Abstract
Background
Neoadjuvant chemoradiotherapy (NCRT) or neoadjuvant chemotherapy (NCT) followed by surgery are two standard strategies in treating locally advanced esophageal cancer (EC). We aim to compare NCRT and NCT in the management of locally advanced EC patients.
Methods
MEDLINE, Embase, CENTRAL, and conferences were systematically searched for clinical trials published up to September 2021. Pairwise comparisons and Bayesian network meta-analyses were conducted to compare overall survival (OS) and disease-free survival (DFS) by reporting the hazard ratio (HR) and 95% credible intervals (CrIs). The study was registered at PROSPERO (CRD42020170619).
Findings
25 trials with 4563 EC patients met inclusion criteria. NCRT improved OS (HR: 0·72, 95%CrI: 0·63–0·82) and DFS (HR: 0·72, 95%CrI: 0·63–0·81) compared to surgery alone. NCRT improved OS (HR: 0·83, 95%CrI: 0·69–0·99) and DFS (HR: 0·83, 95%CI: 0·69–0·99) compared to NCT. Subgroup analysis demonstrated that both NCRT (HR: 0·77, 95%CrI: 0·65–0·90) and NCT (HR: 0·81, 95%CrI: 0·67–0·99) improved OS than surgery in esophageal squamous cell carcinoma (ESCC) patients. No significant differences were observed between NCRT and NCT regarding OS (HR: 0·95, 95%CrI: 0·75–1·19) and DFS (HR: 0·90, 95%CrI: 0·50–1·62) in ESCC. The short-term outcomes were similar between NCRT and NCT. The three treatment strategies were comparable in esophageal adenocarcinoma (EAC) subpopulations.
Interpretation
The study corroborated current guidelines in addressing the importance of analysing EC according to histopathological types. The analysis suggested that in locally advanced ESCC patients, both NCRT and NCT improved OS as compared to surgery alone, whereas no clear evidence supported the optimal strategies between NCRT and NCT. More RCTs comparing different therapeutic strategies in EAC patients are warranted.
Funding
Köln Fortune Program, University of Cologne.
Keywords: Neoadjuvant chemoradiotherapy, Neoadjuvant chemotherapy, Esophageal carcinoma, Network meta-analysis
Research in context.
Evidence before this study
Neoadjuvant chemoradiotherapy (NCRT) or neoadjuvant chemotherapy (NCT) followed by surgery are two standard therapeutic strategies in treating locally advanced esophageal cancer. Till now, only four well-designed randomized controlled trials have directly compared NCRT and NCT whereas there have been several landmarked trials compared NCRT or NCT with surgery alone. Therefore, the relative effectiveness of NCRT and NCT can be better estimated using network meta-analysis.
Added value of this study
We addressed the importance of studying esophageal cancer independently according to the histopathological types. Our results suggest that both NCT and NCRT improve overall survival (OS) in esophageal squamous cell carcinoma (ESCC) compared to surgery alone, whereas no differences were found between NCRT and NCT regarding OS, disease-free survival (DFS) and short-term outcomes.
Implications of all the available evidence
Results corroborated current guidelines and the importance of analysing EC according to histopathological types. The study suggests that in locally advanced ESCC patients, both NCRT and NCT improved OS as compared to surgery alone, butno clear evidence supported the optimal strategies between NCRT and NCT. More RCTs comparing different therapeutic strategies in EAC patients are warranted.
Alt-text: Unlabelled box
1. Introduction
Esophageal carcinoma (EC) is one of the most common and deadly tumors, with about 604,000 newly diagnosed cases and 544,000 deaths in 2020 [1]. Despite new therapeutic strategies and protocols significantly improved in the recent decades, the prognosis of EC remains poor, with an estimated 5-year survival rate of 20% [2,3]. Radical surgery is the cornerstone intervention for EC patients eligible for curative treatment [3]. Based on the JCOG9907 trial, neoadjuvant chemotherapy (NCT) followed by surgery is widely used as a standard therapeutic strategy in advanced esophageal squamous cell carcinoma (ESCC) in Japan [4]. Besides, neoadjuvant chemoradiotherapy (NCRT) followed by esophagectomy achieves significant survival benefits both in the adenocarcinoma-dominant CROSS trial as well as the ESCC-only NEOCRTEC5010 trial [5,6]. Therefore, neoadjuvant therapy combined with radical esophagectomy has gradually become the standard of care for locally advanced EC [7,8]. However, it is still unclear whether adding radiotherapy to NCT is superior to NCT in treating locally advanced EC patients.
Up to date, only four randomized controlled trials (RCTs) have directly compared NRCT with NCT [9], [10], [11], [12]. Two early closed RCTs have compared NRCT with NCT in esophageal adenocarcinoma (EAC), which both showed slightly favorable survival towards NCRT but without statistical significance [10,11]. Similar results were also reported in one trial studying ESCC patients population [12] and another trial with mixed EC tumor types [9]. Overall evaluation of NRCT versus NCT is problematic, being based on one outdated study with quite lower prognosis in both groups (3-year survival rates: 17% and 3% for NCRT and NCT, respectively) [12], two studies with rather small sample sizes (n = 75 and n = 119) which were closed early [10,11], and none of the studies reaching statistical significances in survival outcomes. Therefore, the optimal neoadjuvant treatment remains unclear.
Network meta-analysis (NMA) is a well-accepted method to calculate quantitative estimates of relative effect between multiple treatments [13]. Due to relatively few direct comparisons between NCRT and NCT while there are already many RCTs comparing NCRT or NCT with surgery alone, the relative effectiveness of NCRT and NCT can be better estimated using NMA by synthesizing the direct and indirect evidence across a network of RCTs [14,15]. Here, we performed a comprehensive network meta-analysis to investigate the relative efficacy of NCRT and NCT on long-term and short-term outcomes with the aim of identifying the optimal neoadjuvant therapy options in the management of locally advanced EC patients.
2. Methods
This study followed the PRISMA extension statement for reporting network meta-analysis [16]. The study protocol was registered at PROSPERO in February 2020 (ID: CRD42020170619). An ethics statement is not applicable because all the data included in our analyses were extracted from previous published studies in which informed consents were obtained by primary investigators.
2.1. Search strategy and selection criteria
We systematically searched online databases including MEDLINE, Embase and Cochrane Central Register of Controlled Trials for all the RCTs published up to January 2020. In addition, all abstracts from the American Society of Clinical Oncology, American Association for Cancer Research, European Society for Medical Oncology, European Association for Cancer Research, European Surgical Association, European Association for Cardio-Thoracic Surgery and World Organization for Specialized Studies on Diseases of the Esophagus of the same period were screened as well. Search terms followed the PICOS principle, [14] we used the medical subject headings (if available) of 'esophageal carcinoma', 'chemotherapy', 'radiotherapy', 'surgery' or 'esophagectomy', 'prognosis' or 'survival'. The detailed searching strategies were presented in supplementary information 1. Two reviewers (FN and WZ) reviewed the literature independently. Discrepancies have been resolved by group discussions among FN, WZ and YZ until consensus was reached.
The inclusion criteria of this study included: (a) prospective randomized controlled trials. (b) patients with pathologically proven esophageal squamous cell carcinoma, esophageal adenocarcinoma or adenocarcinoma of the esophagogastric junction (AEGJ). (c) trials that compared any two or more different arms of the following treatment: neoadjuvant chemoradiotherapy followed by surgery, neoadjuvant chemotherapy followed by surgery or surgery alone. (d) trials that reported on overall survival (OS) or disease-free survival (DFS) of the intention-to-treat population. (e) peer-reviewed articles in English. We excluded all studies that did not meet the inclusion criteria. Other exclusion criteria were: (a) high bias RCT. (b) trials compared perioperative chemotherapy. (c) former version of the same trials with updated survival information available. (d) studies with full-text unavailable.
2.2. Quality assessment and data extraction
The quality of included studies was assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB2 tool) [17] by two independent reviews (FN and WZ). Each RCT was judged as low risk of bias, some concerns or high risk of bias. Any discrepancy was resolved in a consensus meeting. Studies with high risk of bias were excluded from final analyses. Publication bias of included studies was evaluated using adjusted funnel plots [18].
The primary outcome was OS, and the secondary outcomes were DFS, pathologic complete response (pCR), grade 3 to 4 adverse events (AEs) for neoadjuvant treatments, resection rate, R0 resection rate and peri‑treatment mortality. For OS and DFS, hazard ratios (HR) with 95% confidence intervals (CIs) were extracted to calculate the natural logarithm hazard ratios (lnHR) and standard error using the HR calculations spreadsheet that was provided by Tierney et al. [19]. Missing data were requested by contacting the authors. When the corresponding author did not respond to our request, the Kaplan-Meier curves were digitized using Engauge Digitizer tool (version 12·1) [20] to extract curve data and to be further computed in the HR calculations spreadsheet. For short-term outcomes, number of events and observations in experimental and control groups were extracted. Data of pCR and grade 3 to 4 AEs was extracted from RCTs comparing NCRT and NCT. Resection rate was defined as the ratio of patients who underwent surgery to the number of patients assigned to that group/arm. Peri-treatment mortality was extracted from RCTs reporting either treatment-related mortality, 30 days mortality or 90 days mortality.
2.3. Data synthesis and statistical analysis
Pairwise meta-analyses were performed for all direct treatment comparisons in R software (version 3·5·3) with RStudio (Boston, MA) using the ‘meta’ package [21]. Heterogeneity between studies were assessed using the Q test and I2 statistic [22]. Fixed effect or random effect model was chosen based on I2 value (<50% or >50%, respectively). The results of the pairwise meta-analysis were reported as HR with 95% CI for OS and DFS, and as relative risk (RR) with 95% CI for pCR and grade 3 to 4 AEs.
Network plots for the comparison of different treatment strategies were generated to depict the network geometry by Stata (version 14·0). Network meta-analyses were performed under the Bayesian framework using JAGS and “gemtc” package in R [23]. Random effects and consistency models were used in network meta-analyses. Noninformative priors were set and posterior distributions were obtained by using 4 Markov chains with 60,000 iterations after 20,000 burn-ins and a thinning interval of 10. The convergence of the chains was confirmed by inspection of the trace plots, density plots and Brooks-Gelman-Rubin statistic (supplementary figure 1). The results of the network meta-analysis were reported as HRs with 95% credible intervals (CrIs) for OS and DFS, and as odds ratio (OR) with 95% CrIs for resection rate, R0 resection and peri‑treatment mortality. The probability of each treatment regarding survival outcomes was ranked according to the HRs and the posterior probabilities. Two-sided p<0·05 indicates statistical significance.
To assess the study consistency, global inconsistency assessment and local inconsistency assessment were performed. The global inconsistency was evaluated through comparing the fit of consistency and inconsistency models using deviance information criterion (DIC), where similar DIC of different models indicates a good consistency [24,25]. The local inconsistency was evaluated through comparing the direct and indirect evidence generated under the Bayesian framework using the node-splitting analysis, where p<0·05 indicates significant inconsistency [26].
We performed subgroup analysis on OS according to the two different histopathological types of esophageal cancer: ESCC and EAC. For DFS, only ESCC subgroup was accessed due to the limited data on EAC. Given the development of radiotherapy technology, chemotherapy regimens and surgical strategy in recent decades, we performed a sensitivity analysis by restricting all the included studies to studies published after the year 2000 to increase the stability of our results. In addition, at the revision stage of our study, there is a time gap between the start of this study (April 2020) and the current time (September 2021). Therefore, we systemically searched the publications from January 2020 to September 2021 using the same strategy described in supplementary information 1. And we performed a sensitivity analysis with all the RCTs published up to September 2021.
2.4. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Study selection and characteristics
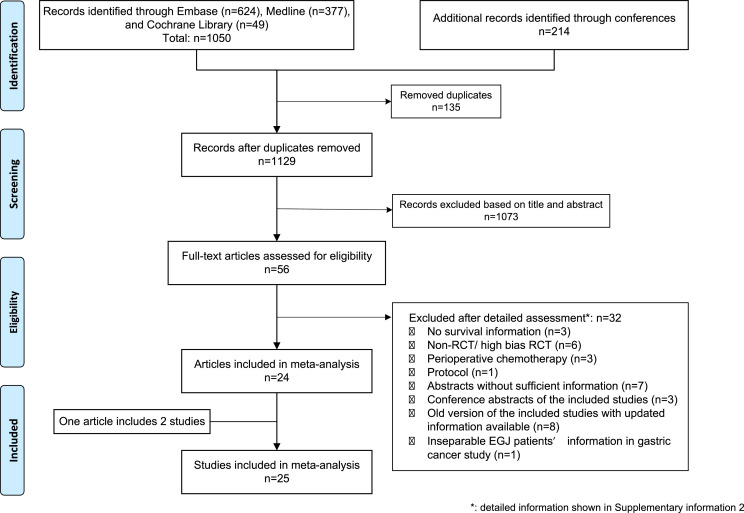
A flowchart of the study selection procedure was showed in Fig. 1. Briefly, 1129 articles were identified from literature search and 56 articles were reviewed in full text. 32 articles were excluded after full-text assessment and 24 articles met our inclusion criteria. The detailed excluded articles with reasons were presented in supplementary information 2. Due to one article reporting two independent RCTs [27], a total of 25 studies with 4563 patients were included in the final analysis. Among 25 included studies, four studies directly compared the NCRT with NCT, thirteen studies compared NCRT with surgery alone, and eight studies compared NCT with surgery alone. The baseline characteristics of all the included studies are shown in Table 1. [5,6,[9], [10], [11], [12],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]] All included studies were judged as low risk of bias or some concerns. The detailed risk of bias assessments of all studies was presented in supplementary figure 2. The adjusted funnel plots for publication bias assessments were presented in supplementary figure 3.
Fig. 1.
PRISMA flowchart diagram of the study selection. Abbreviations: RCT: randomized controlled trial. EGJ: esophagogastric junction carcinoma.+.
Table 1.
Baseline characteristics of studies included in the network meta-analysis. Abbreviations: SCC: squamous cell carcinoma. AC: adenocarcinoma. AEG: adenocarcinoma of the esophagogastric junction. OS: overall survival. DFS: disease-free survival.
| Year started | Radiotherapy schedule | Chemotherapy regimen | Concurrent or sequential | Surgical approach | Tumor type | Tumor stage | Survival data | Sample size | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemoradiotherapy vs. Chemotherapy | ||||||||||
| von Döbeln G.A.9 | 2006 | 40 Gy; 2 Gy per fractions in 20 fractions, 5 days a week. | Cisplatin (100 mg/m2/day) on day 1 and fluorouracil (750 mg/m2/day) on days 1 to 5. Every 3 weeks for 3 cycles. | concurrent | Transthoracic esophagectomy with two-field lymphadenectomy | SCC* AC* | cT1N1M0, cT2–3N0–1M0. | OS DFS | 181 | Everyone was followed up to 60 months |
| Stahl M.10 | 2000 | 30 Gy; 2 Gy per fraction in 15 fractions, 5 days a week after 2 courses of chemotherapy induction. Concurrent 3-week chemotherapy consisted of cisplatin (50 mg/m2/day) on day 1 and day 8, and etoposide (80 mg/m2/day) on days 3 to 5. | Six-week schedule of weekly fluorouracil (2 g/m2, 24-hour infusion) and leucovorin (500 mg/m2, 2-hour infusion) as well as biweekly cisplatin (50 mg/m2, 1-hour infusion). Two courses in chemoradiotherapy group for induction and 2·5 courses in chemotherapy group. | concurrent | Transthoracic esophagectomy with two-field lymphadenectomy or radical transhiatal esophagectomy for AEG type I. Total gastrectomy plus lower esophagus resection with D2 lymphadenectomy for AEG type II. | AC | cT3–4NxM0 | OS DFS | 119 | Median: 126·5 months |
| Burmeister BH11 | 2000 | 35 Gy in 15 fractions over 3 weeks | Cisplatin (80 mg/m2/day) on day1; 5-fluorouracil (1000 mg/m2/d) on days 2 to 4. Every 3 weeks for 2 cycles. | concurrent | Transthoracic esophagectomy. | AC | cT2–3N0–1M0 (stage II-III) | OS DFS | 75 | Median: 94 months |
| Nygaard K12 | 1983 | 35 Gy, 1·75 Gy 20 fractions. 5 days a week over 4 weeks. | Cisplatin (20 mg/m2/day) on days 1 to 5; bleomycin (10 mg/m2/day) on days 1 to 5. Every 2 or 3 weeks for 2 cycles. | sequential | Right-sided Transthoracic esophagectomy. | SCC | cT1–2NxM0 | OS | 97 | Not mention in the manuscript. Speculate median follow -up time: 18 months. |
| Chemoradiotherapy vs. Surgery | ||||||||||
| Yang H5 | 2007 | 40·0 Gy; 2 Gy per fraction in 20 fractions, 5 days a week. | Vinorelbine (25 mg/m2/day) on days 1 and 8; and cisplatin (75 mg/m2) on day 1 or cisplatin (25 mg/m2) on days 1 to 4. Every 3 weeks for 2 cycles. | concurrent | Transthoracic esophagectomy with two-field lymphadenectomy | SCC | cT1–4N1M0, cT4N0M0. (stage IIB or III) | OS DFS | 451 | Median: 41 months in CRT and 36 months in S |
| Shapiro J6 | 2004 | 41·4 Gy; 1·8 Gy per fraction in 23 fractions, 5 days a week. | Carboplatin (AUC 2 mg/mL per min) and paclitaxel (50 mg/m²) were administered intravenously for 5 cycles, starting on days 1, 8, 15, 22, and 29. | concurrent | Transthoracic esophagectomy with two-field lymphadenectomy or transhiatal esophagectomy | SCC* AC* | cT1N1M0, cT2–3N0–1M0. | OS DFS | 366 | Median: 84·1 months |
| Mariette C28 | 2000 | 45 Gy; 1·8 Gy per fraction in 25 fractions, 5 days a week over 5 weeks. | Fluorouracil (800 mg/m2/24 h) on days 1 to 4 and 29 to 32. Cisplatin (75 mg/m2) on day 1 or day 2 and day 29 or 30. | concurrent | Transthoracic esophagectomy with extended two-field lymphadenectomy | SCC AC | cT1–2N0–1M0, cT3N0M0. (stage I-II) |
OS DFS | 170 | Median: 93·6 months |
| Bass G.A.†27 | 1990 | 40 Gy in 15 fractions. | Fluorouracil (15 mg/kg of body weight/day) on day 1–5. Cisplatin (75 mg/m2/day) on day 7. Two cycles. | concurrent | Transthoracic esophagectomy or transhiatal esophagectomy | AC | cT0–4N0–2M0 | OS | 133 | From 0·25–206 months |
| Bass G.A.†27 | 1990 | 40 Gy in 15 fractions. | Fluorouracil (15 mg/kg of body weight/day) on day 1–5. Cisplatin (75 mg/m2/day) on day 7. Two cycles. | concurrent | Transthoracic esophagectomy or transhiatal esophagectomy | SCC | cT0–4N0–2M0 | OS | 98 | From 0·25–206 months |
| Lv J30 | 1997 | 40 Gy; 2 Gy per fraction in 20 fractions. | Paclitaxel (135 mg/m2/day) on day 1, cisplatin (20 mg/m2/day) on days 1 to 3. Every 3 weeks for 2 cycles. | concurrent | Transthoracic esophagectomy with 2-field lymphadenectomy | SCC | Clinical stage II-III | OS DFS | 160 | Median: 45 months |
| Tepper J31 | 1997 | 50·4 Gy; 1·8 Gy in 28 fractions. 5 days a week over 5·5 weeks. | Cisplatin (100 mg/m2/day) on day 1, fluorouracil (1000 mg/m2/day) on days 1 to 4. Every 4 weeks for 2 cycles. | concurrent | Transthoracic esophagectomy | SCC AC | cT1–3NXM0 | OS DFS | 56 | Median: 72 months |
| Natsugoe S33 | 1997 | 40 Gy, 2 Gy in 20 fractions. 5 days a week over 4 weeks | cisplatin (7 mg over 2 h) and 5-fluorouracil (350 mg over 24 h) at the same radiotherapy period. | concurrent | No information in the manuscript | SCC | cT2–3N0–1M0–1 | OS | 43 | Median: 24 months |
| Burmeister BH34 | 1994 | 35 Gy, 15 fractions over 3 weeks | Cisplatin (80 mg/m2/day) on day 1, fluorouracil (800 mg/m2/day) on days 1 to 4. One cycle. | concurrent | According to surgeon’ preference | SCC* AC* | cT1-T3, N0–1 | OS DFS | 256 | Median: 65 months |
| Lee JL35 | 1999 | 45·6 Gy, 1·2 Gy in 38 fractions. | Cisplatin (60 mg/m2/day) on days 1 and 21, 5-fluorouracil (1000 mg/m2/day) on days 2 to 5. | concurrent | Transthoracic esophagectomy with en block lymph node dissection | SCC | cT2–3N0M0, cT1–3N1M0. (stage II-III) | OS DFS | 101 | Median: 25 months |
| Urba SG36 | 1989 | 45 Gy, 1·5 Gy in 30 fractions. 5 days a week over 3 weeks | Cisplatin 20 (mg/m2/d) on days 1 through 5 and 17 through 21; fluorouracil (300 mg/m2/d) on days 1 through 21; and vinblastine (1 mg/m2/d) on days 1 through 4 and 17 through 20. | concurrent | Transhiatal esophagectomy | SCC AC | Resectable | OS DFS | 100 | Median: 98·4 months |
| Bosset JF40 | 1985 | 37 Gy, 3·7 Gy in 10 fractions. 5 days a week over 2 weeks | Cisplatin (80 mg/m2/day) on days 0 to 2. Every week for 2 cycles | concurrent | Transthoracic en bloc esophagectomy with 2-field lymphadenectomy | SCC | cT1–2N0–1M0, cT3N0M0 | OS DFS | 282 | Median: 55·2 months |
| Le Prise E42 | 1988 | 20 Gy, 2 Gy in 10 fractions. 5 days a week over 2 weeks | Cisplatin (100 mg/m2/day) on day 1; 5-fluorouracil (600 mg/m2/day) on days 2 to 5. Every 2 weeks for 2 cycles. | sequential | No information in the manuscript | SCC | Clinical stage I-II | OS DFS | 86 | Median: 16 months |
| Chemotherapy vs. surgery | ||||||||||
| Kelsen DP32 | 1990 | Cisplatin (100 mg/m2/day) on day 1, fluorouracil (1000 mg/m2/day) on days 1 to 5. Every 4 weeks for 3 cycles. | Transthoracic esophagectomy or transhiatal esophagectomy | SCC AC | cT1–3NxM0 | OS DFS | 443 | Median: 105·6 months | ||
| Allum WH44 | 1992 | Cisplatin (80 mg/m2/day) on day 1 and fluorouracil (1000 mg/m2/day) over 4 days. Every 3 weeks for 2 cycles. | According to surgeon’ preference | SCC* AC* | Resectable | OS DFS | 802 | Median: 70·8 months in chemotherapy group and 73·2 months in surgery group. | ||
| Ancona E37 | 1992 | cisplatin (100 mg/m2/day) on day 1, and 5-fluorouracil (1000 mg/m2/day) on Days 1 to 5. Every 3 weeks for 2–3 cycles. | Transthoracic esophagectomy + two-field lymphadenectomy | SCC | cT2–3N0M0, cT1–3N1M0. (Stage II-III) | OS | 94 | A minimum follow-up of 30 months for all the patients | ||
| Baba M38 | 1993 | Cisplatin (70 mg/m2/day) on day 1; 5-Fluorouracil (700 mg/m2/day) and leucovorin (20 mg/m2/day) on days 1 to 5. Every 4 weeks for 2 cycles. | Transthoracic esophagectomy + two-field or three-field lymphadenectomy | SCC | Resectable | OS | 42 | A minimum follow-up of 36 months and the maximum of 72 months | ||
| Law S39 | 1989 | Cisplatin (100 mg/m2/day) on day 1; 5-fluorouracil (500 mg/m2/day) on days 1 to 5. Every 3 weeks for 2 cycles. | Transthoracic esophagectomy or transhiatal esophagectomy | SCC | Resectable | OS | 147 | Median: 17 months |
||
| Boonstra JJ29 | 1989 | Cisplatin (80 mg/m2) on day 1; etoposide (100 mg/m2/d) intravenously on day 1, etoposide (200 mg/m2/d) orally on day 3 and day 5. Every 4 weeks for 2 cycles. | Right-sided thoracic esophagectomy for upper half part of esophagus, transhiatal esophagectomy for lower half part | SCC | cT1–3NxM0 | OS DFS | 169 | Median: 15 moths in the neoadjuvant chemotherapy+ surgery group; 14 months in the surgery group | ||
| Maipang T41 | 1988 | Cisplatin (100 mg/m2/day) on day1; vinblastine (3 mg/m2/day) on days 1, 8, 15, 22; bleomycin (10 mg/m2) on day 3 followed by a 4-day infusion of 10 mg/m2/day. Every 4 weeks for 2 cycles. |
Transthoracic Ivor-Lewis esophagectomy | SCC | Resectable | OS | 46 | Not mention in the manuscript. Speculate median follow -up time: 17 months | ||
| Schlag PM43 | 1988(speculate) | Cisplatin (20 mg/m2/day) on days 1 to 5; fluorouracil (1000 mg/m2/day) on days 1 to 5. Every 3 weeks for 3 cycles. | Abdominothoracic esophagectomy for GEJ. Thoracoabdominocervical approach for all others. | SCC | Resectable | OS | 46 | Not mention in the manuscript. Speculate median follow -up time: 7·5 months | ||
can be extracted separately for subgroup analysis on OS. †: one study reported two independent RCTs.
3.2. Pairwise comparison and network analysis on OS and DFS
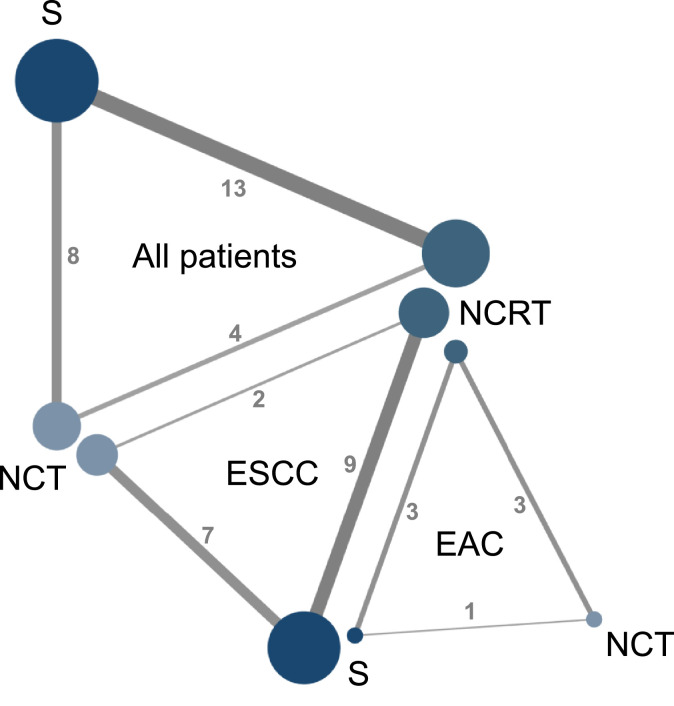
All included studies reported accessible OS information while only 16 studies reported accessible DFS information (Table 1). The network diagrams of the treatment comparisons on OS and DFS were described in Fig. 2 and supplementary figure 4, respectively.
Fig. 2.
Network diagrams of treatment comparisons on overall survival in all included patients and subgroup populations according to the histopathological types. The size of each node represents the number of patients who received the given treatment. Same color indicates same treatment strategy. Each line represents direct comparison of the connected treatments. The thickness of the lines with number represents the number of trails comparing the connected treatments. Abbreviations: NCRT: neoadjuvant chemoradiotherapy. NCT: neoadjuvant chemotherapy. S: surgery. ESCC: esophageal squamous cell carcinoma. EAC: esophageal adenocarcinoma.
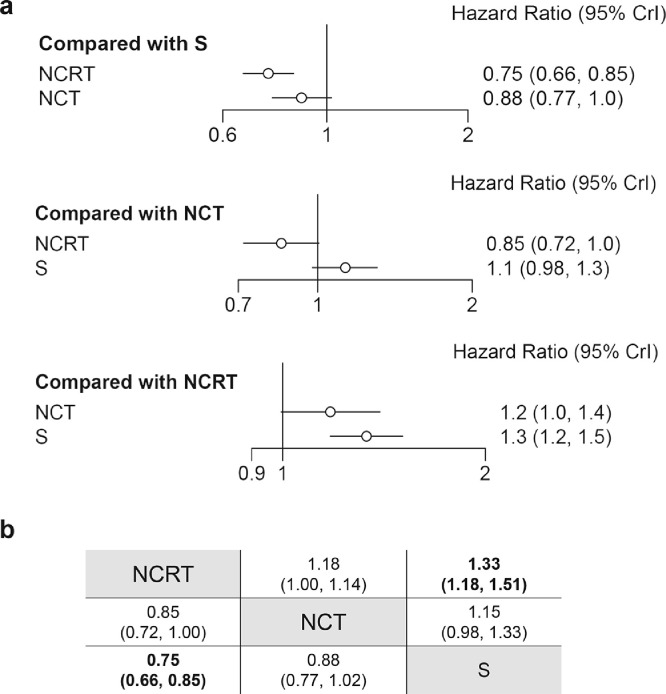
In terms of overall survival, pairwise comparison showed that both NCRT (HR: 0·76, 95%CI: 0·68–0·85) and NCT (HR: 0·88, 95%CI: 0·78–0·98) exhibited a better prognosis as compared to surgery alone, whereas no significant difference was found between NCRT and NCT (HR: 0·82, 95%CI: 0·64–1·04) (supplementary figure 5). Similarly, network meta-analysis on OS showed a better prognosis in NCRT as compared to surgery alone (HR: 0·75, 95%CrI: 0·66–0·85), but the advantage of NCT towards surgery alone diminished after combining direct and indirect comparisons (HR: 0·88, 95%CrI: 0·77–1·02) (Fig. 3). There was no significant difference in the comparison of NCRT with NCT (HR: 0·85, 95%CrI: 0·72–1·00).
Fig. 3.
Network meta-analysis on overall survival. (a). Forest plots of network comparisons between treatments. (b). Pooled hazard ratios with 95% CrI for each comparison arm. The pooled hazard ratio with 95% CrI was estimated under the Bayesian framework. Data in each cell are hazard ratios (95% credible intervals) for the comparison of row-defining treatment versus column-defining treatment. Bold text indicates a statistically significant difference. Abbreviations: NCRT: neoadjuvant chemoradiotherapy. NCT: neoadjuvant chemotherapy. S: surgery. 95% CrI: 95% credible intervals.
In terms of disease-free survival, pairwise comparison showed better therapeutic efficacies in both NCRT (HR: 0·72, 95%CI: 0·64–0·80) and NCT (HR:0·85, 95%CI: 0·76–0·95) than surgery alone. The therapeutic efficacy between NCRT and NCT (HR: 0·79, 95%CI: 0·60–1·05) did not reach significant difference (supplementary figure 6). Similarly, network analysis on DFS showed that NCRT was superior to surgery alone (HR: 0·72, 95%CrI: 0·63–0·81). Besides, NCRT also improved EC patients’ DFS when compared with NCT (HR: 0·83, 95%CrI: 0·69–0·99). No significant difference was found in the comparison of NCT with surgery alone (HR: 0·86, 95%CrI: 0·74–1·02) (supplementary figure 7).
3.3. Inconsistency and heterogeneity assessments
The global inconsistency assessments and local inconsistency assessments in network analyses on OS and DFS were summarized in supplementary figure 8. The global consistency was observed as the fit of the consistency model was similar or better than that of inconsistency model in network analyses (supplementary figure 8, a and c). The local consistency was achieved as the node splitting analysis revealed no significant differences in comparisons of direct and indirect evidence based on the Bayesian framework (supplementary figure 8, b and d).
Heterogeneity estimates of three pairwise comparisons were presented in supplementary figure 5 and 6. There was no significant between-study heterogeneity in all pairwise meta-analysis on OS (supplementary figure 5) as well as DFS (supplementary figure 6).
3.4. Subgroup analysis and sensitivity analysis
Among 25 included studies, 18 studies provided accessible OS information in 2314 ESCC patients while only seven studies with 1380 EAC patients could be extracted for EAC subgroup analysis (Table 1).
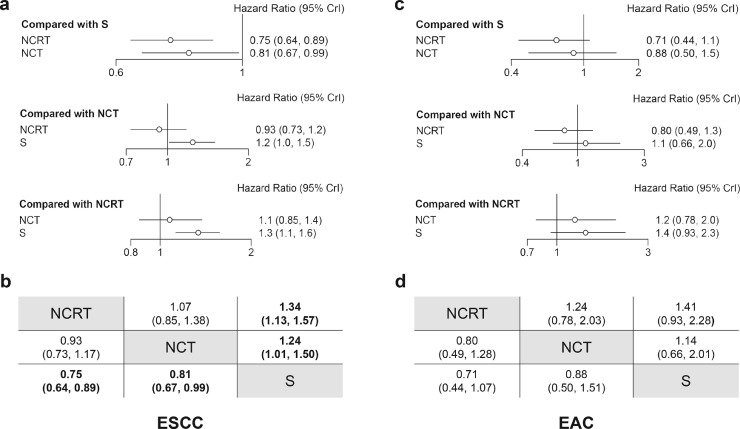
In terms of ESCC subgroup analysis, nine studies directly compared NCRT with surgery alone [5,6,27,30,[33], [34], [35],40,42], seven studies compared NCT with surgery alone [29,[37], [38], [39],41,43,44], and two studies compared NCRT with NCT[9,12] (supplementary figure 9a). Network meta-analysis showed that both NCRT (HR: 0·75, 95%CrI: 0·64–0·89) and NCT (HR: 0·79, 95%CrI: 0·65–0·98) are superior to surgery alone in ESCC, whereas no significance was found in the comparison of NCRT with NCT (HR: 0·94, 95%CrI: 0·74–1·20) (Fig. 4a, b). Similar results were confirmed in pairwise comparisons (supplementary figure 9a).
Fig. 4.
Subgroup network meta-analyses on overall survival. Forest plots of network comparisons between treatments in ESCC (a) and EAC (c). Pooled hazard ratios and 95% CrI for each comparison arm in ESCC (b) and EAC (d). The pooled hazard ratio with 95% CrI was estimated under the Bayesian framework. Data in each cell are hazard ratios (95% credible intervals) for the comparison of row-defining treatment versus column-defining treatment. Bold text indicates a statistically significant difference. Abbreviations: NCRT: neoadjuvant chemoradiotherapy. NCT: neoadjuvant chemotherapy. S: surgery. ESCC: esophageal squamous cell carcinoma. EAC: esophageal adenocarcinoma. 95% CrI: 95% credible intervals.
As to EAC subgroup analysis, three studies compared NCRT with surgery alone [6,27,34], three studies compared NCRT with NCT [9], [10], [11], and only one study compared NCT with surgery alone[44] (supplementary figure 9b). No significant differences were found between any comparable treatments in both network meta-analysis (Fig. 4c, d) and pairwise meta-analysis (supplementary figure 9b).
Supplementary figure 10 summarized the Bayesian ranking profiles of three different treatments in all included patients, ESCC subgroup and EAC subgroup regarding overall survival. The results showed that NCRT was most likely to be ranked first while surgery alone was most likely to be ranked last in all groups.
Eight studies provided accessible DFS information for ESCC subgroup analysis [5,6,11,29,30,35,40,42]. Like EC patient's population, network meta-analysis on ESCC subgroup showed a better DFS in NCRT when compared with surgery alone (HR: 0·64, 95%CrI: 0·51–0·81), whereas no difference was found between NCT and surgery groups (HR: 0·72, 95%CrI: 0·42–1·26). However, the advantage of NCRT over NCT in EC patients was not apparent in ESCC subgroup analysis (HR: 0·89, 95%CrI: 0·49–1·59) (not shown).
Nineteen studies were published after the year 2000 and were included in sensitivity analysis for OS [5,6,[9], [10], [11],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38],44], where 12 out of 19 could be used for the ESCC subgroup analysis [5,6,9,27,29,30,[33], [34], [35],37,38,44]. The EAC subgroup sensitivity analysis was omitted because all the accessible studies for EAC patients were published after the year 2000. Similar results were obtained in the network meta-analyses of studies published after the year 2000 (supplementary figure 11). NCRT is better than surgery alone in all included patients (HR:0·72, 95%CrI: 0·63–0·82) and both NCRT (HR:0·69, 95%CrI: 0·57–0·84) and NCT (HR:0·75, 95%CrI: 0·61–0·96) are superior to surgery alone in the ESCC subgroup. No significant differences were found between NCRT and NCT in both mixed histopathological types of patients and the ESCC subpopulations.
From January 2020 to September 2021, a total of 146 studies (114 from Embase, 26 from Medline, and six from Cochrane Library) were identified from the literature search. 143 studies were excluded after the tittle and abstract screening. Three studies underwent the full-text assessment. [45], [46], [47] One newly published trial that only reported short-term outcomes with one year's follow up was excluded, [45] while two trials from our included studies (the NEOCRTEC5010 trial [5] and the CROSS trial [6]) with updated long-term outcomes were included in the analysis. [46,47] Supplementary figure 12 summarized the network meta-analysis of OS on all included patients [[9], [10], [11], [12],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],46,47], ESCC subpopulation [[9], [10], [11], [12],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],46,47], and EAC subpopulation. [[9], [10], [11],27,34,44,47] Here, network meta-analysis on OS in all included patients shows that NCRT is superior to NCT (HR: 0·83, 95%CrI: 0·69–0·99) and surgery (HR: 0·72, 95%CrI: 0·63–0·82). However, consistent with our main results, both NCRT (HR: 0·77, 95%CrI: 0·65–0·90) and NCT (HR: 0·81, 95%CrI: 0·67–0·99) improve OS than surgery while there is no significant difference between NCRT and NCT (HR:0·95, 95%CrI: 0·75–1·19) in ESCC subpopulation. As to DFS, similarly, NCRT is superior to NCT (HR: 0·83, 95%CrI: 0·69–0·99) and surgery (HR: 0·72, 95%CrI: 0·63–0·81) in all included patients whereas is comparable with NCT (HR: 0·90, 95%CrI: 0·50–1·62) in ESCC subpopulation (not shown).
3.5. Short-term outcomes
All 25 studies were included in the network meta-analyses regarding resection rate and peri‑treatment mortality, and 20 studies can be synthesized for R0 resection (supplementary figure 13a) [5,6,[9], [10], [11], [12],[28], [29], [30], [31], [32],[34], [35], [36], [37],39,40,[42], [43], [44]]. Resection rate was significantly lower in patients assigned to NCRT (OR:0·20, 95%CrI: 0·08–0·43) and NCT (OR:0·29, 95%CrI: 0·10–0·68) groups as compared to surgery group. NCRT achieved higher R0 resection than NCT (OR: 2·47, 95%CrI: 1·48–4·63), and both NCRT and NCT had higher R0 resection than surgery alone (OR: 4·17, 95%CrI: 2·17–6·92 and OR:1·68, 95%CrI: 1·03–2·71, respectively). Peri-treatment mortality was significantly higher in NCRT group than surgery group (OR: 1·81, 95%CrI: 1·20–2·79), whereas no significant differences were found between NCRT and NCT (OR: 1·57, 95%CrI: 0·92–2·57) as well as NCT and surgery (OR: 1·15, 95%CrI: 0·78–1·83).
Pairwise comparison meta-analyses for pCR and grade 3 to 4 AEs after neoadjuvant treatments were shown in supplementary figure 13b-c. Patients undergoing NCRT achieved significantly higher pCR than those undergoing NCT (RR:3·85, 95%CI: 1·88–7·88). Grade 3 to 4 AEs were comparable between NCRT and NCT groups (RR: 1·18, 95%CI: 0·65–2·15).
4. Discussion
The findings of this comprehensive network meta-analysis confirm that NCRT is better than surgery alone regarding OS and DFS in locally advanced EC patients. Patients who underwent NCRT showed longer OS and DFS than those who underwent NCT. Interestingly, the advantages of NCT over surgery alone on OS and DFS in pairwise comparisons were eliminated after synthesizing the direct and indirect evidence by network meta-analysis. More importantly, subgroup analysis on ESCC patients showed that both NCRT and NCT improved OS when compared to surgery alone, whereas no significant differences were observed between NCRT and NCT in both OS and DFS.
The inconsistent results of pairwise comparison and network meta-analysis between NCT and surgery may be attributed to the mixed tumor types as the two major histopathological types of esophageal cancer, ESCC and EAC, vary in epidemiology, etiology and pathogenesis[2,3]. In recent years, several genome analyses on esophageal cancer enriched our understanding that ESCC and EAC are distinct diseases at a molecular level, where ESCC is more similar to squamous cell carcinoma from other organs while EAC resembles a chromosomally unstable variant of gastric cancer [48], [49], [50]. It is rather important to design different treatment strategies and separate clinical trials for treating and studying ESCC and EAC [51]. Our findings support this point of view from the clinical perspective by the subgroup analysis results showed that ESCC and EAC patients exhibit different sensitivities to neoadjuvant therapies when compared with surgery alone.
Network meta-analysis shows little benefit of NCT over surgery alone in EC patients, however subgroup analysis on the ESCC identified an advantage in terms of OS for NCT based on 791 accessible ESCC patients from seven studies. Besides, the advantages of NCRT over NCT regarding OS and DFS in all included patients was diminished in ESCC subpopulation. Here, we found that locally advanced ESCC patients not only benefit from NCRT but also NCT regarding OS when compared with surgery alone while there is no clear evidence supporting which of the two neoadjuvant treatments is a better solution in both short-term and long-term outcomes. With respect to the EAC subpopulation, pairwise comparisons and network meta-analysis showed no significant differences of the three treatment options. Some cautions should be used before making recommendations based on these results. One reason is that only few studies could be included for data synthesizing with only one study comparing NCT with surgery alone. Another reason is that a high heterogeneity exists (I2=80%, p<0·01) in the comparison of NCRT with surgery alone (supplementary figure 9b). The heterogeneity may come from the study by Burmeister et al. [34], which only used one cycle chemotherapy with relatively lower dose of radiotherapy (35 Gy) as neoadjuvant treatment (table 1).
Notably, we excluded the perioperative chemotherapy strategy in this study design. Some previous meta-analyses mixed perioperative chemotherapy with NCT [52,53], but we preferred to regard perioperative chemotherapy as a different therapeutic strategy. Perioperative chemotherapy plus surgery is meanwhile the standard treatment for locally advanced gastric cancer patients [54]. Several important RCTs in gastric cancer research like the MAGIC and FLOT trials included 20%−40% adenocarcinoma of the lower esophagus or AGEJ patients reporting promising survival benefits for perioperative chemotherapy [55,56]. Similar results were also reported in a RCT consisting of 75% EAC and AGEJ patients [57]. Our original study design was to compare NCRT with perioperative chemotherapy on EAC patients. However, as shown in supplementary figure 9, there are relatively few accessible studies on EAC patients. In addition, during the literature searching and data extraction process, it has been found that most of the EAC/AGEJ patients in perioperative chemotherapy studies underwent gastrectomy rather than esophagectomy and their survival results were mixed with results of gastric cancer patients. Therefore, it did not display strong reliability to guide EAC treatment fully based on this rather gastric cancer-based data. There are two ongoing clinical trials comparing perioperative chemotherapy (modified MAGIC regimen and FLOT regimen) with the NRCT (CROSS regimen) focusing mostly on EAC/AGEJ patients [58,59]. Here, we are looking forward to the future results from above trials.
Several limitations of this study should be addressed. Firstly, we only compared the three treatment strategies generally, without considering that there are internal differences within each treatment strategy among studies (eg: chemotherapy regimens, radiation techniques, radiation fractions and total doses). In the history of EC treatment, each therapeutic strategy came along with controversies [3]. Thus, we summarized detailed therapeutic regimens in table 1 for the readers’ reference. There is a three-arm phase III trial (JCOG1109) that comprise a two-drug chemotherapy regimen versus a three-drug chemotherapy regimen versus a chemoradiotherapy regimen in ESCC patients currently ongoing in Japan [60]. With more similar RCTs comparing specific different regimens [58], [59], [60], future network meta-analysis could be conducted with more detailed comparisons between EC treatment regimens. Secondly, we did not perform individual patient data meta-analysis which theoretically could provide higher evidence level than network meta-analysis. However, it is methodologically reliable to use network meta-analysis to compare neoadjuvant treatments of EC due to relatively few direct comparisons (only four studies) between NCRT and NCT while there were already many RCTs comparing NCRT or NCT with surgery alone (indirect evidence). In contrast, using individual patient data meta-analysis to compare NCRT and NCT can only obtain patients’ information from the four studies without considering large data-based indirect evidence. Thirdly, since there is no well-accepted statistic method to test the similarity of included studies, we can only judge the transitivity of our network meta-analysis relatively subjectively. Our network meta-analysis has a strong transitivity because every included study strictly meets the same PICOS principle, and there are similarities among neoadjuvant radiotherapy doses (around 40 Gy), chemotherapy regimens (platinum based) as well as surgical strategies among studies (table 1). In addition, the present study also has potential publication and selection bias. For example, we only included studies reported in English and we could not access the essential information of a few suitable studies (Supplementary information 2). However, adjusted funnel plots showed an acceptable publication bias and its effect is further mitigated by the comprehensive inclusion of RCTs. Despite these limitations, the global and local consistency of our network meta-analysis model as well as the consistency of sensitivity analysis and our main results indicate that our findings are robust.
In conclusion, our study corroborated current guidelines and addressed the importance of independently studying esophageal cancer according to histopathological types. The results of this network meta-analysis demonstrate that locally advanced ESCC patients can benefit from both NCRT and NCT regarding overall survival when compared with surgery alone. There is no clear evidence supporting the optimal neoadjuvant treatment strategies between NCRT and NCT in treating locally advanced ESCC patients. More RCTs comparing different therapeutic strategies in EAC patients are warranted.
Data sharing statement
The raw data of this study were extracted from included RCTs. Derived data supporting the findings of this study are available from the corresponding author on reasonable requests.
Contributors
Conceptualization: Yue Zhao, Christiane Bruns; Methodology: Ningbo Fan, Yue Zhao, Christiane Bruns, Marc Bludau, Gianmarco Contino; Formal analysis and investigation: Ningbo Fan, Zhefang Wang, Chenghui Zhou; Writing - original draft preparation: Ningbo Fan; Writing - review and editing: Zhefang Wang, Chenghui Zhou, Marc Bludau, Gianmarco Contino, Yue Zhao, Christiane Bruns; Funding acquisition: Yue Zhao, Christiane Bruns; Resources: Yue Zhao, Christiane Bruns; Supervision: Yue Zhao, Christiane Bruns. We confirm here that all authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of Competing Interest
The authors declare no conflicts of interest in this work.
Acknowledgments
Funding
This work was supported by Köln Fortune Program/Faculty of Medicine, University of Cologne.
Acknowledgments
Ningbo Fan was financially supported by Guangzhou Elite Scholarship Council (GESC) and Chenghui Zhou was financially supported by CSC scholarship (The China Scholarship Council). The authors thank Jiarong Li and Bin Wan for their assistance in statistical analysis. The authors thank Jiabo Zheng for his assistance in linguistic editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101183.
Appendix. Supplementary materials
Reference
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 2.Thrift A.P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18:432–443. doi: 10.1038/s41575-021-00419-3. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. Lancet Lond Engl. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 4.Ando N., Kato H., Igaki H., Shinoda M., Ozawa S., Shimizu H., et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Liu H., Chen Y., Zhu C., Fang W., Yu Z., et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa Y., Uno T., Oyama T., Kato K., Kato H., Kawakubo H., et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus Off J Jpn Esophageal Soc. 2019;16:25–43. doi: 10.1007/s10388-018-0642-8. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers, version 2.2021. Natl Compr Cancer Netw NCCN n.d; 2021. NCCN clinical practice guidelines in oncology.https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed April 16. [Google Scholar]
- 9.von Döbeln G.A., Klevebro F., Jacobsen A.-.B., Johannessen H.-.O., Nielsen N.H., Johnsen G., et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32 doi: 10.1093/dote/doy078. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 10.Stahl M., Walz M.K., Riera-Knorrenschild J., Stuschke M., Sandermann A., Bitzer M., et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. doi: 10.1016/j.ejca.2017.04.027. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 11.Burmeister B.H., Thomas J.M., Burmeister E.A., Walpole E.T., Harvey J.A., Thomson D.B., et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–360. doi: 10.1016/j.ejca.2010.09.009. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 12.Nygaard K., Hagen S., Hansen H.S., Hatlevoll R., Hultborn R., Jakobsen A., et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104–1109. doi: 10.1007/BF02067069. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 13.Cipriani A., Higgins J.P.T., Geddes J.R., Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159:130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane; 2021. (editors) (updated February 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laws A., Tao R., Wang S., Padhiar A., Goring S. A Comparison of National Guidelines for Network Meta-Analysis. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2019;22:1178–1186. doi: 10.1016/j.jval.2019.05.013. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 16.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 18.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark Mitchell; Baurzhan Muftakhidinov; Tobias Winchen; Bas van Schaik; Alexander Wilms; kylesower; kensington; Zbigniew Jędrzejewski-Szmek; The Gitter Badger; badshah400. Engauge Digitizer Software (Version 12.1). 2019.
- 21.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 22.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkenhoef G van. GeMTC R package. 2020.
- 24.Lu G., Ades A.E. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101:447–459. doi: 10.1198/016214505000001302. https://doi.org/ [DOI] [Google Scholar]
- 25.Dias S., Welton N.J., Sutton A.J., Caldwell D.M., Lu G., Ades A.E. National Institute for Health and Care Excellence (NICE); London: 2014. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. [PubMed] [Google Scholar]
- 26.van Valkenhoef G., Dias S., Ades A.E., Welton N.J. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93. doi: 10.1002/jrsm.1167. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass G.A., Furlong H., O'Sullivan K.E., Hennessy T.P.J., Walsh T.N. Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur J Cancer. 2014;50:1065–1075. doi: 10.1016/j.ejca.2013.12.022. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 28.Mariette C., Dahan L., Mornex F., Maillard E., Thomas P.-.A., Meunier B., et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III Trial FFCD 9901. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.6532. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 29.Boonstra J.J., Kok T.C., Wijnhoven B.P., van Heijl M., van Berge Henegouwen M.I., Ten Kate FJ, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv J., Cao X.F., Zhu B., Ji L., Tao L., Wang D.D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tepper J., Krasna M.J., Niedzwiecki D., Hollis D., Reed C.E., Goldberg R., et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelsen D.P., Winter K.A., Gunderson L.L., Mortimer J., Estes N.C., Haller D.G., et al. Long-term results of RTOG Trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 33.Natsugoe S., Okumura H., Matsumoto M., Uchikado Y., Setoyama T., Yokomakura N., et al. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus Off J Int Soc Dis Esophagus. 2006;19:468–472. doi: 10.1111/j.1442-2050.2006.00615.x. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 34.Burmeister B.H., Smithers B.M., Gebski V., Fitzgerald L., Simes R.J., Devitt P., et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 35.Lee J.-.L., Park S.I., Kim S.-.B., Jung H.-.Y., Lee G.H., Kim J.-.H., et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2004;15:947–954. doi: 10.1093/annonc/mdh219. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 36.Urba S.G., Orringer M.B., Turrisi A., Iannettoni M., Forastiere A., Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 37.Ancona E., Ruol A., Santi S., Merigliano S., Sileni V.C., Koussis H., et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma. Cancer. 2001;91:2165–2174. doi: 10.1002/1097-0142(20010601)91:11<2165::AID-CNCR1245>3.0.CO;2-H. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 38.Baba M., Natsugoe S., Shimada M., Nakano S., Kusano C., Fukumoto T., et al. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis Esophagus Off J Int Soc Dis Esophagus. 2000;13:136–141. doi: 10.1046/j.1442-2050.2000.00101.x. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 39.Law S., Fok M., Chow S., Chu K.-.M., Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 40.Bosset J.-.F., Gignoux M., Triboulet J.-.P., Tiret E., Mantion G., Elias D., et al. Chemoradiotherapy Followed by Surgery Compared with Surgery Alone in Squamous-Cell Cancer of the Esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 41.Maipang T., Vasinanukorn P., Petpichetchian C., Chamroonkul S., Geater A., Chansawwaang S., et al. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J Surg Oncol. 1994;56:191–197. doi: 10.1002/jso.2930560314. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 42.Le Prise E., Etienne P.L., Meunier B., Maddern G., Ben Hassel M., Gedouin D., et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. doi: 10.1002/1097-0142(19940401)73:7<1779::aid−cncr2820730702>3.0.co;2-t. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 43.Schlag P.M. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg Chic Ill 1960. 1992;127:1446–1450. doi: 10.1001/archsurg.1992.01420120080015. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 44.Allum W.H., Stenning S.P., Bancewicz J., Clark P.I., Langley R.E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 45.Wang H., Tang H., Fang Y., Tan L., Yin J., Shen Y., et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156:444. doi: 10.1001/jamasurg.2021.0133. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H., Liu H., Chen Y., Zhu C., Fang W., Yu Z., et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg. 2021;156:721. doi: 10.1001/jamasurg.2021.2373. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eyck B.M., van Lanschot J.J.B., Hulshof M.C.C.M., van der Wilk B.J., Shapiro J., van Hagen P., et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. doi: 10.1200/JCO.20.03614. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 48.Agrawal N., Jiao Y., Bettegowda C., Hutfless S.M., Wang Y., David S., et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2:899–905. doi: 10.1158/2159-8290.CD-12-0189. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, Brigham and Women's Hospital, Broad Institute, Brown University Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin D.-C., Dinh H.Q., Xie J.-J., Mayakonda A., Silva T.C., Jiang Y.-Y., et al. Identification of distinct mutational patterns and new driver genes in oesophageal squamous cell carcinomas and adenocarcinomas. Gut. 2018;67:1769–1779. doi: 10.1136/gutjnl-2017-314607. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burki T.K. Definitions of oesophageal cancer. Lancet Oncol. 2017;18:e71. doi: 10.1016/S1470-2045(17)30018-9. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 52.Chan K.K.W., Saluja R., Delos Santos K., Lien K., Shah K., Cramarossa G., et al. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer. 2018;143:430–437. doi: 10.1002/ijc.31312. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 53.Sjoquist K.M., Burmeister B.H., Smithers B.M., Zalcberg J.R., Simes R.J., Barbour A., et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 54.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet Lond Engl. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 55.Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J.H., Nicolson M., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 56.Al-Batran S.-.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet Lond Engl. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 57.Ychou M., Boige V., Pignon J.-.P., Conroy T., Bouché O., Lebreton G., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 58.Hoeppner J., Lordick F., Brunner T., Glatz T., Bronsert P., Röthling N., et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus ( NCT02509286) BMC Cancer. 2016;16:503. doi: 10.1186/s12885-016-2564-y. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds J.V., Preston S.R., O'Neill B., Baeksgaard L., Griffin S.M., Mariette C., et al. ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS) BMC Cancer. 2017;17:401. doi: 10.1186/s12885-017-3386-2. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura K., Kato K., Igaki H., Ito Y., Mizusawa J., Ando N., et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study) Jpn J Clin Oncol. 2013;43:752–755. doi: 10.1093/jjco/hyt061. https://doi.org/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.