions registry. Eur. Heart J. 2016;37(8):703–712. doi: 10.1093/eurheartj/ehv627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamburino C., Ussia G.P., Maisano F., Capodanno D., La Canna G., Scandura S., Colombo A., Giacomini A., Michev I., Mangiafico S., Cammalleri V., Barbanti M., Alfieri O. Percutaneous mitral valve repair with the MitraClip system: Acute results from a real world setting. Eur. Heart J. 2010;31(11):1382–1389. doi: 10.1093/eurheartj/ehq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzen O., van der Heyden J., Baldus S., Schlüter M., Schillinger W., Butter C., Hoffmann R., Corti R., Pedrazzini G., Swaans M.J., Neuss M., Rudolph V., Sürder D., Grünenfelder J., Eulenburg C., Reichenspurner H., Meinertz T., Auricchio A. MitraClip® therapy in patients with end-stage systolic heart failure. Eur. J. Heart Fail. 2011;13(5):569–576. doi: 10.1093/eurjhf/hfr029. [DOI] [PubMed] [Google Scholar]
- 9.Neuss M., Schau T., Schoepp M., Seifert M., Hölschermann F., Meyhöfer J., Butter C. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur. J. Heart Fail. 2013;15(7):786–795. doi: 10.1093/eurjhf/hfs214. [DOI] [PubMed] [Google Scholar]
- 10.Maisano F., Franzen O., Baldus S., Schäfer U., Hausleiter J., Butter C., Ussia G.P., Sievert H., Richardt G., Widder J.D., Moccetti T., Schillinger W. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, A prospective, multicenter, nonrandomized post-approval study of the Mitraclip therapy in Europe. J. Am. Coll. Cardiol. 2013;62(12):1052–1061. doi: 10.1016/j.jacc.2013.02.094. [DOI] [PubMed] [Google Scholar]
- 11.Keßler M., Seeger J., Muche R., Wöhrle J., Rottbauer W., Markovic S. Predictors of rehospitalization after percutaneous edge-to-edge mitral valve repair by MitraClip implantation. Eur. J. Heart Fail. 2019;21(2):182–192. doi: 10.1002/ejhf.1289. [DOI] [PubMed] [Google Scholar]
- 12.Kalbacher D., Schäfer U., v. Bardeleben R.S., Eggebrecht H., Sievert H., Nickenig G., Butter C., May A.E., Bekeredjian R., Ouarrak T., Kuck K.-H., Plicht B., Zahn R., Baldus S., Ince H., Schillinger W., Boekstegers P., Senges J., Lubos E. Long-term outcome, survival and predictors of mortality after MitraClip therapy: Results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int. J. Cardiol. 2019;277:35–41. doi: 10.1016/j.ijcard.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Osteresch R., Diehl K., Kühl M., Fiehn E., Schmucker J., Backhaus T., Fach A., Wienbergen H., Hambrecht R. Impact of right heart function on outcome in patients with functional mitral regurgitation and chronic heart failure undergoing percutaneous edge-to-edge-repair. J. Intervent. Cardiol. 2018;31(6):916–924. doi: 10.1111/joic.12566. [DOI] [PubMed] [Google Scholar]
- 14.Orban M., Braun D., Orban M., Grebmer C., Sibbing D., Thaler R., Tittus J., Wimbauer F., Lesevic H., Sonne C., Mehilli J., Ott I., Näbauer M., Massberg S., Boekstegers P., Hausleiter J. Long-term outcome of patients with severe biventricular heart failure and severe mitral regurgitation after percutaneous edge-to-edge mitral valve repair. J. Intervent. Cardiol. 2015;28:164–171. doi: 10.1111/joic.12193. [DOI] [PubMed] [Google Scholar]
- 15.Stone G.W., Lindenfeld JoAnn, Abraham W.T., Kar S., Lim D.S., Mishell J.M., Whisenant B., Grayburn P.A., Rinaldi M., Kapadia S.R., Rajagopal V., Sarembock I.J., Brieke A., Marx S.O., Cohen D.J., Weissman N.J., Mack M.J. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018;379(24):2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 16.Maucort-Boulch D., Carrié D., Guerin P., Iung B., Obadia J.-F., Michel N., Teiger E., Donal E., Cormier B., Leurent G., Bonnet G., Mewton N., Nejjari M., Samson G., Barnel C., Boutitie F., Piriou N., Leroux L., Karam N., Ohlmann P., Piot C., Lefèvre T., Leclercq F., Vahanian A., Armoiry X., Saint Etienne C., Messika-Zeitoun D., Gilard M., Rouleau F., Trochu J.-N. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 17.Osteresch R., Diehl K., Schmucker J., Ben Ammar A., Solyom O., Dierks P., Fach A., Wienbergen H., Hambrecht R. Prognostic Impact of the Pulmonary Artery Pulsatility Index in Patients with Chronic Heart Failure and Severe Mitral Regurgitation Undergoing Percutaneous Edge-to-Edge Repair. Cardiology. 2021;146:74–84. doi: 10.1159/000510283. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., Authors/Task Force M and Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi W.A., Adams D., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A., Hahn R.T., Han Y., Hung J., Lang R.M., Little S.H., Shah D.J., Shernan S., Thavendiranathan P., Thomas J.D., Weissman N.J. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Lancellotti P., Moura L., Pierard L.A., Agricola E., Popescu B.A., Tribouilloy C., Hagendorff A., Monin J.L., Badano L., Zamorano J.L., Sicari R., Vahanian A., Roelandt J.R.T.C. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease) Eur. J. Echocardiography. 2010;11(4):307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 21.Foster E., Wasserman H.S., Gray W., Homma S., Di Tullio M.R., Rodriguez L., Stewart W.J., Whitlow P., Block P., Martin R., Merlino J., Herrmann H.C., Wiegers S.E., Silvestry F.E., Hamilton A., Zunamon A., Kraybill K., Gerber I.L., Weeks S.G., Zhang Y., Feldman T. Quantitative Assessment of Severity of Mitral Regurgitation by Serial Echocardiography in a Multicenter Clinical Trial of Percutaneous Mitral Valve Repair. Am. J. Cardiol. 2007;100(10):1577–1583. doi: 10.1016/j.amjcard.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 22.Feldman T., Foster E., Glower D.D., Kar S., Rinaldi M.J., Fail P.S., Smalling R.W., Siegel R., Rose G.A., Engeron E., Loghin C., Trento A., Skipper E.R., Fudge T., Letsou G.V., Massaro J.M., Mauri L. Percutaneous repair or surgery for mitral regurgitation. N Engl. J. Med. 2011;364(15):1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 23.Stone G.W., Vahanian A.S., Adams D.H., Abraham W.T., Borer J.S., Bax J.J., Schofer J., Cutlip D.E., Krucoff M.W., Blackstone E.H., Généreux P., Mack M.J., Siegel R.J., Grayburn P.A., Enriquez-Sarano M., Lancellotti P., Filippatos G., Kappetein A.P. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 1: Clinical Trial Design Principles A Consensus Document from the Mitral Valve Academic Research Consortium. J. Am. Coll. Cardiol. 2015;36:1878–1891. doi: 10.1016/j.jacc.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Zouein F.A., de Castro Brás L.E., da Costa D.V., Lindsey M.L., Kurdi M., Booz G.W. Heart failure with preserved ejection fraction: emerging drug strategies. J. Cardiovasc. Pharmacol. 2013;62(1):13–21. doi: 10.1097/FJC.0b013e31829a4e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper L.B., Mentz R.J., Stevens S.R., Felker G.M., Lombardi C., Metra M., Stevenson L.W., O'Connor C.M., Milano C.A., Patel C.B., Rogers J.G. Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J. Card. Fail. 2016;22(3):182–189. doi: 10.1016/j.cardfail.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley D., Brozena S.C. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. Am. J. Cardiol. 1994;73(5):379–383. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 27.Cameli M., Bernazzali S., Lisi M., Tsioulpas C., Croccia M.G., Lisi G., Maccherini M., Mondillo S. Right ventricular longitudinal strain and right ventricular stroke work index in patients with severe heart failure: Left ventricular assist device suitability for transplant candidates. Transpl. Proc. 2012;44(7):2013–2015. doi: 10.1016/j.transproceed.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Giannini C., Fiorelli F., Colombo A., De Carlo M., Weisz S.H., Agricola E., Godino C., Castriota F., Golino P., Petronio A.S. Right ventricular evaluation to improve survival outcome in patients with severe functional mitral regurgitation and advanced heart failure undergoing MitraClip therapy. Int. J. Cardiol. 2016;223:574–580. doi: 10.1016/j.ijcard.2016.08.189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.