Abstract
There are over 29,000 children and young people (CYP) with Type 1 diabetes mellitus (T1DM) in England and Wales and another 726 with Type 2 diabetes mellitus (T2DM). There is little effect of deprivation on the prevalence of T1DM whereas the association of deprivation on the percentage of CYP with T2DM is striking with 45% of cases drawn from the most deprived backgrounds. A number that has not changed over the last 4 years. Data from the UK and USA as well as other countries demonstrate the impact of deprivation on outcomes in diabetes mellitus with clear effects on measures of long-term control and complications. In the UK black CYP had higher glycosylated haemoglobin (HbA1c) values compared to other groups. Within the black group, CYP from a Caribbean background had a higher mean HbA1c (77.0 mmol/mol (9.2%)) than those from Africa (70.4 mmol/mol (8.6%)). Treatment regimen (multiple daily injections or insulin pump therapy) explained the largest proportion of the variability in HbA1c followed by deprivation. Those in the least deprived areas had an average HbA1c 5.88 mmol/mol (0.5%) lower than those living in the most deprived areas. The picture is complex as UK data also show that deprivation and ethnicity is associated with less use of technology that is likely to improve diabetes control. Increased usage of pump therapy and continuous glucose monitoring was associated with a younger age of patient (less than 10 years of age), living in the least deprived areas and white ethnicity. This gap between pump usage amongst CYP with T1DM living in the most and least deprived areas has widened with time. In 2014/15 the gap was 7.9% and by 2018/19 had increased to 13.5%. To attain an equitable service for CYP with diabetes mellitus we need to consider interventions at the patient, health care professional, community, and health care system levels.
Keywords: Deprivation, Ethnicity, Type 1 diabetes mellitus, Type 2 diabetes mellitus
1. Introduction
Inequalities in health and health care provision in childhood can have lasting effects in adulthood. In paediatric diabetes ethnicity and deprivation impact on health outcomes such as access to technologies that facilitate diabetes care and diabetes control. Health care professionals dealing with paediatric diabetes need to be aware of how they approach care provision in ethnic groups and in those from more deprived backgrounds.
Diabetes Mellitus is a chronic health problem. Type 1 Diabetes Mellitus (T1DM) results from a lack of insulin production by the pancreatic beta cells of the Islets of Langerhans whereas Type 2 Diabetes Mellitus (T2DM) arises from a reduced beta cell mass along with a reduced responsivity to insulin usually associated with obesity [1].
Type 1 Diabetes Mellitus in both children and adults requires continuous monitoring/input, involves a variety of health and non-health care providers in a variety of settings and a high level of patient/parent involvement. Both T1DM and T2DM require considerable behavioural change. The consequences of deferred immediate motivation in self-care in T2DM are lower than in T1DM where the consequences are more immediate in terms of metabolic decompensation and the development of Diabetic Ketoacidosis.
As medical science and technology have advanced at pace, health care delivery systems have struggled to provide consistent high-quality care. There is a shortfall between knowledge acquisition and translation into safe practice. The Institute of Medicine (now the National Academy of Medicine) recognised in its seminal 2001 report “Crossing the Quality Chasm” [2] that healthcare has safety and quality problems because the system that is utilised is outmoded.
The medical model was largely based on acute care, which utilised a linear approach to consultation with diagnosis, treatment and outcome. This approach does not work well for chronic conditions, such as Diabetes Mellitus, which require long term monitoring and input from healthcare and non-healthcare providers in a variety of settings such as home, work, further education, schools and leisure facilities. Central to the development of a high-quality health system “Crossing the Quality Chasm” identified six areas to be addressed: safety, timeliness, effectiveness and efficient services that are patient centred and equitable.
Equity operates at the level of the population and at the level of the individual. At the population level, a health system should improve health status in a way that reduces health disparities among subgroups. Primarily, this is about access to health care which varies considerably between countries depending on their finance model and this issue of health insurance in whatever form, private or public, is directly associated with increased morbidity and mortality [3,4]. At the individual level, the availability of care and quality of services should be based on individuals needs not on personal characteristics. Characteristics such as age, gender, ethnicity, income, education, disability, sexual orientation or simply where they live should not matter in the provision of quality care [4], [5], [6], [7].
In this review article we look at equitable care provision in the way that children and young people, (CYP), aged between 0 and 19 years, with diabetes are managed in the United Kingdom and what effects ethnicity and disparities in socioeconomic standing have on health related outcomes in these individuals. In particular, we will focus on outcomes of treatments such as glycosylated haemoglobin (HbA1c) and, because this outcome is dependent on the means of controlling blood glucose, we will also address access to technologies that are known to improve glycaemic control. The literature on this topic is broad and for the purposes of this review we have focussed predominantly on UK/USA literature.
2. Ethnicity and socioeconomic status
Ethnicity refers to social groupings that are based on some combination of shared language, history, religion and culture. Race refers to the person's identification with a group or identity ascribed on the basis of physical characteristics and skin colour [8]. Ethnic groups often overlap with racial groups in such things as shared historical experience. At the same time, we need to be mindful that race is also directly associated with genetic ancestry, genetic data which estimates the geographic origins of a person's recent ancestors, and indirectly to genetic variants that may affect disease and health outcomes.
Ethnic disparities in healthcare have been widely reported [9], [10], [11]. Intertwined with these observations is the impact of deprivation, which may explain better the observed patterns of ethnic disparities and health status [12,13]. Disentangling race/ethnicity from socioeconomic status is difficult because they are often concurrent. Deprivation per se covers a broad range of issues and refers to unmet needs caused by a lack of resources of all kinds, not just financial. The English Indices of Deprivation 2019 [14]. Attempt to measure a broader concept of multiple deprivation, made up of several distinct domains of deprivation. Each domain represents a specific form of deprivation experienced by people and each can be measured individually using a number of indicators. The domains identified include income, employment, health and disability, education skills and training, barriers to housing and services, environment and crime. This approach covers a number of the social determinants of health yielding a more comprehensive assessment of the situation faced by individuals and communities and one that we utilised in our work on deprivation and T1DM in CYP [15].
3. Epidemiology of types 1 and 2 diabetes mellitus
T1DM in children and young people occurs in individuals with genetic susceptibility who subsequently have a second ‘hit’, often assumed to be viral, which triggers an autoimmune process with progressive immune-mediated destruction of the beta cells in the pancreatic islets. Beta cell loss over 90% leads to sustained hyperglycaemia which causes polydipsia, polyuria and weight loss, leading to the diagnosis. Treatment is with exogenous insulin, which needs to be given to provide background insulin throughout the 24 h period along with bolus injections of short acting insulin to cope with the carbohydrate content of food and drink as well as correction bolus injections to ensure blood glucose is maintained within target ranges. This therapy can be delivered with multiple daily injections of short and long-acting insulins or with insulin pump technology.
In contrast, the traditional view of T2DM is of a primary problem in insulin action, which over time leads to beta cell decompensation from persistent hyperglycaemia and hyperlipidaemia [16]. There is probably also a component of beta cell dysfunction coupled with an early loss of beta cell mass or failure to form adequate number of beta cells in earlier development that compromises insulin production so that it loses its natural pulsatility [17]. To overcome this more insulin is needed, and increased production in turn results in peripheral insulin insensitivity in liver and muscle cells but less so in adipose tissue. This would then lead to an increase in adipose mass and further insulin insensitivity, establishing a vicious circle leading to long term beta cell “stress”. Treatment is with diet, exercise, oral hypoglycaemic agents such as metformin and injection therapies with GLP-1 and DPP-4 modalities. Despite the use of a variety of agents, insulin therapy is usually required to control blood glucose concentrations.
3.1. Incidence
The incidence of T1DM is 24.5 per 100,000 children aged 0–15 years in the UK [18] but the worldwide incidence of T1DM varies by at least 100-fold, being highest in Finland and Sardinia (Italy) and lowest in Venezuela and China [19]. The incidence of T1DM has been increasing worldwide at an annual rate of approximately 3–5% [20], [21], [22], [23], [24]. There is also an increasing incidence over the last 30 years of T2DM worldwide [25], [26], [27]. The underlying reasons for this overall increase in CYP diabetes is not well understood and we will examine some of the genetic, ethnic, environmental and socioeconomic factors that may interplay and contribute to this phenomenon.
3.2. Genetics
It is generally accepted that some individuals may have a genetic predisposition to diabetes. In T1DM, the major genetic contribution comes from loci within the human leukocyte antigen (HLA) complex, in particular HLA class II. In T2DM approximately 143 susceptibility genes or variants have been identified to date, to be associated with T2DM but all the genetic loci identified so far account for only about 10% of the overall heritability of T2DM [28]. Other loci must be involved given the strong hereditary component to the disease and differences in the prevalence of T2DM [29]. The majority of studies have been conducted in the white population and where different ethnic groups have been studied the sample sizes are too small to be able to draw firm conclusions. However, the rate of increase of T1DM and T2DM globally is too quick to be the result of increased gene frequency and/or altered gene pool, which leads to consideration of environmental factors.
The interaction between environment and genetics is seen in the field of epigenetics. Epigenetic changes may also be involved in development of T1DM and T2DM and there is some evidence that epigenetic changes may be involved in the intergenerational risks associated with obesity and T2DM [30,31].
3.3. Geographical variation
Genetic factors might explain some of the geographic variability in the occurrence of T1DM. For instance, migration from a small area of the United Kingdom to New Zealand may be one factor that has led to a higher incidence of T1DM in the New Zealand population of European decent compared to the population who identify as Maori [32].
The rapid increase in frequency of T1 and T2DM varies between countries. Generally, countries that have the lowest incidence rates have the highest increases [33]. These increases in incidence may have peaked in those countries with traditionally high incidences such as Finland, although this is mainly confined to the younger age group and requires further evaluation with a longer time frame for data collection to establish a trend [23]. The European data of conversion from low to high incidence is centred on Central and Eastern European countries (Poland 5.2/105 person years in 1989–93 to 20.1 /105 person years in 2009–13; Lithuania 7.3/105 person years in 1989–93 to 19.9 /105 person years 2009–13) who have undergone the largest shift in economic make up [23].
Comparisons between countries and regions with low and high-incidence rates have suggested that higher socioeconomic status and degree of urbanization are among the environmental factors that play a role in the rising incidence of T1DM. Of the many factors that may influence the risk of T1DM, high birth weight, accelerated early growth, low exposure to pathogens and dietary factors such as breastfeeding, all might plausibly be explained in terms of higher socioeconomic standing [34].
Studies from Northern Ireland and Scotland showed that the incidence of T1DM was lowest in areas with the highest deprivation (for Northern Ireland the standardised incidence ratio was 83 in the most deprived areas compared to 118 in the least deprived areas) [35,36], a finding replicated in Australia [37] and other countries [38,39]. The role of socioeconomic factors is the best explanation of the difference seen between rural and urban areas, but this will depend on the economic gradient between such areas as well as the population density which may well vary in different countries. There remains a difficulty with many of these studies in that place of residence at diagnosis may differ from place of residence when the individual was exposed to the putative environmental factor(s). Further, there is considerable variation in what is defined as rural and urban even in the same country [39,40].
Geographical environment may also contribute to the prevalence of T2DM. Because the cause of T1DM includes the second “hit” the geographical component will differ from that in T2DM. In T1DM social contact will be a determinant of whether the person comes in contact with the viral second “hit.” In T2DM the geographical environment will more reflect change from rural subsistence farming to more easily accessible cheaper highly refined carbohydrate in the urban setting. The prevalence of T2DM in Asian Indians living in rural India is considerably lower than Asian Indians living in the UK or USA. The prevalence of T2DM in Black Africans is 12 times lower than the prevalence of the same condition in African Americans [41].
These environmental differences are likely to be heavily influenced by socioeconomic factors, living conditions and poverty. These are all intertwined and have been explored further by Rosling [42]. We can understand the effect of diet on a daily income of $1 on T2DM in that lack of access to refined carbohydrate in the rural subsistence farming case might be viewed as protective. A glucose toxic environment is avoided. A step up to the next income level ($4 per day) although seemingly an improvement, the minimal increase means that the food purchased is of the refined carbohydrate type. It is only when a certain income is generated of $16 per day that varied foodstuffs can be purchased and stored safely that less reliance would be placed on the cheaper refined carbohydrate commodities [42].
Consideration also has to be given to other confounding factors. For example, the low incidence rates in sub-Saharan Africa may reflect higher mortality from other conditions such as infectious diseases before the age is reached where a diagnosis of T1DM would be made.
3.4. Deprivation
The National Paediatric Diabetes Audit (NPDA) provides a yearly account of care and outcomes for children and young people (CYP) in England and Wales [18]. The main strength is 100% submissions from all units managing paediatric diabetes and a population size of approximately 29,000 CYP aged between 0 and 19 years. This large comprehensive sample size allows for categorising into deprivation status as well as ethnicity overcoming the limitations of size present in many of the studies cited above. It also has the advantage that there is free and easy access to care within a single provider – the National Health Service, and service delivery which aims to harmonise care through the Best Practice Tariff [43].
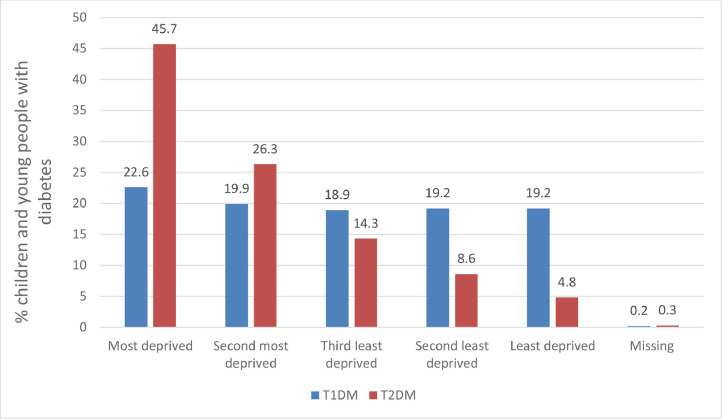
The effect of deprivation on the percentage of CYP with T1DM and T2DM are shown in Fig. 1, which is derived from the NPDA data. These NPDA data have been consistent on the impact of deprivation for each audit since 2015. In contrast to data that has suggested a preponderance of T1DM in higher socioeconomic groups, there is little effect of deprivation with a gradient if anything towards a higher percentage (22.6%) in the most deprived cohort compared to the least deprived (19.2%). Unlike T1DM, the association of deprivation on the percentage of CYP with T2DM is striking. There is an over representation of deprivation with 45% of cases drawn from the most deprived backgrounds (Fig. 1), a number that has not changed over the last 4 years [18,44].
Fig. 1.
Percentage of children and young people with T1DM and T2DM out of the total with diabetes in England and Wales over the period 2018 to 2019 according to level of deprivation.
Data adapted from the 2018/19 National Paediatric Diabetes Audit [18]
This may reflect the effects of ongoing income inequality in England and Wales. By 2020, the income of the richest 20% of people was over six times higher than the poorest 20%, while the richest 10% received 50% more income than the poorest 40%. Income inequality, has been high, at 34.6%, but broadly stable over the past ten years after a step increase in 1989 [45]. Household poverty and the implications for food choices (cheaper highly refined carbohydrate based foods) remains a major barrier to the community health proposals to tackle T2DM and rise in UK childhood obesity.
The adverse effect of obesity on glucose metabolism and potential development of T2DM is evident early in childhood. Obese children are hyperinsulinemic and have approximately 40% lower insulin-stimulated glucose metabolism compared with non-obese children [46]. Furthermore, the inverse relationship between insulin sensitivity and abdominal fat is stronger for visceral than for subcutaneous fat [46,47].
The likelihood of developing T2DM increases with body mass index but the level of risk associated with a given body mass index differs in ethnic populations. For example in a review from North America, in adults, a 10% incidence of developing T2DM per 1000 patient years is associated with a body mass index of 30 kg/m2 in whites, 26 kg/m2 in blacks, 25 kg/m2 in Chinese and 24 kg/m2 in South Asians [48] and sex also modifies the relationship [49]. Racial differences in insulin sensitivity are also evident in childhood. African-American 7- to 11-year-old children have significantly higher insulin levels than age-matched white children [50].
Table 1 derived from the 2018/19 NPDA Annual report [18] provides a breakdown by ethnic background of CYP diagnosed with T1 and T2 DM compared with the National Census data from 2011 for England and Wales. Whilst the percentage proportions with T1DM in each ethnic group reflects the proportions of the same groups in the overall population, the distribution of T2DM according to ethnicity is completely different to the background population, with a particular preponderance in Asians.
Table 1.
Ethnic group of children and young people diagnosed with T1DM and T2DM in England and Wales 2018/19
| Ethnic Group | Number with T1DM | Percentage of T1DM sample with known ethnicity | Number with T2DM | Percentage of T2DM sample with known ethnicity | Ethnic group as a percentage of total population* |
|---|---|---|---|---|---|
| White | 22,733 | 84.4 | 291 | 40.3 | 86.0 |
| Asian | 1,795 | 6.7 | 281 | 38.9 | 7.5 |
| Black | 1,044 | 3.9 | 84 | 11.6 | 3.3 |
| Mixed | 836 | 3.1 | 42 | 5.8 | 2.2 |
| Other | 529 | 2.0 | 24 | 3.3 | 1.0 |
| Not stated/unknown/missing | 1,660 | 68 |
These data suggest that particular ethnic groups (South Asian, Chinese and blacks) may have a predisposition to insulin resistance, which may increase their risk for T2DM. This emphasises the importance of not using blanket body mass cut-offs derived from the white population to define intervention strategies. It is also important when considering these cut-offs to back extrapolate the values onto the BMI Reference Charts used in paediatric practice. For example, the body mass index of 30 kg/m2 in whites equates to the 95th centile on the paediatric BMI charts. Focusing on obesity seems reasonable as the trajectory of T2DM lags the increase in obesity in the population.
4. Acute and chronic complications of type 1 and 2 diabetes mellitus
4.1. Acute complications
Hyper- and hypo-glycaemia constitute the two major acute complications of T1DM whilst hyperglycaemia is the major issue in T2DM. There is a worldwide variation in the incidence of diabetic ketoacidosis (DKA) at presentation [51]. DKA arises from loss of insulin or insulin action, leading to raised blood glucose with polyuria and dehydration along with breakdown of fat as an energy source releasing ketoacids which contribute along with circulatory collapse to the acidosis. A systematic review of studies that included the frequency of DKA in children at diagnosis ranged from 12.8 to 80%, lowest in Sweden and Canada and highest in the United Arab Emirates and Saudi Arabia [51]. Frequency of DKA was inversely associated with national gross domestic product and background incidence of T1DM [51]. The differences may be partially explained by variations in the definition of DKA, although this effect was minimised in review [51].
Health care professional access to information plays a role in DKA definition as does general access and responsiveness of health care systems. Disease awareness amongst both health care professionals and parents plays a role in DKA diagnosis as it is often assumed that young children do not develop diabetes. The frequency of DKA reported in different studies from the United States is quite striking, which may reflect variation in delivery and access to healthcare. Within ethnic groups there was also a variation in the presentation at diagnosis with DKA ranging from 15.8 to 28.9% in non-Hispanic whites, 16.7–30.8% in African Americans, 25% overall in Hispanics, 26.1% in Asian and Pacific Islanders and highest in the Navajo population at 40%.
Data are lacking for comparisons of other measures of acute complications, such as hypoglycaemia in childhood. Generally, hypoglycaemia rates are inversely related to glycosylated haemoglobin (HbA1c) which in turn tracks with both ethnicity and deprivation and which we will consider further below. As people strive for lower glycosylated haemoglobin and therefore better and tighter diabetes control the risk of hypoglycaemia increases as it is not always possible to match insulin need for food for example with insulin delivery so that over treatment can occur. Over treatment is less likely with insulin pump therapy and closed loop insulin delivery systems [52].
DKA is an uncommon presentation of T2DM in white CYP, who are generally asymptomatic at diagnosis. However, minority populations tend to have higher insulin concentrations at diagnosis, with up to 33% developing ketonuria and 5−25% presenting with DKA [16]. Hyperosmolar hyperglycaemia is a rarer presentation of T2DM in childhood but carries with it a high mortality [53].
4.2. Chronic complications
As alluded to, the demands of T1DM require a lot of attention to avoid short term problems such as DKA and chronic problems affecting the micro- and macro-vasculature. The major chronic complications of T1DM and T2DM can be subdivided into microvascular (diabetic nephropathy, retinopathy, and neuropathy) and macrovascular complications, manifesting as cardiovascular disease, in particular ischemic heart disease, but also cerebrovascular disease, and peripheral vascular disease. The Diabetes Control and Prevention Trial (DCCT) designed in the 1980s to address whether intensive insulin therapy could delay or avoid long-term diabetes complications showed that more intensive insulin therapy resulted in better glycaemic control. This in turn was associated with a marked reduction in the long-term complications of T1DM [54], in particular proliferative retinopathy (PDR), but also nephropathy, and cardiovascular disease. A similar outcome in T2DM came from the UK Prospective Diabetes Study [55].
This translates into a series of practical demands particularly in T1DM; frequent blood testing 7–10 times per day with readjustment of any high or low blood glucose back into the target range, managing insulin dosing based on carbohydrate intake and likely exercise and careful monitoring of night time blood glucose to avoid nocturnal hypoglycaemia. These demands are in addition to normal parenting and are time consuming and relentless. Recent smart meters that do many of these calculations help as do semi-automated pump systems for delivering insulin.
When families are juggling work patterns to make ends meet, time is often limited and managing the daily demands of T1DM may not be the top priority. In addition, access to high quality, lower glycaemic index carbohydrate foods is often further limited by time and cost.
Children and young people with T1DM and T2DM have a higher risk for complications when compared to adult onset diabetes mellitus, partly because of the younger age at diagnosis. The risk of developing early complications is higher in CYP with T2DM than those with T1DM. This higher level of risk does not appear to be related to overall glycaemic control or duration of disease but rather to the occurrence of hypertension and dyslipidaemia [56].
The earlier onset and aggressive nature of complications places an additional burden on families and households who are already under strain because of associated deprivation. In turn, less time may be available to attend to the management of these problems on a day to day basis. There may also be a limit on what the family can achieve in terms of changes to carbohydrate and fat consumption due to the cost of food. Access to exercise may be limited, particularly in high density, high-rise housing.
4.3. Glycosylated haemoglobin
In adult diabetes practice there are clear effects of ethnicity on rates of chronic complications. The overall complication rate in childhood is very low compared with adults so for the purposes of this article we will focus more on glycosylated haemoglobin (HbA1c) as a surrogate for longer term complications. The problem with extrapolating from DCCT forwards, is that the majority of studies with HbA1c are cross sectional and do not account for potential long-term change. In T1DM children in the UK, ethnic differences in glycaemic control have been demonstrated at diagnosis and shown to track in the first six months post diagnosis [57].
A further potential problem when analysing HbA1c is that haemoglobin variants exist in ethnic groups. Glycosylated haemoglobin reflects glucose exposure and red cell turnover. In a meta-analysis of HbA1c in ethnic groups overall values when compared to the White group were 2.8 mmol/mol (0.25%) higher in blacks, 2.6 mmol/mol (0.24%) higher in Asians and 0.9 mmol/mol (0.08%) in Hispanics [58]. These differences do not matter when the differences reported in studies of HbA1c in CYP with T1DM amount to between 9 and 17 mmol/mol (0.8−1.6%) [15,59].
Geographical differences in HbA1c have become more evident with the increasing use of diabetes registries. A comparison of data from 64,666 CYP from seven European regions and the USA demonstrated a 1.2 fold difference in HbA1c across countries [60]. Variations between centres within some countries was even larger than across countries. In Sweden, the mean HbA1c was 59 mmol/mol (7.6%) with a variation between centres of 4% whilst in the USA the mean HbA1c was a lot higher at 72 mmol/mol (8.7%) as was the variation between centres at 7.9%. Some of these variations could be related to differences in patient case mix such as greater or lesser number of deprived groups in each country as well as differences in quality of, or access to, diabetes care. These unwarranted variations raise concerns about the equity of health care systems. Unfortunately, the reporting of ethnicity was highly variable between countries and at best ranged from 6% of the study population in Norway to 27% in England.
Studies in London suggest that the level of HbA1c was better explained by ethnicity as opposed to socioeconomic status [15,59] reflecting the difficulties often encountered teasing out ethnicity from deprivation. Only when the larger white population was studied alone did the impact of socioeconomic status become apparent and appeared to be mediated by intensity of insulin therapy offered [15]. Of note, clinic attendance was no different between the ethnic groups in both studies.
The study by Thompson et al [15], was able to categorise further the Asian group into origin from India and elsewhere. Asians of Indian origin had the same HbA1c as the White population whereas those not originating in India had values similar to the black population. These findings echo those from other paediatric diabetes centres around the world [61], [62], [63]. Carter et al. [63] did detect an independent effect of socioeconomic status influencing HbA1c, which might reflect that the clinic population of 555 patients was predominantly of European descent (75%).
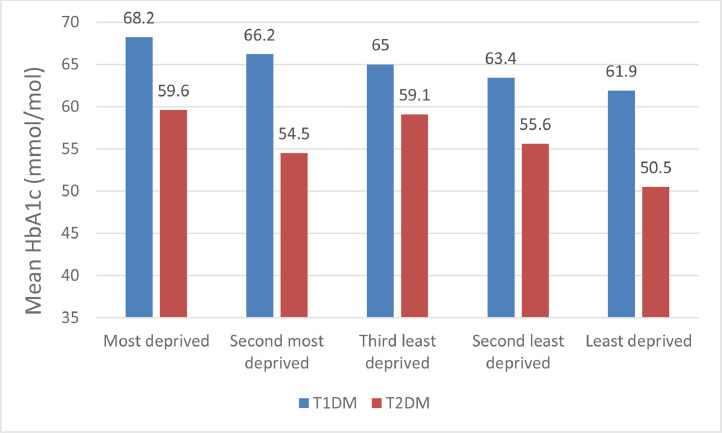
The NPDA data from England and Wales finds that in both T1DM and T2DM, black CYP had higher HbA1c values compared to the other groups (Table 2). Within the black group, CYP from a Caribbean background had a higher mean HbA1c (77.0 mmol/mol (9.2%)) than those from Africa (70.4 mmol/mol (8.6%)) [18]. However, these data should be viewed as descriptive and viewed alongside data from the same audit which shows the relationship between deprivation and HbA1c (Fig. 2) and treatment regimens (Figs. 3 and 4). Overall treatment regimen (multiple daily injections or insulin pump therapy) explained the largest proportion of the variability in HbA1c followed by deprivation. Those in the least deprived areas having an average HbA1c 5.88 mmol/mol (0.5%) lower than those living in the most deprived areas. Only 8–15% of the variation in HbA1c was explained by the patient factors included in the models chosen for analysis and this is similar to other studies with a similar construction [64,65].
Table 2.
Glycosylated haemoglobin (HbA1c) by ethnicity in children and young people with T1DM and T2DM
| Ethnic Group | T1DMMean HbA1c |
T2DMMean HbA1c |
||
|---|---|---|---|---|
| (%) | (mmol/l) | (%) | (mmol/mol) | |
| White | 8.1 | 64.6 | 7.1 | 54.1 |
| Asian | 8.2 | 66.2 | 7.5 | 58.0 |
| Black | 8.7 | 71.4 | 8.3 | 66.9 |
| Mixed | 8.3 | 67.5 | 7.7 | 60.2 |
| Other | 8.0 | 63.7 | 7.3 | 56.7 |
| Not stated/unknown/missing | 8.0 | 63.9 | 6.5 | 47.1 |
Fig. 2.
Effect of deprivation on mean glycosylated haemoglobin (HbA1c) in children and young people with T1DM and T2DM in England and Wales 2018–19.
Data adapted from the 2018/19 National Paediatric Diabetes Audit [18]
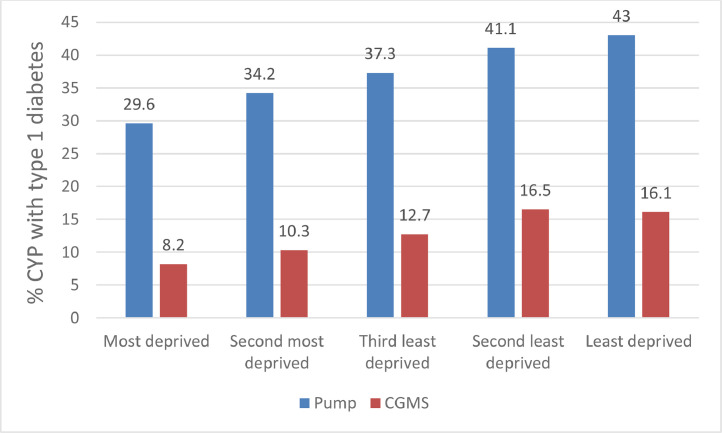
Fig. 3.
Proportion of children and young people (CYP) with T1DM mellitus using insulin pump therapy and continuous glucose monitoring (CGMS) in England and Wales in 2018–19 by deprivation.
Data adapted from the 2018/19 National Paediatric Diabetes Audit [18]
Fig. 4.
Proportion of children and young people (CYP) with T1DM mellitus using insulin pump therapy and continuous glucose monitoring (CGMS) in England and Wales in 2018–19 by ethnicity.
Data adapted from the 2018/19 National Paediatric Diabetes Audit [18]
5. Access to treatment and modalities
As a result of the DCCT, intensification of insulin treatment by using basal–bolus multiple daily injection regimens or continuous subcutaneous insulin infusion pumps has increased in the 25 years since publication. Newer insulin analogues with fast, intermediate, or long action provide better metabolic control. In addition, self-monitoring of blood glucose or continuous glucose monitoring (CGM) have become established tools in improving glycaemic control.
In England and Wales 36.7% of CYP with T1DM were using an insulin pump and when used with CGM were more likely to achieve HbA1c targets of <48 mmol/mol (6.5%) or <58 mmol/mol (7.5%) [18,66]. Use of pump therapy and CGM was not uniformly distributed across the population of CYP with T1DM. Increased usage of pump therapy and CGM was associated with a younger age of patient (less than 10 years of age), living in the least deprived areas and white ethnicity echoing a number of observations [67,68]. Even more concerning was that this gap between pump usage amongst CYP with T1DM living in the most and least deprived areas has widened with time. In 2014/15 the gap was 7.9% and by 2018/19 had increased to 13.5% (Fig. 3). The reason for this is not clear and needs to be explored further. The recruitment of ethnic minorities to technology trials and equity of access to all treatment options need further consideration [69,70].
In a large UK cohort of adults with T2DM, there was a delay in identification of uncontrolled HbA1c and intensification of diabetes therapy in Black and South Asian groups compared to white groups [71]. A study of US adults with diabetes found that self-reported racial discrimination was associated with poorer diabetes quality of care and complications, but not self-management [72]. Whilst the reasons for this difference in diabetes care may be multifactorial, reducing the disparity is essential to improve long term outcomes in these groups.
It is in the hands of the clinics and health care professionals themselves to challenge the perception that most health care providers have implicit bias in terms of positive attitudes toward white people and negative attitudes towards ethnic minority groups. The NPDA data demonstrate that some clinics can and do challenge the stereotypes with several demonstrating equal if not skewed towards people of colour in providing access to technology. This would imply that more work in this area is required but the observations in the NPDA that staff can have very strong consciousness of these issues.
A similar picture pertains in the audit of access to psychology support which plays an important role in supporting diabetes care [73,74]. Whilst the number of CYP with T1DM who had mental health assessed in England and Wales was high (91%) there was a higher proportion of adolescent girls recorded as requiring additional psychological support compared to adolescent boys. Numbers were highest amongst those who were either newly or recently diagnosed and amongst those living in the most deprived areas (36.1% in least deprived areas versus 41.1% in most deprived areas). Those requiring additional support were equally spread across the ethnic groups. What is not clear is the ability to distinguish between “offered” and “accepted” care. As clinical attendance rates are generally good for CYP with T1DM the issue is not so much access to care but to potential variability in utilisation of care and treatments.
Both T1DM and T2DM require a lot of behavioural change. Motivation in self-care management may be lower in T2DM because the consequences are less immediate compared to T1DM where there is the risk of metabolic decompensation and the development of Diabetic Ketoacidosis. Incorporating changes needed to manage T1DM or T2DM poses a challenge for many families particularly where there are financial constraints, unemployment, associated mental health problems within the family along with ease of access to high quality food stuffs and exercise. Overall in England and Wales the NPDA reports good engagement of all families with the education programmes on offer but work needs to be undertaken not so much on motivation itself but on how CYP, parents and carers can maintain motivation over long periods of time whilst avoiding “diabetes burnout.”
6. Strategies to improve the situation
Ethnicity and deprivation are two factors that recur frequently in the diabetes literature in explaining health outcomes. Along with geography, ethnicity and deprivation are viewed as factors beyond the control and/or influence of the inter-disciplinary teams managing diabetes mellitus. As health care professionals we are often more concerned with factors that we may have most control over such as resources, approach to the patient and family and training. Improving deprivation and income inequality seem beyond what we can easily achieve in the UK although this is not the case globally where the numbers in poverty have declined considerably over the last 30 years.
We can construct likely mechanisms to explain the contribution of ethnicity. One explanation might be differences in the glycosylation of haemoglobin albeit a small effect. Another might be the cumulative stress that operates within certain ethnic groups resulting from deprivation. This might lead to alterations in allostatic load (the physiologic response to chronic exposure to stress) [75], which, by influencing cortisol secretion, would lead to differences in glucose regulation [76]. These ideas are testable but less amenable to modification.
To attain an equitable service for CYP with diabetes mellitus then we need to consider interventions at the patient, health care professional, community and health care system levels. Awareness and acceptance of the issue is the starting point.
A medicine-centric approach to the variation in diabetes outcomes considers insulin therapy, the role of technology, duration of diabetes and demographic factors such as age, gender, family structure as well as ethnicity and socioeconomic status. Important as they are collectively, they only explain a small fraction of the patient-to-patient variation [64,65,77]. Other factors that play a role but are harder to assess include patient education, expectations, empowerment, attitudes of the management team, psychological support and the overall use of resources by the diabetes team. How these operate within different ethnic groups and how they can be delivered to varying deprived and disadvantaged people needs to be considered. There is evidence supporting the use of interventions that target patients (primarily through culturally tailored programs) and providers (through face to face feedback and education), and health systems (particularly with nurse case managers and nurse clinicians) [78]. This must also include widening the ethnic remit to groups other than those currently reported and who will have their own needs, medical and societal beliefs and customs.
Successful interventions are frequently culturally tailored to meet patients’ needs and employ multidisciplinary teams of care providers including lay expert patients to deliver face to face interventions with individualised assessment of learning style. Feedback is direct and of high intensity (more than 10 contacts) over a period of time greater than 6 months and is aimed at increasing knowledge, skills, attitudes and behaviour. Health education that uses interactive techniques to deliver skills training and is mindful of language, cultural context and literacy appears to be more effective than traditional didactic approaches. Furthermore, engaging family and community members in the care process may improve outcomes for minority patients [78].
The key steps outlined by Chin et al. [78] consist of the following: (a) recognize disparities and commit to reducing them; (b) implement a basic quality improvement structure and process; (c) make equity an integral component of quality improvement efforts; (d) design the intervention(s); (e) implement, evaluate, and adjust the intervention(s) and (f) sustain the intervention(s). The use of quality improvement concepts into diabetes care has been valuable in improving care. Care needs to be exercised however, as many studies do not include impact on equity and in fact minimise ethnicity and deprivation in the randomisation process often employed [79]. Applying these principles is important not only as we put the patient at the centre of the care process but also when we look at our own short comings as health care providers and when we consider the broader aspects of the patient's life in terms of schooling and education, the built environment and social support networks.
One area which interfaces with socioeconomic factors and ethnicity is health literacy and numeracy. The latter, is particularly important in diabetes where much of self-management is dependent on the numerical skills of the person, carer or parents to determine insulin dosing.
Health literacy has been defined as the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions. Each of the three components of health literacy, reading, numeracy and accessing resources are modifiable. There are not many studies on how health literacy affects diabetes outcomes, and many are inconsistent. Among adults with diabetes, increased risk of hospitalization and increased health costs have been independently associated with health literacy [78,79].
In adolescents using intensive insulin regimens, higher parents’ health literacy was associated with better diabetes management and control [80]. In particular, parental diabetes related numeracy, but not reading skills were inversely correlated with the child's glycaemic control [81] and numeracy skills in adolescents have been associated with diabetes outcomes [82,83].
Studies such as these need to be broadened to other age cohorts in paediatric and adolescent diabetes practice. It can be said however, that health care professionals need to consider the role of parental numeracy, in health outcomes for children with T1 and T2DM.
7. Conclusions
Managing T1 and T2DM in children and young people by families requires the acquisition of practical skills, motivation and long-term resilience. This is a two-way process between the health care team and the family and carers. Such engagement needs to be meaningful and respect the culture, background and beliefs of the family. Health care professionals have a responsibility to identify disparities in service delivery and to minimise variations in access to services, technology and outcomes that are meaningful to both the professionals and the family.
Understanding the socioeconomic environment that families function within is important as that may determine priorities within the household. Data from the UK and USA as well as other countries demonstrate the impact of deprivation on outcomes in diabetes mellitus with clear effects on measures of long-term control and complications. The UK and USA are two large and quite unequal countries with high levels of ethnic diversity amongst children and young people. They have very different health systems, which from the UK perspective is a strength of the NPDA as coverage is 100% of all clinics operating in a single organisation which is free to all at the point of access. This reduces the variability in other datasets which may be attributed to the varying health insurance systems encountered. It is also clear from the UK NPDA that deprivation and ethnicity (as defined by the NHS assessment system) is associated with less use of technology that is likely to improve diabetes control. It is not clear whether this reflects health care professional reluctance to offer such access or to reduced uptake. Using the steps outlined by Chin et al. [78] (recognize and reduce disparities by making equity an integral and sustainable component of quality improvement efforts) is the way to address these issues.
Going forward we need to better define and integrate what we mean by the various terms encountered in this review. For example, it is easy to correlate race with a number of measures, but we should not portray race (consciously or not) as an essential biological causal mechanism. It is often used as a poor proxy for genetic ancestry, which in itself needs to be measured better. This means getting better inclusion of non-white patients in clinical studies particularly where we can better define genetic background. This is important because the race/ethnicity categories used in research and clinical practice are broad and less precise than genetic ancestry. Genetic ancestry may bring more precise disease classification. Both variables are important, however, as they cpture different exposures, genetic and environmental.
These observations highlight, also, the need to refine the different statistical models to evaluate the impact of ethnicity and/or socioeconomic status. Two approaches might be helpful in this respect. First, conceptual frameworks are dynamic in the sense that they allow for a variety of ways of arriving at the same end point. This approach may be of more value than multivariate analysis as it does not treat all explanatory variables as if they belong to the same hierarchical level and considers whether an effect is direct or mediated through other factors. This evolves into the second approach using causality modelling which is suited to this difficult type of analysis. Classic modelling using linear models are often not appropriate in these situations [84].
This paper has reviewed the impact of deprivation and ethnicity on CYP with T1 and T2DM. It is clear that further work is required to determine how health care professionals and systems can work to minimise the impact that these two factors have on the development of diabetes mellitus particularly T2DM. Helping CYP, families and carers to manage the condition to maximise their health and well-being requires a broader holistic approach rather than the current medico-centric one.
Search strategy and selection criteria
We undertook a computerized search of MEDLINE from 1966 to December 2020 and supplemented this with hand searches of the reference lists within the relevant articles identified from the electronic search. We concentrated on articles predominantly from the United States of America and the United Kingdom and also included articles from countries with similar health care systems. We used the following search terms, type 1 and type 2 diabetes mellitus; socioeconomics; race; ethnicity; equitable; diabetes technology; diabetes audit; diabetes registers.
Contributors
Peter Hindmarsh and Catherine Peters undertook the literature search and figure construction and with Russell Viner the writing of the review.
Funding
No funding source was involved in this piece
Declaration of Competing Interest
CJP, RV and PCH have no conflicts of interest to declare and the review was undertaken with no supporting funding source.
References
- 1.Cho N.H., Shaw J.E., Karuranga S., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Medicine Io . National Academy Press; Washington: 2001. Crossing the quality chasm: a new health system for the 21st century. [PubMed] [Google Scholar]
- 3.Chen J., Vargas-Bustamante A., Mortensen K., Ortega A.N. Racial and ethnic disparities in health care access and utilization under the affordable care act. Med Care. 2016;54(2):140–146. doi: 10.1097/MLR.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manuel J.I. Racial/ethnic and gender disparities in health care use and access. Health Serv Res. 2018;53(3):1407–1429. doi: 10.1111/1475-6773.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccardi C., Detollenaere J., Vanden B.P., Willems S. Social disparities in patient safety in primary care: a systematic review. Int J Equity Health. 2018;17(1):114. doi: 10.1186/s12939-018-0828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakkeeran N., Nakkeeran B. Disability, mental health, sexual orientation and gender identity: understanding health inequity through experience and difference. Health Res Policy Syst. 2018;16(Suppl 1):97. doi: 10.1186/s12961-018-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockwell D.C., Landrigan C.P., Toomey S.L., et al. Racial, ethnic, and socioeconomic disparities in patient safety events for hospitalized children. Hosp Pediatr. 2019;9(1):1–5. doi: 10.1542/hpeds.2018-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrell L.N., Elhawary J.R., Fuentes-Afflick E., et al. Race and genetic ancestry in medicine - a time for reckoning with racism. N Engl J Med. 2021;384(5):474–480. doi: 10.1056/NEJMms2029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbrook R. Disparities in health care–from politics to policy. N Engl J Med. 2004;350(15):1486–1488. doi: 10.1056/NEJMp048060. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs S.L., Schroeder S.A. Class - the ignored determinant of the nation's health. N Engl J Med. 2004;351(11):1137–1142. doi: 10.1056/NEJMsb040329. [DOI] [PubMed] [Google Scholar]
- 11.Caraballo C., Massey D., Mahajan S., et al. Racial and ethnic disparities in access to health care among adults in the United States: a 20-year national health interview survey analysis, 1999-2018. medRxiv: the preprint server for health sciences 2020.
- 12.Stringhini S., Carmeli C., Jokela M., et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet. 2017;389(10075):1229–1237. doi: 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tajeu G.S., Safford M.M., Howard G., et al. Black-white differences in cardiovascular disease mortality: a prospective US study, 2003-2017. Am J Public Health. 2020;110(5):696–703. doi: 10.2105/AJPH.2019.305543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLennan D.N., S. Noble, M. Plunkett, E. Wright, G. Gutacker, N. The English indices of deprivation 2019. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833951/IoD2019_Technical_Report.pdf (accessed 18th November 2020).
- 15.Thompson R.J., Agostini K., Potts L., et al. Deprivation and ethnicity impact on diabetes control and use of treatment regimen. Diabetic Med. 2013;30(4):491–494. doi: 10.1111/dme.12023. [DOI] [PubMed] [Google Scholar]
- 16.Arslanian S.A. Type 2 diabetes mellitus in children: pathophysiology and risk factors. JPEM. 2000;13(Suppl 6):1385–1394. doi: 10.1515/jpem-2000-s612. [DOI] [PubMed] [Google Scholar]
- 17.O'Rahilly S., Turner R.C., Matthews D.R. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318(19):1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 18.Health RCoPaC. National paediatric diabetes audit. Annual report 2018-19: Care processes and outcomes. 2020. https://www.rcpch.ac.uk/sites/default/files/2020-03/final_npda_core_report_2018-2019.pdf (accessed 6th December 2020).
- 19.Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabetic Med. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 20.Newhook L.A., Grant M., Sloka S., et al. Very high and increasing incidence of type 1 diabetes mellitus in Newfoundland and Labrador, Canada. Pediatr Diabetes. 2008;9(3 Pt 2):62–68. doi: 10.1111/j.1399-5448.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Xia W., Yu Q., et al. Increasing incidence of type 1 diabetes in children aged 0-14 years in Harbin, China (1990-2000) Prim Care Diabetes. 2008;2(3):121–126. doi: 10.1016/j.pcd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Mayer-Davis E.J., Lawrence J.M., Dabelea D., et al. Incidence trends of Type 1 and Type 2 diabetes among Youths, 2002-2012. N Engl J Med. 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson C.C., Harjutsalo V., Rosenbauer J., et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–417. doi: 10.1007/s00125-018-4763-3. [DOI] [PubMed] [Google Scholar]
- 24.Norris J.M., Johnson R.K., Stene L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020;8(3):226–238. doi: 10.1016/S2213-8587(19)30412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez B.L., Fujimoto W.Y., Mayer-Davis E.J., et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29(8):1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 26.Awa W.L., Boehm B.O., Rosinger S., et al. HLA-typing, clinical, and immunological characterization of youth with type 2 diabetes mellitus phenotype from the German/Austrian DPV database. Pediatr Diabetes. 2013;14(8):562–574. doi: 10.1111/pedi.12043. [DOI] [PubMed] [Google Scholar]
- 27.Candler T.P., Mahmoud O., Lynn R.M., Majbar A.A., Barrett T.G., Shield J.P.H. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabetic Med. 2018;35(6):737–744. doi: 10.1111/dme.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue A., Wu Y., Zhu Z., et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(1):2941. doi: 10.1038/s41467-018-04951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 30.Ling C., Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028–1044. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommese L., Benincasa G., Lanza M., et al. Novel epigenetic-sensitive clinical challenges both in type 1 and type 2 diabetes. J Diabetes Complicat. 2018;32(11):1076–1084. doi: 10.1016/j.jdiacomp.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Campbell-Stokes P.L., Taylor B.J. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005;48(4):643–648. doi: 10.1007/s00125-005-1697-3. [DOI] [PubMed] [Google Scholar]
- 33.Parviainen A., But A., Siljander H., Knip M. Decreased incidence of type 1 diabetes in young Finnish children. Diabetes Care. 2020;43:2953–2958. doi: 10.2337/dc20-0604. [DOI] [PubMed] [Google Scholar]
- 34.Borchers A.T., Uibo R., Gershwin M.E. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9(5):A355–A365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Patterson C.C., Carson D.J., Hadden D.R. Epidemiology of childhood IDDM in Northern Ireland 1989-1994: low incidence in areas with highest population density and most household crowding. Northern Ireland Diabetes Study Group. Diabetologia. 1996;39(9):1063–1069. doi: 10.1007/BF00400655. [DOI] [PubMed] [Google Scholar]
- 36.Patterson C.C., Waugh N.R. Urban/rural and deprivational differences in incidence and clustering of childhood diabetes in Scotland. Int J Epidemiol. 1992;21(1):108–117. doi: 10.1093/ije/21.1.108. [DOI] [PubMed] [Google Scholar]
- 37.Haynes A., Bulsara M.K., Bower C., Codde J.P., Jones T.W., Davis E.A. Independent effects of socioeconomic status and place of residence on the incidence of childhood type 1 diabetes in Western Australia. Pediatr Diabetes. 2006;7(2):94–100. doi: 10.1111/j.1399-543X.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 38.Pundziute-Lyckå A., Urbonaite B., Ostrauskas R., Zalinkevicius R., Dahlquist G.G. Incidence of type 1 diabetes in Lithuanians aged 0-39 years varies by the urban-rural setting, and the time change differs for men and women during 1991-2000. Diabetes Care. 2003;26(3):671–676. doi: 10.2337/diacare.26.3.671. [DOI] [PubMed] [Google Scholar]
- 39.Castillo-Reinado K., Maier W., Holle R., et al. Associations of area deprivation and urban/rural traits with the incidence of type 1 diabetes: analysis at the municipality level in North Rhine-Westphalia, Germany. Diabetic Med. 2020;37:2089–2097. doi: 10.1111/dme.14258. [DOI] [PubMed] [Google Scholar]
- 40.Staines A., Bodansky H.J., McKinney P.A., et al. Small area variation in the incidence of childhood insulin-dependent diabetes mellitus in Yorkshire, UK: links with overcrowding and population density. Int J Epidemiol. 1997;26(6):1307–1313. doi: 10.1093/ije/26.6.1307. [DOI] [PubMed] [Google Scholar]
- 41.Goff L.M. Ethnicity and type 2 diabetes in the UK. Diabetic Med. 2019;36(8):927–938. doi: 10.1111/dme.13895. [DOI] [PubMed] [Google Scholar]
- 42.Rosling H. Think about factfulness. London: Hodder and Stoughton Ltd; 2019. Chapter 1: the gap instinct.https://www.gapminder.org/fw/income-levels/last accessed October 18 2021, [Google Scholar]
- 43.Randell T. Developing a best practice tariff in paediatric diabetes: can we improve services and outcomes for children and young people with diabetes in England? Diabetes Care Child Young People. 2012;1(1):23–26. [Google Scholar]
- 44.Khanolkar A.R., Amin R., Taylor-Robinson D., Viner R.M., Warner J., Stephenson T. Inequalities in glycemic control in childhood onset type 2 diabetes in England and Wales-A national population-based longitudinal study. Pediatr Diabetes. 2019;20(7):821–831. doi: 10.1111/pedi.12897. [DOI] [PubMed] [Google Scholar]
- 45.Statistics UOoN. Household inequality, UK; Financial year ending 202 (provisional). 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/personalandhouseholdfinances/incomeandwealth/bulletins/householdincomeinequalityfinancial/financialyearending2020provisional (accessed 18th November 2020).
- 46.Taksali S.E., Caprio S., Dziura J., et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57(2):367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 47.Roth C.L., Reinehr T. Roles of gastrointestinal and adipose tissue peptides in childhood obesity and changes after weight loss due to lifestyle intervention. Arch Pediatr Adolesc Med. 2010;164(2):131–138. doi: 10.1001/archpediatrics.2009.265. [DOI] [PubMed] [Google Scholar]
- 48.Chiu M., Austin P.C., Manuel D.G., Shah B.R., Tu J.V. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–1748. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ntuk U.E., Gill J.M., Mackay D.F., Sattar N., Pell J.P. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care. 2014;37(9):2500–2507. doi: 10.2337/dc13-2966. [DOI] [PubMed] [Google Scholar]
- 50.Uwaifo G.I., Fallon E.M., Chin J., Elberg J., Parikh S.J., Yanovski J.A. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–2087. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 51.Usher-Smith J.A., Thompson M., Ercole A., Walter F.M. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–2894. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Beers C.A.J., Caris M.G., DeVries J.H., Serné E.H. The relation between HbA1c and hypoglycemia revisited; a secondary analysis from an intervention trial in patients with type 1 diabetes and impaired awareness of hypoglycemia. J Diabetes Complicat. 2018;32(1):100–103. doi: 10.1016/j.jdiacomp.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Bagdure D., Rewers A., Campagna E., Sills M.R. Epidemiology of hyperglycemic hyperosmolar syndrome in children hospitalized in USA. Pediatr Diabetes. 2013;14(1):18–24. doi: 10.1111/j.1399-5448.2012.00897.x. [DOI] [PubMed] [Google Scholar]
- 54.Nathan D.M., Bayless M., Cleary P., et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stratton I.M., Adler A.I., Neil H.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ Clin Res Ed. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eppens M.C., Craig M.E., Cusumano J., et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 57.Khanolkar A.R., Amin R., Taylor-Robinson D., et al. Ethnic differences in early glycemic control in childhood-onset type 1 diabetes. BMJ Open Diabetes Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2017-000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavagnolli G., Pimentel A.L., Freitas P.A., Gross J.L., Camargo J.L. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0171315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dias R.P., Brown F., Wyatt C., Cheema S., Allgrove J., Amin R. The effect of insulin intensification in children and young persons with type 1 diabetes differs in relation to ethnic group; a prospective observational study. Diabetic Med. 2013;30(4):495–501. doi: 10.1111/dme.12022. [DOI] [PubMed] [Google Scholar]
- 60.Charalampopoulos D., Hermann J.M., Svensson J., et al. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care. 2018;41(6):1180–1187. doi: 10.2337/dc17-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weil E.J., Curtis J.M., Hanson R.L., Knowler W.C., Nelson R.G. The impact of disadvantage on the development and progression of diabetic kidney disease. Clin Nephrol. 2010;74(Suppl 1):S32–S38. doi: 10.5414/cnp74s032. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leese G.P., Boyle P., Feng Z., Emslie-Smith A., Ellis J.D. Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care. 2008;31(11):2131–2135. doi: 10.2337/dc08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter P.J., Cutfield W.S., Hofman P.L., et al. Ethnicity and social deprivation independently influence metabolic control in children with type 1 diabetes. Diabetologia. 2008;51(10):1835–1842. doi: 10.1007/s00125-008-1106-9. [DOI] [PubMed] [Google Scholar]
- 64.Cardwell C.R., Patterson C.C., Allen M., Carson D.J. Diabetes care provision and glycaemic control in Northern Ireland: a UK regional audit. Arch Dis Child. 2005;90(5):468–473. doi: 10.1136/adc.2004.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Group S.S. Factors influencing glycemic control in young people with type 1 diabetes in Scotland: a population-based study (DIABAUD2) Diabetes Care. 2001;24(2):239–244. doi: 10.2337/diacare.24.2.239. [DOI] [PubMed] [Google Scholar]
- 66.Sherr J.L., Hermann J.M., Campbell F., et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87–91. doi: 10.1007/s00125-015-3790-6. [DOI] [PubMed] [Google Scholar]
- 67.Senniappan S., Hine P., Tang W., et al. The effect of socioeconomic deprivation on efficacy of continuous subcutaneous insulin infusion: a retrospective paediatric case-controlled survey. Eur J Pediatr. 2012;171(1):59–65. doi: 10.1007/s00431-011-1482-x. [DOI] [PubMed] [Google Scholar]
- 68.Viner R.M., White B., Amin R., et al. Impact of deprivation, ethnicity, and insulin pump therapy on developmental trajectories of diabetes control in COB type 1 diabetes. Pediatr Diabetes. 2017;18(5):384–391. doi: 10.1111/pedi.12407. [DOI] [PubMed] [Google Scholar]
- 69.Barnard-Kelly K.D., Cherñavvsky D. Social inequality and diabetes: a commentary. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2020;11(4):803–811. doi: 10.1007/s13300-020-00791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerr D., Klonoff D.C. Digital diabetes data and artificial intelligence: a time for humility not hubris. J Diabetes Sci Technol. 2019;13(1):123–127. doi: 10.1177/1932296818796508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathur R., Farmer R.E., Eastwood S.V., Chaturvedi N., Douglas I., Smeeth L. Ethnic disparities in initiation and intensification of diabetes treatment in adults with type 2 diabetes in the UK, 1990-2017. PLoS Med. 2020;17(5) doi: 10.1371/journal.pmed.1003106. A cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peek M.E., Wagner J., Tang H., Baker D.C., Chin M.H. Self-reported racial discrimination in health care and diabetes outcomes. Med Care. 2011;49(7):618–625. doi: 10.1097/MLR.0b013e318215d925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hilliard M.E., Harris M.A., Weissberg-Benchell J. Diabetes resilience: a model of risk and protection in type 1 diabetes. Curr Diabetes Rep. 2012;12(6):739–748. doi: 10.1007/s11892-012-0314-3. [DOI] [PubMed] [Google Scholar]
- 74.Christie D. Radcliffe Publishing; London: 2012. Psychological approaches to working with families: an example of solution-focused brief therapy. [Google Scholar]
- 75.Szanton S.L., Gill J.M., Allen J.K. Allostatic load: a mechanism of socioeconomic health disparities? Biol Res Nurs. 2005;7(1):7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weigensberg M.J., Toledo-Corral C.M., Goran M.I. Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab. 2008;93(4):1372–1378. doi: 10.1210/jc.2007-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Health RCoPaC. National paediatric diabetes audit 2018/19. 2020. https://www.rcpch.ac.uk/resources/npda-annual-reports.
- 78.Chin M.H., Clarke A.R., Nocon R.S., et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012;27(8):992–1000. doi: 10.1007/s11606-012-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu J.B., Danko K.J., Elfassy M.D., Welch V., Grimshaw J.M., Ivers N.M. Do quality improvement initiatives for diabetes care address social inequities? Secondary analysis of a systematic review. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-018826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janisse H, C, Naar-King S, Ellis D. Brief report: Parent’s HL among high-risk adolescents with insulin dependent diabetes. J Pediatr Psychol. 2010;35:436–440. doi: 10.1093/jpepsy/jsp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasan K, Heptulla R. Glycemic control in pediatric Type 1 diabetes: role of caregiver literacy. Pediatrics. 2010;94:e1104–1108. doi: 10.1542/peds.2009-1486. [DOI] [PubMed] [Google Scholar]
- 82.Pulgaron RE, Sanders ML, Patino-Fernandez MA, et al. Glycemic control in young children with diabetes: The role of parental health literacy. Patient Educated Couns. 2014;94 doi: 10.1016/j.pec.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulvaney S, Lilly J, Cavanaugh K, Rothman R., L Validation of the Diabetes Numeracy Test with adolescents. J Health Comm. 2013;18:795–804. doi: 10.1080/10810730.2012.757394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pearl J. Causality: Models, reasoning and inference. 2nd ed. Cambridge University Press; New York: 2009. [Google Scholar]