Abstract
Background
There is currently no commonly accepted method of stratifying complexity of prosthetic joint infection (PJI). This study assesses a new classification, the Joint-Specific, Bone involvement, Anti-microbial options, Coverage of the soft tissues, Host status (JS-BACH) classification, for predicting clinical and patient reported outcomes in PJI.
Methods
Patients who received surgery for PJI at two centres in the UK between 2010 and 2015 were classified using JS-BACH as ‘uncomplicated’, ‘complex’ or ‘limited treatment options’. Patient reported outcomes were recorded at 365-days following the index operation and included the EuroQol EQ-5D-3L index score and the EQ-visual analogue score (VAS). Clinical outcome data were obtained from the most recent follow-up appointment.
Findings
220 patients met the inclusion criteria. At 365-days following the index operation, patients with ‘uncomplicated’ PJI reported similar EQ-index scores (0.730, SD:0.326) and EQ-VAS (79.4, SD:20.9) compared to the age-matched population. Scores for ‘uncomplicated’ PJI were significantly higher than patients classified as having ‘complex’ (EQ-index:0.515 SD:0.323, p = 0.012; EQ-VAS:68.4 SD:19.4, p = 0.042) and ‘limited treatment options’ PJI (EQ-index:0.333 SD:0.383, p < 0.001; EQ-VAS:60.2, SD:23.1, p = 0.005). The median time to final follow-up was 4.7 years (inter-quartile range 2.7–6.7 years) where there were 74 cases (33.6%) of confirmed recurrence. Using death as a competing risk, the Cox proportional-hazards ratio of recurrence for ‘complex’ versus ‘uncomplicated’ PJI was 23.7 (95% CI:3.23–174.0, p = 0.002) and having ‘limited options’ verses ‘uncomplicated’ PJI was 57.7 (95% CI:7.66–433.9, p < 0.001).
Interpretation
The JS-BACH classification can help predict likelihood of recurrence and quality of life following surgery for PJI. This will aid clinicians in sharing prognostic information with patients and help guide referral for specialist management of PJI.
Research in context.
Evidence before this study
Periprosthetic joint infection (PJI) occurs in 1, 2% of joint arthroplasties and is a frequent cause for revision surgery. There is currently no commonly accepted method of stratifying the complexity of PJI, or method to determine when best to refer to a specialist centre.
Added value of this study
Added value of this study A classification for PJI was developed, termed the Joint-Specific, Bone involvement, Anti-microbial options, Coverage of the soft tissues, Host status (JS-BACH) classification, which was adapted from the BACH classification, a recently developed classification for bone infection. This study validates the JS-BACH classification for the stratification of cases of PJI using 220 patients across 2 specialist centres for bone and joint infection in the UK. Patients were classified as ‘uncomplicated’, ‘complex’ or as having ‘limited treatment options’ available. Patients who were classified as ‘Uncomplicated’ had reduced recurrence rates and higher self-reported quality of life compared to ‘complex’ or ‘limited options’ PJI.
Implications of all the available evidence
JS-BACH can be applied to patients with PJI to help predict likelihood of recurrence and quality of life following surgery. This will aid clinicians in sharing prognostic information with patients and help guide referral for specialist management in PJI.
Alt-text: Unlabelled box
1. Introduction
Joint arthroplasty is a common procedure, with over 235,000 performed in the UK in 2019 and numbers increasing each year [1,2]. Periprosthetic joint infection (PJI) occurs in 1, 2% of joint arthroplasties and is a frequent cause for revision [3,4]. PJI is challenging, causing significant morbidity for patients and carrying a major financial burden for health services [3]. Successful treatment of bone and joint infections requires a committed multi-disciplinary team (MDT), including (but not limited to) orthopaedic surgeons, plastic surgeons and infectious disease physicians [5], [6], [7]. Despite the recent proposal of a national referral network for bone and joint infections, approaches to managing PJI vary between centres, with no accepted criteria for referral to specialist services [8]. The most commonly used PJI classification was presented by McPherson et al. in 2002, but it is not widely used and does not guide clinicians on the timing of referral to a specialist centre [9].
The BACH classification (Bone involvement, Anti-microbial options, Coverage of the soft tissues, Host status) was introduced to identify ‘complex’ or ‘limited options’ cases of long bone osteomyelitis that may require early referral to a specialist unit [10]. BACH was also correlated with patient reported outcome measures (PROMs) at one year following surgery [11]. Based on this framework, a new classification was developed to identify patients with ‘complex’ or ‘limited options’ PJI. This replaced the ‘bone involvement’ variable from the osteomyelitis classification with a ‘joint-specific’ variable for classification of PJI cases (JS-BACH), (Fig. 1).
Fig. 1.
The JS-BACH classification of prosthetic joint infection and long bone osteomyelitis.
ST = soft tissues. *Anderson orthopaedic research institute (AORI) [37] stage 1 or 2A in the knee and Paprosky type 1 [38] in the hip; **AORI stage 2B in the knee and Paprosky type 2A, 2B or 2C in the hip; *** AORI stage 3 in the knee and Paprosky type 3A or 3B in the hip.
This study investigated the feasibility of classifying cases of PJI using the new classification system: JS-BACH. The classification system has been assessed as a clinical tool for predicting clinical outcomes such as risk of recurrence and patient reported outcomes following surgery for PJI.
2. Methods
2.1. Prosthetic joint-specific characteristics
The BACH classification allows a validated assessment of the health of the host, microbiological and soft tissue features of a bone infection. These features are common to osteomyelitis and PJI [10,12,13], so were retained in the joint-specific analysis. Based on our experience with the BACH classification [14], a provisional list of the radiological features of infected prosthetic joints was compiled by five authors (BK, AA, AT, MMcN, AH), with extensive experience of PJI and infection classification. These included the presence of loosening, bone loss, periprosthetic fracture, the nature of the implant in situ. This list was refined to remove duplicates and to simplify the number of variables to be analysed. The ‘JS’ variable was classified into J1 (uncomplicated), J2 (complex) and J3 (limited options) to align with the BACH classification. The final version was reviewed by the five authors and the text modified to allow a clear description of the features which characterize each level of classification (Fig. 1).
This version was evaluated using a ‘definition cohort’ of 14 cases. These cases were classified by 4 independent PJI and bone infection surgeons (BK, AA, AT, MMcN) who were not involved in the case selection. The evaluation showed ‘almost perfect agreement’ between surgeons in the classification of cases (Fleiss Kappa 0.85). Based on the feedback from this evaluation, the definition of ‘associated periprosthetic fracture’ in the ‘J2’ class was modified to also include any previous history of periprosthetic fracture. This initial inter-observer evaluation produced the key components for validation in this study.
Similar to the original BACH classification, the overall JS-BACH status (Uncomplicated, Complex or Limited Options) was determined from the highest score (x, 1, 2 or 3) in each variable. A level 2 score, in any variable, indicated ‘Complex PJI’ and any level 3 score indicated ‘Limited Option PJI’ [10,11].
3. Validation of the JS-BACH classification
3.1. Patient identification
Patients with suspected PJI were identified prospectively at one of two university teaching hospitals within the UK (Oxford University Hospitals NHS Foundation Trust and Cambridge University Hospitals). Both are tertiary centres for treatment of bone infection. All patients received surgery for PJI between 2010 and 2015. Following identification, patients were retrospectively screened using the 2021 EBJIS definition for PJI [15] and only patients who met the ‘infection confirmed’ criteria were included for analysis (Fig. 2).
Fig. 2.
Flow diagram showing number of patients included.
PROMs = Patient reported outcome measures.
3.2. Patient and public involvement
Four patients with confirmed PJI, but not included in the study, were consulted at Cambridge University Hospitals. These patients provided insights into their experience and concerns regarding PJI, particularly around the certainty of outcome and prediction of prognosis. Their views contributed to the study design and presentation of the data. The study was performed in accordance with the 2013 Declaration of Helsinki and was approved by the hospital governance boards (ISRCTN91566927, OUH 2021 6739 and CUH ID 3559). Informed patient consent was obtained at prior to data collection.
3.3. Management options
All patients received surgery for PJI, determined by the treating surgeon. Both centres had access to plastic surgeons and infection disease physicians, who specialised in bone and joint infection. All patients were treated with systemic antibiotics for a minimum of 6 weeks after the index operation.
3.4. Clinical outcomes
Two major clinical outcomes were measured: (i) recurrence and (ii) the status of the prosthetic joint at the most recent follow-up appointment prior to January 2021.
-
(i)
Recurrence
Recurrence within the first 365-days following the index procedure was assessed by a trained study nurse at face to face or telephone appointment. Recurrence occurring after 365-days was retrospectively assessed using the medical notes and diagnosed based on clinical, histological, microbiological and serological data according to the 2021 EBJIS definition [15].
-
(ii)
Joint status at final follow-up
Patients were assessed at their most recent follow-up as either (i) treatment success: a functional, infection free prosthesis in situ (including patients who have had a successfully treated recurrence) or (ii) treatment failure: patient awaiting further surgery for infection; died secondary to infection (and PJI was on the death certificate); had an excision arthroplasty (including patients awaiting reimplantation); amputation or arthrodesis of the limb due to infection, or was on long term suppressive antibiotics. In the event where patients had died, the status of their limb at their last follow-up was noted as being either a treatment success or treatment failure. All assessments were made by two independent reviewers blinded to classification.
3.5. Patient reported outcome measures (PROMs)
Patients were invited to complete the EuroQol EQ-5D-3L [16] questionnaire and an EQ-visual analogue score (VAS) (worst = 0, best = 100) on day 0, day 14, day 42, day 120 and day 365 following the index procedure. From the EuroQol EQ-5D-3L scores, the EQ-index score was calculated (worst - 0.543, best = 1.000). At day 0, day 120 and day 365, patients who had a knee PJI were asked to complete the Oxford knee score (OKS) [17] (worst = 0, best = 48) and patients with a hip PJI, the Oxford hip score (OHS) [18] (worst = 0, best = 48). Patients who had missed one follow-up time remained in the study and were invited for follow-up at later time points.
3.6. Classification
The ‘JS-BACH’ classification was applied retrospectively to each patient by two independent reviewers who were blinded to clinical and patient reported outcomes. Any discrepancy in classification was adjudicated by a third reviewer who was also blinded to outcome. None of these three reviewers were involved in the surgery or recording of outcome.
3.7. Statistical analysis
A power calculation was performed at 80% and alpha of 0.05 to detect a significant difference in the patient reported outcomes difference of 14 points between groups at a standard deviation of 15, the sample size of the smallest group should be 19 patients. Preliminary work suggests that the smallest group would be the limited options PJI group and account for approximately 19% the cohort. Therefore, with an expected PROM response rate of 50% and 20% of patients not meeting inclusion criteria, the minimum number of included patients should be 240. For all statistical tests, a significance level of < 0.05 was used. Statistical analyses were performed using R in RStudio (Version 4.0.2, 22/06/2020, Boston, MA).
Continuous variables were reported using means and standard deviations, if normally distributed, and medians and inter-quartile ranges (IQR) if non-normally distributed. Categorical variables were presented as a percentage. Univariate analysis was performed using a simple logistic regression approach for a binary outcome, Kruskal-Wallis test for non-parametric continuous variables with Dunn post-hoc and one-way analysis of variance (ANOVA) with Tukey post-hoc was performed for parametric variables. Inter-observer assessment was performed using either Fleiss kappa for multiple observers or Cohen's kappa for two observers and interpreted according to Landis and Koch [19].
To assess individual predictors of recurrence, a Cox proportional-hazards model was built and proportional hazard ratios (HRs) for each variable were calculated. The variables included in the model were those that achieved or approached significance in the univariate analysis (p < 0.100) and were deemed by the senior authors as being clinically significant. To assess recurrence over time, a competing risks model was built to account for death prior to recurrence as a confounding factor. Proportional HRs were presented with 95% confidence intervals. To assess PROMs, a Tobit linear regression model was built to account for the ceiling and floor effects of PROMs, using both left and right censoring. The EQ-VAS, EQ-index, OHS and OKS were treated as continuous variables as they represent a continuous range of health states.
3.8. Role of funding source
The funder has no role in the study design, data collection, analysis, or writing the paper. AJH, ERW, MGW and MMcN had access to the data.
4. Results
4.1. Inclusion and demographics
271 patients were included from the prospectively collected database. Following screening, 51 entries were excluded due to patients either not meeting the diagnostic criteria for PJI (n = 18), being diagnosed with osteomyelitis (n = 18), fracture related infection (n = 8), or septic arthritis (n = 7) in the absence of a prosthetic joint. Therefore, 220 patients met the inclusion criteria and had a confirmed diagnosis of PJI. Patient demographics are shown in Table 1. Patients were followed for a median of 4.7 years (IQR 2.7–6.7). There were 51 patients (23.2%) who were lost to follow-up at 2 years.
Table 1.
Demographics, surgical treatment performed as the index operation and site of infection, clinical outcomes including all-cause mortality, recurrence (defined using the EBJIS 2021 criteria), and status at final follow-up, and patient reported outcomes. Results are given for the whole cohort (total) and sub-classified by the overall JS-BACH classification (either uncomplicated, complex or limited options).
| Total | ‘Uncomplicated’ | ‘Complex’ | ‘Limited options’ | ||
|---|---|---|---|---|---|
| Overall (%) | 220 (100.0%) | 52 (23.6%) | 127 (57.7%) | 41 (18.6%) | |
| Demographics, treatment and site of infection | |||||
| Gender | |||||
| Male | 121 | 36 | 66 | 19 | |
| Female | 99 | 16 | 61 | 22 | |
| Age at index procedure (median, IQR in years) |
70.0 years (62.0–78.0) |
67.2 years (60.0–74.4) |
71.6 years (63.7–77.6) |
69.5 years (61.1–77.9) |
|
| Treatment at index op. | |||||
| DAIR (+/- exchange) | 97 (44.1%) | 33 (63.5%) | 52 (41.0%) | 12 (29.3%) | |
| 1st stage of 2-stage | 84 (38.2%) | 11 (21.2%) | 54 (42.5%) | 19 (46.3%) | |
| Single stage revision | 35 (15.9%) | 8 (15.4%) | 20 (15.7%) | 7 (17.1%) | |
| Amputation or fusion | 4 (1.8%) | 0 | 1 (0.8%) | 3 (7.3%) | |
| Site of infection | |||||
| Upper limb: shoulder | 3 (1.4%) | 1 | 1 | 1 | |
| Upper limb: elbow | 4 (1.8%) | 0 | 3 | 1 | |
| Lower limb: hip | 102 (46.4%) | 29 | 56 | 17 | |
| Lower limb: knee | 111 (50.5%) | 22 | 67 | 22 | |
| Clinical Outcome: | |||||
| Duration of final follow-up (median, IQR in years) |
4.7 years (2.7–6.7) |
5.4 years (4.7–7.1) |
4.2 years (2.1–6.2) |
4.9 years (3.0–6.9) |
|
| All-cause mortality | 32 (14.6%) | 6 (11.5%) | 21 (16.5%) | 5 (12.2%) | |
| At 1-year | 6 (2.7%) | 0 | 5 (4.0%) | 1 (2.4%) | |
| Secondary to PJI | 8 (3.6%) | 0 | 5 (3.9%) | 3 (7.3%) | |
| Recurrence events (EBJIS 2021 definition) | |||||
| At any point | 74 (33.6%) | 1 (1.9%) | 45 (35.4%) | 28 (68.3%) | |
| At 1-year | 40 (18.2%) | 0 | 23 (18.1%) | 17 (41.5%) | |
| Outcome at final follow-up | |||||
| Infection free | 166 (75.5%) | 52 (100.0%) | 100 (78.7%) | 14 (34.2%) | |
| Excision arthroplasty | 25 (11.4%) | 0 | 15 (11.8%) | 10 (24.4%) | |
| Amputation | 12 (5.5%) | 0 | 2 (1.6%) | 10 (24.4%) | |
| Chronic suppressive antibiotics | 9 (4.1%) | 0 | 6 (4.7%) | 3 (7.3%) | |
| Active infection | 8 (3.6%) | 0 | 4 (3.2%) | 4 (9.8%) | |
| Patient reported outcomes at 365 days: | |||||
| EQ-index mean (SD) |
0.359 (0.359) |
0.730 (0.326) |
0.515 (0.323) |
0.333 (0.383) |
|
| EQ-VAS mean (SD) |
69.5 (21.4) |
79.4 (20.9) |
68.4 (19.4) |
60.2 (23.1) |
|
| OHS mean (SD) |
32.1 (14.3) |
40.2 (12.9) |
32.3 (14.4) |
19.5 (14.5) |
|
| OKS mean (SD) |
25.1 (12.0) |
33.0 (10.0) |
22.9 (11.3) |
23.7 (13.9) |
|
DAIR = debridement and implant retention; IQR = interquartile range; EBJIS = European bone and joint infection society; VAS = Visual analogues score; OHS = Oxford hip score; OKS = Oxford knee score.
4.2. Treatment and classification
All patients received a surgical procedure for PJI. Procedures included debridement and implant retention (DAIR) (97/220, 44.1%), two-stage revision (84/220, 38.2%), single stage revision (35/220, 15.9%) or salvage procedure such as amputation or joint fusion (4/220, 1.8%).
Applying the JS-BACH classification, 52 patients were classified as ‘uncomplicated’, 127 as ‘complex’ and 41 as having ‘limited options’ available (Table 2). For the new ‘JS’ variable, there was a Cohen's kappa of 0.84 across observers and sites, which suggests an ‘almost perfect’ inter-user agreement [19].
Table 2.
Distribution of the individual categories of the 220 patients across the 4 variables of the JS-BACH classification and the resultant overall classification. The overall classification was made based on the highest individual score from the 4 variables. Scoring a ‘1′ (or ‘x’) in all variables indicated ‘uncomplicated’ PJI, a ‘2′ in any one variable indicated ‘complex’ PJI and a ‘3′ in any one variable indicated ‘limited options’ PJI.
| Joint-Specific (JS) | Antimicrobial options (A) | Coverage of soft tissue (C) | Host status (H) | Overall classification | |
|---|---|---|---|---|---|
| Uncomplicated | 97 | 148 | 179 | 80 | 52 |
| Complex | 84 | 72 | 41 | 138 | 127 |
| Limited options | 39 | 0 | – | 2 | 41 |
4.3. Clinical outcomes: recurrence
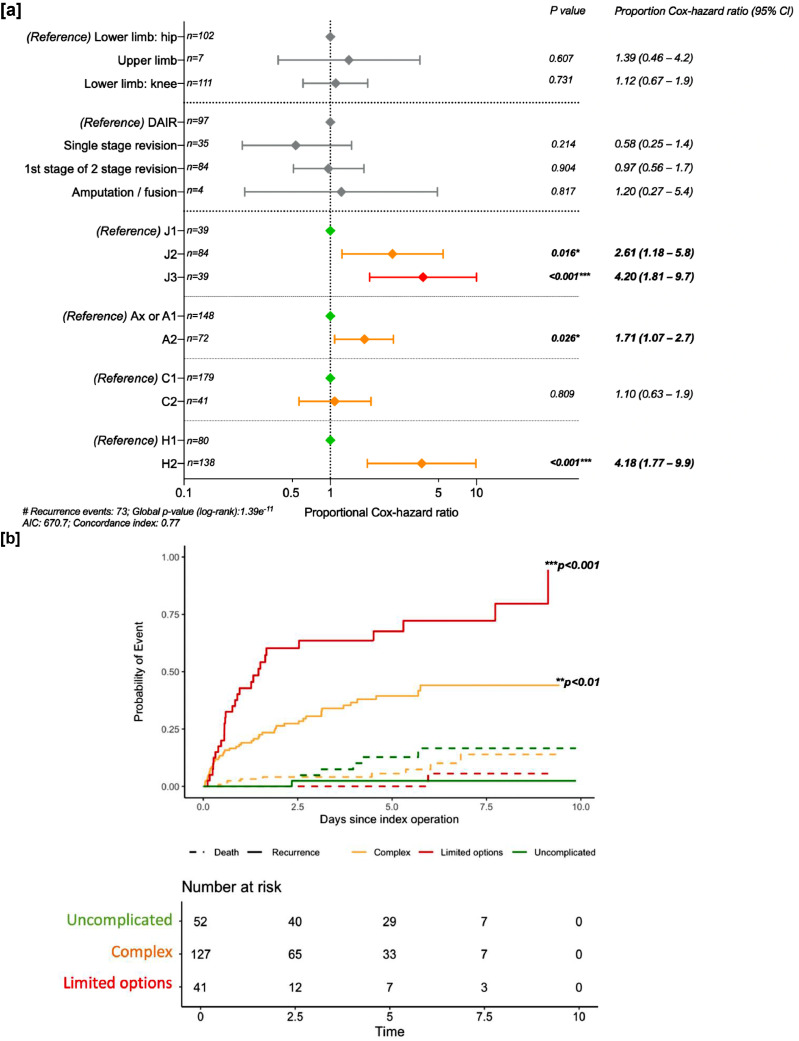
There were 74 cases (33.6%) of recurrence during the follow-up period. In univariate analysis, increasing severity in all 4 variables in the classification (‘JS’, ‘A’, ‘C’, and ‘H’) was significantly associated with increased risk of recurrence. Other factors that were deemed clinically important included two-stage revision (hazard ratio (HR): 1.6 [95% CI 0.98–2.60]) and the site of infection being in the upper limb (HR 3.13 [95% CI 1.10–8.95]), (Table 3). Controlling for index procedure type and site of infection, the Cox proportional-hazards ratio for risk of recurrence following surgery were calculated for each of the variables in the classification. Increasing severity of the ‘joint-specific’, ‘anti-microbial options’, and ‘host status’ variables were independent predictors of recurrence following the index operation (Fig. 3a). The Cox proportional-hazards ratio of recurrence using death as a competing risk for being classified as complex versus uncomplicated was 23.7 (95% CI 3.23–174.0, p = 0.002) and having limited options verses uncomplicated was 57.7 (95% CI 7.66–433.9, p < 0.001) (Fig. 3b).
Table 3.
Univariate analysis of demographic and surgical variables collected with all-follow-up recurrence. Increasing severity of classification in all JS-BACH variables were associated with increased odds of recurrence. Significant p-values are highlighted in bold.
| Univariate hazard ratio | 95% Confidence interval | p-value | ||
|---|---|---|---|---|
| Age at the time of surgery | 0.99 | 0.97–1.01 | p = 0.728 | |
| Male gender | 1.10 | 0.69–1.74 | p = 0.703 | |
| Index procedure: | ||||
| DAIR (reference) | – | – | – | |
| 2-stage revision | 1.60 | 0.98–2.60 | p = 0.060 | |
| Single stage revision | 0.72 | 0.32–1.70 | p = 0.445 | |
| Amputation / fusion | 3.01 | 0.71–12.7 | p = 0.134 | |
| Site of infection: | ||||
| Lower limb: hip (reference) | – | – | – | |
| Upper limb | 3.13 | 1.10–8.95 | p = 0.033 | |
| Lower limb: knee | 1.47 | 0.91–2.36 | p = 0.120 | |
| Overall classification: | ||||
| Uncomplicated (reference) | – | – | – | |
| Complex | 25.8 | 3.60–187.4 | p = 0.001 | |
| Limited options | 61.8 | 8.39–455.0 | p<0.001 | |
| Joint classification: | ||||
| J1 (reference) | – | – | – | |
| J2 | 4.79 | 2.44–9.43 | p<0.001 | |
| J3 | 8.86 | 4.40–17.9 | p<0.001 | |
| Anti-microbial options: | ||||
| A1 or axe (reference) | – | – | – | |
| A2 | 2.17 | 1.37–3.45 | p<0.001 | |
| Coverage of soft tissue | ||||
| C1 (reference) | – | – | – | |
| C2 | 2.44 | 1.50– 4.00 | p<0.001 | |
| Host status#: | ||||
| H1 (reference) | – | – | – | |
| H2 | 8.09 | 3.70–17.7 | p<0.001 | |
H3 patients not included due to low numbers (n = 2).
Fig. 3.
Summary of the Cox-proportional Hazard ratios and competing risk cumulative incidence curve for recurrence versus all cause mortality in patients with prosthetic joint infection (PJI) according to the EBJIS 2021 definition.
[a] Forest plot showing the variables that were deemed to be potential confounders in the univariate testing. DAIR = debridement and implant retention. #H3 patients not included in host status analysis due to low numbers (n = 2).
[b] Cumulative incidence curve for recurrence (solid line) with death (dashed line) as a competing interest, stratified by JS-BACH classification: uncomplicated (green), complex (amber) and limited options (red) PJI. Time 0 (years) is the index operation. P-values derived from Cox-proportional-hazard ratio for recurrence controlling for site of infection and index procedure type, using death as a competing risk. No censored data. *p < 0.05; **p < 0.01; ***p < 0.001.
4.4. Clinical outcomes: status at final follow-up
At final follow-up (median: 4.7 years, IQR: 2.7–6.7 years), 75.5% of patients were categorised as having ‘treatment success’ (166/220) and 24.5% had ‘treatment failure’, defined by one of: excision arthroplasty, amputation, chronic suppressive antibiotic therapy or were receiving treatment for active infection at final follow-up (Table 1). Univariate predictors of treatment failure included increased severity in any of the four classification variables as well as two stage revision performed as the index operation (Supplementary Table 1). Age, gender and the site of infection were not significantly associated with treatment failure (Supplementary Table 1). Controlling for the operation performed as the index procedure and each variable in the classification system, the factors that independently predicted ‘treatment failure’ were classification as J3 (p < 0.001) and H2 (p = 0.002) (Table 4). None of the patients who were classified as ‘uncomplicated’ PJI (0/52) had treatment failure at final follow-up, which compared to 21.3% (27/127) of patients classified as ‘complex’ PJI and 65.9% (27/41) of patients classified as ‘limited options’ PJI.
Table 4.
Multivariate analysis of treatment success (infection free with prosthetic joint in situ) vs. treatment failure (either excision arthroplasty, amputation, chronic antibiotic suppression or active infection awaiting further surgery) at latest follow-up for each of the JS-BACH variables, controlling for index procedure performed.
| Multivariate odds ratio | 95% Confidence interval | p-value | ||
|---|---|---|---|---|
| Index procedure | ||||
| DAIR (reference) | – | – | – | |
| 2-stage revision | 1.36 | 0.57–3.26 | p = 0.491 | |
| Single stage revision | 0.32 | 0.085–1.22 | p = 0.100 | |
| Joint classification | ||||
| J1 (reference) | – | – | – | |
| J2 | 1.43 | 0.49–4.15 | p = 0.516 | |
| J3 | 8.20 | 2.50–27.1 | p<0.001 | |
| Antimicrobial options | ||||
| Axe or A1 (reference) | – | – | – | |
| A2 | 2.01 | 0.92–4.42 | p = 0.079 | |
| Coverage of soft tissues | ||||
| C1 (reference) | – | – | – | |
| C2 | 1.11 | 0.45–2.71 | p = 0.825 | |
| Host status# | ||||
| H1 (reference) | – | – | – | |
| H2 | 12.0 | 2.60–55.1 | p = 0.002 | |
H3 patients not included due to low numbers (n = 2).
4.5. Patient reported outcome measures
53.3% (586/1100) of patients completed the PROMs assessments across all five timepoints (Supplementary Table 2). Specifically, at the 365-day timepoint following surgery, there was a total of 54.1% (119/220) responses.
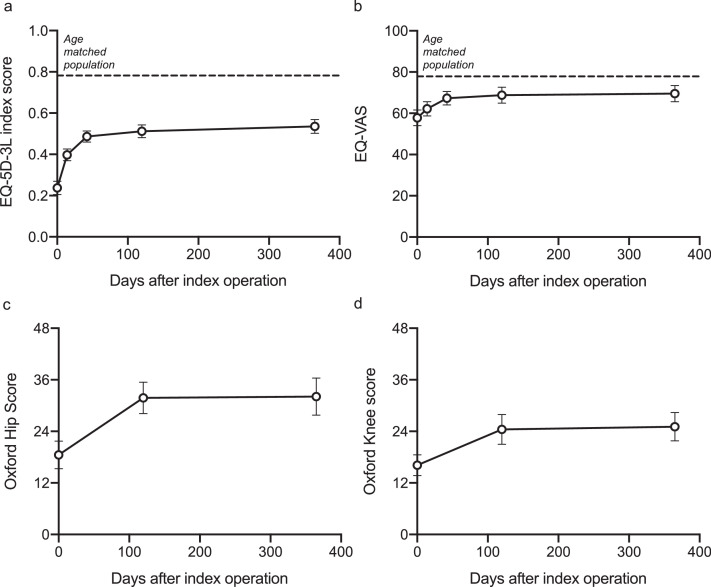
Overall, there was a significant improvement from baseline to 365-days following the index procedure in EQ-index score and EQ-VAS (Fig. 4a, b). For patients with PJI of the hip, the OHS improved over 365 days and for patients with PJI of the knee, the OKS improved over 365 days (Fig. 4c, d).
Fig. 4.
. Patient reported outcomes for all patients.
[a] EQ-index score, [b] EQ-Visual analogue score, [c] Oxford hip score and [d] Oxford knee score.
Mean and 95% confidence intervals are shown. Dotted line: age matched population.
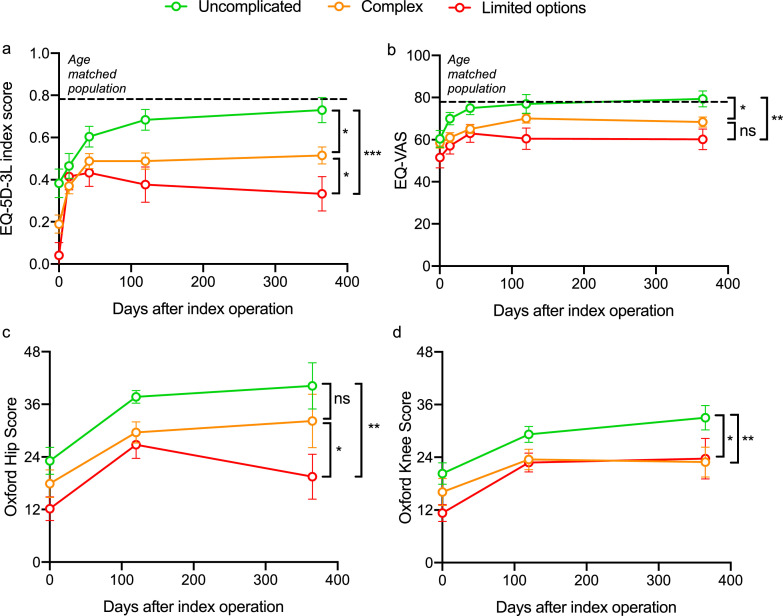
Compared to the age-matched population (age-matched population EQ-index: 0.782 and age matched population EQ-VAS: age-matched: 77.9), patients with ‘uncomplicated’ PJI reported similar EQ-index scores (‘uncomplicated’: 0.730, SD 0.326) and EQ-VAS (‘uncomplicated’ PJI: 79.4, SD 20.9). These scores were significantly higher when compared to patients classified as complex (EQ-index: 0.515 SD 0.323, p < 0.012; EQ-VAS: 68.4 SD 19.4, p = 0.042) and limited options (EQ-index: 0.333 SD 0.383, p < 0.001; EQ-VAS: 60.2, SD 23.1, p = 0.005, ANOVA with Tukey post-hoc comparison) Table 1 and Fig. 5a, b. Similarly, patients with ‘uncomplicated’ PJI gave significantly higher OHS and OKS compared to patients classified as having ‘limited options’ and ‘complex’ PJI (Fig. 5c, d). When exploring the individual classification variables in a univariate analysis, increasing severity in the ‘JS’, ‘C’ and ‘H’ variables had a significant correlation with worse patient reported outcomes at one year post-operatively (Supplementary Table 3).
Fig. 5.
Patient reported outcomes for each of the classification types of JS-BACH: uncomplicated, complex and limited options PJI.
[a] EQ-5D-3L index score; [b] EQ-visual analogue score (VAS); [c] Oxford hip score and [d] Oxford knee score. Error bars are the standard error of the mean. *p < 0.05; **p < 0.01, ***p < 0.001, one-way analysis of variance (ANOVA) with Tukey post-hoc test. Dotted line: age matched population.
A multivariate Tobit regression model was built to assess confounders hypothesised to influence patient reported outcome. When controlling for age and gender, patients classified as ‘complex’ and ‘limited options’ reported significantly lower EQ-index, EQ-VAS, OHS and OKS compared to ‘uncomplicated’ PJI, Table 5.
Table 5.
Multivariate Tobit regression for each of the patient reported outcome measures assessing the classification of PJI controlling for increasing age and gender. Regression coefficient is shown with the standard error (se).
| EQ-5D index | EQ-VAS | OHS | OKS | |
|---|---|---|---|---|
| Number included | 119 | 119 | 43 | 54 |
| Regression coefficient (se) | Regression coefficient (se) | Regression coefficient (se) | Regression coefficient (se) | |
| Increasing age | 0.006 (0.003) | 0.174 (0.171) | 0.215 (0.186) | - 0.162 (0.186) |
| Female gender (reference) | ||||
| Male gender | 0.144 (0.070)* | - 0.153 (3.95) | 0.162 (4.25) | 0.314 (3.40) |
| Uncomplicated (reference) | ||||
| Complex | - 0.271 (0.083)⁎⁎ | - 11.0 (4.69)* | - 10.1 (4.91)* | - 9.81 (3.93)* |
| Limited options | - 0.424 (0.106)⁎⁎⁎ | - 18.7 (5.97)⁎⁎ | - 23.2 (6.68)⁎⁎⁎ | - 8.36 (5.17)* |
EQ-5D index, EQ-5D visual analogue score (VAS), Oxford hip score (OHS) and Oxford knee score (OKS) shown.
p < 0.05.
p < 0.01.
p < 0.001.
5. Discussion
This study presents clinical and patient reported outcomes for patients who have undergone surgery for PJI in two specialist centres within the UK. These outcomes were stratified with the JS-BACH classification and this revealed the classification system is able to predict the likelihood of recurrence, favourable clinical outcomes and patient reported quality of life following the index operation for PJI.
The risk of recurrence correlated with an increase in severity of the ‘joint-specific’, ‘anti-microbial options’ and ‘host status’ variables of the JS-BACH classification. Patients classified as having multi-drug resistant (MDR) isolates (classified as A2) had an increased risk of recurrence compared to patients who had a negative culture (axe) or non-MDR isolates (A1). Specific MDR isolates such as methicillin-resistant Staphylococcus aureus (MRSA) have been previously associated with recurrence in 2-stage revision knee arthroplasty [20], [21], [22] and patients with calcaneal infection [23].
Underlying co-morbidity, classified using the ‘host’ variable of JS-BACH, is a well-established risk factor for recurrence in bone infection [24] and PJI [25]. Specifically, poorly controlled or severe comorbidity such as obesity, current smoking, liver cirrhosis, end stage renal disease, peripheral vascular disease and hyperlipidaemia are all implicated [25], [26], [27], [28], [28], [30], [31]. Furthermore, receiving a previous revision for infection has been shown to be an independent risk factor for recurrence [27,[32], [33], [34]], which is also accounted for in the ‘H’ variable of the classification.
In addition to predicting recurrence, the classification had a strong correlation with prediction of treatment failure at a follow-up of almost 5 years following the index procedure. Almost two thirds (65.9%) of patients with PJI classified as having ‘limited treatment options’ had received an amputation, joint fusion, excision arthroplasty, were on chronic suppressive antibiotics, had died from sepsis secondary to their PJI, or were awaiting treatment for an active infection. This compares to an absence of treatment failure amongst patients classified as having uncomplicated PJI and 21.3% amongst patients classified as having complex PJI. Previously identified risk factors for treatment failure include raised BMI, liver cirrhosis, quality of the soft tissues and the general co-morbidity of the host status [29,30,35,36]. Our findings support the use of the JS-BACH classification system in predicting medium to long-term outcomes for PJI in patients treated surgically at a tertiary bone infection centre.
Overall, we found that self-reported quality of life in patients who were classified as having uncomplicated PJI, returned to the level of the age-matched population, by one year for a general measure of activities of daily living (EQ-index) and by day 42 for global quality of life (EQ-VAS). In terms of EQ-index, we observed that this had plateaued for patients with complex PJI by day 42, and for patients classified as having limited options for treatment started to decline from day 42. This may be partly explained by the higher likelihood of later recurrence in those classified as having complex disease or limited options for treatment with a resulting significant impact on quality of life. The individual variables of the classification that were significant in predicting PROMs were specific to the joint, the soft tissue cover and the health of the host (JS, C and H). In long bone osteomyelitis, the requirement for a soft tissue procedure (C2) was not associated with a poor outcome [11]. In PJI, patients classified as C2 reported significantly lower quality of life compared to patients who did not require plastic surgical expertise. This could reflect a limitation in function that is associated with a microvascular tissue transfer that involves a joint compared to a long bone.
The primary aim of the JS-BACH classification is to aid the triage of patients who have PJI that is complex or has limited options for treatment to an appropriate centre early in their management pathway. The present study included two UK centres, both of which were tertiary referral centres for bone infection, with access to a full bone infection multidisciplinary team. Therefore, further evaluation of the application of the JS-BACH classification in other clinical settings is required.
This is the first study to use the 2021 EBJIS Definition of PJI [8] for inclusion of cases and definition of recurrence. The 2021 EBJIS definition for PJI includes three categories to define whether cases are either “infection unlikely”, “infection likely” or “infection confirmed”. It was decided to only include cases that met the “infection confirmed” criteria, firstly to ensure that the JS-BACH classification was validated in confirmed cases of PJI and secondly due to concern that cases considered either “infection unlikely” or “infection likely” being poorly recorded in prospective data collection. Therefore it may be useful, in future work to assess the applicability of JS-BACH to cases of “infection likely”; this may help ensure any cases that may be viewed as complex receive early referral.
A further limitation of this study is the proportion of patients for whom PROM data were available (53.3%). However, the completeness of the PROM data is similar to other recently reported studies [28] and all patients (who had not died) had data available for at least 1-year follow-up for clinical outcomes. Furthermore, the final follow-up duration was similar for the classification sub-groups. Our power calculation was based on a 50% return of PROM data, so this criterion was met.
The JS-BACH classification was designed to evaluate all infected prosthetic joints, using features which are common to all cases. In our cohort, there were only seven PJIs of upper limb prostheses, so our conclusions must be interpreted with caution in the upper limb. However, in both the definition and validation cohorts, the joint-specific features of the classification (prosthetic loosening, bone loss, implant type and presence of fracture) are equally applicable were applied equally in the upper and lower limbs with good interobserver agreement.
The correlation of the JS-BACH classification to clinical and patient reported outcomes could be used to create a clinical assessment tool based on the factors found to influence outcome. Known factors could be entered, such as classification, age, gender, site of infection and index operation performed and this could then be used to give a percentage risk of recurrence, quality of life and chance of treatment success. Knowing this information at the time of surgery was identified as a major priority for patients during the study design.
In summary, this study has demonstrated that the BACH classification of osteomyelitis can be adapted for use in PJI by adding to the ‘bone involvement’ variable a ‘joint-specific’ variable. The JS-BACH classification showed good performance in predicting clinical outcome and PROMs and so may be used to offer prognostic information to patients and predict clinical outcome. In addition, the JS-BACH classification could be used preoperatively to identify patients with complex PJI or PJI with limited treatment options who may benefit from timely referral to a specialist centre to expedite specialist treatment.
Author contributions
AJH conceptualised, designed, collected and interpreted the data. Drafted and edited the final manuscript.
MGW, ERW, SMM, BK, AT, AA collected and interpreted data. All edited the final manuscript.
MMcN conceptualised, designed the study and interpreted the data. Drafted and edited the final manuscript.
Data sharing statement
Data available from the KTWRP Wellcome-Trust Harvard database upon reasonable request.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Funding
Data acquisition was funded by the National Institute for Health Research (NIHR), Trials No. ISRCTN91566927. AJH is funded by a Versus Arthritis clinical research fellowship. ERW is funded by a national institute of health research (NIHR) academic clinical fellowship. SMM and AA are funded by a NIHR University lectureship.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101192.
Appendix. Supplementary materials
References
- 1.N.J. Registry. 17th annual report: the national joint registry. (2020). [PubMed]
- 2.Kapadia B.H., et al. Periprosthetic joint infection. Lancet. 2016;387:386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 3.Sabah S.A., Alvand A., Price A.J. Prosthetic joint infection of revision knee replacement: epidemiology, outcomes and health economic analysis. Knee. 2021 doi: 10.1016/j.knee.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Lenguerrand E., et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis. 2019;19:589–600. doi: 10.1016/S1473-3099(18)30755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchen A.J., McNally M.A., Sendi P. The classification of long bone osteomyelitis : a systemic review of the literature. J Bone Jt Infect. 2017;2:167–174. doi: 10.7150/jbji.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziran B.H., Rao N., Hall R.A. A dedicated team approach enhances outcomes of osteomyelitis treatment. Clin Orthop Relat Res. 2003:31–36. doi: 10.1097/01.blo.0000087320.60612.86. [DOI] [PubMed] [Google Scholar]
- 7.Salvana J., et al. Chronic osteomyelitis: results obtained by an integrated team approach to management. Conn Med. 2005;69:195–202. [PubMed] [Google Scholar]
- 8.2013/14 NHS standard contract for bone and joint infection service specification (ADULT) Section B part 1-service specifications service specification No. B07/S/e service bone and joint infections (ADULT) commissioner lead provider lead period 12 months D. 2021. https://www.england.nhs.uk/wp-content/uploads/2017/04/b07-bone-joint-infec.pdf
- 9.McPherson E.J., et al. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002:8–15. doi: 10.1097/01.blo.0000030172.56585.c6. [DOI] [PubMed] [Google Scholar]
- 10.Hotchen A.J., Dudareva M., Ferguson J.Y., Sendi P., McNally M.A. The BACH classification of long bone osteomyelitis. Bone Jt Res. 2019;8 doi: 10.1302/2046-3758.810.BJR-2019-0050.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchen A.J., Dudareva M., Corrigan R.A., Ferguson J.Y., McNally M.A. Can we predict outcome after treatment of long bone osteomyelitis? A study of patient-reported quality of life stratified with the BACH classification. Bone Jt J. 2020;102:1587–1596. doi: 10.1302/0301-620X.102B11.BJJ-2020-0284.R1. [DOI] [PubMed] [Google Scholar]
- 12.Izakovicova P., Borens O., Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4:482–494. doi: 10.1302/2058-5241.4.180092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton R., Khan T., Alvand A. Update on the diagnosis and management of prosthetic joint infection in hip and knee arthroplasty. Bone Jt. 2019;360(8):5–13. [Google Scholar]
- 14.Hotchen A.J., Dudareva M., Ferguson J.Y., Sendi P., McNally M.A. The BACH classification of long bone osteomyelitis. Bone Jt Res. 2019;8 doi: 10.1302/2046-3758.810.BJR-2019-0050.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNally M., et al. The EBJIS definition of periprosthetic joint infection. Bone Jt J. 2021;103-B:18–25. doi: 10.1302/0301-620X.103B1.BJJ-2020-1381.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabin R., de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 17.Dawson J., Fitzpatrick R., Murray D., Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Jt Surg Br. 1998;80:63–69. doi: 10.1302/0301-620x.80b1.7859. [DOI] [PubMed] [Google Scholar]
- 18.Dawson J., Fitzpatrick R., Murray D., Carr A. Comparison of measures to assess outcomes in total hip replacement surgery. Qual Health Care. 1996;5:81–88. doi: 10.1136/qshc.5.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis J.R., Koch G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement amongst multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 20.Mortazavi S.M.J., Vegari D., Ho A., Zmistowski B., Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011;469:3049–3054. doi: 10.1007/s11999-011-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasso M., Schiavone Panni A., De Martino I., Gasparini G. Prosthetic knee infection by resistant bacteria: the worst-case scenario. Knee Surg Sports Traumatol Arthrosc. 2016;24:3140–3146. doi: 10.1007/s00167-016-4010-8. [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa K., Stulberg B.N., Wilde A.H., Bauer T.W., Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplast. 1998;13:22–28. doi: 10.1016/s0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 23.Cook J., et al. A Retrospective assessment of partial calcanectomies and factors influencing postoperative course. J Foot Ankle Surg. 2007;46:248–255. doi: 10.1053/j.jfas.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg. 2011;127:190S–204S. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q., et al. Two-stage exchange arthroplasty for periprosthetic joint infection: the rate and reason for the attrition after the first stage. J Arthroplast. 2019;34:2749–2756. doi: 10.1016/j.arth.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Watts C.D., et al. Morbid obesity : a significant risk factor for arthroplasty for infection. J Bone Jt Surg. 2014;96:1–7. doi: 10.2106/JBJS.M.01289. [DOI] [PubMed] [Google Scholar]
- 27.Chen S.Y., et al. Two-stage revision arthroplasty for periprosthetic hip infection: mean follow-up of ten years. Biomed Res Int. 2015;2015 doi: 10.1155/2015/345475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barshes N.R., Mindru C., Ashong C., Rodriguez-Barradas M., Trautner B.W. Treatment failure and leg amputation amongst patients with foot osteomyelitis. Int J Lower Extrem Wounds. 2016;15:303–312. doi: 10.1177/1534734616661058. [DOI] [PubMed] [Google Scholar]
- 28.Jhan S.W., et al. The risk factors of failed reimplantation arthroplasty for periprosthetic hip infection. BMC Musculoskelet Disord. 2017;18:1–7. doi: 10.1186/s12891-017-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancienne J.M., Werner B.C., Bolarinwa S.A., Browne J.A. Removal of an infected total hip arthroplasty: risk factors for repeat debridement, long-term spacer retention, and mortality. J Arthroplast. 2017;32:2519–2522. doi: 10.1016/j.arth.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad S.S., et al. Obesity and smoking predict the results of two-stage exchange in septic revision hip arthroplasty: a cohort study. Orthop Traumatol Surg Res. 2019;105:467–471. doi: 10.1016/j.otsr.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Petis S.M., et al. Two-stage exchange protocol for periprosthetic joint infection following total knee arthroplasty in 245 knees without prior treatment for infection. J Bone Jt Surg Am. 2019;101:239–249. doi: 10.2106/JBJS.18.00356. [DOI] [PubMed] [Google Scholar]
- 33.Faschingbauer M., et al. Difficult to treat: are there organism-dependent differences and overall risk factors in success rates for two-stage knee revision? Arch Orthop Trauma Surg. 2020 doi: 10.1007/s00402-020-03335-4. [DOI] [PubMed] [Google Scholar]
- 34.Corona P.S., Vicente M., Carrera L., Rodríguez-Pardo D., Corró S. Current actual success rate of the two-stage exchange arthroplasty strategy in chronic hip and knee periprosthetic joint infection. Bone Jt J. 2020;102-B:1682–1688. doi: 10.1302/0301-620X.102B12.BJJ-2020-0792.R1. [DOI] [PubMed] [Google Scholar]
- 35.Son M.S., et al. What are the frequency, associated factors, and mortality of amputation and arthrodesis after a failed infected TKA? Clin Orthop Relat Res. 2017;475:2905–2913. doi: 10.1007/s11999-017-5285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam A., et al. Chronic osteomyelitis of the tibia and ankle treated with limb salvage reconstruction. J Bone Jt Infect. 2019;4:306–313. doi: 10.7150/jbji.40337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonanzinga T., et al. Are trabecular metal cones a valid option to treat metaphyseal bone defects in complex primary and revision knee arthroplasty? Joints. 2018;6:58–64. doi: 10.1055/s-0037-1608950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valle C.J.D., Paprosky W.G. Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J Bone Jt Surg Ser A. 2003;85:1–6. doi: 10.2106/00004623-200300004-00001. Journal of Bone and Joint Surgery Inc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.