Abstract
Amyloid proteins are widely studied, both for their unusual biophysical properties and their association with disorders such as Alzheimer’s and Parkinson’s disease. Fluorescence-based methods using site-specifically labeled proteins can provide information on the details of their structural dynamics and their roles in specific biological processes. Here, we describe the application of different labeling methods and novel fluorescent probe strategies to the study of amyloid proteins, both for in vitro biophysical experiments and for in vivo imaging. These labeling tools can be elegantly used to answer important questions on the function and pathology of amyloid proteins.
Graphical Abstract

Introduction
Amyloids are peptide/protein aggregates possessing cross β-sheet structure which are implicated in a variety of significant diseases, including many of the most prevalent neurodegenerative diseases [1,2]. These peptides/proteins include amyloid-beta (Aβ) and tau in Alzheimer’s disease, α-synuclein (αS) and tau in Parkinson’s disease, and huntingtin in Huntington’s disease. Fluorescence methods can be valuable for studying amyloid proteins due to their sensitivity, enabling them to detect low concentrations of transient species, and to their ability to be used in real time and in physiological environments, complementing high resolution structural methods. While aggregation has conventionally been monitored with amyloid-binding dyes such as thioflavin T or Congo Red, these dyes have limitations in their accuracy [3,4] and sensitivity to earlier-stage oligomers [5,6], which are typically composed of 2–10 monomers and are more structurally heterogeneous than fibrils. Oligomers have been shown to be important in toxicity and pathogenicity of amyloids [7]. Small molecule dyes customized for specific proteins or types of aggregates (e.g. oligomer-sensors) can overcome some of these disadvantages, but generally cannot report on the details of protein structure and have limited specificity in cells [8,9]. In contrast, fluorescently-labeled proteins can be used to obtain precise structural information in biophysics or biochemistry experiments,[10–16] and can report on specific functional or pathological processes in microscopy experiments [17,18].
Many in vitro studies with labeled proteins have focused on aggregation, conformational dynamics, and interactions with lipids and/or other proteins, exploiting sensitive physical properties of fluorophores. Distance-dependent fluorescent interactions like Förster resonance energy transfer (FRET) [19] or environmentally-sensitive processes like excited-state intramolecular proton-transfer (ESIPT) [20] can track protein structural change and thus reveal complex aggregation mechanisms. Moreover, fluorescent measurements can be made at the single molecule level to observe heterogeneity in monomer or aggregate structures, rather than only averaged information. For both single molecule and ensemble measurements, the ability to label at specific protein sites is essential to obtaining detailed structural information.
Fluorescent labeling of amyloid proteins also allows one to obtain real-time, high-resolution cellular imaging, and “smart” probes can be designed to report on specific biological processes. These events include interactions with other proteins, cellular uptake of amyloid proteins to seed aggregation, changes in protein microenvironments, proteolytic processing, and effects on cellular metabolism or protein synthesis. Proteins labeled at specific sites with small, synthetic probes can overcome disruptions caused by more commonly used fluorescent fusion proteins.
In this review, we describe recent applications of site-specific protein labeling methods to the study of amyloid proteins. These methods vary in their specificity, scope of modifications that can be introduced, and level of perturbation to the wild type (WT) protein. Thus, some methods are more appropriate for certain types of labeling problems, and combinations of methods are often most efficient, particularly when multiple modifications are required.
Methods for site-specifically incorporating probes
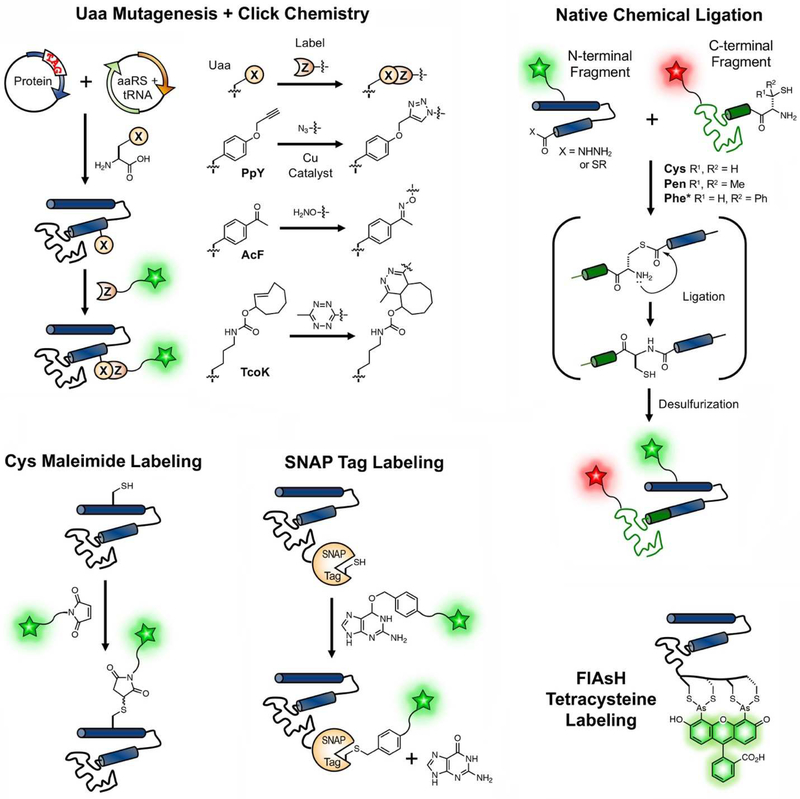
Among the labeling methods, unnatural amino acid (Uaa) mutagenesis is perhaps the most elegant, combining non-perturbing, site-specific labels with the ease of cellular expression [21,22]. Uaa incorporation is made possible by orthogonal aminoacyl tRNA synthetases (aaRSs) and tRNAs through suppression of stop codons (typically TAG) or non-standard codons. While the development of new aaRS/tRNA pairs requires specialized expertise in directed evolution, the use of existing aaRR/tRNA pairs for established Uaas can be easily adopted by non-developer laboratories. Site-specific probe incorporation can be accomplished either by direct introduction of fluorescent Uaas or by incorporating Uaas with handles for bioorthogonal “click” reactions with fluorophores (Figure 1, Top Left) [22]. These Uaas include propargyl tyrosine (PpY), used with azide partners in copper-catalyzed cycloadditions; acetylphenylalanine (AcF), used with hydroxylamine or hydrazine partners; and trans-cyclooctenyl lysine (TcoK), used with tetrazine partners. Although there are examples of multiple Uaa incorporation in E. coli as well as in mammalian cells [21], chemical protein synthesis is generally better suited to multiple modifications, at least for in vitro experiments.
Figure 1.
Strategies for site-specific protein labeling. (Top Left) Unnatural amino acid (Uaa) mutagenesis can be used to genetically incorporate click chemistry handles such as propargyltyrosine (PpY), p-acetylphenylalanine (AcF), and trans-cyclooctene lysine (TcoK) which are reacted with azide, alkoxyamine, and tetrazine, respectively. (Top Right) Native chemical ligation (NCL) and other peptide ligation chemistries easily allow incorporating multiple modifications. Thiol-containing amino acids such as cysteine (Cys), penicillamine (Pen) and mercaptophenylalanine (Phe*) are used for NCL, and can then be desulfurized to form Ala, Val, or Phe, respectively. (Bottom Left) Cys-maleimide chemistry is a conventional, simple approach for in vitro labeling. (Bottom Middle) The genetically encodable SNAP tag is conjugated with labeling agents bearing a O-benzyl guanine moiety. (Bottom Right) Bisarsenical dyes such as FlAsH bind to tetracysteine (-C-C-X-X-C-C-) motifs with a turn-on of fluorescence.
Chemical protein synthesis or semi-synthesis combines fragments of a target protein made by solid phase peptide synthesis (SPPS) or cellular expression, followed by ligation to assemble the protein (Figure 1, Top Right) [23]. Native chemical ligation (NCL), the most widely used peptide ligation, involves attack of a thiol from Cys or a Cys analog in the C-terminal peptide on a thioester in the N-terminal peptide, followed by an S-to-N acyl shift to form the native amide bond. Desulfurization can be performed as needed to regenerate the native sequence. NCL and expressed protein ligation strategies provide the highest level of chemical control, and can be used to introduce multiple labels as well as post-translational modifications (PTMs).* However, NCL is more labor-intensive than Uaa mutagenesis, and with a few exceptions that make use of enzymatic protein splicing [24,25], NCL cannot be applied in cells.
Several other genetically encodable, site-specific labeling methods have been employed which, although they are either less selective or more perturbing than the above methods, are often easier to employ. Cysteine mutation by site-directed mutagenesis enables labeling via maleimides (Figure 1, Bottom Left) [26] or other thiol-reactive groups [27], but requires the use of protecting strategies for multiple labeling and is not suitable for cellular applications. For live cell imaging, the tetracysteine motif (-C-C-X-X-C-C-) can be bound by bisarsenical dyes such as FIAsH with high affinity and fluorescence turn-on (Figure 1, Bottom Right) [28]. FlAsH labeling has been successfully applied to Aβ, αS, and TDP-43 [29–31]. More commonly used are larger, encodable protein tags that self-label through reactions with specific substrates. These include the SNAP tag which reacts with derivatives of O-benzylguanine to label the protein via a benzyl linker (Figure 1, Bottom Middle), as well as the related CLIP tag which reacts with O-benzylcytosine derivatives, and the HALO tag which reacts with haloalkanes [32,33].** SNAP and HALO methods are generally favored by laboratories with less chemical synthesis expertise as the labeling reagents are often commercially available, but the size of the tags restricts their use to the N- and C-termini of proteins. Therefore, they are not useful for experiments like site-specific FRET, but are well-suited to microscopy imaging.
In vitro probes for biophysics studies
Single labeling with fluorescent probes can report on conformational changes and aggregation through the environmental sensitivity of the probes or through fluorescence polarization. Double labeling with chromophore pairs can report on folding through FRET or quenching interactions, in either ensemble or single molecule measurements. These are well-established methods in the study of disordered and/or amyloidogenic proteins, where single or double labeling can be accomplished by selective Cys labeling, NCL, and/or Uaa mutagenesis [10,13,14,34–41].
Among these examples, Cys-selective reactions are most commonly used, particularly with maleimide dye derivatives, and is a very useful way of labeling a protein such as αS that lacks endogenous Cys [10,13,14,35–37,39,40]. Cys-maleimide reactions can typically be driven to completion, which is important for larger proteins, where separation of excess dye is much easier than separation of unlabeled protein. However, these allow only single-site labeling unless protecting groups are used. In a protein such as tau that contains native Cys, perturbation of these sites (i.e. mutation to Ser) may be necessary [14]. Uaa mutagenesis can be effectively combined with Cys-selective reaction to directly incorporate a second fluorophore or a click chemistry handle for efficient multiple labeling for FRET studies [10,13,42]. However, the efficiency of Uaa mutagenesis varies a lot with different aaRS/tRNA pairs and target sequences. Therefore, optimization of growth conditions is often required. While incorporation of two Uaas can conceivably be used for FRET labeling,[43,44] reports of double labeling in this way have been largely restricted to developer laboratories and this strategy has not yet been applied to amyloid proteins. Chemical protein synthesis can also potentially be used to incorporate FRET modifications, but it is labor-intensive and can be low-yielding compared to direct protein expression [45]. One’s choice of ligation sites is quite important not only in terms of ligation efficiency, but also in considering the aggregation propensity of the NCL fragments, such as N-terminal truncations of αS, since NCL usually requires the peptide components to be highly concentrated (~1 mM). Inteins used to produce the protein fragments can aid in solubilizing protein fragments, or even the full-length proteins, for handling [10,46,47]. Currently, combinations of Cys labeling, Uaa mutagenesis, and NCL must be considered judiciously when designing a labeling strategy, but double Uaa mutagenesis is likely to emerge as the method of choice if it can be made routine and efficient.
Examples of fluorescence-enabled experiments using these proteins include ensemble or single molecule FRET (smFRET) measurements, fluorescence correlation spectroscopy (FCS), fluorescence polarization (FP) assays, and ESIPT or other environmental probe experiments. Ensemble and smFRET that can be used to measure distances changes between specific regions of the protein during conformational transitions of the monomer protein in response to small molecules [10,40], covalent modifications of the protein (below), or binding to vesicles and other proteins [35,48]. Ensemble FRET has also been used to study the dynamics of regions of fibrils that are outside of the amyloid core [13]. FCS (single molecule) and FP (ensemble) can be used to monitor binding to lipid vesicles or other proteins to determine affinity [12,14,49], as well as local dynamics around the fluorescent label [12,35]. FP can also be used to monitor fibril formation and disaggregation by small molecules without concern for artifacts due to competition of the small molecule with the fluorescent probe that comes from trying to perform the same types of experiments with thioflavin T [12,40,50]. Environmental probes can be used to determine which regions of a protein are solvent exposed and which regions become buried upon association with another protein [14]. Many of these methods have been published prior to the last few years, therefore we here highlight three recent studies with unusual probes or synthetic strategies.
One important consideration in any fluorescence experiment is whether the fluorescent label perturbs the protein’s structure and function. While an exhaustive review of these effects is beyond the scope of this article, a few examples are illustrative. In the context of a long peptide (or short protein) like the 42 amino acid Aβ, labeling N-terminally may be a relatively minor perturbation to the system [51], but if the dye is added N-terminally to the shorter, 22 amino acid Amyloid Alpha/p3, this same dye can cause a larger perturbation [52]. Even in a larger protein, such as the 140 amino acid αS, dye labeling at some positions was shown to alter aggregation kinetics [12,13,46], so care must be taken to validate labeling positions as non-perturbing in structure-sensitive experiments. These can include Congo Red aggregation assays (Congo Red used because fluorophores will interfere with Thioflavin T assays), circular dichroism (CD) spectra, or transmission electron microscopy (TEM) imaging, as well as cell-based fibril seeding assays [46].
The first type of experiment that we wish to highlight uses FRET to study the effects of PTMs on protein folding. FRET studies can provide dynamic measurements of rearrangements induced by the PTM that complement structural studies such as X-ray crystallography, cryo-EM, or NMR. In theory, the entire protein, including PTMs and fluorophores, can be assembled through SPPS. However, in spite of some recent successes in the synthesis of large proteins [53], this can often involve an impractical number of fragment ligations, so combining NCL with Uaa mutagenesis can enable more efficient syntheses (Figure 2, Top Left). This is exemplified by our study of the role of phosphorylation at tyrosine 39 (pY39) in αS, employing a chemoenzymatic synthesis with an improved yield compared to SPPS routes that allowed us to prepare αS with the pY39 PTM and two fluorophores for smFRET [54]. The smFRET experiments revealed conformational changes in αS-pY39 that contributed to a dose-dependent, bidirectional effect on aggregation. Using a similar combination of NCL and Uaa mutagenesis, we generated αS with multiple glutamate arginylation PTMs and PpY fluorophore modification for single molecule studies [55]. The Lashuel group has incorporated FRET probes as well as site-specific phosphorylation into exon 1 of huntingtin by semi-synthetic [56] or chemoenzymatic [57] approaches, enabling smFRET studies of the effects of increasing polyglutamine repeats on protein conformation. Rather than using Uaa mutagenesis, their synthesis used selective protection of the terminal Cys.
Figure 2.
In vitro probe applications to study conformational dynamics and aggregation of amyloid proteins. (Top Left) Combined PTM and FRET pair labeling through SPPS or chemoenzymatic synthesis, Uaa mutagenesis and NCL to give homogenous αS pY39 for smFRET studies. (Top Right) Three color smFRET in αS, which was site-specifically labeled with three distinct dyes all via cysteine-maleimide chemistry, by combining thiazolidine capture of the N-terminal cysteine in the C-terminal fragment with dextran-aldehyde resin and NCL. (Bottom) αS labeled with ESIPT probe 7FME that populates excited states NH*, N*, and T* is used to monitor the levels of monomers (M), intermediates (I), and fibrils (F) based on contributions to the spectra indicated by the fluorophore colors. Spectra at different time points and the derived speciation of αS are shown for an aggregation reaction. (Top Left) Adapted from ref. [54], Copyright 2020, the American Chemical Society. (Top Right) Adapted from ref. [58], Copyright 2018, Cell Press. (Bottom) Adapted from ref. [60], Copyright 2018, Springer Nature.
The second type of study uses three color smFRET for more complex analysis of protein dynamics and interactions between protein domains. Dawson, Deniz, and coworkers labeled αS at three Cys positions with different fluorophores using maleimide chemistry made selective by capturing the N-terminal Cys of one fragment as a thiazolidine using an aldehyde resin (Figure 2, Top Right) [58]. Three color smFRET data were consistent with an extended αS C-terminus and structural change to a hairpin-like structure upon addition of sodium dodecyl sulfate (SDS). The Chung group performed three color smFRET of a protein/protein interaction, with two sites labeled in the trans-activation domain of the tumor suppressor p53 using AcF/hydroxylamine and Cys/maleimide chemistries. They combined this with a Cys/maleimide labeled nuclear coactivator binding domain of CREB binding protein, and observed multiple transition paths of these proteins on folding and binding [59].
A third class of experiments uses a probe of local environment based on ESIPT, a rapid photophysical process where the initial excited state undergoes proton transfer to populate multiple tautomeric states with variations in spectral shape and quantum yield [20]. This is exemplified by hydroxychromones such as [7-(3-maleimido-N-propanamide)-2-(4-diethyaminophenyl)-3-hydroxychromone (7MFE, Figure 2, Bottom) where the excited enol (N* or hydrogen-bonded NH*) form tautomerizes to the keto form (T*). Conformational changes create different microenvironments, which have different T*/N*/NH* ratios, allowing one to track the aggregated species based on spectral changes. The Jovin group applied 7MFE to monitor the various species formed during αS aggregation that cannot be detected by thioflavin-based dyes [60]. The Yushchenko group also used maleimide ESIPT probes to study αS, where one of their probes, 7-amino-3-hydroxyflavone, clearly distinguished unstructured, membrane-bound and fibrillar states [61].
Cell-based imaging probes
Many labeling methods can also be used to study amyloid proteins in live cells and even in organisms, where a more physiologically-relevant understanding of disease mechanism may be achieved. Labeled proteins can be used to study activities such as cellular uptake of fibrils, protein processing, and effects on cellular metabolism [17,62–70].
Tracking the processing of proteins can be important to understanding their function in health and disease, particularly in the case of Aβ, where proteolysis by β- and γ-secretases determines the levels of the amyloidogenic Aβ40 or 42 peptides. The Sakmar, Elsässer, and Tjernberg groups performed live cell dual labeling of amyloid-β precursor protein (APP), where the Aβ42 segment was labeled with a genetically encoded TcoK and a BODIPY tetrazine, and the APP C-terminus was labeled with a SNAP tag and a rhodamine O-benzyl guanine (Figure 3, Top) [70]. The small size of TcoK allowed them to label the relatively short Aβ42 segment without disrupting its recognition by the secretase machinery, and they used their optimal APP-TcoK-SNAP construct to monitor the timecourse of γ-secretase activity as a change in signal colocalization.
Figure 3.
Probe applications in live cell imaging to study biological processing of amyloid proteins. (Top) Live cell double labeling of APP to monitor proteolytic processing that generates Aβ42 and N- and C-terminal APP fragments, enabled by labeling at TcoK inserted by Uaa mutagenesis and at a SNAP tag on the APP C-terminus. Sites of β- and γ-secretase cleavage and Aβ42 segment are indicated with sites of TcoK incorporation indicated in green. Change in fluorescence colocalization can be observed over time. Blue arrow indicates nucleus, pink arrow indicates subcellular locations where APP is highly expressed. (Bottom) Photoconvertible CPX probe attached to PpY inserted in αS by Uaa mutagenesis was used for real-time tracking of neuronal uptake and distribution of single αS aggregates. Photoconversion using a UV laser switches CPX from green to red emission, marking single aggregates.
(Top) Adapted from ref. [70], Copyright 2019, IOS Press. (Bottom) Adapted from ref. [68], Copyright 2019, the American Chemical Society.
Attachment of synthetic fluorophores also enables super-resolution microscopy to gain more precise protein co-localization information. Rizzoli and coworkers used different aaRS/tRNA pairs to incorporate propargyl lysine in a protein in one cell and TcoK in a different protein in another cell, labeled by Star635 azide and Star580 tetrazine, respectively [69]. They then fused the cells to combine their contents and used stimulated emission depletion (STED) microscopy to assess co-localization of αS with proteins involved in vesicle secretion, such as SNAP-25 and syntaxin, as well as cytoskeletal elements like actin.
In addition to co-localization studies, insights into amyloid protein function and pathology can be gained with fluorescent probes designed for specific functions. This is exemplified by our αS studies with the Lee and Chenoweth laboratories. After showing that αS fibrils were endosomally internalized by neurons [17], questions remained as to the mechanism of fibril entry into the cytosol to seed endogenous αS aggregation. We developed an experiment to track the fates of single aggregated clusters using a clickable photo-convertible diazaxanthilidene probe (CPX) (Figure 3, Bottom) [68]. The CPX azide was attached to αS by reaction with genetically encoded PpY and then fibrils formed by co-aggregating αS-CPX with WT αS were applied to neurons and internalized. Specific aggregates in endosomes were tagged by photo-conversion of CPX from green to red emission so that their location could be tracked over periods of several hours.
In similar studies of the uptake of tau monomers and aggregates in neurons, Livesey and coworkers used tau modified with the pH-sensitive dye, pHrodo, to monitor the acidification of endosomes/lysosomes [67]. Time series imaging of neuronal uptake of monomeric or aggregated tau with and without a dynamin inhibitor revealed that aggregated tau lost pHrodo fluorescence, unlike monomeric tau, suggesting that its entry primarily involves dynamin-dependent endocytosis (Figure 4, Left). In contrast, monomeric tau entry exploits both rapid, dynamin-dependent endocytosis and a slow, dynamin independent mechanism.
Figure 4.
Amyloid protein probes used to study the mechanism and effects of aggregation. (Left) Low pH reporter dye pHrodo was used to label tau and reveal neuronal entry mechanisms by turn-on as endosomes acidify and mature. The fluorescence of aggregated pHrodo tau decreased in the presence of a dynamin inhibitor, suggesting that aggregated tau internalizes via dynamin dependent endocytosis. (Right) Met replacement was used in WT and pathological tau Tg mice to incorporate Aha into newly synthesized proteins, which can be functionalized for fluorescent non-canonical amino acid tagging (FUNCAT) or bio-orthogonal non-canonical amino acid tagging (BONCAT) for MS analysis. The combined results show that pathological tau decreases protein synthesis overall, with expression of specific clusters of proteins modulated collectively. (Left) Adapted from ref. [67], Copyright 2018, Cell Press. (Right) Adapted from ref. [66], Copyright 2019, John Wiley and Sons.
In addition to labeling amyloid proteins, labeling other proteins in the cell can provide understanding of disease mechanism. The Bodea and Götz groups used the “non-canonical amino acid” (i.e. Uaa) tagging approach developed by Tirrell [71] to determine how pathological tau affects protein synthesis [66]. Azidohomoalanine (Aha), a methionine surrogate, was incorporated at Met positions in newly synthesized proteins in tau transgenic (Tg) mice using the permissivity of the endogenous Met aaRS. Aha can be functionalized for fluorescent imaging or biotin enrichment for mass spectrometry (MS) analysis (Figure 4, Right). The fluorescence signal in certain brain regions was reduced in tau Tg mice compared to WT mice, showing that tau aggregation inhibits protein synthesis. MS analysis further revealed that specific proteins, such as those involved in ribosomal protein synthesis, are affected in the context of pathology.
Conclusion
We have briefly reviewed different approaches to fluorescently label amyloid proteins and their use in biophysical and cell-based studies of their folding and aggregation. Multiple labeling at specific sites enables both in vitro FRET and cell-based activity or colocalization experiments. Probe labels can also include smart fluorophores that report on their local environment or can be converted for tracking specific aggregates. Some labeling approaches can even be applied in animals to bring in vivo insights to functional or pathological roles of amyloids. The efficient preparation of labeled protein probes will continue to expand our understanding of the biophysics and biology of amyloid proteins.
Acknowledgements
This work was supported by the National Institutes of Health (NIH NS103873 to E.J.P.). M.S. thanks the Nakajima Foundation for scholarship funding.
Abbreviations
- 7MFE
7-(3-maleimido-N-propanamide)-2-(4-diethyaminophenyl)-3-hydroxychromone
- AcF
acetylphenylalanine
- aaRSs
aminoacyl tRNA synthetases
- Aβ
amyloid-beta
- APP
amyloid-β precursor protein
- Aha
azidohomoalanine
- CPX
clickable photo-convertible diazaxanthilidene
- ESIPT
excited-state intramolecular proton-transfer
- FRET
Förster resonance energy transfer
- MS
mass spectrometry
- NCL
native chemical ligation
- PpY
propargyl tyrosine
- smFRET
single molecule FRET
- SDS
sodium dodecyl sulfate
- SPPS
solid phase peptide synthesis
- STED
stimulated emission depletion
- αS
α-synuclein
- TcoK
trans-cyclooctenyl lysine
- Tg
transgenic
- WT
wild type
Footnotes
See a review of the preparation of amyloid proteins with PTMs by Pratt and coworkers in this issue.
See a review including the use of SNAP, CLIP, and Halo tags in combination with small molecule probes by Zhang and coworkers in this issue.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Chiti F, Dobson CM: Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86:27–68. [DOI] [PubMed] [Google Scholar]
- 2.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE: A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19:755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akter R, Zhyvoloup A, Zheng BQ, Bhatia SR, Raleigh DP: The triphenylmethane dye brilliant blue g is only moderately effective at inhibiting amyloid formation by human amylin or at disaggregating amylin amyloid fibrils, but interferes with amyloid assays; implications for inhibitor design. PLoS One 2019, 14:e0219130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong AG, Wu C, Hannaberry E, Watson MD, Shea JE, Raleigh DP: Analysis of the amyloidogenic potential of pufferfish (takifugu rubripes) islet amyloid polypeptide highlights the limitations of thioflavin-t assays and the difficulties in defining amyloidogenicity. Biochemistry 2016, 55:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar H, Singh J, Kumari P, Udgaonkar JB: Modulation of the extent of structural heterogeneity in alpha-synuclein fibrils by the small molecule thioflavin t. J. Biol. Chem. 2017, 292:16891–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray S, Singh N, Kumar R, Patel K, Pandey S, Datta D, Mahato J, Panigrahi R, Navalkar A, Mehra S, et al. : Alpha-synuclein aggregation nucleates through liquid-liquid phase separation. Nat. Chem. 2020, 12:705–716. [DOI] [PubMed] [Google Scholar]
- 7.Verma M, Vats A, Taneja V: Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verwilst P, Kim HS, Kim S, Kang C, Kim JS: Shedding light on tau protein aggregation: The progress in developing highly selective fluorophores. Chem. Soc. Rev. 2018, 47:2249–2265. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Wang W, Wang X, Yang F, Xie L, Shen J, Brimble MA, Xiao Q, Yao SQ: Fluorescent probes for bioimaging of potential biomarkers in parkinson’s disease. Chem. Soc. Rev. 2021. [DOI] [PubMed] [Google Scholar]
- 10.Ferrie JJ, Haney CM, Yoon J, Pan B, Lin Y-C, Fakhraai Z, Rhoades E, Nath A, Petersson EJ: Using a fret library with multiple probe pairs to drive monte carlo simulations of -synuclein. Biophys. J. 2018, 114:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath A, Sammalkorpi M, DeWitt David C, Trexler Adam J, Elbaum-Garfinkle S, O’Hern Corey S, Rhoades E: The conformational ensembles of α-synuclein and tau: Combining single-molecule fret and simulations. Biophys. J. 2012, 103:1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haney CM, Cleveland CL, Wissner RF, Owei L, Robustelli J, Daniels MJ, Canyurt M, Rodriguez P, Ischiropoulos H, Baumgart T, et al. : Site-specific fluorescence polarization for studying the disaggregation of -synuclein fibrils by small molecules. Biochemistry 2017, 56:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haney CM, Petersson EJ: Fluorescence spectroscopy reveals n-terminal order in fibrillar forms of -synuclein. Chem. Commun. 2018, 54:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung HYJ, McKibben KM, Ramirez J, Gupta K, Rhoades E: Structural characterization of tau in fuzzy tau:Tubulin complexes. Structure 2020, 28:378–384.e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKibben KM, Rhoades E: Independent tubulin binding and polymerization by the proline-rich region of tau is regulated by tau’s n-terminal domain. J. Biol. Chem. 2019, 294:19381–19394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baughman HER, Clouser AF, Klevit RE, Nath A: Hspb1 and hsc70 chaperones engage distinct tau species and have different inhibitory effects on amyloid formation. J. Biol. Chem. 2018, 293:2687–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpowicz RJ, Haney CM, Mihaila TS, Sandler RM, Petersson EJ, Lee VMY: Selective imaging of internalized proteopathic -synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies. J. Biol. Chem. 2017, 292:13482–13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birol M, Wojcik SP, Miranker AD, Rhoades E: Identification of n-linked glycans as specific mediators of neuronal uptake of acetylated -synuclein. PLOS Biology 2019, 17:e3000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas E: The study of protein folding and dynamics by determination of intramolecular distance distributions and their fluctuations using ensemble and single-molecule fret measurements. ChemPhysChem 2005, 6:858–870. [DOI] [PubMed] [Google Scholar]
- 20.Sedgwick AC, Wu LL, Han HH, Bull SD, He XP, James TD, Sessler JL, Tang BZ, Tian H, Yoon J: Excited-state intramolecular proton-transfer (esipt) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47:8842–8880. [DOI] [PubMed] [Google Scholar]
- 21.de la Torre D, Chin JW: Reprogramming the genetic code. Nat. Rev. Genetics 2020. [DOI] [PubMed] [Google Scholar]
- 22.Lang K, Chin JW: Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 2014, 114:4764–4806. [DOI] [PubMed] [Google Scholar]
- 23.Conibear AC, Watson EE, Payne RJ, Becker CFW: Native chemical ligation in protein synthesis and semi-synthesis. Chem. Soc. Rev. 2018, 47:9046–9068. [DOI] [PubMed] [Google Scholar]
- 24.Khoo KK, Galleano I, Gasparri F, Wieneke R, Harms H, Poulsen MH, Chua HC, Wulf M, Tampé R, Pless SA: Chemical modification of proteins by insertion of synthetic peptides using tandem protein trans-splicing. Nat. Commun. 2020, 11:2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton AJ, Haugbro M, Parisi E, Muir TW: Live-cell protein engineering with an ultrashort split intein. Proc. Natl. Acad. Sci. U.S.A. 2020, 117:12041–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravasco J, Faustino H, Trindade A, Gois PMP: Bioconjugation with maleimides: A useful tool for chemical biology. Chem.-Eur. J. 2019, 25:43–59. [DOI] [PubMed] [Google Scholar]
- 27.Gunnoo SB, Madder A: Chemical protein modification through cysteine. ChemBioChem 2016, 17:529–553. [DOI] [PubMed] [Google Scholar]
- 28.Griffin BA, Adams SR, Tsien RY: Specific covalent labeling of recombinant protein molecules inside live cells. Science 1998, 281:269–272. [DOI] [PubMed] [Google Scholar]
- 29.Ng JSW, Hanspal MA, Matharu NS, Barros TP, Esbjörner EK, Wilson MR, Yerbury JJ, Dobson CM, Kumita JR: Using tetracysteine-tagged tdp-43 with a biarsenical dye to monitor real-time trafficking in a cell model of amyotrophic lateral sclerosis. Biochemistry 2019, 58:4086–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Culyba EK, Powers ET, Kelly JW: Amyloid- forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011, 7:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberti MJ, Bertoncini CW, Klement R, Jares-Erijman EA, Jovin TM: Fluorescence imaging of amyloid formation in living cells by a functional, tetracysteine-tagged -synuclein. Nat. Methods 2007, 4:345–351. [DOI] [PubMed] [Google Scholar]
- 32.Gautier A, Juillerat A, Heinis C, Corrêa IR, Kindermann M, Beaufils F, Johnsson K: An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 2008, 15:128–136. [DOI] [PubMed] [Google Scholar]
- 33.Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K: Labeling of fusion proteins with synthetic fluorophores in live cells. Proc. Natl. Acad. Sci. U. S. A. 2004, 101:9955–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haney CM, Wissner RF, Petersson EJ: Multiply labeling proteins for studies of folding and stability. Curr. Opin. Chem. Biol. 2015, 28:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhasne K, Jain N, Kamawat R, Arya S, Majumdar A, Singh A, Mukhopadhyay S: Discerning dynamic signatures of membrane-bound alpha-synuclein using site-specific fluorescence depolarization kinetics. J. Phys. Chem. B 2020, 124:708–717. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee S, Ghosh S, Mishra S, Das Saha K, Banerji B, Chattopadhyar K: Efficient detection of early events of alpha-synuclein aggregation using a cysteine specific hybrid scaffold. Biochemistry 2019, 58:1109–1119. [DOI] [PubMed] [Google Scholar]
- 37.Goluguri RR, Sen S, Udgaonkar J: Microsecond sub-domain motions and the folding and misfolding of the mouse prion protein. eLife 2019, 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pramanik U, Chakraborty S, Bhattacharyya K, Mukherjee S: An intrinsically disordered protein in f127 hydrogel: Fluorescence correlation spectroscopy and structural diversity of beta casein. Chem. Phys. Lett. 2021, 762:7. [Google Scholar]
- 39.Whiten DR, Cox D, Horrocks MH, Taylor CG, De S, Flagmeier P, Tosatto L, Kumita JR, Ecroyd H, Dobson CM, et al. : Single-molecule characterization of the interactions between extracellular chaperones and toxic alpha-synuclein oligomers. Cell Rep. 2018, 23:3492–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels MJ, Nourse JB, Kim H, Sainati V, Schiavina M, Murrali MG, Pan B, Ferrie JJ, Haney CM, Moons R, et al. : Cyclized ndga modifies dynamic -synuclein monomers preventing aggregation and toxicity. Sci. Rep. 2019, 9:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batjargal S, Walters CR, Petersson EJ: Inteins as traceless purification tags for unnatural amino acid proteins. J. Am. Chem. Soc. 2015, 137:1734–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrie JJ, Ieda N, Haney CM, Walters CR, Sungwienwong I, Yoon J, Petersson EJ: Multicolor protein fret with tryptophan, selective coumarin-cysteine labeling, and genetic acridonylalanine encoding. Chem. Commun. 2017, 53:11072–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann H, Wang KH, Davis L, Garcia-Alai M, Chin JW: Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature 2010, 464:441–444. [DOI] [PubMed] [Google Scholar]
- 44.Wan W, Huang Y, Wang Z, Russell WK, Pai P-J, Russell DH, Liu WR: A facile system for genetic incorporation of two different noncanonical amino acids into one protein in escherichia coli. Angew. Chem.-Int. Edit. 2010, 49:3211–3214. [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni SS, Sayers J, Premdjee B, Payne RJ: Rapid and efficient protein synthesis through expansion of the native chemical ligation concept. Nat. Rev. Chem. 2018, 2:0122. [Google Scholar]
- 46.Haney CM, Wissner RF, Warner JB, Wang YJ, Ferrie JJ, J. Covell D, Karpowicz RJ, Lee VMY, James Petersson E: Comparison of strategies for non-perturbing labeling of -synuclein to study amyloidogenesis. Org. Biomol. Chem. 2016, 14:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones CM, Venkatesh Y, Petersson EJ: Protein labeling for fret with methoxycoumarin and acridonylalanine. In Chemical tools for imaging, manipulating, and tracking biological systems: Diverse methods for optical imaging and conjugation. Edited by Chenoweth DM: Academic Press Ltd-Elsevier Science Ltd; 2020:37–69. Methods in enzymology, vol 639.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trexler AJ, Rhoades E: -synuclein binds large unilamellar vesicles as an extended helix. Biochemistry 2009, 48:2304–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middleton ER, Rhoades E: Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys. J 2010, 99:2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrie JJ, Lengyel-Zhand Z, Janssen B, Lougee MG, Giannakoulias S, Hsieh C-J, Pagar VV, Weng C-C, Xu H, Graham TJA, et al. : Identification of a nanomolar affinity -synuclein fibril imaging probe by ultra-high throughput in silico screening. Chem. Sci 2020, 11:12746–12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutta S, Finn TS, Kuhn AJ, Abrams B, Raskatov JA: Chirality dependence of amyloid cellular uptake and a new mechanistic perspective. ChemBioChem 2019, 20:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhn AJ, Abrams BS, Knowlton S, Raskatov JA: Alzheimer’s disease “non-amyloidogenic” p3 peptide revisited: A case for amyloid-. ACS Chem. Neurosci. 2020, 11:1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartrampf N, Saebi A, Poskus M, Gates ZP, Callahan AJ, Cowfer AE, Hanna S, Antilla S, Schissel CK, Quartararo AJ, et al. : Synthesis of proteins by automated flow chemistry. Science 2020, 368:980. [DOI] [PubMed] [Google Scholar]
- 54. Pan Y, Rhoades E, Petersson EJ: Chemoenzymatic semisynthesis of phosphorylated alpha-synuclein enables identification of a bidirectional effect on fibril formation. ACS Chem. Biol. 2020, 15:640–645. The advantage of combining different labeling approaches was demonstrated by chemoenzymatic semi-synthesis of αS-pY39 with a FRET pair through Uaa mutagenesis, Cys-maleimide chemistry, and NCL.
- 55.Pan B, Kamo N, Shimogawa M, Huang Y, Kashina A, Rhoades E, Petersson EJ: Effects of glutamate arginylation on -synuclein: Studying an unusual post-translational modification through semisynthesis. J. Am. Chem. Soc. 2020, 142:21786–21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warner JB, Ruff KM, Tang PS, Lemke EA, Pappu RV, Lashuel HA: Monomeric huntingtin exon 1 has similar overall structural features for wild-type and pathological polyglutamine lengths. J. Am. Chem. Soc. 2017, 139:14456–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiki A, Ricci J, Hegde R, Abriata LA, Reif A, Boudeffa D, Lashuel HA: Site-specific phosphorylation of huntingtin exon 1 recombinant proteins enabled by the discovery of novel kinases. ChemBioChem 2021, 22:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee TC, Moran CR, Cistrone PA, Dawson PE, Deniz AA: Site-specific three-color labeling of alpha-synuclein via conjugation to uniquely reactive cysteines during assembly by native chemical ligation. Cell Chem. Biol. 2018, 25:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JY, Chung HS: Disordered proteins follow diverse transition paths as they fold and bind to a partner. Science 2020, 368:1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fauerbach JA, Jovin TM: Pre-aggregation kinetics and intermediates of alpha-synuclein monitored by the esipt probe 7mfe. Eur. Biophys. J. Biophys. Lett. 2018, 47:345–362. Site-specific incorporation of an environment-sensitive ESIPT probe into αS enabled reporting on subtle microenvironment changes to identify changes in aggregated species over time.
- 61.Kucherak OA, Shvadchak VV, Kyriukha YA, Yushchenko DA: Synthesis of a fluorescent probe for sensing multiple protein states. Eur. J. Org. Chem. 2018, 2018:5155–5162. [Google Scholar]
- 62.De Cecco E, Celauro L, Vanni S, Grandolfo M, Bistaffa E, Moda F, Aguzzi A, Legname G: The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells. J. Neurochem. 2020, 155:577–591. [DOI] [PubMed] [Google Scholar]
- 63.Gibbons GS, Kim SJ, Wu QH, Riddle DM, Leight SN, Changolkar L, Xu H, Meymand ES, O’Reilly M, Zhang B, et al. : Conformation-selective tau monoclonal antibodies inhibit tau pathology in primary neurons and a mouse model of alzheimer’s disease. Mol. Neurodegener. 2020, 15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin WS, Di J, Cao Q, Li B, Seidler PM, Murray KA, Bitan G, Jiang L: Amyloid beta-protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimers Res. Ther. 2019, 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang XL, Wesen E, Kumar R, Bernson D, Gallud A, Paul A, Wittung-Stafshede P, Esbjorner EK: Correlation between cellular uptake and cytotoxicity of fragmented alpha-synuclein amyloid fibrils suggests intracellular basis for toxicity. ACS Chem. Neurosci. 2020, 11:233–241. [DOI] [PubMed] [Google Scholar]
- 66. Evans HT, Benetatos J, van Roijen M, Bodea L-G, Götz J: Decreased synthesis of ribosomal proteins in tauopathy revealed by non-canonical amino acid labelling. EMBO J. 2019, 38:e101174. The power of in vivo labeling tools in understanding the pathological roles of amyloids is shown by non-canonical amino acid tagging of newly synthesized proteins in tau Tg mice and subsequent labeling both for fluorescence imaging and MS analysis.
- 67.Evans LD, Wassmer T, Fraser G, Smith J, Perkinton M, Billinton A, Livesey FJ: Extracellular monomeric and aggregated tau efficiently enter human neurons through overlapping but distinct pathways. Cell Rep. 2018, 22:3612–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jun JV, Haney CM, Karpowicz RJ, Giannakoulias S, Lee VMY, Petersson EJ, Chenoweth DM: A “clickable” photoconvertible small fluorescent molecule as a minimalist probe for tracking individual biomolecule complexes. J. Am. Chem. Soc. 2019, 141:1893–1897. This work demonstrates the utility of CPX-labeled proteins in tracking single aggregate clusters through spatially-resolved tagging by photo-conversion.
- 69. Saal K-A, Richter F, Rehling P, Rizzoli SO: Combined use of unnatural amino acids enables dual-color super-resolution imaging of proteins via click chemistry. ACS Nano 2018, 12:12247–12254. The authors site-specifically labeled two different proteins with different fluorophores through Uaa mutagenesis by expressing them in separate sets of cells and fusing the cells, followed by super-resolution microscopy imaging of co-localization of the proteins.
- 70. van Husen LS, Schedin-Weiss S, Trung MN, Kazmi MA, Winblad B, Sakmar TP, Elsasser SJ, Tjernberg LO: Dual bioorthogonal labeling of the amyloid-beta protein precursor facilitates simultaneous visualization of the protein and its cleavage products. J. Alzheimers Dis. 2019, 72:537–548. Uaa mutagenesis and enzymatic (SNAP) tagging for live cell dual labeling was used to monitor proteolytic cleavage of APP to generate toxic Aβ42 and co-localization of the products.
- 71.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM: Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (boncat). Proc. Natl. Acad. Sci. U.S.A. 2006, 103:9482–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]