Abstract
Background
PB-201, a partial, pancreas/liver-dual glucokinase activator, showed good tolerance and glycaemic effects in multinational studies. This study determined its optimal dose, safety, pharmacokinetics, and pharmacodynamics in Chinese patients with type 2 diabetes.
Methods
In this double-blind, randomised, four-period, crossover, phase 1 trial in China, conducted at the Peking University Third Hospital, adult patients with drug-naive type 2 diabetes were randomised (1:1:1:1) to four sequence groups using a computer-generated randomisation table. In each period, they received oral placebo or PB-201 (50+50, 100+50, or 100+100 mg split doses) for 7 days. Investigators and patients were masked to treatment assignment. The primary endpoints were safety and pharmacokinetics. Continuous glucose monitoring was used to delineate the glucose excursion profile. Trial registration number: NCT03973515.
Findings
Between August 27, 2019 and December 19, 2019, 16 patients were randomised. PB-201 showed a dose-proportional pharmacokinetic profile without apparent accumulation in the body and induced dose-dependent lowering of blood glucose. PB-201 at 50+50, 100+50, and 100+100 mg increased mean time in range (49·210% [standard deviation 27], 56·130% [25], and 63·330% [20] with three doses, respectively) versus placebo (49·380% [27]) and reduced estimated glycated haemoglobin from baseline (−0·5445% [1·654], −1·063% [1·236], and −1·888% [1·381] vs −0·581% [1·200]). Fifteen patients (93·8%) had treatment-emergent adverse events, which were mild. No patients had hypoglycaemia with venous/capillary glucose <3·9 mmol/L or nocturnal hypoglycaemia.
Interpretation
PB-201 100 mg twice daily is identified as the optimal dose, which shows promising glucose-lowering effects and low risks of hypoglycaemia and other side effects. Further investigation of PB-201 100 mg twice daily in confirmatory trials is warranted.
Funding
PegBio.
Keywords: Continuous glucose monitoring, Glucokinase activator PB-201, Type 2 diabetes
Research in context.
Evidence before the study
Most glucokinase activators did not enter late development phases because of loss of efficacy over time and safety concerns such as hypoglycaemia, hyperlipidaemia, and impairment of liver function. We searched ClinicalTrials.gov using “glucokinase” and “diabetes mellitus” and found one study drug in phase 3 trials; we also searched PubMed using keywords “glucokinase” and “phase 3” in title/abstract but found no publications for clinical trials. PB-201 is a partial, pancreas- and liver-dual activator of glucokinase that was designed to have sustainable glucokinase-stimulating activity and low risk of hypoglycaemia, and it shows promising glucose-lowering effects and no signals of toxicity in previous phase 1 and phase 2 studies conducted in countries outside China.
Added value of this study
Results from this phase 1 study added to previous evidence that PB-201 was a safe, well tolerated, and potentially efficacious antidiabetic drug. We also found a dosage regimen (100 mg twice daily) that might have a larger glucose-lowering effect than those used in previous phase 2 studies while remaining well tolerated. The 24-h continuous glucose monitoring data provided convincing evidence that the risk of hypoglycaemia and other side effects reported with previous glucokinase activators were low with PB-201.
Implications of all the available evidence
Altogether, the evidence shows that PB-201 is likely to be associated with a low risk of side effects. Its effective glucose-lowering potential is of clinical significance. These results support phase 3 trials to further investigate its efficacy and safety.
Alt-text: Unlabelled box
1. Introduction
Hyperglycaemia is the hallmark of type 2 diabetes, caused by insulin resistance and impaired insulin secretion. Treating diabetes to glycaemic targets is the cardinal goal in diabetic care because of the macrovascular and microvascular complications associated with hyperglycaemia [1]. Although metformin is well established as the first-line treatment for type 2 diabetes and various other glucose-lowering options are available [2], only a proportion of patients (less than 35% in a large-scale survey in China [3]) can achieve glycaemic goals, and secondary failure is common among patients on oral antidiabetic drugs due to progressive decline in β-cell function [4]. Moreover, side effects and risks associated with these agents, such as gastrointestinal side effects with metformin and glucagon-like peptide-1 receptor agonists and risks of bone fractures, genitourinary infections, and volume depletion with sodium-glucose co-transporter-2 inhibitors, not only impact patient's quality of life, but also influence choice of treatment and adherence [2].
Glucokinase activators (GKAs) are being pursued as next-generation antidiabetic therapeutics, which have a novel mechanism of action and may provide effective glycaemic control while having a low risk of side effects. Their mechanism of action builds on the role of glucokinase as a glucose sensor and a central regulator of systemic glucose haemostasis [5]. In a glucose-dependent manner, glucokinase stimulates insulin secretion in the pancreas and regulates glucose production and utilization in the liver. Up to now, a number of GKA candidates have been developed; however, only four candidates are currently being investigated globally (TTP399 [6,7], dorzagliatin [8], globalagliatin [9], and PB-201 [10,11]). Issues prohibiting the entry of most GKAs into late clinical phases include hypoglycaemia [12, 13], hyperlipidaemia [12,13], risk of fatty liver [14], and loss of efficacy over time [13,15].
PB-201 was designed as a partial, pancreas- and liver-dual activator of glucokinase that has glucose-dependent, moderate binding affinity and fast dissociation [16,17], thereby having a low risk of hypoglycaemia while conferring sustained glucokinase activation. Its pharmacological properties, safety, tolerability, and exploratory efficacy have been investigated in previous studies [10,11]. In two phase 2 trials, the maximal dose (100 mg once daily) demonstrated a glycated haemoglobin (HbA1c)-lowering effect similar to that with sitagliptin and less than that with glimepiride, as well as good tolerance [10]. This study sought to identify the dose of PB-201 that can provide the optimal benefit/risk ratio to be used for future confirmatory trials, and to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of PB-201 in drug-naïve Chinese patients with type 2 diabetes. A range of doses up to 100 mg twice daily, higher than those used in phase 2 trials, were tested. Continuous glucose monitoring (CGM) was utilised in this study as a robust evaluation tool for delineating the glucose excursion profile, providing evidence in relation to safety in terms of hypoglycaemia as well as glycaemic effects.
2. Methods
2.1. Study design
This double-blind, placebo-controlled, randomised, four-period, four-sequence, crossover, phase 1 study was conducted at Peking University Third Hospital. After screening, eligible patients entered a run-in period of 28–35 days, during which they were instructed to follow a standardised lifestyle programme and their concomitant medications, if any, were stabilised. Following the run-in period, patients were randomly assigned (1:1:1:1) to a sequence group in which they received four sequential treatments, consisting of placebo and three dose levels of PB-201 and separated by washout periods (table 1). Patients were admitted and stayed at the study centre for 10 days (designated as days −1 to 9) per treatment period, when they wore the CGM device throughout the 10 days and took the study drug from days 1 to 7.
Table 1.
Treatment sequence in four sequence groups
| Group number | Number of patients | Treatment in Period 1 | Treatment in Period 2 | Treatment in Period 3 | Treatment in Period 4 |
|---|---|---|---|---|---|
| 1 | 4 | Placebo | 50+50 mg PB-201 | 100+50 mg PB-201 | 100+100 mg PB-201 |
| 2 | 4 | 50+50 mg PB-201 | Placebo | 100+50 mg PB-201 | 100+100 mg PB-201 |
| 3 | 4 | 50+50 mg PB-201 | 100+50 mg PB-201 | Placebo | 100+100 mg PB-201 |
| 4 | 4 | 50+50 mg PB-201 | 100+50 mg PB-201 | 100+100 mg PB-201 | Placebo |
All subjects gave their written informed consent before any study-related procedures. This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, and other applicable regulatory requirements. The study protocol and informed consent information were approved by the ethics committee at Peking University Third Hospital (ethics approval reference, 037-02). This trial was registered with ClinicalTrials.gov, number NCT03973515.
2.2. Patients
Patients were eligible if they were diagnosed with type 2 diabetes according to 1999 World Health Organization diagnostic criteria for diabetes mellitus [18], aged 18–65 years, with a body mass index of 18·5–35·0 kg/m2, had not received any glucose-lowering agents within 2 months, had an HbA1c of 7·5–11·0% (58–97 mmol/mol) during screening and 7·0–10·0% (53–86 mmol/mol) before randomisation, had a fasting plasma glucose (FPG) of 7·0–11·1 mmol/L during screening and before randomisation, and had a fasting C-peptide level of ≥0·8 ng/mL during screening. Key exclusion criteria included diagnosis of type 1 diabetes, clinically significant concomitant diseases or laboratory test abnormalities, concurrent malignancy, hypersensitivity, concomitant treatments that might interfere with the conduct of the study or pose an unacceptable risk to subjects, alcoholism, drug abuse, and fever within 5 days. Details of exclusion criteria are provided in the appendix.
2.3. Procedures
Placebo or PB-201 in split doses of 50+50, 100+50, and 100+100 mg PB-201 were administered orally, one dose 30 min before breakfast and the other 30 min before lunch. Patients were treated for 7 days per treatment period, and between two treatment periods had a washout period of 7–14 days, which were deemed sufficient to eliminate carryover effects on pharmacokinetics and pharmacodynamics. Patients had standard meals (designed by the study site following dietary recommendations for patients with type 2 diabetes [19]) and were not allowed to have food or drinks other than those provided by the study centre during the treatment periods. The standard meal for postprandial glucose measurements provided 540.7 kcal/meal, consisted of 60.4 g carbohydrate, 28.8 g fat, and 10.7 g protein (details of the standard meal are provided in the appendix). Prohibited concomitant pharmacological treatments included any antidiabetic medications and medications that might interfere with the conduct of the study or pose an unacceptable risk to subjects. Treatment was to be interrupted (for no longer than 48 h) within one treatment period in the event of patients experiencing two episodes of hypoglycaemia with glucose ≤3·9 mmol/L or two episodes of hypoglycaemia that resolved with oral carbohydrate. Patients could freely withdraw their consent at any time. In the case of major protocol deviation, noncompliance, severe hypoglycaemia (i.e. with severe cognitive impairment requiring assistance for recovery) or two episodes of hypoglycaemia with glucose ≤3·0 mmol/L within one treatment period, repeated hyperglycaemia (FPG ≥13·3 mmol/L measured over 3 consecutive days), or other circumstances considered in the patient's best interests, or if treatment had been interrupted for more than 48 h, the investigator could withdraw patients from the study.
2.4. Outcomes
Primary endpoints were safety, tolerability, and pharmacokinetics. Secondary endpoints included pharmacodynamics and pharmacokinetics of WI-0800, the main metabolite of PB-201 [20].
Blood samples for pharmacokinetic assessments were collected on days 1–9 in each treatment period; they were taken at 30 min predose and 0·5, 1, 2, 3, 4·5, 6, 8,10, 12, and 14 h postdose on days 1 and 7; at 30 min predose on days 2, 5, and 6; at 24 h postdose on day 8; and at 48 h postdose on day 9. Plasma concentrations of PB-201 and WI-0800 were measured using a validated ultrafast liquid chromatography-tandem mass spectrometry method (MDS Sciex, Toronto, Canada; Shimadzu Corp., Kyoto, Japan). Pharmacokinetic parameters included AUC0–inf (area under the concentration–time curve from time zero to infinity), AUC0–last (area under the concentration–time curve from time zero to the last measurable concentration), CL/F (apparent clearance), Cmax (maximum plasma concentration), tmax (time to maximum plasma concentration), t½ (plasma half-life), Rac,AUC0–inf (accumulation ratio based on AUC0–inf), Rac,Cmax (accumulation ratio based on Cmax), and Vz/F (apparent volume of distribution).
Safety was monitored throughout the study and for up to 14 days after the last dose. Adverse events (during the study and for up to 14 days after the last dose), clinical laboratory tests (day −1, 7, and 9 of each period and 7–14 days after the last dose), vital signs (day −1 to day 9 of each period and 7–14 days after the last dose), electrocardiogram (ECG) parameters (day −1, 1, 7, and 9 of each period and 7–14 days after the last dose), and physical examination (day −1 and 9 of each period and 7–14 days after the last dose) were assessed. Adverse events were coded and classified according to Medical Dictionary for Regulatory Activities version 22.0. Treatment-emergent adverse events (TEAEs), defined as any events that began or worsened in severity during or after study drug administration until the last day of follow-up, were evaluated and compared between treatments. Their relationship to the study drug was investigated (unrelated, possibly unrelated, possibly related, probably related, or definitely related). Hypoglycaemia was defined and classified in accordance with guidelines for clinical trials of antidiabetic agents and biologics released by the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA), where hypoglycaemia was diagnosed and classified as severe hypoglycaemia (characterised by hypoglycaemia with severe cognitive impairment that requires assistance for recovery), documented symptomatic hypoglycaemia (with symptoms and glucose ≤ 3·9 mmol/L), asymptomatic hypoglycaemia (glucose ≤3·9 mmol/L but without symptoms), undocumented symptomatic hypoglycaemia (with symptoms and glucose level unknown), and relative hypoglycaemia (with symptoms and glucose reduced but >3·9 mmol/L) based on a combination of symptoms and blood glucose levels (measured with either venous or capillary glucose) [21].
Measures for assessing pharmacodynamics included FPG, 2-h postprandial plasma glucose (PPG), fasting insulin, 2-h postprandial insulin, fasting C-peptide, 2-h postprandial C-peptide, and CGM metrics. Blood samples for analysing pharmacodynamics were taken under fasting state (before breakfast) and 2 h after a standard meal at baseline and day 7. CGM metrics, including mean glucose, time in range (TIR; 3·9–10·0 mmol/L), time below range (TBR; <3·9 or <3·0 mmol/L), and time above range (TAR; >10 mmol/L) calculated as per international consensus recommendations [22] and estimated HbA1c [23], were evaluated based on average values of glucose concentrations over 7 days from days 1 to 7. The FreeStyle Libre flash glucose monitoring system (Abbott Diabetes Care, Witney, UK) was used, which measures glucose concentration with a mean absolute relative difference (MARD) of 10.0% vs capillary blood glucose measures and a MARD of 10.7% vs venous blood glucose measures [24]. Data from this blinded CGM device were retrospectively reviewed by investigators.
2.5. Randomisation and masking
A random number table containing 24 sequentially numbered codes was generated with the SAS 9.4 PLAN procedure with a block of four by an independent statistician. Patients were randomly assigned to a code, which corresponded to a group. Placebo or PB-201, provided as identical tablets, were sealed in a code-labelled package and dispensed by pharmacists. PK data were collected by unblinded personnel and handled and integrated by unblinded data manager, and these persons were not directly involved in the conduct of the study or patient care. The investigators, site personnel, and sponsor had no access to the randomisation assignment and were not involved in medication distribution. The investigators, site personnel, sponsor, and patients were masked to treatment assignment during the trial. After database lock, the data were unblinded and transferred to the statistician for analysis.
2.6. Statistical analysis
The sample size of this study was estimated not based on statistical inference but rather in accord with sample size for phase 1 trials recommended by CDE of China NMPA (8–12 subjects are recommended for each dose level) [25] as well as empirical evidence. To ensure that 12 patients completed four treatment periods and assuming a withdrawal rate of 25%, 16 patients were to be enrolled. The pharmacokinetic analysis set (PKS) included all patients who received at least one dose of PB-201 and had at least one pharmacokinetic assessment. The pharmacodynamic analysis set (PDS) included all patients who received at least one dose of study drug and had at least one baseline and one post-baseline pharmacodynamic assessment within one treatment period. Safety analysis set included all patients who received at least one dose of study drug. Descriptive statistics were used to summarise safety and pharmacokinetic results. Pharmacokinetic parameters were calculated with a noncompartmental method using Phoenix WinNonlin version 6.4 (Pharsight Corp., Mountain View, CA, USA). Dose proportionality of pharmacokinetic parameters was assessed with a power model, where linearity was claimed if 90% CI of the coefficient (β) fell within a predefined dose-adjusted range ([, ], where ) [26]. Details of pharmacokinetic analyses are provided in the appendix. Change from baseline in FPG, PPG, plasma insulin, or C-peptide was analysed using a generalised linear model. In the model, plasma glucose was included as a repeated effect, treatment sequence, treatment period, dose, and baseline data were included as fixed effects, and subject within sequence was included as a random effect. Plasma insulin or C-peptide was included as a repeated effect, treatment sequence, treatment period, days, and dose-time interaction were included as fixed effects, and subject within sequence was included as a random effect. No imputation for missing data was conducted. Least-square mean (LSM) was estimated for change from baseline with two-sided 90% confidence interval (90% CI). Two-sided p-values were reported for these pharmacodynamic variables to flag any potential differences between placebo and PB-201 treatments (p<0·05 considered statistically significant). The p-values were not adjusted for multiplicity. HbA1c level was estimated using a previously published equation relating it to average glucose (AG) measured by CGM: or [23]. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
2.7. Role of the funding source
The funder designed the study, developed the protocol, and supervised the conduct of the trial, data collection, data review, and data analysis, in accord with the Good Clinical Practice guidelines. All authors had full access to the raw data and the corresponding author had final responsibility for the decision to submit for publication.
3. Results
Between August 27, 2019 and December 19, 2019, 75 patients were screened, and 16 eligible patients were enrolled and randomised to one of the four crossover sequence groups (n=4 each group; figure 1). The most common reason for exclusion after screening was not meeting the eligibility criteria (n=58). All patients completed four-period treatment as per protocol, except for one in Group 4 who experienced predefined repeated hyperglycaemia and was withdrawn from the study after being treated with 50+50 mg PB-201 for 7 days. Analysis sets (full analysis set, per-protocol set, PKS, PDS, and safety set) for 50+50 mg PB-201 included 16 patients, while those for placebo and 100+50 or 100+100 mg PB-201 included 15 patients. Most patients were male (11 [68·8%]), with a median duration of disease of 2 years (range, 0–8·67; table 2). No patients had taken anti-diabetic pharmacological treatments within 2 months before randomisation. Prohibited concomitant medications were not used during the study. There were no major protocol violations.
Figure 1.
Trial profile
Table 2.
Baseline characteristics of the full analysis set
| Full analysis set (n=16) | |
|---|---|
| Male, n (%) | 11 (68·8) |
| Age, years, median (IQR) | 50·5 (49·0 to 54·0) |
| Chinese, n (%) | 16 (100) |
| BMI, kg/m2, mean (SD) | 25·9 (2·92) |
| Duration of type 2 diabetes mellitus since first diagnosis, years, median (range) | 2 (0 to 8·67) |
BMI=body mass index. IQR=interquartile range. SD=standard deviation.
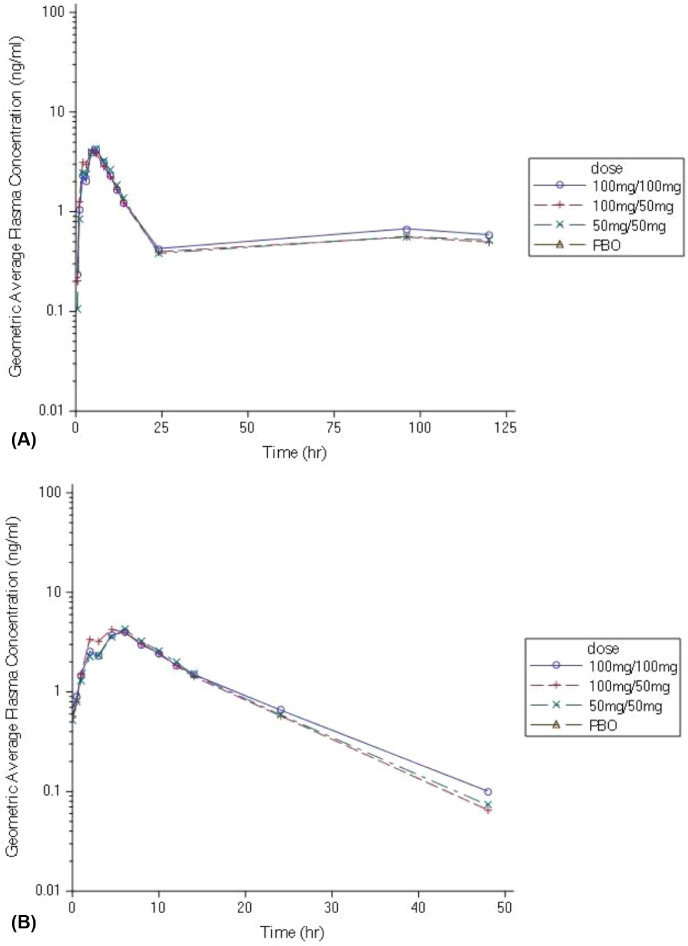
On day 1, PB-201 was well absorbed upon oral administration and reached a peak plasma concentration 5·25, 4·50, 6·00 h after the morning dose, with a geometric mean Cmax of 469, 646, and 905 ng/mL with 50+50, 100+50, and 100+100 mg PB-201, respectively (figure 2 and appendix table S1). It had a wide tissue distribution in the body and was cleared within 24 h of the morning dose.
Figure 2.
Dose-normalised plasma PB-201 concentration–time profiles on days 1 and 7
Geometric mean (coefficient of variation) of dose-normalised plasma concentration of PB-201 versus time curves on day 1 (A) and day 7 (B) on a logarithmic scale.
The steady state of PB-201 was reached on day 4 based on the plateau of the trough plasma concentration after 96 h. On day 7, the absorption rate and maximum exposure of PB-201 were similar to those on day 1 (figure 2 and appendix table S1). It was cleared within 48 h on day 7. No apparent accumulation in the body was observed after multiple dosing (Rac,max and Rac,AUC0–inf around 1 with three dose levels).
The systemic exposure to the main metabolite of PB-201, WI-0800 [20], was similar to the parent drug on day 1 and higher than it on day 7 (appendix figure S1 and table S2).
Systemic exposure to PB-201 and its metabolite showed dose proportionality, with either completely or strongly linear kinetics (90% CI of the coefficient β included 1 for Cmax, AUC0–last, and AUC0–inf, table 3). No differences were found between males and females with regards to key pharmacokinetic parameters of PB-201 and WI-0800 (similar Cmax, AUC0–last, and AUC0–inf).
Table 3.
Linear regression analysis of dose-proportionality for PB-201 and WI-0800
| β point estimate | 90% CI of β | Predefined criterion of linearity | |
|---|---|---|---|
| Day 1, PB-201 | |||
| Cmax | 1·04 | 0·714–1·500 | 0·678–1·320 |
| AUC0–last | 1·12 | 0·790–1·590 | 0·678–1·320 |
| AUC0–inf | 1·12 | 0·781–1·590 | 0·678–1·320 |
| Day 1, WI-0800 | |||
| Cmax | 1·27 | 0·886–1·810 | 0·678–1·320 |
| AUC0–last | 1·31 | 0·923–1·860 | 0·678–1·320 |
| Day 7, PB-201 | |||
| Cmax | 1·15 | 0·867–1·530 | 0·678–1·320 |
| AUC0–last | 0·98 | 0·734–1·310 | 0·678–1·320 |
| AUC0–inf | 0·96 | 0·708–1·290 | 0·678–1·320 |
| Day 7, WI-0800 | |||
| Cmax | 1·11 | 0·827–1·490 | 0·678–1·320 |
| AUC0–last | 1·19 | 0·835–1·700 | 0·678–1·320 |
AUC0–inf=area under the concentration–time curve from time zero to infinity. AUC0–last=area under the concentration–time curve from time zero to the last measurable concentration. Cmax=maximum plasma concentration. β=linear correlation coefficient.
On day 7, the LSM change from baseline in FPG was 0·306 mmol/L (90% CI −0·463 to 1·076), −0·738 mmol/L (−1·714 to 0·239), −1·227 mmol/L (−2·033 to −0·421), and −1·473 (−2·307 to −0·640) with placebo, 50+50 mg, 100+50 mg, and 100+100 mg PB-201, respectively (table 4). A more substantial reduction in FPG from baseline was observed with 100+50 and 100+100 mg PB-201 compared with placebo (placebo-adjusted difference in LSM change from baseline, −1·044 mmol/L [90% CI −2·038 to −0·051], −1·533 mmol/L [−2·543 to −0·254], and −1·780 mmol/L [−2·789 to 0·771]; p value, 0·084, 0·014, 0·005 with three dose levels of PB-201, respectively).
Table 4.
Change in FPG and 2-h PPG from baseline in the pharmacodynamic analysis set
| Placebo (n=15) | 50+50 mg PB-201 (n=16) | 100+50 mg PB-201 (n=15) | 100+100 mg PB-201 (n=15) | |
|---|---|---|---|---|
| FPG, mmol/L | ||||
| Baseline, mean (SD) | 9·047 (1·157) | 9·656 (1·283) | 9·207 (1·545) | 9·087 (1·442) |
| Day 7, mean (SD) | 9·353 (1·627) | 8·919 (2·350) | 7·980 (1·032) | 7·613 (1·250) |
| Change from baseline, mean (SD) | 0·306 (1·029) | −0·737 (2·336) | −1·227 (1·833) | −1·473 (0·928) |
| LSM change from baseline (90% CI) | 0·306 (−0·463 to 1·076) | −0·738 (−1·714 to 0·239) | −1·227 (−2·033 to −0·421) | −1·473 (−2·307 to −0·640) |
| Placebo-adjusted difference in LSM change from baseline (90% CI) | NA | −1·044 (−2·038 to −0·051) | −1·533 (−2·543 to −0·254) | −1·780 (−2·789 to 0·771) |
| P value* | NA | 0·084 | 0·014 | 0·005 |
| 2-h PPG, mmol/L | ||||
| Baseline, mean (SD) | 15·773 (2·776) | 16·550 (2·073) | 15·727 (2·519) | 15·780 (2·747) |
| Day 7, mean (SD) | 16·600 (2·450) | 15·681 (4·233) | 14·140 (2·795) | 13·900 (3·155) |
| Change from baseline, mean (SD) | 0·827 (1·390) | −0·869 (3·635) | −1·587 (1·209) | −1·880 (3·131) |
| LSM change from baseline (90% CI) | 0·827 (−0·800 to 2·453) | −0·869 (−2·869 to 1·131) | −1·587 (−3·239 to 0·066) | −1·880 (−3·717 to −0·043) |
| Placebo-adjusted difference in LSM change from baseline (90% CI) | NA | −1·696 (−3·250 to −0·138) | −2·413 (−3·996 to −0·831) | −2·707 (−4·289 to −1·124) |
| P value* | NA | 0·007 | 0·014 | 0·006 |
FPG=fasting plasma glucose. LSM=least-square mean. NA=not applicable. SD=standard deviation. 2-h PPG=2-h postprandial plasma glucose. 90% CI= 90% confidence interval.
Compared with placebo.
The LSM change in 2-h PPG on day 7 from baseline was 0·827 mmol/L (90% CI −0·800 to 2·453) with placebo and −0·869 mmol/L (−2·869 to 1·131), −1·587 mmol/L (−3·239 to 0·066), and −1·880 mmol/L (−3·717 to −0·043) with three dose levels of PB-201, respectively (table 4). PB-201 at three dose levels markedly reduced 2-h PPG from baseline compared with placebo (placebo-adjusted difference in LSM change from baseline, −1·696 mmol/L [90% CI −3·250 to −0·138], −2·413 mmol/L [−3·996 to −0·831], and −2·707 mmol/L [−4·289 to −1·124]; p value, 0·007, 0·014, and 0·006, respectively). A dose-dependent reduction in FPG and 2-h PPG with PB-201 was observed. No differences were observed in the change in plasma levels of C-peptide and insulin at either fasting or postprandial state between placebo and any dose levels of PB-201 (appendix table S3).
Based on readings of CGM, daily blood glucose dynamics took a lower trajectory with increasing doses of PB-201 compared with placebo (figure 3A). Mean glucose was 10·680 mmol/L (SD 1·990) with placebo and 10·820 mmol/L (2·670), 10·020 mmol/L (2·010), and 8·700 mmol/L (2·140) with three dose levels of PB-201. TIR increased as the dose of PB-201 increased, and TIRs in those given 100+50 and 100+100 mg were higher than that with placebo (mean TIR, 49·380% [SD 27], 49·210% [27], 56·130% [25], and 63·330% [20] with placebo and the three dose levels of PB-201, respectively; figure 3B). Only 0·310%, 0, 0·070%, and 4·600% of time on average respectively were found to be TBR (<3·9 mmol/L), indicating low frequencies of hypoglycaemia with both placebo and PB-201; the corresponding percentage of TBR (<3·0 mmol/L) was 0 with all four regimens. TBR (<3·9 mmol/L) during the night was not detected. Baseline HbA1c was 8.931%, 8.979%, 8.993%, and 8.993% for placebo and three dose levels of PB-201, respectively, and mean estimated HbA1c after treatment was 8·350% (68 mmol/mol) with placebo, and 8·435% (69 mmol/mol), 7·930% (63 mmol/mol), and 7·105% (54 mmol/mol) with the three dose levels of PB-201. The reduction in estimated HbA1c from baseline was dose-dependent and numerically greater in patients treated with 100+50 mg and 100+100 mg PB-201 compared with placebo (−0·581% [SD 1·200] or −6·4 mmol/mol [13·1], −0·5445% [1·654] or −5·9 mmol/mol [18·1], −1·063% [1·236] or −11·6 mmol/mol [13·5], and −1·888% [1·381] or −20·6 mmol/mol [15·1] with placebo, 50+50 mg, 100+50 mg, and 100+100 mg PB-201, respectively).
Figure 3.
CGM profiles of patients on placebo or PB-201
(A) Daily blood glucose dynamics measured by CGM. Solid lines represent mean values of glucose, and dotted lines represent the first and third quantiles. (B) Percentages of TIR, TBR, and TAR. CGM=continuous glucose monitoring. TAR=time above range. TBR=time below range. TIR=time in range.
Thirty-four TEAEs were reported by 15 patients (93·8%) during this study, including 11 (32·4%), 5 (14·7%), 6 (17·7%), and 12 (35·3%) patients treated with placebo, 50+50 mg, 100+50 mg, and 100+100 mg PB-201, respectively (table 5). All TEAEs were considered mild (grade 1), and no serious TEAEs or death occurred. One TEAE (6·3%) led to treatment discontinuation, which was predefined repeated hyperglycaemia occurring after treatment with 50+50 mg PB-201. There were no TEAEs in terms of vital signs or ECG. All TEAEs occurred in no more than two patients with either placebo or any PB-201 dose levels.
Table 5.
Incidence of treatment-emergent adverse events in the safety analysis set
| Placebo (n=15) | 50+50 mg PB-201 (n=16) | 100+50 mg PB-201 (n=15) | 100+100 mg PB-201 (n=15) | |
|---|---|---|---|---|
| Any TEAEs | 11 (32·4) | 5 (14·7) | 6 (17·7) | 12 (35·3) |
| Related to study drug* | 0 | 2 (12·5) | 0 | 0 |
| Gastrointestinal disorders | ||||
| Constipation | 2 (13·3) | 1 (6·3) | 2 (13·3) | 0 |
| Diarrhoea | 1 (6·7) | 0 | 0 | 1 (6·7) |
| Mouth ulcers | 0 | 0 | 0 | 1 (6·7) |
| Toothache | 1 (6·7) | 0 | 1 (6·7) | 0 |
| Injury, poisoning, and procedural complications | ||||
| Fall | 0 | 0 | 1 (6·7) | 0 |
| Bruising | 0 | 1 (6·3) | 0 | 0 |
| Laboratory tests | ||||
| Bacteraemia | 0 | 0 | 0 | 1 (6·7) |
| Haematuria | 0 | 0 | 0 | 2 (13·3) |
| Glomerular filtration rate decreased | 0 | 0 | 1 (6·7) | 1 (6·7) |
| HDL decreased | 0 | 0 | 0 | 1 (6·7) |
| Haemoglobinuria | 0 | 0 | 0 | 2 (13·3) |
| Urine ketone positive | 0 | 0 | 0 | 2 (13·3) |
| Urine WBC positive | 2 (13·3) | 0 | 0 | 0 |
| Metabolism and nutrition disorders | ||||
| Hyperglycaemia† | 0 | 1 (6·3) | 0 | 0 |
| Hypertriglyceridemia | 0 | 0 | 1 (6·7) | 1 (6·7) |
| Relative hypoglycaemia‡ | 0 | 2 (12·5) | 0 | 0 |
| Hypokalaemia | 2 (13·3) | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Pain in extremity | 1 (6·7) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Hyperhidrosis | 1 (6·7) | 0 | 0 | 0 |
| Vascular disorders | ||||
| Hypertension | 1 (6·7) | 0 | 0 | 0 |
HDL=high-density lipoprotein. TEAE=treatment-emergent adverse event. WBC=white blood cell.
These two events were considered definitely related to the study drug. †Hyperglycaemia was defined by fasting plasma glucose ≥13·3 mmol/L measured over 3 consecutive days. ‡Hypoglycaemia was defined in accord with guidelines for clinical trials of antidiabetic agent and biologics released by the Center for Drug Evaluation of China National Medical Products Administration, and relative hypoglycaemia was defined by having classic hypoglycaemia symptoms and glucose reduced but >3·9 mmol/L .
Among the TEAEs, only two events were considered related to the study drug—both episodes of symptom-based relative hypoglycaemia (presenting with symptoms and reduced blood glucose at a level of >3.9 mmol/l) in two patients with 50+50 mg PB-201, with a blood glucose of 6·4 and 6·3 mmol/L, respectively. Both cases were resolved with oral glucose or a meal.
4. Discussion
Based on the dose-dependent improvements in FPG, PPG, TIR, and estimated HbA1c from baseline with PB-201 and the low rate and mild severity of TEAEs at all dose levels, 100 mg twice daily was identified as the optimal dose. This dosage regimen might induce a more robust glucose-lowering effect compared with that achieved with 100 mg once daily in previous phase 2 trials (placebo-adjusted change in estimated HbA1c from baseline −1·307% [−14·3 mmol/mol] vs that measured with 100 mg once daily −0·45% [−4·9 mmol/mol] or −0·47% [−5·1 mmol/mol] [10]) while being without clinically significant safety issues. Safety concerns with other GKAs, in particular, hypoglycaemia that meets the American Diabetes Association (ADA) criterion of a venous/capillary glucose of <3·9 mmol/L [1], were not noted. Multiple dosing of PB-201 did not result in drug accumulation in the body, as opposed to globalagliatin, which showed an accumulation ratio of >2 [9]. PB-201 presented a favourable pharmacokinetic and safety profile in Chinese patients with type 2 diabetes, consistent with that found in non-Chinese populations. Based on the totality of evidence, it is worthwhile to confirm the efficacy and safety of 100 mg twice-daily PB-201 in future studies.
One principal goal of this study was dose-finding, because the maximal dose used in previous phase 2 trials (100 mg once daily), though well tolerated, resulted in only modest glucose-lowering effects [10]. Dose selection in this study was also based on previous findings that 300 mg once-daily PB-201 was the maximal tolerated dose (unpublished data) and that fewer adverse events were reported with split doses than with once-daily dosing [11]. PB-201 at 100 mg twice daily demonstrated a potential of better glucose-lowering effects; meanwhile, PB-201 given at this dosing regimen was safe and well tolerated.
The design of this study has three features: (1) drug-naive patients, instead of those with poorly controlled type 2 diabetes on metformin, were enrolled after consulting with China CDE in order to reveal the genuine safety, and pharmacokinetic/pharmacodynamic characteristics of PB-201; (2) one strength of the study was the four-period, crossover design, which improved the precision for assessing change from baseline in glycaemic parameters and reduced potential confounding compared with parallel-group design because patients served as their own control; (3) CGM was utilised, which provided rigorous evidence on safety with regard to hypoglycaemia (especially asymptomatic hypoglycaemia) and the effect of the study drug on 24-h glucose profiles synthesised from 7 days of CGM data.
Based on existing evidence, the alarming side effects with previous GKA candidates—eg, hypoglycaemia [12,13], hyperlipidaemia [12,13], and impairment of liver function [14]—are unlikely to be of concern with PB-201. The mild severity and low incidences of TEAEs with PB-201 in this study were consistent with previous findings [10,11]. In two phase 2 clinical trials comparing PB-201, placebo, and active comparators as an add-on to metformin in patients with type 2 diabetes, hypoglycaemia occurred in 3·0% (12/405) of patients with PB-201, 34·4% (21/61) with glimepiride, 1·8% (1/55) with sitagliptin, and 2·5% (3/118) with placebo; all resolved with oral carbohydrates [10]. In comparison, hypoglycaemia was reported at an incidence rate ranging from 0·6% (1/169) to 33·8% (68/201) with other GKAs [27]. In a phase 2 trial in Chinese patients with type 2 diabetes, hypoglycaemia was reported in 5·4% (11/205) of patients with dorzagliatin [8]. However, in this study of PB-201, only two (12·5%) patients reported symptom-based relative hypoglycaemia, and no episodes were confirmed to be with a venous/capillary glucose of <3·9 mmol/L, which was used to define hypoglycaemia by the ADA guideline [1]. The low risk of hypoglycaemia was further supported by evidence from CGM, which also eliminated the concern over nocturnal hypoglycaemia. It has been reported that FreeStyle Libre Pro Flash CGM System tends to overestimate the risk of hypoglycaemia as compared with point-of-care capillary blood glucose testing [28]. The fact that no hypoglycaemia events were detected by CGM in this study provides further evidence that PB-201 treatment was not associated with risk of hypoglycaemia. Although the short treatment duration, small number of patients, and inclusion of treatment-naïve patients might have contributed to the decreased risk of hypoglycaemia for PB-201 in this study, safety findings were similar in phase 2 trials involving previously treated patients [10].
Standard CGM metrics are endorsed by the ADA as a complement to HbA1c for evaluating glycaemic control [22]. PB-201 dose-dependently improved CGM metrics, including mean glucose, TIR, TAR, and estimated HbA1c. The marked reduction in estimated HbA1c with higher doses of PB-201 predicted the long-term glucose-lowering effect of PB-201, which could be even larger after prolonged treatment. With 100 mg twice-daily PB-201, mean TIR was 63·330%, corresponding well with an estimated HbA1c of 7·105% [54 mmol/mol] [29,30]. Although caution should be taken when making direct comparisons, the glycaemic effect with 100 mg twice-daily PB-201 (placebo-adjusted change from baseline in estimated HbA1c, −1·307% [−14·3 mmol/mol]) can be inferred to be greater than those shown in previous phase 2 trials with 100 mg once daily (placebo-adjusted LSM change from baseline in HbA1c, −0·45% [−4·9 mmol/mol] or −0·47% [−5·1 mmol/mol]) [10] and possibly comparable to or larger than that with other GKAs (placebo-adjusted LSM change from baseline in HbA1c ranging from −0·04% [−0·4 mmol/mol] to −0·9% [−9·8 mmol/mol] with dorzagliatin [8], TTP399 [7], and AZD1656 [31]); this hypothesis will need to be tested in studies with larger sample sizes and longer durations.
The daily blood glucose curve with PB-201 had a similar shape but shifted downwards compared with placebo, indicating that PB-201 may have an impact on fasting glucose rather than postprandial glucose. This trajectory is in contrast with the flattened curve under treatment with mitiglinide/voglibose, which lowered glucose fluctuations postmeal [32]. The mechanism of action behind this may be PB-201’s constant activation of glucokinase in the liver. Functional glucokinase in the liver regulates glucose metabolism in a glucose-dependent manner: at low glucose concentration (<10 nM), glucokinase binds to its inhibitor glucokinase regulatory protein (GKBP) and remains inactive; when glucose concentration increases, glucokinase dissociates form GKBP and becomes activated, promoting hepatic glucose utilisation while inhibiting hepatic glucose release. The durable effect throughout 24 hours shown in the daily blood glucose curve suggests that the biological half-life is longer than the pharmacological half-life. Blood glucose reached the trough in late afternoon before dinner. These observations give us clues that prolonging the dosing interval (ie, one dose before breakfast and the other before dinner) or administering once daily/weekly with slow releasing preparations at a higher dose might provide more stable glycaemic control and reduce the risk of hypoglycaemia, which will be considered in future studies. Since the number of daily does is inversely related to compliance [33], once-daily/weekly dosing may improve patient compliance compared with twice-daily dosing.
This study has several limitations. First, the treatment duration for each period was only 7 days, determined based on the previous finding that this was the shortest duration to observe a stable glycaemic effect (unpublished data), but the estimated HbA1c based on CGM data offered hint on long-term effects. Second, HbA1c was estimated rather than measured given the short duration of treatment. Third, drug administration and evaluation were carried out in an inpatient setting, which differs from ambulatory care of diabetes but ensures compliance and data collection accuracy. Fourth, patients were asked to follow a standardised lifestyle programme during the study, which controlled the influence of diet and lifestyle. Fifth, we did not use a William design, and escalating doses of PB-201 were administered sequentially; therefore, the crossover design was not balanced for first-order carryover effects.
In conclusion, increasing the dose of PB-201 to 100 mg twice daily is safe and well tolerated and potentially provides better glycaemic control than doses tested in previous phase 2 trials. This dosage regimen is also supported by the favourable pharmacokinetic profile of PB-201 in Chinese patients, which is consistent with that in non-Chinese populations. PB-201 at 100 mg twice daily will be selected for confirmatory trials to further investigate its efficacy and safety.
5. Contributors
XY was responsible for the acquisition, analysis, or interpretation of data. CC was responsible for the statistical analysis. All authors contributed to the study concept, design, and supervision; interpretation of the data; and preparation of the manuscript. All authors approved the final version. All authors verified the underlying data.
6. Data sharing
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymised, and study documents will be redacted to protect the privacy of our trial participants. Further details on PegBio's data sharing criteria, eligible studies, and process for requesting access can be found at: http://www.clinicalstudydatarequest.com/
Supplementary material
Inclusion/exclusion criteria
Nutritional composition of standard meal
Pharmacokinetic analyses
Figure S1: Dose-normalised plasma WI-0800 concentration–time profiles on Day 1 and Day 7.
Geometric mean (coefficient of variation) of dose-normalised plasma concentration of WI-0800 versus time curves on day 1 (A) and day 7 (B) on a logarithmic scale
Table S1: Summary of plasma PB-201 pharmacokinetic parameters
Table S2: Summary of plasma WI-0800 pharmacokinetic parameters
Table S3: Change in C-peptide and insulin from baseline in the pharmacodynamic analysis set
Declaration of Competing Interest
YD and MX are employees of PegBio, and HZ is an ex-employee of PegBio. LJ has received fees for lecture presentations from AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis and Takeda; consulting fees from AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis and Takeda; and grants/research support from AstraZeneca, Bristol-Myers Squibb, Merck, Novartis and Sanofi-Aventis. All other authors declare no competing interests.
Acknowledgments
Funding
This work was funded by PegBio Co., Ltd.
Acknowledgements
The authors thank Lei Wang and Ke Ding of PegBio for medical writing assistance.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101185.
Contributor Information
Haiyan Li, Email: haiyanli1027@hotmail.com.
Linong Ji, Email: jiln@bjmu.edu.cn.
Appendix. Supplementary materials
References
- 1.American Diabetes Association. 6 Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 3.Ji LN, Lu JM, Guo XH, et al. Glycemic control among patients in China with type 2 diabetes mellitus receiving oral drugs or injectables. BMC Public Health. 2013;13:602. doi: 10.1186/1471-2458-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care. 2010;33(3):501–506. doi: 10.2337/dc09-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front Physiol. 2019;10:148. doi: 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein KR, Freeman JLR, Dunn I, et al. The SimpliciT1 Study: a randomized, double-blind, placebo-controlled phase 1b/2 adaptive study of TTP399, a hepatoselective glucokinase activator, for adjunctive treatment of type 1 diabetes. Diabetes Care. 2021;44(4):960–968. doi: 10.2337/dc20-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vella A, Freeman JLR, Dunn I, Keller K, Buse JB, Valcarce C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci Transl Med. 2019;11(475):eaau3441. doi: 10.1126/scitranslmed.aau3441. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D, Gan S, Liu Y, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6(8):627–636. doi: 10.1016/S2213-8587(18)30105-0. [DOI] [PubMed] [Google Scholar]
- 9.Zheng S, Shao F, Ding Y, et al. Safety, pharmacokinetics, and pharmacodynamics of globalagliatin, a glucokinase activator, in Chinese patients with type 2 diabetes mellitus: a randomized, phase Ib, 28-day ascending dose study. Clin Drug Investig. 2020;40(12):1155–1166. doi: 10.1007/s40261-020-00971-x. [DOI] [PubMed] [Google Scholar]
- 10.Amin NB, Aggarwal N, Pall D, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab. 2015;17(8):751–759. doi: 10.1111/dom.12474. [DOI] [PubMed] [Google Scholar]
- 11.Denney WS, Denham DS, Riggs MR, Amin NB. Glycemic effect and safety of a systemic, partial glucokinase activator, PF-04937319, in patients with type 2 diabetes mellitus inadequately controlled on metformin-a randomized, crossover, active-controlled study. Clin Pharmacol Drug Dev. 2016;5(6):517–527. doi: 10.1002/cpdd.261. [DOI] [PubMed] [Google Scholar]
- 12.Katz L, Manamley N, Snyder WJ, et al. AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(2):191–195. doi: 10.1111/dom.12586. [DOI] [PubMed] [Google Scholar]
- 13.Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(12):2560–2566. doi: 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Ceuninck F, Kargar C, Ilic C, et al. Small molecule glucokinase activators disturb lipid homeostasis and induce fatty liver in rodents: a warning for therapeutic applications in humans. Br J Pharmacol. 2013;168(2):339–353. doi: 10.1111/j.1476-5381.2012.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilding JP, Leonsson-Zachrisson M, Wessman C, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 in patients with type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2013;15(8):750–759. doi: 10.1111/dom.12088. [DOI] [PubMed] [Google Scholar]
- 16.Pfefferkorn JA, Guzman-Perez A, Oates PJ, et al. Designing glucokinase activators with reduced hypoglycemia risk: discovery of N,N-dimethyl-5-(2-methyl-6-(5-methylpyrazin-2-yl)-carbamoyl)benzofuran-4-yloxy)pyrimidine-2-carboxamide as a clinical candidate for the treatment of type 2 diabetes mellitus. MedChemComm. 2011;2(9):828–839. [Google Scholar]
- 17.Borzilleri KA, Pfefferkorn JA, Guzman-Perez A, et al. Optimizing glucokinase activator binding kinetics to lower in vivo hypoglycemia risk. MedChemComm. 2014;5(6):802–807. [Google Scholar]
- 18.World Health Organization . World Health Organization; Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. [Google Scholar]
- 19.Society CD. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition) Chinese Journal of Practical Internal Medicine. 2018;38(4):292–344. [Google Scholar]
- 20.Sharma R, Litchfield J, Atkinson K, et al. Metabolites in safety testing assessment in early clinical development: a case study with a glucokinase activator. Drug Metab Dispos. 2014;42(11):1926–1939. doi: 10.1124/dmd.114.060087. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines for clinical trials of anti-diabetic agents and biologics. https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/fgwj/gzwj/gzwjyp/20120515120001975.html (accessed 24 December 2020).
- 22.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayed A, Alyafei F, De Sanctis V, Soliman A, Elgamal M. Translating the HbA1c assay into estimated average glucose values in children and adolescents with type 1 diabetes mellitus. Acta Biomed. 2018;89(S5):22–26. doi: 10.23750/abm.v89i5.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A Multicenter Evaluation of the Performance and Usability of a Novel Glucose Monitoring System in Chinese Adults With Diabetes. J Diabetes Sci Technol. 2017;11(2):290–295. doi: 10.1177/1932296816662884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidelines for clinical pharmacokinetic studies. Center for Drug Evaluation of China National Medical Products Administration. http://www.cde.org.cn/zdyz.do?method=largePage&id=8b461127bccfdd5e (accessed 24 December 2020).
- 26.Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–1283. doi: 10.1023/a:1026451721686. [DOI] [PubMed] [Google Scholar]
- 27.Diao H, Yu X, Li C, Guo Y, Shen B, Zhao W. The effects and safety of activators of glucokinase versus placebo in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Endocr J. 2021;68(2):189–194. doi: 10.1507/endocrj.EJ20-0286. [DOI] [PubMed] [Google Scholar]
- 28.Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro Flash Continuous Glucose Monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43(11):2730–2735. doi: 10.2337/dc19-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 31.Kiyosue A, Hayashi N, Komori H, Leonsson-Zachrisson M, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(10):923–930. doi: 10.1111/dom.12100. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K, Shibayama Y, Yamaguchi E, Honjo S, Hamasaki A, Hamamoto Y. Glucose excursions and hypoglycemia in patients with type 2 diabetes treated with mitiglinide/voglibose versus glimepiride: a randomized cross-over trial. J Diabetes. 2018;10(8):675–682. doi: 10.1111/1753-0407.12658. [DOI] [PubMed] [Google Scholar]
- 33.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.