Abstract
Background
Studies have shown the efficacy of asthma biologics in real-world settings, confirming the generalizability of randomized controlled trial (RCT) results, but studies on more than one biologic are scarce. Accordingly, little is known about the different background characteristics in users of asthma biologics. This study aimed to describe the backgrounds of asthma patients using biologics (omalizumab, mepolizumab, benralizumab, and dupilumab) and examine the effectiveness of these biologics for reducing asthma exacerbations and total systemic corticosteroid doses.
Methods
We conducted a retrospective cohort study using self-controlled methods to evaluate the association between the use of biologics and reduction in exacerbations and hospitalizations using a large-scale health insurance claims database in Japan.
Results
Of 355 continuously treated asthma patients using biologics, 119, 82, 69, and 85 patients were assigned to the omalizumab, mepolizumab, benralizumab, and dupilumab groups, respectively. The baseline characteristics differed among users of biologics. The incidence ratios of exacerbations and hospitalizations during biologics use were 0.68 (95% confidence interval, 0.62–0.74) and 0.65 (0.55–0.77) compared with the period before biologics use. The total systemic corticosteroid dose equivalent to prednisolone per person-year was reduced from a median of 600 [interquartile range, 90–1713] mg to 164 [0–1010] mg (P < .001). Similar results were obtained for individual biologics with a few exceptions.
Conclusions
The background characteristics of biologics users differed in a real-world setting. Our results confirmed findings from RCTs demonstrating that each biologic (omalizumab, mepolizumab, benralizumab, and dupilumab) is associated with decreased exacerbation numbers and corticosteroid-sparing effects, even outside of the controlled settings of RCTs.
Keywords: Asthma biologics, Exacerbation, Patient backgrounds, Severe asthma, Steroids
Introduction
Asthma affects approximately 235 million people worldwide, accounting for 338 000 deaths and 15 million disability-adjusted life years lost annually.1,2 Patients with severe asthma are defined as individuals who require treatment with high-dose inhaled corticosteroids (ICS) plus a second controller and/or systemic corticosteroids to prevent them from becoming “uncontrolled” or who remain “uncontrolled” despite this therapy.3 Severe asthma, which is estimated to be present in 5–10% of the entire population of patients with asthma,3 is a high-burden condition because frequent exacerbations can occur, impairing quality of life.4, 5, 6 Furthermore, systemic corticosteroids, often prescribed for patients with severe asthma, can lead to additional complications related to their use.7,8 New add-on therapies are needed for reducing this burden, and in the last decade, several biologics targeting type 2 inflammation9 have become available for use in clinical practice.
Type 2 inflammation is an important molecular mechanism of asthma, and as the understanding of it has progressed, biologic agents have been developed to inhibit the activity of mediators involved in this type of inflammation, such as immunoglobulin E (IgE), interleukin (IL)-4/5/13/33, and thymic stromal lymphopoietin.10 Several randomized controlled trials (RCTs) demonstrated that these biologics reduce asthma exacerbation rates11, 12, 13, 14, 15, 16, 17 and enable corticosteroid-sparing therapies.18, 19, 20 Biologics that were available in Japan as of July 2020 include anti-immunoglobulin E (omalizumab), anti-interleukin-5 (mepolizumab), anti-interleukin-5 receptor (benralizumab), and anti-interleukin-4 receptor (dupilumab) antibodies.
Considering the limited generalizability of RCTs, the real-world effectiveness of biologics has been verified using various registries and databases.21, 22, 23, 24, 25, 26, 27, 28, 29 However, these registries were usually created for a specific biologic, and the validation using a single registry containing multiple types of biologics, which may be a less biased approach especially when describing the patients’ backgrounds, has rarely been attempted. Therefore, differences in patient backgrounds in a real-life clinical setting also remain uncertain. The present study using a large-scale health insurance claims database in Japan aimed to describe the backgrounds of patients using asthma biologics and examine the effectiveness of biologics for reducing asthma exacerbations and total doses of systemic corticosteroids in a real-world setting.
Methods
Data source
We used the health insurance claims database managed by JMDC Inc. (Tokyo, Japan).30 The database includes anonymized data of medical (inpatient and outpatient) and pharmacy (dispensing) claims from 9.8 million employees and their family members (aged <75 years), covering approximately 10% of the total population of Japan (as of June 2020). Patient demographics, as well as date-stamped claims information about in- and outpatient services (eg, diagnoses, procedures, costs, and medical institutions), are included. Unless the employee withdraws from the participating health insurance programs (ie, by quitting or changing jobs), the individual's medical and treatment history of all healthcare procedures with insurance compensation can be traced using this database.
Patient selection
Of all patients in the database who had an asthma diagnosis (ICD-10 code J45/46) and were prescribed ICS or ICS with long-acting beta2-agonist (LABA), we included initially those who received an asthma biologic at least once between March 1, 2009, and July 31, 2020. The index date was defined as the date of the first administration of an asthma biologic (omalizumab, mepolizumab, benralizumab, or dupilumab).
Patients were included if they met all of the following criteria: at least 1 administration record of an asthma biologic; aged ≥12 years at the index date; asthma diagnosis 12 months or more before the index date; and either at least 4 separate prescriptions of ICS or ICS/LABA within the 12 months before the index date, or 3 separate prescriptions of ICS or ICS/LABA within the abovementioned period, and at least 1 prescription of ICS or ICS/LABA within 4 months preceding this period. These study inclusion criteria were established to exclude seasonal effects on asthma status or other diseases such as influenza infections. Patients who met any of the following criteria were excluded: combined usage of biologics; diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA); and either final observation or withdrawal from the database or discontinuation of biologics within 3 months after the index date.
Comorbidities
We identified the following comorbidities within 12 months before the index date based on the ICD-10 codes: 1) allergic rhinitis (J30); 2) gastroesophageal reflux disease (GERD; K21); 3) chronic obstructive pulmonary disease (J43/44); 4) diabetes mellitus (DM; E10-14); 5) chronic paranasal sinusitis (J32); 6) atopic dermatitis (L20); 7) osteoporosis (M80/81); 8) sleep apnea (G47.3); 9) nasal polyp (J33); 10) EGPA (M30.1); 11) allergic bronchopulmonary mycosis (B44.1, B49); and 12) aspirin intolerance (T88.7, J45.1, L50.8).
Drug prescriptions and dosages
Identification of the treatment drugs for asthma and dosage calculation of inhaled and systemic corticosteroids were based on the following period: from the date of the most recent asthma visit with ICS or ICS/LABA prescription between 12 and 16 months before the index date to the index date (the prescription of the index date was not included). If no such date existed, the start date was set as 12 months before the index date. Dosage calculations of systemic corticosteroids during biologics use were based on the following period: from the index date to the “termination date” mentioned below. As is often done in database research on asthma, ICS dosages per day were calculated according to the following formula: (sum of the ICS dosages in the abovementioned period)÷(number of days of the period × 0.8).
The approved dosages of asthma biologics in Japan as of July 31, 2020 are as the following: omalizumab is administered every 2–4 weeks according to the baseline total IgE level and body weight; mepolizumab is administered at a dosage of 300 mg every 4 weeks; benralizumab is administered at a dosage of 30 mg every 4 weeks for the first 3 times and then every 8 weeks thereafter; dupilumab is administered at an initial dosage of 600 mg followed by 300 mg every 2 weeks.
Study design and statistical analyses
We conducted this cohort study using self-controlled methods to evaluate the incidence rate ratios of asthma exacerbation events and exacerbation-related admissions. An asthma exacerbation event was defined as an event requiring oral corticosteroids (OCS) equivalent to prednisolone 15 mg per day for 3 to 9 days or an event requiring corticosteroid injection. Events within 14 days were treated as one event. A hospitalization due to asthma exacerbation was identified either by the primary disease of the hospitalization being asthma (ICD-10 code J45/46) or by having at least 1 prescription of an injectable corticosteroid if the primary disease name was blank.
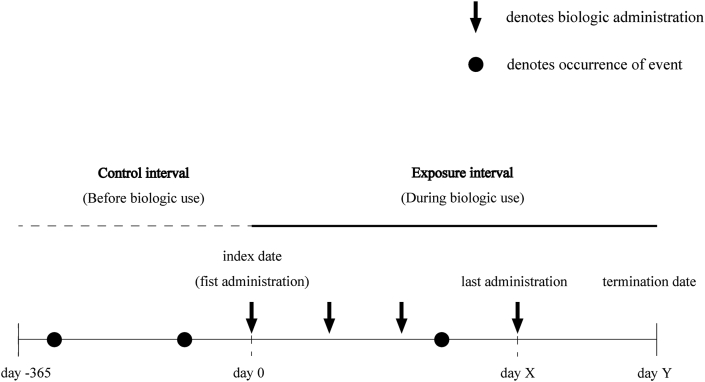
The statistical analysis design based on the self-controlled method is shown in Fig. 1. The date of the first administration of biologics served as the index date for defining exposure. The beginning of the observation time was 12 months before the index date, and the termination of the observation time was defined as the later date of either 60 days after the last administration of biologics or the withdrawal date from the database. If the administration interval exceeded 3 months, the time point immediately before the interval was considered as the last administration date. We defined “exposure interval” as the interval from the index date to the termination date of the observation time.
Fig. 1.
Schematic of the study design. The incidence rates of exacerbations and hospitalizations (numbers per patient-year) were calculated based on the control and exposure intervals. The termination date (day Y) is the latest time point among the date of last drug administration (X) + 60 days, withdrawal date, and final observation date.
We estimated the incidence rate ratios of asthma exacerbation events and exacerbation-related admissions during the exposure interval compared with the control interval using a conditional Poisson regression model with the logarithm of the length of either exposure or control interval as the offset.31 As a sensitivity analysis, we estimated the incidence rate ratio as above after limiting the study population to patients with at least 12 months from the index date to the termination date and defining “exposure interval” as the interval from the index date to 12 months after the index date. It should be noted that some patients were switched to another biologic, but as we focused on the first biologic, the second or third biologic use was not included in the “exposure interval.”
In addition, we compared the total doses of systemic corticosteroids per person-year using the paired Wilcoxon signed-rank test.
We performed an additional sensitivity analysis to assess the robustness of the study findings. We limited the study population to those who had at least 1 of the comorbidities associated with type 2 inflammation (allergic rhinitis, sinusitis, atopic dermatitis, nasal polyp, and aspirin intolerance).
Normally distributed continuous variables were presented as mean (SD), and non-normally distributed as median [IQR]. Continuous variables were compared among groups using the analysis of variance (ANOVA), and categorical variables were compared using the Kruskal-Wallis test. A P-value <.05 was considered statistically significant. All statistical analyses were performed using R software version 4.0.2.
The article was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (See eTable S1).
Results
Clinical characteristics of the study population
We identified 1699 patients who were administered any of the defined asthma biologics at least once between March 2009 and July 2020. Of the 570 patients who met our inclusion criteria, one patient (0.1%) was excluded due to combined usage of omalizumab and mepolizumab, 60 patients (10.5%) due to EGPA comorbidity, and 154 (27.0%) because of withdrawal or discontinuation of biologics within 3 months after the index date (Fig. 2). Finally, 119, 82, 69, and 85 patients were categorized into the omalizumab, mepolizumab, benralizumab, and dupilumab groups, respectively.
Fig. 2.
Patient flowchart. After applying the inclusion and exclusion criteria, 119, 82, 69, and 85 patients were classified as omalizumab, mepolizumab, benralizumab, and dupilumab users. EGPA, eosinophilic granulomatosis with polyangiitis; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist.
Table 1 summarizes the demographic data and comorbidities within 12 months before the index date (control interval). The mean ages in the omalizumab, mepolizumab, benralizumab, and dupilumab groups were 43.5 (16.0), 53.1 (13.4), 51.4 (11.1), and 44.8 (11.8) years, respectively. Male sex was less predominant in the omalizumab group (33.6%) in contrast to the dupilumab group (61.2%). Common comorbidities (≥10%) were allergic rhinitis (omalizumab, mepolizumab, benralizumab, and dupilumab: 87.4%, 82.9%, 85.5%, and 80.0%), GERD (47.9%, 53.7%, 55.1%, and 37.6%), DM (16.8%, 34.1%, 24.6%, and 23.5%), chronic paranasal sinusitis (31.1%, 46.3%, 49.3%, and 25.9%), and atopic dermatitis (18.5%, 22.0%, 14.5%, and 75.3%). Comorbid nasal polyp was more common in the mepolizumab and benralizumab groups (6.1% and 13.0%, respectively) than in the omalizumab and dupilumab groups (4.2% and 2.4%, respectively).
Table 1.
Demographic characteristics of the study population.
| Omalizumab (n = 119) |
Mepolizumab (n = 82) |
Benralizumab (n = 69) |
Dupilumab (n = 85) |
P-valuea | |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 43.5 (16.0) | 53.1 (13.4) | 51.4 (11.1) | 44.8 (11.8) | <.001 |
| Male, n (%) | 40 (33.6) | 38 (46.3) | 28 (40.6) | 52 (61.2) | .001 |
| Comorbidities, n (%) | |||||
| Allergic rhinitis | 104 (87.4) | 68 (82.9) | 59 (85.5) | 68 (80.0) | .53 |
| GERD | 57 (47.9) | 44 (53.7) | 38 (55.1) | 32 (37.6) | .11 |
| COPD | 26 (21.8) | 15 (18.3) | 16 (23.2) | 5 (5.9) | .011 |
| DM | 20 (16.8) | 28 (34.1) | 17 (24.6) | 20 (23.5) | .045 |
| Sinusitis | 37 (31.1) | 38 (46.3) | 34 (49.3) | 22 (25.9) | .003 |
| Atopic dermatitis | 22 (18.5) | 18 (22.0) | 10 (14.5) | 64 (75.3) | <.001 |
| Osteoporosis | 17 (14.3) | 30 (36.6) | 19 (27.5) | 7 (8.2) | <.001 |
| Sleep apnea | 4 (3.4) | 2 (2.4) | 2 (2.9) | 1 (1.2) | .80 |
| Nasal polyp | 5 (4.2) | 5 (6.1) | 9 (13.0) | 2 (2.4) | .031 |
| ABPM | 2 (1.7) | 1 (1.2) | 6 (8.7) | 0 (0.0) | .003 |
| Aspirin intolerance | 2 (1.7) | 2 (2.4) | 7 (10.1) | 2 (2.4) | .016 |
ABPM, allergic bronchopulmonary mycosis; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GERD, gastroesophageal reflux disease; SD, standard deviation
Analysis of variance for continuous variables and Kruskal-Wallis test for categorical variables
Regarding prescription records, leukotriene receptor antagonists (omalizumab, mepolizumab, benralizumab, and dupilumab: 95.0%, 81.7%, 82.6%, and 70.6%), antihistamines (78.2%, 67.1%, 72.5%, and 94.1%), and xanthines (eg, theophylline, 67.2%, 54.9%, 46.4%, and 32.9%) were commonly used as a second controller in all groups (See Table S2). The percentages of daily OCS therapy (prescription days ≥183 days per year) were different among groups (12.6%, 35.4%, 31.9%, and 7.1%; P < .001).
Median ICS dosages per day equivalent to budesonide were 1,480, 1,563, 1,409, and 944 μg in the omalizumab, mepolizumab, benralizumab, and dupilumab groups, respectively (P < .001; See Table S3). The median dosages of daily OCS equivalent to prednisolone were approximately 5.0 mg in all groups of patients who required daily OCS.
Risk of asthma exacerbation and admission
There were 337 asthma exacerbation events per 100 person-years during the control interval and 218 during the exposure interval (incidence ratio, 0.68; 95% confidence interval [CI], 0.62–0.74; Table 2). For all 4 biologics, significant reductions were detected; the incidence rate ratios in the omalizumab, mepolizumab, benralizumab, and dupilumab groups were 0.65 (0.55–0.77), 0.66 (0.57–0.76), 0.67 (0.56–0.81), and 0.86 (0.73–0.99), respectively. The results were robust in a sensitivity analysis in which the study population was limited to patients with at least 12 months exposure interval, and the exposure interval was truncated at 12 months. Under these conditions, there were 261 asthma exacerbation events per 100 person-years during the control interval and 147 during the exposure interval (0.68; 0.63–0.74; See Table S4).
Table 2.
Rates and incidence ratios of asthma exacerbations and admissions during control and exposure intervals.
| Rate per 100 person-y |
Incidence ratioa | P-valuea | |||
|---|---|---|---|---|---|
| Total | Before biologic use (Control interval) |
During biologic use (Exposure interval) |
|||
| Asthma exacerbations | |||||
| All biologics (n = 355; before biologic use: 355.0 person-y, during biologic use: 447.9 person-y) |
270.5 | 337.4 | 217.5 | 0.68 (0.62–0.74) | <.001 |
| Omalizumab (n = 119; before biologic use: 119.0 person-y, during biologic use: 188.3 person-y) |
298.1 | 397.5 | 235.2 | 0.65 (0.55–0.77) | <.001 |
| Mepolizumab (n = 82; before biologic use: 82.0 person-y, during biologic use: 120.3 person-y) |
252.2 | 337.8 | 193.8 | 0.66 (0.57–0.76) | <.001 |
| Benralizumab (n = 69; before biologic use: 69.0 person-y, during biologic use: 71.3 person-y) |
303.0 | 362.3 | 245.6 | 0.67 (0.56–0.81) | <.001 |
| Dupilumab (n = 85; before biologic use: 85.0 person-y, during biologic use: 68.0 person-y) |
209.8 | 232.9 | 180.8 | 0.86 (0.73–0.99) | .048 |
| Hospitalizations | |||||
| All biologics (n = 355; before biologic use: 355.0 person-y, during biologic use: 447.9 person-y) |
20.7 | 25.9 | 16.5 | 0.65 (0.55–0.77) | <.001 |
| Omalizumab (n = 119; before biologic use: 119.0 person-y, during biologic use: 188.3 person-y) |
26.7 | 31.8 | 23.9 | 0.82 (0.60–1.12) | .21 |
| Mepolizumab (n = 82; before biologic use: 82.0 person-y, during biologic use: 120.3 person-y) |
15.8 | 23.2 | 10.8 | 0.64 (0.45–0.89) | .009 |
| Benralizumab (n = 69; before biologic use: 69.0 person-y, during biologic use: 71.3 person-y) |
20.0 | 27.5 | 12.6 | 0.45 (0.31–0.66) | <.001 |
| Dupilumab (n = 85; before biologic use: 85.0 person-y, during biologic use: 68.0 person-y) |
15.7 | 20.0 | 10.3 | 0.48 (0.33–0.69) | <.001 |
Conditional Poisson regression model including the logarithm of the interval time as the offset
Regarding hospitalizations due to asthma exacerbation, there were 26 hospitalizations per 100 person-years during the control interval and 17 during the exposure interval (incidence ratio, 0.65; 95% CI, 0.55–0.77; Table 2). All biologics apart from omalizumab significantly reduced hospitalization rates; the incidence rate ratios in the omalizumab, mepolizumab, benralizumab, and dupilumab groups were 0.82 (0.60–1.12), 0.64 (0.45–0.89), 0.45 (0.31–0.45), and 0.48 (0.33–0.69), respectively. In the sensitivity analysis, this result was confirmed as robust; there were 35 hospitalizations per 100 person-years during the control interval and 22 during the exposure interval (0.63; 0.50–0.78; See Table S4).
Total dose of systemic corticosteroids
The total dose of systemic corticosteroids equivalent to prednisolone per person-year was significantly reduced from 600 [90–1713] mg during the control interval to 164 [0–1010] mg during the exposure interval (P < .001; Table 3). In patients who required regular treatment with OCS, the dose was also reduced from 2310 [1381–3924] mg to 831 [248–3053] mg (P = .013). In the sensitivity analysis, the results were similar. The corticosteroid dose was reduced in all patients from 615 [93–1738] mg to 150 [0–889] mg (P < .001) and in patients with regular use of OCS from 2482 [1722–4440] mg to 953 [305–2151] mg (P < .001; See Table S5).
Table 3.
Total exposure to systemic corticosteroids during control and exposure intervals.
| mg per person-y |
P-valuea | ||
|---|---|---|---|
| Before biologic use (Control interval) |
During antibody use (Exposure interval) |
||
| All patients (n = 355; before biologic use: 355.0 person-y, during biologic use: 447.9 person-y) |
600.0 [90.0–1713.0] |
163.6 [0.0–1010.1] |
<.001 |
| Patients with regular OCS (n = 72; before biologic use: 72.0 person-y, during biologic use: 98.9 person-y) |
2310.0 [1381.0–3924.0] |
830.8 [248.0–3052.6] |
.013 |
| Omalizumab (n = 119; before biologic use: 119.0 person-y, during biologic use: 188.3 person-y) |
721.2 [180.0–1927.5] |
355.6 [23.7–1388.7] |
.055 |
| Mepolizumab (n = 82; before biologic use: 82.0 person-y, during biologic use: 120.3 person-y) |
936.1 [340.4–1781.1] |
392.8 [21.3–1256.7] |
.008 |
| Benralizumab (n = 69; before biologic use: 69.0 person-y, during biologic use: 71.3 person-y) |
1024.0 [224.0–2328.0] |
219.5 [0.0–1047.6] |
<.001 |
| Dupilumab (n = 85; before biologic use: 85.0 person-y, during biologic use: 68.0 person-y) |
75.0 [0.0–375.0] |
0.0 [0.0–23.8] |
<.001 |
OCS, oral corticosteroids.
The cumulative dosage of systemic corticosteroids is indicated as the prednisolone equivalent.
Paired Wilcoxon signed-rank test
The total doses of systemic corticoids were significantly different at baseline among the examined biologics. The doses were 721.2 [180.0–1927.5] mg, 936.1 [340.4–1781.1] mg, 1024.0 [224.0–2328.0] mg, and 75.0 [0.0–375.0] mg in the omalizumab, mepolizumab, benralizumab, and dupilumab groups, respectively (Table 3). Although the reduction in corticosteroid use did not reach statistical significance in the omalizumab group, significant reductions were achieved in the other 3 groups of biologics.
Additional sensitivity analysis
After limiting the study population to those who had at least 1 of the comorbidities associated with type 2 inflammation, 106, 73, 61, and 83 patients were categorized into the omalizumab, mepolizumab, benralizumab, and dupilumab groups, respectively. All biologics apart from dupilumab significantly reduced exacerbation rates, and all biologics including omalizumab significantly reduced hospitalization rates (See Table S6). Significant reductions were achieved in all biologics including omalizumab (See Table S7).
Discussion
We found that in a real-world setting, the incidence rates of asthma exacerbation events and admission from exacerbation were significantly reduced during biologics use compared to the rates before their use. Patient backgrounds were shown to be different in a real-life setting as discussed below; nonetheless, the numbers of exacerbation events were significantly decreased during use of all examined biologics as were the numbers of admissions during usage of all biologics except for omalizumab. We also found that the total dose of systemic corticosteroids during biologics use was reduced to about one-third of that before administering biologics. These results were robust in the sensitivity analyses.
Asthma exacerbations are still a major health risk, associated with substantial healthcare costs and psychological burden, and an indicator of treatment efficacy.32 According to a recent systematic review assessing 14 RCTs (5 for omalizumab, 3 for mepolizumab, 3 for benralizumab, and 3 for dupilumab) including patients aged 12–75 years, all biologics reliably reduce exacerbation rates; the pooled incidence ratios were calculated as 0.56 (0.40–0.77) for omalizumab, 0.49 (0.38–0.66) for mepolizumab, 0.53 (0.39–0.72) for benralizumab, and 0.43 (0.32–0.59) for dupilumab.33 In addition, real-world data analyses have associated biologics with a reduction in exacerbation events.21, 22, 23, 24, 25, 26, 27, 28, 29 In the present study, the incidence ratios of asthma exacerbation events and hospitalizations for exacerbation during biologics use were in total 0.68 (95% CI: 0.62–0.74) and 0.65 (0.55–0.77), respectively, compared with the period before their use. Further analyses associated all individual biologics with a reduction in exacerbation events and all biologics apart from omalizumab with a reduction in hospitalizations. These results suggest that in terms of exacerbations, severe asthma patients benefit from all examined biologics. It should be noted that the incidence ratio in one type of biologic should not be compared directly with that in another because the patient backgrounds were different as mentioned below.
We found a significant reduction in the total dose of systemic corticosteroids in all patients and even in patients who required maintenance OCS. For individual biologics, significant reductions were detected in all biologics except for omalizumab. Each biologic included in this study had been shown to have OCS-sparing effects in several RCTs.18, 19, 20,34 A recent systematic review investigating the real-world extent and burden of systemic corticosteroids for asthma showed that oral/systemic corticosteroids are commonly used for asthma control and that both long-term and repeated short-term use of oral/systemic corticosteroids are associated with an increased risk of acute and chronic adverse events.35 Clinicians have to balance the benefits of corticosteroid use against these risks. Prior to the present study, data evaluating the corticosteroid-sparing effects by biologics were scarce except for omalizumab. Our findings demonstrate that the use of a biologic other than omalizumab was also related to a decrease in the systemic corticosteroid dose, with each patient having a different baseline dose.
Regarding the patients’ age and sex, users of non-anti-eosinophilic biologics (omalizumab and dupilumab) were comparably younger than users of anti-eosinophilic biologics (mepolizumab and benralizumab), and omalizumab users were predominantly female which contrasted with the male predominance in the dupilumab group. Although few studies assessed more than one type of biologics at once, this result is in agreement with the findings of previous studies showing that omalizumab users are younger than mepolizumab users in real-world settings.36,37 Higher ages in patients using anti-eosinophilic biologics might reflect that in the population with severe asthma, the patient cluster characterized by eosinophilia is older.38 Likewise, the female predominance in omalizumab patients in our analysis may be explained by the reported female dominance in the population with allergic severe asthma, as demonstrated by various cluster analyses.38, 39, 40 We cannot give a clear explanation for the male predominance in dupilumab patients, but considering that dupilumab was the only biologic agent approved for self-injection as of July 2020 in Japan, and thereby contributed to less frequent hospital visits, dupilumab might have been preferred by males in employment.
A previous study using the same data source as ours assessed the prevalences of controlled and uncontrolled severe asthma in Japan and described their characteristics.41 This study determined the percentages of comorbidities as follows: allergic rhinitis (controlled, uncontrolled: 58.1%, 65.2%), GERD (34.9%, 34.5%), DM (20.5%, 27.7%), chronic paranasal sinusitis (10.3%, 16.5%), atopic dermatitis (8.3%, 9.4%), and nasal polyp (0.5%, 1.5%). Compared with these data, users of biologics targeting type 2 inflammation in our study were more commonly comorbid with diseases associated with allergic/eosinophilic inflammation (allergic rhinitis, chronic paranasal sinusitis, atopic dermatitis, and nasal polyp). Omalizumab and dupilumab are approved for allergic rhinitis and atopic dermatitis, respectively, and these comorbidities were, as expected, more frequently present in patients using omalizumab and dupilumab, respectively. Although dupilumab had been approved in March 2020 in Japan for chronic sinusitis with nasal polyps, chronic paranasal sinusitis and nasal polyps were less frequently detected in dupilumab patients. By contrast, the rates of comorbid sinusitis and nasal polyps were higher in anti-eosinophilic biologic users. As the largest cluster analysis to date revealed that the cluster characterized by eosinophilia is strongly associated with a history of nasal polyps and sinusitis,38 and nasal polyps were identified as a predictor of good response in a pooled analysis of two RCTs (the SIROCCO and CALIMA studies),42 our results suggest that comorbid nasal polyps and sinusitis affected the clinical decision-making, favoring anti-eosinophilic biologics.
Regarding asthma treatment patterns, ICS/LABA was selected for almost all patients (97.7% in total). Although some differences in prescription rates among types of biologics were observed, leukotriene receptor antagonists, antihistamines, and xanthines were prescribed at high rates in all study groups. Interestingly, more patients with anti-eosinophilic biologics needed regular OCS compared to patients with non-anti-eosinophilic biologics, which reflected the tendency in exposure to systemic corticosteroids. Considering that this corresponds with the finding that populations characterized by eosinophilia require more frequent corticosteroid use,38 even in real-world settings, anti-eosinophilic biologics were properly selected for patient populations characterized by eosinophilia. The ICS dosage in the dupilumab group was lower than the dosages in all other groups. This is probably due to the circumstance specific for Japan that only for dupilumab the use of biologics has been approved for patients with medium-dose ICS.
This study has several limitations. First, the age of the enrolled patients was limited to 75 years because the data used in this study (JMDC database) were collated by health insurance societies. Accordingly, the study population might be younger than the general population of biologics users in Japan, causing bias to some extent. Second, as benralizumab and dupilumab became available later (2018 and 2019 years, respectively) than omalizumab and mepolizumab (2009 and 2016 years, respectively), the observational periods were shorter for the former drugs. To address this problem, we employed the interval time as an offset term in Poisson regression models. Furthermore, we truncated the exposure interval (during biologics use) to 12 months in the sensitivity analysis, which showed similar results. Third, the possibility of confounding by time-dependent variables (eg, seasonal and prescription patterns) must be considered. It was difficult to take different prescription patterns into consideration because intervals of hospital visits varied from patient to patient. To adjust for seasonal effects, we performed a sensitivity analysis that excluded seasonal effects. Finally, patients might have been treated with another asthma biologic before their inclusion in the database, affecting the incidence rates of the outcomes. To address this problem, the study protocol ensured that no asthma biologic was administered for at least 1 year prior to the index date, and the benefits of biologics were shown even with the possibility of administration of any type of biologic before entry into the database.
In conclusion, we have clarified background differences among patients using the biologics (omalizumab, mepolizumab, benralizumab, and dupilumab) and demonstrated a significant association between the use of biologics and reduction in asthma exacerbations in a real-world setting. Moreover, we confirmed the corticosteroid-sparing effects of those biologics. Additional research using registries containing more than 1 type of biologic is needed to confirm the reproducibility of our findings.
Abbreviations
ANOVA, analysis of variance; CI, confidence interval; DM, diabetes mellitus; EGPA, eosinophilic granulomatosis with polyangiitis; GERD, gastroesophageal reflux disease; ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting beta2-agonist; OCS, oral corticosteroids; RCT, randomized controlled trial; SD, standard deviation.
Funding
This study was funded by Research Institute of Healthcare Data Science. The funding body had no role in the design of the study; collection, analysis, or interpretation of the data; or writing of the manuscript.
Ethics statement
This study was approved by the Institutional Review Board of the National Hospital Organization Tokyo National Hospital. The requirement for informed consent was waived owing to the retrospective nature of this study.
Authorship
Conceptualization, Y.K.; methodology, Y.K., N.I., S.I. and M.A.; software, Y.K., N.I.; validation, Y.K., N.I.; formal analysis, Y.K., N.I.; investigation, Y.K., N.I.; writing original draft preparation, Y.K.; writing review and editing, Y.K., M.S., N.I., S.I., M.A. and H.M.; supervision, H.M. All authors have read and agreed to the published version of the manuscript.
Submission declaration
Our manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Availability of data and materials
All data related with the study are included in this published article and its Supplemental file. The data are not publicly available due to JMDC Inc. requirements and requirement for IRB approval for data release.
Declaration of competing interest
Yuya Kimura, Norihiko Inoue, Shinobu Imai, Manabu Akazawa, and Hirotoshi Matsui declare no conflict of interest. Maho Suzukawa received research funding from Shionogi, MSD, Sanofi, Kyorin, AstraZeneca, and GlaxoSmithKline.
Acknowledgements
This study was funded by Research Institute of Healthcare Data Science. The funding body had no role in the design of the study; collection, analysis, or interpretation of the data; or writing of the manuscript. We would like to express our gratitude to Mr. Masayoshi Kurihara (a health information manager of the National Hospital Organization Tokyo National Hospital) for advice on ICD-10 codes. We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Footnotes
Full list of author information is available at the end of the article. https://doi.org/10.1016/j.waojou.2021.100600
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100600.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organisation . 2017. Asthma. Fact sheet No. 307. [Google Scholar]
- 2.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 4.Chupp G., Laviolette M., Cohn L., et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.00017-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiles S.A., Harvey E.S., McDonald V.M., et al. Working while unwell: workplace impairment in people with severe asthma. Clin Exp Allergy. 2018;48(6):650–662. doi: 10.1111/cea.13153. [DOI] [PubMed] [Google Scholar]
- 6.McDonald V.M., Gibson P.G. Exacerbations of severe asthma. Clin Exp Allergy. 2012;42(5):670–677. doi: 10.1111/j.1365-2222.2012.03981.x. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre P., Duh M.S., Lafeuille M.H., et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney J., Patterson C.C., Menzies-Gow A., et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie A.N. Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc. 2014;11(Suppl 5):S263–S270. doi: 10.1513/AnnalsATS.201403-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleecker E.R., FitzGerald J.M., Chanez P., et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 12.FitzGerald J.M., Bleecker E.R., Nair P., et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 13.Haldar P., Brightling C., Hargadon B., et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanania N., Alpan O., Hamilos D., et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ortega H.G., Liu M.C., Pavord I.D., et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 16.Pavord I.D., Korn S., Howarth P., et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel S., Castro M., Corren J., et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 18.Bel E.H., Wenzel S.E., Thompson P.J., et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 19.Nair P., Wenzel S., Rabe K.F., et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 20.Rabe K.F., Nair P., Brusselle G., et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 21.Brusselle G., Michils A., Louis R., et al. Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103(11):1633–1642. doi: 10.1016/j.rmed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Deschildre A., Marguet C., Langlois C., et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46(3):856–859. doi: 10.1183/09031936.00008115. [DOI] [PubMed] [Google Scholar]
- 23.Deschildre A., Marguet C., Salleron J., et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42(5):1224–1233. doi: 10.1183/09031936.00149812. [DOI] [PubMed] [Google Scholar]
- 24.Grimaldi-Bensouda L., Zureik M., Aubier M., et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations?: results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143(2):398–405. doi: 10.1378/chest.12-1372. [DOI] [PubMed] [Google Scholar]
- 25.Harvey E.S., Langton D., Katelaris C., et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.02420-2019. [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh J.E., Hearn A.P., Dhariwal J., et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506. doi: 10.1016/j.chest.2020.08.2083. [DOI] [PubMed] [Google Scholar]
- 27.Llanos J.P., Ortega H., Bogart M., et al. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. J Asthma Allergy. 2020;13:77–87. doi: 10.2147/JAA.S236609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molimard M., de Blay F., Didier A., Le Gros V. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med. 2008;102(1):71–76. doi: 10.1016/j.rmed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Taille C., Chanez P., Devouassoux G., et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.02345-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai K., Tanaka T., Kodaira N., Kimura S., Takahashi Y., Nakayama T. Data resource profile: JMDC claims database sourced from health insurance societies. J Gen Fam Med. 2021;22(3):118–127. doi: 10.1002/jgf2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitaker H.J., Farrington C.P., Spiessens B., Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 32.Bourdin A., Bjermer L., Brightling C., et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J. 2019;54(3) doi: 10.1183/13993003.00900-2019. [DOI] [PubMed] [Google Scholar]
- 33.Agache I., Beltran J., Akdis C., et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 34.Siergiejko Z., Świebocka E., Smith N., et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opin. 2011;27(11):2223–2228. doi: 10.1185/03007995.2011.620950. [DOI] [PubMed] [Google Scholar]
- 35.Bleecker E.R., Menzies-Gow A.N., Price D.B., et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. doi: 10.1164/rccm.201904-0903SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fong W.C.G., Azim A., Knight D., et al. Real-world Omalizumab and Mepolizumab treated difficult asthma phenotypes and their clinical outcomes. Clin Exp Allergy. 2021;51(8):1019–1032. doi: 10.1111/cea.13882. [DOI] [PubMed] [Google Scholar]
- 37.Llanos J.P., Bell C.F., Packnett E., et al. Real-world characteristics and disease burden of patients with asthma prior to treatment initiation with mepolizumab or omalizumab: a retrospective cohort database study. J Asthma Allergy. 2019;12:43–58. doi: 10.2147/JAA.S189676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu W., Bleecker E., Moore W., et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haldar P., Pavord I.D., Shaw D.E., et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore W.C., Meyers D.A., Wenzel S.E., et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagase H., Adachi M., Matsunaga K., et al. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69(1):53–60. doi: 10.1016/j.alit.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 42.FitzGerald J.M., Bleecker E.R., Menzies-Gow A., et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51–64. doi: 10.1016/S2213-2600(17)30344-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related with the study are included in this published article and its Supplemental file. The data are not publicly available due to JMDC Inc. requirements and requirement for IRB approval for data release.