Abstract
Background
The diagnostic approach for beta-lactam (BL) drug hypersensitivity reactions (DHR) is based on the history, clinical signs, skin tests (ST), in vitro tests, and drug provocation tests (DPT). The aim of this study was to assess the performance of an allergy workup with ST in a real-world use.
Methods
In this cross-sectional study the rate of positive ST in subjects with suspected DHR to penicillins and cephalosporins was investigated. Of special interest were correlations of ST positivity: 1) to the time intervals between index reaction and the allergic work-up, 2) time interval from drug exposure to the onset of signs, 3) pattern of manifestation in delayed DHR and involvement of test area in the index reaction, and 4) potential advantage of patch testing in delayed DHR.
Results
175 patients were included between January 2018 and April 2019 (63.4% female), 45 (25.7%) with immediate DHR manifestation and 130 with delayed DHR manifestation (74.3%). A total of 44 patients (25.1%) had a positive ST (immediate DHR 37.8% versus 20.0% in delayed DHR). ST positivity decreased in both groups after 3 years from 47.8% [95%CI 29.2–67] to 23.5% [95%CI 9.6–47.3] in immediate DHR and 23.0% [95%CI 15-4-32.9] to 12.9% [95%CI 5.1–28.9] in delayed DHR. The proportion of positive ST was higher in patients with more severe forms of delayed DHR, and in subjects with a shorter latency period of onset of symptoms after drug exposure: 0-3d: 29.5% [95%CI 19.6–41.9] vs. >3d: 11.6% [95%CI 6.0–21.2]). No sensitization was shown in delayed urticaria or angioedema. ST done outside the skin area involved during the index reaction were negative in all cases (0/38 vs. 26/84 in cases with involved area). The combination of patch test and intradermal test (IDT) revealed an additional positive result in 2/77 cases. Additional in vitro testing reduced the proportion of negative test results to 72%.
Conclusion
In most patients with negative test results, we could not clarify the cause of the BL-associated adverse events even with further investigations (including DPT). How to prevent new drug-induced adverse events in such patients has hardly been investigated yet. Corresponding cohort studies could improve the data situation.
Keywords: Drug hypersensitivity, Drug allergy, Beta-lactams, Penicillins, Amoxicillin, Cephalosporins, Skin test, T-cell, IgE, Anaphylaxis, Maculopapular exanthema, Intradermal skin test, Patch test, Lymphocyte transformation test, Basophil activation test, Drug provocation test
Introduction
The revised nomenclature of the World Allergy Organization (WAO) describes hypersensitivity as objectively reproducible symptoms or signs initiated by exposure to a defined stimulus at a dose tolerated by normal persons.1 It differentiates between allergy and nonallergic hypersensitivity: Allergy is a hypersensitivity reaction initiated by specific immunologic mechanisms. When the reaction is caused by other mechanisms, the term nonallergic hypersensitivity should be used.1 This distinction may play an important role in advising patients with drug hypersensitivity reactions (DHR). In order to avoid subsequent DHR, the triggering drug as well as cross-reactive substances must be identified.2 Cross-reactivity depends on the mechanism of the DHR. In the case of allergy to beta-lactam antibiotics (BL), the chemical structure plays a key role.2 In case of nonallergic DHR, other factors may be relevant (eg, change of arachidonic acid metabolism in COX1 inhibition in aspirin hypersensitivity3). The diagnostic approach for a suspected allergic DHR is mainly based on assessing the history, clinical signs, skin tests (ST), in vitro tests and partly drug provocation tests (DPT).4 For BL, ST have a good negative and a good positive predictive value for immediate type reactions, while the negative predictive value is considered to be less reliable for delayed DHR.5, 6, 7 This assumption is based mainly on findings in selected patients with unequivocal history, signs and test results. However, prevalence of true positive tests influences the predictive values. Therefore, transferring predictive values to a population with a different prevalence of drug allergy can lead to wrong conclusions. We conducted a cross-sectional study in patients with suspected DHR to BL in order to assess the impact of an allergy work-up based on the medical history and ST. Specifically, how conclusive were positive ST, and did ST positivity correlate with the clinic patterns as well as with the type ST performed.
Methods
Study design and population
This is a cross-sectional study from two university allergy out-patient centers, the Division of Allergology and Clinical Immunology, Inselspital Bern and the Allergy Unit, University Hospital Zurich. All patients with a history of a DHR to a penicillin or cephalosporin antibiotic who were referred for an allergy work-up between January 2018 and April 2019 (Inselspital Bern) and June 2018 and December 2018 (University Hospital Zurich) were included.
Patient assessment
For each DHR case, the following information was recorded and analysed with regard to the index-reaction: date, clinical pattern, chronological sequence regarding involved drugs, development of the clinical course, type of reaction — anaphylaxis according to the classification of H.L. Mueller, delayed type reactions by consensus judgment (general practitioner, dermatologist, allergologist, or based on photo documentation), ST with the incriminated BL as well as with a set of penicillins and cephalosporins according to the EAACI/ENDA standards, and when available, additional in vitro tests.8
Skin test procedures
ST were done intradermally on the volar forearm with the suspected drugs, as described by EAACI/ENDA.8 Readings were done after 15–20 min (immediate reactions), and after 24 and 48 h (late reading for non-immediate reactions). As a standard, amoxicillin (25 mg/ml), amoxicillin/clavulanic acid (25/5 mg/ml), penicillin G (10,000 UI/ml), cefuroxime (7.5 mg/ml and 2 mg/ml) and partly penicilloyl-polylysine (PPL) (0.04 mg/ml), minor determinant mixture (MDM) (0.5 mg/ml), and ampicillin (125 mg/ml, only prick) were tested. In cephalosporin DHR, cefazolin (4 mg/ml), ceftazidime (10 mg/ml and 2 mg/ml), ceftriaxone (10 mg/ml and 2 mg/ml), and cefepime (3.3 mg/ml) were evaluated in addition. In patients with a history of a severe immediate reaction (anaphylaxis/acute urticaria/angioedema), intradermal tests (IDT) were not performed unless skin prick tests with the incriminated drug were negative. In some children and in patients with a history of severe delayed DHR (eg, Drug reaction with eosinophilia and systemic symptoms — DRESS, Stevens-Johnson Syndrome — SJS /Toxic Epidermal Necrolysis — TEN, bullous exanthema) IDT were not performed. These patients were tested by patch tests only. For patch tests, the involved drugs were diluted at 5–10% in petrolatum, fixed on the upper back of the patient using Finn Chambers for 2 days, and read after 2 and 3 days.9
In vitro tests
In some cases with severe DHR, discrepant or negative ST complementary in vitro tests were performed such as determination of specific IgE,10 basophil activation test (BAT), or lymphocytic transformation test (LTT) (read out 3(H)-thymidine incorporation or Enzyme-linked Immunosorbent Assay for IL-5, IL-13, INF-gamma, granulysin, and granzyme B11,12).
Drug provocation test
DPT were suggested to all persons with immediate DHR: either the trigger in negative tests, or a BL from another group (usually amoxicillin or cefuroxime) in positive test results was challenged. The starting challenge dose was 1/100 in mild and moderate reactions, 1/1000 in severe reactions. A tenfold dose increase was done every 30–45 min up to a full therapeutic dose. All DPT were performed by oral route. In delayed DHR, no controlled DPT was performed. However, in the further course therapeutic exposures to BL occurred.
Evaluation and analysis
Results are summarized in Table 1, Table 2, Table 3, Table 4, Table 5, statistics and figures were created by using Graphpad Prism 9 (GraphPad Software, Inc, La Jolla, Calif). The 95% confidence intervals from the proportions of patients with positive test-results in a specific group were calculated as Wilson/Brown interval. Statistical inferential analyses were not performed. Results of the in vitro tests and DPT are summarized.
Table 1.
Patient characteristics
| All |
DHR to penicillins |
DHR to cephalosporins |
|
|---|---|---|---|
| N = 175 | N = 152 | N = 23 | |
| Demographics | |||
| Age | 47.0 (32.0; 62.0) | 46.0 (29.0; 61.8) | 49.0 (40.0; 68.0) |
| Gender (female) | 111 (63.4%) | 98 (64.5%) | 13 (56.5%) |
| Type of DHR | |||
| Anaphylaxis/acute urticaria | 45 (25.7%) | 33 (21.7%) | 12 (52.2%) |
| Delayed urticaria/angioedema | 13 (7.4%) | 10 (6.6%) | 3 (13.0%) |
| Macular exanthema | 18 (10.3%) | 17 (11.2%) | 1 (4.3%) |
| MPE | 71 (40.6%) | 65 (42.8%) | 6 (26.1%) |
| SJS/TEN/bullous exanthema | 4 (2.3%) | 4 (2.6%) | 0 |
| AGEP/pustular exanthema | 4 (2.3%) | 4 (2.6%) | 0 |
| FDE | 3 (1.7%) | 2 (1.3%) | 1 (4.3%) |
| SDRIFE | 1 (0.6%) | 1 (0.7%) | 0 |
| DRESS | 4 (2.3%) | 4 (2.6%) | 0 |
| Unspecified delayed exanthema | 9 (5.1%) | 9 (5.9%) | 0 |
| Other | 3 (1.7%) | 3 (5.9%) | 0 |
| Cases with MDH | 1 (0.6%) | 1 (0.7%) | 0 |
| Latency period anaphylaxis/acute urticaria (min) | 30.0 (5.0; 90.0) | 45.0 (11.3; 165.0) | 5.0 (3.0; 25.0) |
| Latency period delayed DHR (days) | 4.0 (3.0; 7.0) | 5.0 (3.0; 7.0) | 3.0 (2.0; 3.0) |
| Time interval till testing (months) | 6.0 (3.0; 48.0) | 7.0 (3.0; 60.0) | 3.0 (2.0; 6.0) |
| Rate of positive skin tests % | 44/175 (25.1%) | 36/125 (23.7%) | 8/23 (34.8%) |
| Affected test area in delayed DHR (yes) | 88/140 (62.9%) | 83/130 (63.8%) | 5/10 (50.0%) |
Values are median and interquartile ranges (IQR) for continuous variables. Categorical variables reported as n (%).
Multiple drug hypersensitivity syndrome (MDH), drug reaction with eosinophilia and systemic symptoms (DRESS), maculopapular exanthema (MPE), anaphylaxis (ANA), acute generalized exanthematous pustulosis (AGEP), symmetrical drug related flexural and intertriginous exanthema (SDRIFE), stevens johnson syndrome (SJS), toxic epidermal necrolysis (TEN), fixed drug eruption (FDE)
Table 2.
Numbers of skin-test-positivity depending on type of tests and clinical manifestations (%); [95%CI]
| immediate reading positivea (n) | late reading positiveb (n) | |
|---|---|---|
| Anaphylactic manifestationsc n = 45 | n = 17 (37.8%), [25.1%–52.4%] | n = 0 (0%), [0%–7.9%] |
| Delayed DHR-manifestationd n = 106 | n = 1 (0.9%), [0.05%–5.2%] | n = 25 (23.6%), [16.5 %-32.5%] |
| Unclassifiable/othere n = 24 | n = 0 (0%), [0%–13.8%] | n = 1 (4.2%), [0.2%–20.2%] |
IDT with immediate reading and/or prick test.
IDT with late reading and/or patch test.
including cases with bronchospasm, acute urticaria/angioedema.
Macular/maculopapular exanthema, pustular exanthema, bullous exanthema, DRESS, SDRIFE, FDE.
including delayed urticaria/angioedema
Table 3.
Proportions of positive immediate reading skin-test-results and 95% confidence intervals in patients with various anaphylactic manifestations (%); [95%CI]
| Characteristics | Proportion immediate reading positive | |
|---|---|---|
| All anaphylaxis manifestations including acute urticaria/angioedema n = 45 | 17/45, (37.8%), [25.1%–52.4%] | |
| Anaphylaxis Grade I (H.L.Mueller) (urticaria only) n = 4 | 1/4, (20.0%), [1.0%–62.4%] | |
| Anaphylaxis Grade II-IV (H.L.Mueller) n = 41 | 16/41, (39.0%), [25.7%–54.3%] | |
| interval from drug exposure to the onset | 0–1 h n = 35 | 16/35 (45.7%), [30.5%–61.8%] |
| 1–24 h n = 10 | 1/10 (10%), [0.5%–40.4%] | |
| interval between the index reaction and the allergic work-up | <2 months, | 2/5 (40.0%), [7.1–76.9%] |
| 2–6 months | 8/16 (50.0%), [28.0–72.0%] | |
| 2 months to 3 years | 11/23 (47.8%), [29.2–67.0%] | |
| >3years | 4/17 (23.5%), [9.6%–47.3%] | |
Table 4.
Proportion of the different late reading skin-test-results and theirs 95% confidence intervals in patients with various delayed DHR-manifestation (%); [95%CI]
| Characteristics | Proportion combined late skin-test positive (intradermal or patch) | Proportion intradermal skin-test positive | Proportion patch test positive | Proportion positive results only patch test performed | |
|---|---|---|---|---|---|
| All Delayed DHR-manifestation n = 130 | 26/130 (20.0%), [14.0%–27.7%] | 15/111 (13.5%) [8.4%–21.1%] | 18/96 (18.8%) [12.2%–27.7%] | 9/19 (47.4% [27.3%–68.3%] | |
| Macular/maculopapular exanthema n = 92 | 18/86 (20.9%), [13.7–30.7%] | 12/76 (15.8%), [9.3%–25.6%] | 11/63 (17.5%) [10.0%–28.6%] | 4/10 (40.0%), [16.8–68.7%] | |
| Pustular exanthema, n = 4 | 2/4 (50%) [8.9–91.1%] | 2/4 (50%) [8.9–91.1%] | 2/4 (50%) [8.9–91.1%] | n/a | |
| bullous exanthema, n = 4 | 2/4 (50%) [8.9–91.1%] | 1/1 (100%), [5.1–100%] | 1/3 (33.3), [1.7–88.2%] | 1/3 (33.3), [1.7–88.2%] | |
| DRESS n = 4 | 3/4 (75.0%), [30.1%–98.7%] | n/a | 3/4 (75.0%), [30.1%–98.7%] | 3/4 (75.0%), [30.1%–98.7%] | |
| fixed drug eruption n = 3 | 0/3 (0%), [0.0–56.2%] | 0/2 (0%), [0.0–82.2%] | 0/3 (0%), [0.0–56.2%] | 0/1 (0%), [0.0–94.9%] | |
| Delayed urticaria/angioedema n = 13 | 0/13 (0%), [0.0–22.8%] | 0/13 (0%), [0.0–22.8%] | 0/6 (0%), [0.0–39.0%] | n/a | |
| Skin eruption including test site n = 84 | 26/84 (31.0%), [22.1–41.5%] | 15/71 (21.1%), [13.2–32.0%] | 18/55 (32.7%), [21.8–45.9%] | 9/13 (69.2%), [42.4–87.3%] | |
| Skin eruption excluding test site n = 38 | 0/38 (0%), [0.0–9.2%] | 0/34, (0%), [0.0–10.2%] | 0/35 (0%), [0.0–9.9%] | 0/4 (0%), [0.0–49.0%] | |
| interval from drug exposure to the onset | 0-3d | 18/61 (29.5%), [19.6–41.9%] | 12/54 (22.2%), [13.2–34.9%] | 11/43 (25.6%), [14.9–40.2%] | 4/7 (57.1%), [25.0–84.2%] |
| >3d | 8/69 (11.6%), [6.0–21.2%] | 3/57 (5.3%), [1.4–14.4%] | 7/51 (13.2%), [6.5–24.8%] | 5/12 (41.7%), [19.3–68.0%] | |
| interval between index reaction and allergy work-up | <2 m | 2/12 (16.7%), [3.0–44.8] | 1/8 (12.5%), [0.6–47.1%] | 1/10 (10.0%), [0.5–40.4%] | 1/4 (25.0%), [1.3–69.9] |
| 2–6 m | 14/61 (23.0%), [14.2–34.9%] | 5/48 (10.4%), [4.5–22.2%] | 13/48 (27.1%), [16.6–41.0%] | 8/13 (61.5%), [35.5–82.3%] | |
| 2 m -3 y | 20/87 (23.0%), [15.4–32.9%] | 11/73 (15.1%), [8.6–25.0%] | 15/64 (23.4%), [14.8–25.1%] | 8/14 (57.1%), [32.6–78.6%] | |
| >3years | 4/31 (12.9%), [5.1–28.9%] | 3/30 (10.0%), [3.5–25.6%] | 2/22 (9.1%), [1.6–27.8%] | 0/1 (SJS, LTT positive) | |
Table 5.
Summary of in vitro tests and corresponding skin test (ST) results
| A |
B |
|||
|---|---|---|---|---|
| ST + | ST - | ST + | ST - | |
| LTT + | 5 | 11 | 1 | 0 |
| LTT - | 1 | 7 | 0 | 4 |
| BAT + | 1 | 1 | 2 | 0 |
| BAT - | 1 | 2 | 0 | 3 |
A: Drug hypersensitivity reaction (DHR) to penicillins B: DHR to cephalosporins.
Basophil activation test (BAT), Lymphocyte transformation test (LTT)
Results
Study patients and skin test results
A total of 192 patients were screened at both study sites. Of these, 17 subjects had to be excluded because of missing data (Fig. 1). One hundred thirty-eight 138 out of 175 (78.9%) study subjects were from the Division of Allergology and Clinical Immunology, Inselspital Bern and 37 from the Allergy Unit, University Hospital Zurich. One hundred fifty-two of 175 (86.9%) patients were admitted due to a suspected DHR to penicillins, and 23 (13.1%) to cephalosporins (Table 1). The median age of the patients was 47 years (IQR 32.0; 62.0); 111 (63.4%) were female. Clinically, most patients had a maculopapular exanthema (MPE) (71, 40.6%), and 45 (25.7%) an immediate DHR manifestation. A single case had a diagnosis of multiple drug hypersensitivity (MDH), based on published criteria,13 that initially developed from MPE. Prick- and/or IDT with immediate reading were carried out in 156 (89.1%) patients, IDT with late reading with or without patch test in 153 (87.4%) subjects and patch test alone in 19 (10.8%) persons. Because of severe skin reactions IDT was not performed in 10 (5.7%) subjects, and in 9 children (fear of needles) (5.1%). The overall positivity rate of all ST (immediate and late reading) was 25.1% (44/175) (Table 1, Table 2). Individual information on the results for immediate and delayed reading as well as the correlation to clinical manifestations and time interval from drug exposure to the onset of DHR are shown in Table 2, Table 3, Table 4 and Fig. 1, Fig. 2, Fig. 3.
Fig. 1.
Study workflow. a One additional positive skin test in immediate reading. Basophil activation test (BAT), lymphocyte transformation test (LTT), skin test (ST)
Fig. 2.
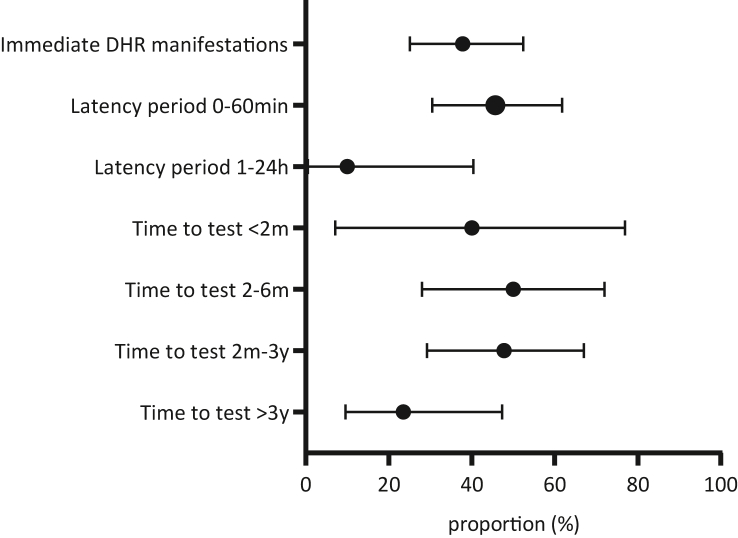
Proportions of positive immediate reading skin-test-results and their 95% confidence intervals in patients with immediate DHR manifestations. The proportion of positive skin test is plotted for the latency period from the first drug intake to the onset of signs and the period from the index reaction to the allergy workup. The propriton is calculated for various time thresholds. 95% confidence intervals (95%CI) of the proportions were calculated by Wilson/Brown method. Drug hypersensitivity reaction (DHR)
Fig. 3.
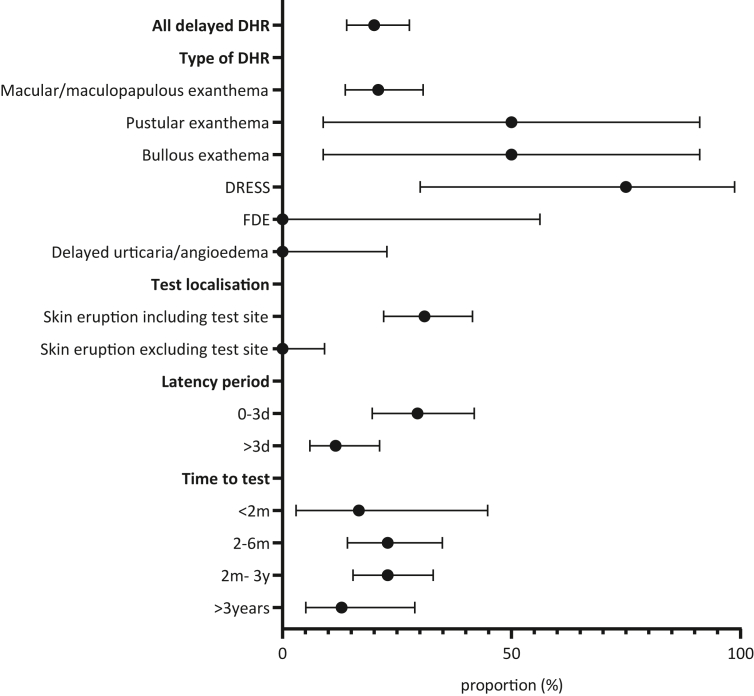
Rate of positive delayed skin test reading with corresponding 95% confidence intervals (95%CI) in various characteristics of delayed DHR manifestations. The plotted data is based on the combined skin test positivity of IDT and patch tests. 95%CI of the proportions were calculated by Wilson/Brown method. Drug hypersensitivity reaction (DHR), intradermal skin test (IDT), Maculopapular exanthema (MPE), drug reaction with eosinophilia and systemic symptoms (DRESS), fixed drug eruption (FDE)
Time interval between the index reaction and the allergic work-up
The time interval between the index reaction and the allergological work-up was assessed in all 175 patients (Table 3, Table 4, Fig. 2, Fig. 3). The shortest test time-point was 4 weeks, the longest interval 30 years. Proportions of positive ST for various time thresholds were assessed as follows: The positivity rate of prick and/or IDT with immediate reading in immediate DHR manifestations to penicillins and cephalosporins was 40.0% [7.1–76.9%] for the interval 0–2 months, 50.0% [28.0–72.0%] for 2–6 months and 47.8%, [29.2–67.0%] for 2 months to 3 years. After 3 years, the positivity rate dropped to 23.5%, [9.6%–47.3%] (Table 3, Fig. 2). Similar results were obtained in penicillins alone (data not shown). In delayed skin test (IDT with late reading and/or patch test), the positivity rate was lower than in immediate reading: 16.7% [3.0–44.8] for the interval 0–2 months, 23.0% [14.2–34.9%] for 2–6 moths, 23.0% [15.4–32.9%] for 2 months to 3 years, and a decrease to 12.9% [5.1–28.9%] after 3 years (Fig. 3, Table 3). Interestingly, all subjects with unknown time interval to the allergy workup (n = 17, all excluded) had negative skin test results.
Time interval from drug exposure to the onset of signs
The time interval from drug exposure to the onset of index signs was assessed for each patient. For immediate DHR manifestations, the positivity rate of ST with immediate reading was 45.7% [95%CI 30.5%–61.8%] in latency periods below 1 h and 10% [95%CI 0.5%–40.4%] for the time interval 1–24 h. For combined delayed ST (IDT with late reading and/or patch), the positivity rate for delayed DHR with a latency period ≤3 days was 29.5% [95%CI 19.6–41.9%] and higher than reactions with a latency period of more than 3 days (11.6% [95%CI 6.0–21.2]; Fig. 3, Table 4). Of note, some delayed DHR with positive delayed skin test reading developed as soon as 2 h after drug intake. Delayed DHR with a long latency period of over 15 days had again a higher positivity rate (mostly DRESS cases).
Age and skin test reactivity
In immediate DHR, the proportion of positive ST in persons aged 18–65 years was 13/35 (37.1%, [23.2–53.7]) versus 4/10 (40.0%, [16.8–68.7]) in persons over 65 years. In delayed DHR, we found the following data: 18–45 years: 7/52 (13.5%, [6.7–25.3]), 45–65 years: 6/38 (15.8%, [7.4–30.4]), and >65 years: 8/26 (30.8%, [16.5–50.0]). It should be noted, that significantly more severe DHR fell on individuals >65 years of age, especially all 4 subjects with DRESS.
Pattern of manifestation in delayed DHR and duration of DHR
The numbers of positive and negative ST for various types of exanthema/hypersensitivity reactions were evaluated. The proportion of positive test results tended to increase as the severity of the reactions increased. Macular exanthema and MPE (20.9%, [95%CI 13.7–30.7]), DRESS (75.0%, [95%CI 30.1%-98.7]), pustular exanthema (50%, [95%CI 8.9–91.1]), and even bullous exanthema (50%, [95%CI 8.9–91.1%]) were found to have the highest ST positivity rates. On the other hand, no sensitization was found in all subjects with delayed urticaria (onset after 24 h) or unspecified delayed exanthema (Fig. 3, Table 4). The proportion of positive ST increased with the duration auf the DHR (DHR 1-7d: 14.3%, [95%CI 7.4–25.7]) vs. DHR >7d (40.0%, [95%CI 26.3–55.4]).
Influence of the test area
In delayed DHR, we found 84 of 130 subjects whose test area was involved in the index reaction. The positivity rate of combined ST with late reading (IDT and/or patch) for these patients was 31.0%, [95%CI 22.1–41.5], whereas the rate was zero if the index-reaction did not involve the test area (Fig. 3, Table 4).
Additional benefit of patch test in addition to intradermal late reading in delayed DHR
Based on the study protocol it was up to the examiner whether or not to conduct IDT only or combined IDT with patch test (n = 77) or patch test alone (n = 19). The combined test showed an additional positive result for the patch test in 2/77 cases, which would not have been recorded with a single IDT.
In vitro tests
In vitro tests were performed in 44 (25.1%) cases (Table 5): In patients with immediate DHR to penicillins, specific IgE to amoxicillin was determined once, which was positive in accordance with the ST. In 5 patients BAT was performed, which was positive twice: once matching the ST, and once showing sensitization to clavulanic acid in negative ST. A single subject with positive ST had a negative BAT to the same drugs. In subjects with immediate DHR to cephalosporins, 5 BATs were performed: 3 tests were negative, 2 were positive, all matching the ST.
In 24 (18.5%) patients with a delayed DHR to penicillins LTT were performed: 5 by measurement of cell proliferation by 3(H)-thymidine and 19 by enzyme-linked Immunosorbent assay for IL-5, IL-13, INF-gamma, granulysin and granzyme B. 16 of 24 (66.7%) LTT were positive: five-times matching ST, and in 11 cases with negative ST. Out of eight negative LTTs, only 1 subject had a positive ST. For delayed DHR to cephalosporins, all 5 LTTs did match the ST (with one positive ST).
Drug provocation tests/reexposure/exposure to possibly cross-reactive drugs
Eighteen DPT were performed in subjects with an immediate DHR to penicillin. In 9 patients who tested positive with penicillins, DPT were performed with cephalosporins. All did tolerate the given drug. In the 9 patients with a negative ST to pencillins, DPT was performed with amoxicillin. In this group, 2 out of 9 (22.2%) patients developed an acute urticaria within 60 and 120 min. In subjects who suffered an immediate DHR to cephalosporins, four DPT were performed: two-times with amoxicillin (ST positive to cefuroxime), and two-times with cefuroxime (ST negative to cefuroxime). All 4 DPT were well tolerated.
In delayed DHR, no controlled DPT were performed. However, in the further course exposures to BL occurred in 4 patients with penicillin DHR and positive ST results, cefuroxime/ceftriaxone was well tolerated later. In 6 subjects with a negative ST to penicillin, amoxicillin/clavulanic acid was administered with no adverse reaction. In 1 case with MPE and documented sensitization to amoxicillin, an accidental re-exposure to amoxicillin led to a recurrence of the skin reaction. In 1 subject with delayed DHR to cefuroxime and negative IDT, the re-exposure was well tolerated.
Discussion
The work-up with a compatible history for an allergic reaction due to BL revealed positive ST results in 25% (43/175) of the patients tested. Patients with an immediate-type DHR had a higher rate of positive ST results than those with clinical manifestations of a delayed type DHR (38% versus 20%). In 28 out of 45 patients with immediate DHR manifestations, IDT with immediate reading were negative. In 1 patient, reactivity to clavulanic acid was observed in an additional in vitro assay (BAT). Overall, even with the inclusion of additional in vitro tests, we were still unable to make a conclusive diagnosis in 27/45 patients (Fig. 1).When evaluating different manifestations in delayed DHR, the positivity rate of ST increased with the severity of the index reaction and with the duration of the DHR. In the vast majority (104/130) patients with delayed DHR or unclassifiable delayed manifestations, IDT with late reading and/or patch tests were negative. Amongst those, positive results however were found in LTT in 11 subjects. Overall, we were unable to establish a conclusive diagnosis in 93/130 patients even with the inclusion of additional in vitro tests.
How can we improve skin test results?
In delayed DHR with generalized skin eruptions affecting the skin area but tested later, the tests showed positive results in 31% (26/84) cases. If the skin test area was not involved in the index reaction, we did not find a single positive ST (0/38). This difference may be due to a presumably more pronounced index reaction with spreading also to the forearms (usual test area in IDT). However, it may indicate that the sensitivity of these tests could be improved if performed in skin areas affected in the index reaction. Such correlation has been described for fixed drug eruption14 and is probably attributable to resident T memory cells.15
A combination of IDT with late reading and patch test was performed in 77 patients. Patch tests showed an additional positive result in two cases that would not have been detected by IDT alone. Based on these results, combined testing might be useful in delayed DHR, because it may increase sensitivity.
It is known that ST sensitivity decreases over time after an allergic reaction.16,17 Moreover, IgE mediated penicillin allergies wane over time.18 In our study the rate of positive ST decreased significantly after 3 years for both immediate and delayed DHR. Although current guidelines advise to perform drug testing within 6 months after the index reaction,5 based on our data in BL, this period could possibly be extended to 3 years.
How to manage patients depending on their test-results?
-
•
Patients with a suitable positive test result: In such patients, cross-reactivity with other BL has been investigated in various studies in skin tests and partly also with DPT. These literature data allow evidence-based recommendations regarding potentially cross-reactive BL for exposure prophylaxis and for presumed well-tolerated alternatives.2 Patients with a negative test result: Such patients are not well investigated in the literature and in everyday practice it is usually unclear whether a false negative or true negative test result should be assumed. True negative test results may be due to "adverse events" that were clinically classified as "BL DHR" but which were due to concurrent diseases, other drugs or non-specific mechanisms/interactions. Typical examples are an underlying urticarial disease or new onset urticaria in immediate DHR19 or concomitant viral infections in delayed DHR, especially in children.20 Nevertheless, false negative tests do not seem to be rare.21 A non-allergic mechanism might have been involved which poses a risk for a subsequent reaction upon exposure.22,23 In the case of negative test results, we considered the following options for recommending exposure prophylaxis: In cases with false negative ST, the same recommendations as in cases with positive ST would be appropriate.
-
•
In nonallergic unspecific DHR, like BL induced neuro/nephrotoxicity,22 causally involved drugs must usually also be avoided. Nevertheless, in such cases the same cross-reactivities cannot be expected as in allergic DHR.
-
•
In cases with no causal relationship to the BL, the initially suspected drug could be used as needed.
However, we were usually not able to distinguish between these 3 reasons for negative test results. In the case of anamnestically moderate or severe reactions, we therefore recommended not only the avoidance of the triggering agent, but also the avoidance of potentially cross-reactive BL (as in the case of skin test positive patients). Only in few patients could we find out through DPT or further follow-up whether such recommendations were adequate.
Drug provocation test
DPT should be used to further evaluate DHR if the diagnostic gain outweighs the risk.24 Since anaphylaxis can be treated with early intervention by the administration of epinephrine, intravenous volume supply as well as intensive care measures,24 DPT with cautious gradual dose increase is a decent approach in patients with a clear immediate DHR.25 In our study, 11 patients were challenged with the causative BL, and 11 patients had DPT with a BL antibiotic expected to be not cross-reactive, as described under the methods section. Of the remaining 16 patients with negative ST, most had mild to moderate index reactions and refused DPT. In such patients, we recommended in the allergy passport an exposition prophylaxis with avoidance of the triggering agent and potentially cross-reactive BLs. In the case of a severe index reaction, we additionally recommended monitoring at the first administration of presumably tolerated BL, and a ‘graded challlenge’ in the case of a threatening reaction.
In cases with delayed DHR, a further approach by DPT is more problematic than in immediate DHR: Unlike anaphylaxis, the natural progression is unpredictable, even with early recognition and intervention. Therefore, in severe delayed reactions (eg, acute generalized exanthematous pustulosis—AGEP, DRESS, SJS/TEN), most relevant guidelines advise against DPT.24,26 In addition, since delayed DHR manifestations usually occur many hours to days after exposure, a cautious dose increase to a full therapeutic dose would take days to weeks, which would not be justifiable from an infectiological point of view. There are no standardized protocols for DPT in delayed DHR.14 In mild or moderate delayed DHR (only skin eruption) with negative ST, direct therapeutic administration, if necessary instead of a DPT, may be preferable.27 In our study, all patients with unexplained delayed DHR or unclassifiable manifestations were not challenged. Most of these patients had mild or moderate index reactions, limited to the skin or an alternative explanation for the reaction (eg, viral infection). In these subjects a direct therapeutic reexposure when necessary was proposed. In severe delayed DHR or cases with systemic involvement and negative test results, we recommended (assuming a false negative test result), to avoid the suspected drug and BL with a high risk of cross-reactivity. All patients received an allergy passport with information of alternative treatments. Unfortunately, for true negative tests, this is not ideal: we unnecessarily limit options of antibiotics in patients whose reaction was not caused by BL. Furthermore, in patients with a non-allergic mechanism, a new DHR might occur in the case of reexposure.
We recorded the outcome of re-exposure to the causative agent in 7 patients with mostly mild index reactions and negative test results; all were well tolerated.
Weaknesses of the study and possibilities for improvement
The limitation of this study is the rather small number of included patients. The proportion of severe DHR was higher than described by other authors.27,28 Skin test positivity may be overestimated in our study. Recent studies found indeed a lower skin test positivity rate, patients were, however, recruited from a retrospective chart28 or were prescreend for mild DHR.27 In addition, the follow-up time was short. Moreover, in some cases only IDT was performed without additional patch test. A larger patient cohort and a longer observation period of these study patients with systematic evaluation on drug exposure could improve the recommendations, especially in those subjects with negative test results. Fortunately, participation in our study did not impose any additional burden for patients. For the medical staff, the additional workload for the study was minimal compared with a routine allergy workup. We are therefore planning a larger longitudinal study with defined recommendations regarding drugs to be avoided or allowed. After the initial work-up, patients will be interviewed in 2-year intervals about the further course of the disease. Sufficiently large data sets may reveal patterns for better detection of true negative test results.
Conclusion
The management of patients with DHR but negative ST remains uncertain, especially in delayed DHR. Combined IDT and patch tests, ideally done within 3 years after the index reaction and tested within the affected skin area might improve ST positivity. In immediate DHR, DPT is a suitable option to offer alternative drugs for future treatments. However, most patients in our collective had a delayed DHR with negative ST in 80%. DPT may be unnecessary in these patients because the effort may exceed the diagnostic benefit. Additional in vitro tests reduced the proportion of negative tests to 72%. In case of severe delayed DHR, we recommended to avoid the causative agent and all BL with high risk of cross-reactivity, analogue to T-cell mediated drug allergy. With this procedure, we might unnecessarily deprive these patients of important drugs. However, in case of mild to moderate DHR, we recommended direct reexposure with the suspected drug. The benefit/risk ratio of this approach should be verified in larger longitudinal studies.
Abbreviations
95%CI, 95% confidence interval; AGEP, acute generalized exanthematous pustulosis; BAT, basophil activation test; BL, beta-lactam antibiotic; DHR, drug hypersensitivity reaction; DPT, drug provocation test; DRESS, drug reaction with eosinophilia and systemic symptoms; FDE, fixed drug eruption; LTT, lymphocyte transformation test; MDH, multiple drug hypersensitivity syndrome; MPE, maculopapular exanthema; IDT, intradermal skin test; SDRIFE, symmetrical drug related flexural and intertriginous exanthema; SJS, Stevens-Johnson syndrome; ST, skin test; TEN, toxic epidermal necrolysis.
Ethics approval and consent to participate
The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich and Bern, ID 2017–01518). All patients included gave informed consent for study participation and data publication.
Role of the funding source
This study was funded by the Ulrich-Mueller-Gierok Allergy Foundation Bern, Switzerland and CK Care (Christine Kühne – Center for Allergy Research and Education, Davos, Switzerland). Their representatives were not involved in the design of the study or any data collection, analysis, or interpretation.
Confirmation of unpublished work
This manuscript is an original work and is not under consideration by any other journal. All authors approved the content of the manuscript and this submission. All authors listed have contributed sufficiently to the project to be included as authors.
Author contribution
LJ, BS, MG and PSG designed and planned the study, LJ, SH, AG, CM, TMN, MG, BS, AH and PSG acquired the data, LJ, MH and BS analysed the data and participated in the interpretation of the data, LJ and BS wrote the manuscript. All authors critically reviewed the manuscript and gave final approval of the submitted work.
Availability of data and materials
The raw datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors report no competing interests.
Acknowledgements
The authors acknowledge Prof. Werner Pichler for editing the manuscript.
References
- 1.Johansson S.G., Bieber T., Dahl R., Friedmann P.S., Lanier B.Q., Lockey R.F., et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol. May 2004;113(5):832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Caruso C., Valluzzi R.L., Colantuono S., Gaeta F., Romano A. β-Lactam allergy and cross-reactivity: a clinician's guide to selecting an alternative antibiotic. J Asthma Allergy. 2021 Jan 18;14:31–46. doi: 10.2147/JAA.S242061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastalerz L., Setkowicz M., Sanak M., Szczeklik A. Hypersensitivity to aspirin: common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004;113:771–775. doi: 10.1016/j.jaci.2003.12.323. [DOI] [PubMed] [Google Scholar]
- 4.Broyles A.D., Banerji A., Castells M. Practical guidance for the evaluation and management of drug hypersensitivity: general concepts. J Allergy Clin Immunol Pract. 2020 Oct;8(9S):S3–S15. doi: 10.1016/j.jaip.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Wurpts G., Aberer W., Dickel H., et al. Guideline on diagnostic procedures for suspected hypersensitivity to beta-lactam antibiotics: guideline of the German society for allergology and clinical immunology (DGAKI) in collaboration with the German society of allergology (AeDA), German society for pediatric allergology and environmental medicine (GPA), the German contact dermatitis Research group (DKG), the Austrian society for allergology and immunology (ÖGAI), and the Paul-ehrlich society for chemotherapy (PEG) Allergol Select. 2020 May 28;4:11–43. doi: 10.5414/ALX02104E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano A., Atanaskovic-Markovic M., Barbaud A., et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams - an EAACI position paper. Allergy. 2020 Jun;75(6):1300–1315. doi: 10.1111/all.14122. [DOI] [PubMed] [Google Scholar]
- 7.Jeimy S., Ben-Shoshan M., Abrams E.M., Ellis A.K., Connors L., Wong T. Practical guide for evaluation and management of beta-lactam allergy: position statement from the Canadian Society of Allergy and Clinical Immunology. Allergy Asthma Clin Immunol. 2020 Nov 10;16(1):95. doi: 10.1186/s13223-020-00494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockow K., Garvey L.H., Aberer W., Atanaskovic-Markovic M., Barbaud A., Bilo M.B., et al. Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper Allergy. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 9.Barbaud A., Gonçalo M., Bruynzeel D., Bircher A., European Society of Contact Dermatitis Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45(6):321–328. doi: 10.1034/j.1600-0536.2001.450601.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Poorten M.M., Van Gasse A.L., Hagendorens M.M., et al. Serum specific IgE antibodies in immediate drug hypersensitivity. Clin Chim Acta. 2020 May;504:119–124. doi: 10.1016/j.cca.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Mayorga C., Celik G., Rouzaire P., et al. Vitro tests for drug allergy task force of EAACI drug interest group. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI drug allergy interest group position paper. Allergy. 2016 Aug;71(8):1103–1134. doi: 10.1111/all.12886. [DOI] [PubMed] [Google Scholar]
- 12.Mayorga C., Ebo D.G., Lang D.M., et al. Controversies in drug allergy: in vitro testing. J Allergy Clin Immunol. 2019 Jan;143(1):56–65. doi: 10.1016/j.jaci.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Jörg L., Yerly D., Helbling A., Pichler W. The role of drug, dose, and the tolerance/intolerance of new drugs in multiple drug hypersensitivity syndrome. Allergy. 2020 May;75(5):1178–1187. doi: 10.1111/all.14146. [DOI] [PubMed] [Google Scholar]
- 14.Phillips E.J., Bigliardi P., Bircher A.J., et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. 2019 Jan;143(1):66–73. doi: 10.1016/j.jaci.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaide O., Emerson R.O., Jiang X., et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015 Jun;21(6):647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanca M., Torres M., Garcia J., et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5 Pt 1):918–924. doi: 10.1016/s0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
- 17.Romano A., Gaeta F., Valluzzi R.L., Zaffiro A., Caruso C., Quaratino D. Natural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporins. Allergy. 2014 Jun;69(6):806–809. doi: 10.1111/all.12390. [DOI] [PubMed] [Google Scholar]
- 18.Trubiano J.A., Adkinson N.F., Phillips E.J. Penicillin allergy is not necessarily forever. J Am Med Assoc. 2017 Jul 4;318(1):82–83. doi: 10.1001/jama.2017.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman S., Localio R., Apter A.J. Association between chronic urticaria and self-reported penicillin allergy. Ann Allergy Asthma Immunol. 2016 Apr;116(4):317–320. doi: 10.1016/j.anai.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Caubet J.C., Kaiser L., Lemaître B., Fellay B., Gervaix A., Eigenmann P.A. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011 Jan;127(1):218–222. doi: 10.1016/j.jaci.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransson S., Mosbech H.F., Elberling J., Kappel M., Garvey L.H. Intradermal testing identifies 1 in 4 patients with nonimmediate penicillin allergy. Int Arch Allergy Immunol. 2021 Apr 19:1–8. doi: 10.1159/000515080. [DOI] [PubMed] [Google Scholar]
- 22.Roger C., Louart B. Beta-Lactams toxicity in the intensive care unit: an underestimated collateral damage? Microorganisms. 2021 Jul 14;9(7):1505. doi: 10.3390/microorganisms9071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y., Han Y., Zhang X., et al. Penicillin causes non-allergic anaphylaxis by activating the contact system. Sci Rep. 2020 Aug 25;10(1):14160. doi: 10.1038/s41598-020-71083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aberer W., Bircher A., Romano A., et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman P., Nicklas R.A., Randolph C., et al. Anaphylaxis--a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015 Nov;115(5):341–384. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Jörg L., Yerly D., Pichler W. Multiple drug hypersensitivity syndrome (MDH) should not be diagnosed by drug provocation tests. J Allergy Clin Immunol Pract. 2020 Feb;8(2):822–823. doi: 10.1016/j.jaip.2019.10.050. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa S.S., Conn K., Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract. 2019;7(7):2163–2170. doi: 10.1016/j.jaip.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Wöhrl S., Ostermayer C., Sesztak-Greinecker G., Jarisch R., Hemmer W., Wantke F. Drug-specific history, skin and in vitro tests can reduce the need for drug provocation tests in betalactam-hypersensitivity. Allergol Int. 2021 Apr;70(2):244–251. doi: 10.1016/j.alit.2020.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets used and analysed during the current study are available from the corresponding author upon reasonable request.