Abstract
Objective:
To develop and validate a pre-conception risk prediction index for severe maternal morbidity (SMM), defined by the CDC as indicators of a life-threatening complication, among infertile patients.
Design:
Retrospective analysis of livebirths and stillbirths from 2007–2017 among infertile women.
Setting:
National commercial claims database.
Patients:
Infertile women identified based on diagnosis, testing, or treatment codes.
Interventions:
None
Main Outcome Measures:
The primary outcome was SMM, identified as any indicator from the CDC Index except blood transfusion alone which has been found to overestimate cases. Twenty preconception comorbidities associated with risk of SMM were selected from prior literature. Targeted ensemble learning methods were used to rank the importance of comorbidities as potential risk factors for SMM. The independent strength of association between each comorbidity and SMM was then used to define each comorbidity’s risk score.
Results:
Among 94,097 infertile women with a delivery, 2.3% (n=2,181) experienced an SMM event. The highest risk of SMM was conferred by pulmonary hypertension, hematologic disorders, renal disease, and cardiac disease. Associated significant risks were lowest for substance abuse disorders, prior cesarean section, age > 40 years, gastrointestinal disease, anemia, mental health disorders and asthma. The ROC-AUC for the developed comorbidity score was 0.66. Calibration plots showed good concordance between predicted and actual risk of SMM.
Conclusion:
We developed and validated an index to predict the probability of SMM in patients with infertility based on preconception comorbidities. This tool may inform preconception counseling of infertile women and support maternal health research initiatives.
Keywords: severe maternal morbidity, infertility, preconception counseling
Capsule:
Development and validation of a risk prediction index for severe maternal morbidity based on preconception comorbidities among infertile patients. This tool may inform preconception counseling of infertile women.
Introduction
Severe maternal morbidity (SMM) refers to a life-threatening diagnosis or the need to undergo a potentially life-saving procedure in close proximity to birth (1). SMM continues to be an issue of national and global concern and is associated with maternal mortality, increased health care costs, and significant family burden (2, 3). While deaths attributed to direct obstetric causes such as hemorrhage, infection and hypertensive disorders of pregnancy have decreased, there has been a sharp increase in the United States in maternal deaths due to indirect obstetric causes including complications related to chronic health conditions (4). SMM, which serves as a proxy for maternal mortality, has several recognized risk factors including age, co-morbid health conditions and infertility (5–7).
Infertility affects 15% of US women overall and 26% of women 40–44 years of age (8) and the utilization of Assisted Reproductive Technology (ART) has also tripled since 1996 (9). There is substantial evidence that both short- and long-term health outcomes of infertile women are poorer than among fertile women (10, 11). Infertile women who succeed in conceiving face higher risks of adverse pregnancy outcomes including SMM (12, 13). The goal of pre-pregnancy care is to reduce the risk of adverse health effects for a woman and her neonate by providing education about healthy pregnancy and addressing modifiable risk factors (14). By nature of their inability to conceive, many infertile patients have preconception clinical interactions that provide an opportunity to consider risks to a woman’s health to inform her choices and medical care. Adverse pregnancy outcomes, SMM and maternal mortality have been consistently associated with poor pre-pregnancy health (2, 4, 15, 16). Furthermore, individual comorbidities such as chronic hypertension (17) and obesity (18, 19) have been associated with increased risk of maternal morbidity in a dose-response fashion, but the interaction of multiple underlying health conditions and infertility on a woman’s risk of experiencing SMM is poorly understood. Currently, no algorithm accounts for multiple contributing factors to help physicians counsel infertile women on the health risks associated with carrying a pregnancy.
Obstetric comorbidity indices are used in the general obstetrics population but are primarily designed to evaluate the risk of adverse pregnancy outcomes upon admission to labor and delivery and are derived from nationally acquired samples of obstetric patients at low risk for infertility (20, 21). Neither has considered pre-conception comorbidities together nor studied them among infertile women, who are known to face higher risk of SMM than fertile women (12). This increased risk for SMM among women with infertility is likely multifactorial, due to beginning pregnancy at older ages and with more comorbidities than fertile women, as well an independent association of infertility and increased risk of adverse pregnancy outcomes, and therefore warrants analysis separate from the general obstetric population (5, 6, 10–13). Furthermore, pregnancy-related conditions (in particular placental disorders) rank high in these obstetric risk prediction indices, but are not informative for preconception counseling. We hypothesized that the risk of SMM among infertile women can be predicted and this can serve as a preconception physician counseling tool.
Methods
We analyzed livebirths and stillbirths among infertile women in the Optum® Clinformatics DataMart from 2007–2017. Optum’s Clinformatics DataMart is derived from a database of administrative health claims that are verified, adjudicated, adjusted and deidentified prior to inclusion. 23% of the US population is covered by a plan included in CDM, and the distribution of ethnicity and region is similar to the US population overall. Ethics approval was obtained from the Stanford University Institutional Review Board. We followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis) statements in the reporting of our study (22).
Definition of Study Cohort
The study cohort was constructed by first identifying women in the database who underwent a livebirth or stillbirth during the enrollment period using a previously developed method for claims databases during 2007 to 2017 (23). The diagnosis and procedure codes used to identify births have been previously described by our group(24). For the 20% of patients who had more than one delivery during the enrollment period, only the first delivery was included. Twin and higher order gestations were excluded because they are associated with SMM but not known before conception. In the delivery cohort, patients with infertility were then identified using a claims-based characterization that our group has described and used in prior publications (10–12). In brief, the group of infertile women was comprised of women receiving any of the following during enrollment: 1) an infertility diagnosis, 2) fertility testing, or 3) fertility treatment. Women with an infertility diagnosis were identified by ICD-9/10 diagnosis codes detailed in Supplemental Table 1. Fertility testing was identified through diagnosis codes (V26.21, Z31.41) or the presence of a procedure code (CPT) for hysterosalpingogram (HSG) (74740). We included patients undergoing fertility testing with the understanding that they would already meet criteria for an infertility diagnosis. However, physicians may use testing instead of diagnosis codes for reimbursement purposes thus we sought to capture these women for inclusion in the infertile group. Women receiving fertility treatment were identified by the presence of a CPT code for intra-uterine artificial insemination (58322), follicular puncture for oocyte retrieval (58970), or intrauterine embryo transfer (58974). The presence of a pharmacy claim for a prescription for clomiphene citrate or a gonadotropin (Follicle Stimulating Hormone, FSH, Human Menopausal Gonadotropin, HMG, Human Chorionic Gonadotropin, HCG) was also used to identify women receiving fertility treatment.
Definition of Severe Maternal Morbidity
The primary outcome was SMM, identified by the CDC SMM Index of 21 indicators and corresponding ICD-CM codes (Supplemental Table 2), except for blood transfusion alone. Severe maternal morbidity excluding blood transfusion-only cases, which we refer to hereafter as SMM, was studied as the primary outcome because blood transfusion is the only indicator of SMM in approximately half of cases, suggesting that some transfusion-related events may represent less severe forms of SMM. The CDC and others now commonly report both forms of SMM (1, 20). As a secondary outcome, we looked at all SMM cases including those in which blood transfusion was the only indicator, and have included these results as supplementary information.
Selection of Comorbidities
Preconception comorbidities associated with risk of SMM were selected a priori based on relevant literature and available data (19, 21, 25–30). We used claims from 2006 through 2017, one year prior to the delivery cohort period, to identify the comorbidities. Women were classified as having a preconception comorbidity if there was any claim specific to the condition recorded prior to the delivery date. However, identification of anemia and hematologic disorders was restricted to diagnoses on claims at least 6 months (187 days) before the delivery to ensure it was not associated with gestation. A total of 20 preconception comorbidities were included that have been associated with increased risk of SMM in a general obstetrics population, as well as chronic conditions and diagnoses that are prevalent among infertile women, including uterine fibroids, endometriosis and polycystic ovarian syndrome. The ICD-CM diagnosis codes used to identify each predictor are detailed in Supplemental Table 3.
Development of Comorbidity Index
We used a casual inference approach to rank the importance of comorbidities as potential risk factors for SMM based on adjusted risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CI). Targeted ensemble learning methods previously used by our group and others have been shown to produce more accurate and precise estimates than conventional statistical approaches (24). Analyses were performed using the Varimpact package in R Version 4.0.2 (https://github.com/ck37/varimpact). Performance of the index was assessed by randomly selecting 75% of the cohort as training data, and validation was assessed in the remaining 25% of the data. Four-fold cross-validation was also used in the variable importance analysis.
Comorbidities with significant P values (<0.05) were retained in the risk prediction index after adjustment for multiple comparisons using the Benjamini-Hochberg false discovery rate procedure (31). In brief, points were assigned to each comorbidity retained in the risk prediction index by dividing the log of the risk ratio by the log of the smallest comorbidity risk ratio. This approach results in a comorbidity assigned a score of 5 points as 5 times “more important” in increasing the risk of SMM than a comorbidity assigned a score of 1 point. More detailed information on development of the comorbidity index is presented in a prior publication (24).
Performance of the Comorbidity Index
Discrimination and calibration of the risk prediction index was assessed to determine accuracy. Discrimination was determined by plotting a receiver operator characteristic (ROC) curve and calculating the area under the curve (AUC), using a ROC-AUC of 0.5 as baseline and a value of 1.0 for perfect discrimination. Precision-recall (P-R) curves were also plotted, which show how the positive predictive value (precision) varies with the true positive rate (recall). P-R curves are informative when predicting rare outcomes, which can cause ROC statistics to be misleadingly high (32). We also assessed calibration of the comorbidity scores using Lowess-smoothed and grouped visualizations comparing the actual with the predicted risk of SMM in accordance with TRIPOD guidelines on prediction model development (22).
Results
Among 94,097 infertile women with a delivery, 91,916 (97.7%) did not experience an SMM event and 2,181 (2.3%) experienced SMM. 3,027 (3.2%) experienced SMM including transfusion-only events. The majority of women in the cohort were White and approximately one-third of the cohort attained a bachelor degree or higher level of education (Table 1). Preconception comorbidities varied in prevalence; the most common comorbidities among infertile women without any SMM events were thyroid disease (16.1%), major mental health disorder (14.9%) and age 40 years or older (13.8%). Among infertile women who experienced SMM, the most common comorbidities were age 40 years or older (19.7%), major mental health disorder (20.2%), uterine fibroids (17.9%), and thyroid disease (17.6%). In both groups, the least common comorbidities were pulmonary hypertension (0.09% vs 1.2%, respectively), epilepsy (0.5% vs 1.0%, respectively), and substance use disorder (2.0% vs 3.4%, respectively).
Table 1.
Descriptive characteristics of the study population, patients with singleton live birth and history of infertility, 2006–2017 (N = 94,097)
| Infertile patients with delivery and no SMM* event N=91.916 |
Infertile patients with delivery and any SMM* event N=2.181 |
||
|---|---|---|---|
| Maternal Age (years) | Mean (SD) | 34.2 (4.8) | 35.2 (5.2) |
| Year of Delivery | % | ||
| 2007–2008 | 16.7 | 16.9 | |
| 2009–2011 | 26.7 | 27.7 | |
| 2012–2014 | 26.9 | 22.6 | |
| 2015–2017 | 29.7 | 32.9 | |
| Maternal Race | % | ||
| Asian | 12.5 | 8.5 | |
| Black | 7.8 | 12.7 | |
| Hispanic | 10.7 | 10.3 | |
| White | 69.0 | 68.6 | |
| Highest Education | % | ||
| High School Diploma or less | 15.0 | 17.4 | |
| < Bachelor Degree | 49.3 | 52.6 | |
| ≥ Bachelor Degree | 35.8 | 30.0 | |
| Maternal age ≥ 40 | 13.8 | 19.7 | |
| Preconception anemia | 8.7 | 12.8 | |
| Asthma | 6.4 | 8.9 | |
| Cardiac disease | 5.4 | 14.6 | |
| Chronic hypertension | 6.7 | 16.5 | |
| Chronic renal disease | 1.4 | 3.6 | |
| Connective tissue disease | 1.7 | 3.7 | |
| Diabetes mellitus | 4.6 | 7.8 | |
| Endometriosis | 6.2 | 7.8 | |
| Epilepsy | 0.5 | 1.0 | |
| Gastrointestinal disease | 10.7 | 16.1 | |
| Hematologic disorder | 1.7 | 4.9 | |
| Mental health disorder | 14.9 | 20.2 | |
| Polycystic ovarian syndrome | 12.9 | 12.5 | |
| Prior cesarean delivery | 5.8 | 9.5 | |
| Prior uterine surgery (non-cesarean) | 5.0 | 9.2 | |
| Pulmonary hypertension | 0.09 | 1.2 | |
| Substance abuse disorder | 2.0 | 3.4 | |
| Thyroid disease | 16.1 | 17.6 | |
| Uterine fibroids | 10.4 | 17.9 | |
SMM, severe maternal morbidity
Excluding cases with blood transfusion as the only SMM indicator
Variable importance analyses were performed in a training cohort (75% of full sample) to identify the preconception comorbidities that conferred the highest risk of SMM. The independent strength of association between each comorbidity and SMM outcome was then used to define each comorbidity’s risk score. Comorbidity scores ranged from 1 to 7. Patients received a total risk score ranging from 0 to 26 based on the presence or absence of individual preconception diagnoses with a mean score of 1.7 and median score of 1. Among patients who experienced an SMM event, the mean score was 3.3 and the median score was 2. Among patients who did not experience an SMM event, the mean score was 1.7 and the median score was 1. Pulmonary hypertension, hematologic disorders, renal disease, cardiac disease and chronic hypertension conferred the highest risk of SMM (Table 2). Associated significant risks were lowest for substance abuse disorders, prior cesarean section, age > 40 years, gastrointestinal disease, anemia, mental health disorders and asthma. Certain variables dropped out of the analysis due to small and inconsistent point estimates; these preconception conditions included endometriosis, thyroid disease, and polycystic ovarian syndrome. Comorbidity scores for the secondary outcome of SMM including cases with blood transfusion as the only indicator are shown in Supplemental Table 4.
Table 2.
Associations and derived risk scores for comorbidities in relation to non-transfusion severe maternal morbidity (using training set of births, n = 70,572)
| Risk factor for SMM* | Rate of SMM* (%) | Crude RR | aRR** (95% CI) | Risk Score |
|---|---|---|---|---|
| Pulmonary Hypertension | 21.4 | 11.5 (6.9–19.2) | 4.8 (4.5–5.2) | 7 |
| Hematologic Disorder | 7.0 | 3.3 (2.6–4.1) | 2.9 (2.5–3.4) | 5 |
| Chronic Renal Disease | 6.1 | 2.8 (2.1–3.7) | 1.9 (1.7–2.2) | 3 |
| Cardiac Disease | 5.9 | 2.9 (2.5–3.3) | 2.3 (2.0–2.7 | 4 |
| Chronic Hypertension | 5.4 | 2.7 (2.3–3.1) | 2.4 (2.1–2.7) | 4 |
| Connective Tissue Disease | 4.6 | 2.1 (1.6–2.7) | 1.5 (1.2–1.8) | 2 |
| Prior Uterine Surgery (Non-Cesarean) | 4.4 | 2.0 (1.7–2.4) | 1.6 (1.4–1.9) | 2 |
| Epilepsy | 4.1 | 1.8 (1.1–3.1) | 1.5 (1.3–1.7) | 2 |
| Uterine Fibroids | 4.0 | 1.9 (1.7–2.1) | 1.6 (1.4–1.9) | 2 |
| Diabetes Mellitus | 3.6 | 1.6 (1.3–2.0) | 1.4 (1.2–1.8) | 2 |
| Substance Use Disorder | 3.9 | 1.7 (1.3–2.2) | 1.4 (1.1–1.7) | 1 |
| Prior Cesarean Delivery | 3.7 | 1.7 (1.4–2.0) | 1.3 (1.1–1.6) | 1 |
| Age 40 Years or Older | 3.4 | 1.6 (1.4–1.8) | 1.4 (1.2–1.6) | 1 |
| Gastrointestinal Disease | 3.4 | 1.6 (1.4–1.8) | 1.3 (1.2–1.6) | 1 |
| Preconception anemia | 3.3 | 1.5 (1.3–1.7) | 1.2 (1.1–1.5) | 1 |
| Major Mental Health Disorder | 3.2 | 1.5 (1.3–1.6) | 1.3 (1.1–1.4) | 1 |
| Asthma | 3.1 | 1.4 (1.2–1.7) | 1.3 (1.1–1.5) | 1 |
| Endometriosis | 3.1 | 1.4 (1.2–1.6) | NS | NS |
| Thyroid disease | 2.6 | 1.1 (0.99–1.3) | NS | NS |
| Polycystic Ovarian Syndrome | 2.2 | 0.96 (0.8–1.1) | NS | NS |
SMM, severe maternal morbidity; aRR, adjusted risk ratio
Excluding cases with blood transfusion as the only SMM indicator
Adjusted for delivery year and other comorbidities using data-adaptive algorithms
The discrimination of the comorbidity scores in prediction of SMM was assessed based on ROC curves and corresponding AUC statistics (AUROC). As shown in Figure 1a, the AUROC for SMM was 0.66. We further assessed discrimination of the model using P-R curves because SMM and non-transfusion SMM are rare outcomes, which can skew the ROC and AUROC to conflate the predictive ability of the model. The baseline value for the PR ROC is 0.023 (the incidence of SMM). As shown in Figure 1b, the PR ROC for SMM was 0.054. The shape of the PR curve indicates good detection of outcomes that are strongly correlated with the outcome of interest. We also assessed calibration as a goodness of fit; that is, to what extent the index correctly estimates absolute risk of SMM. As shown in the calibration plot in Figure 2, the predicted values of the outcomes using the comorbidity index scores were concordant with the actual values for the vast majority of patients. In the small subset of patients with an actual risk of SMM between 5–10%, the model over-estimated risk.
Figure 1a:
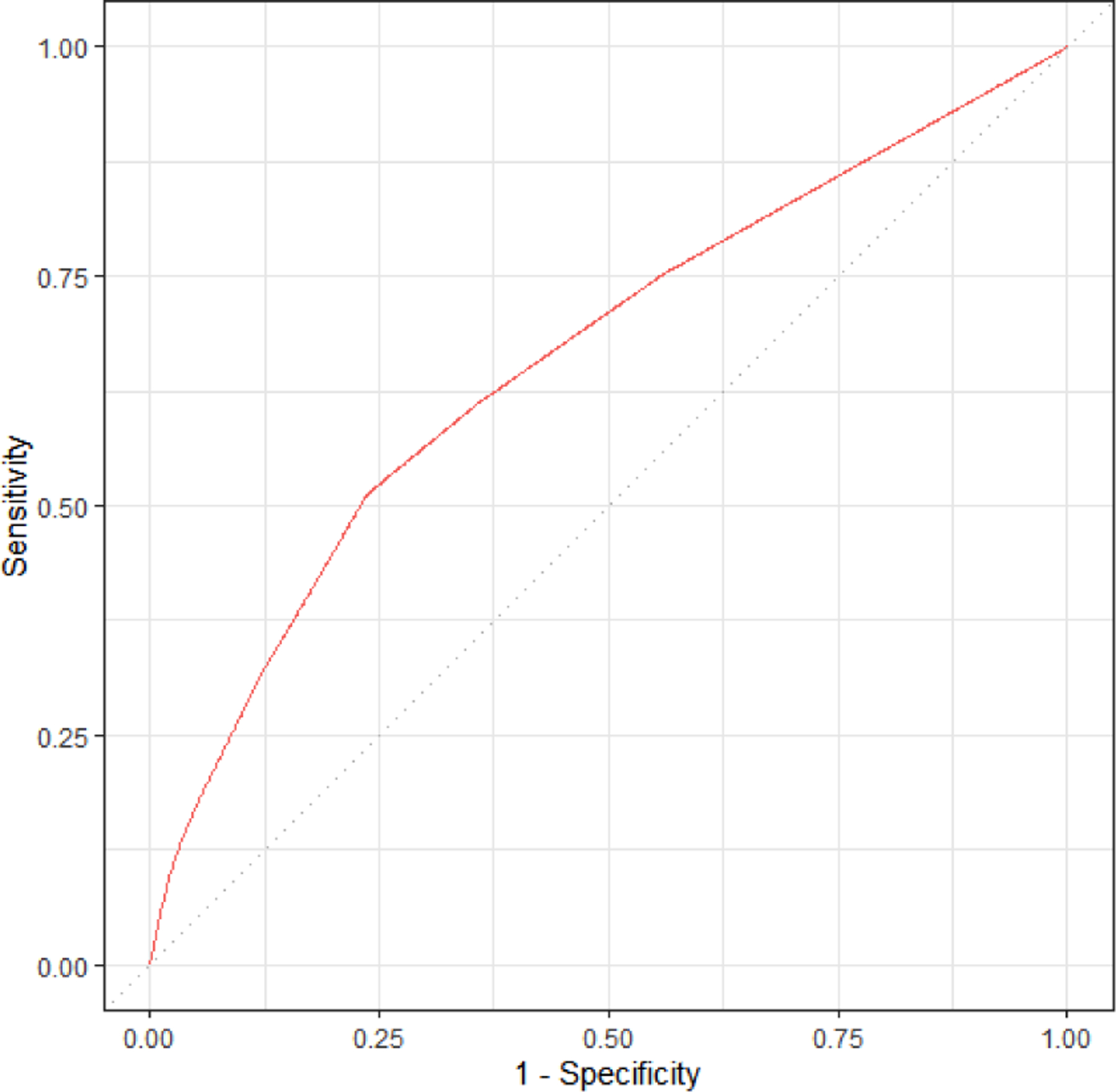
Receiver operating characteristic (ROC) curve for expanded comorbidity index in predicting SMM excluding transfusion-only cases (AUC=0.66). Assessed using validation set of births, n = 23,525.
Figure 1b.
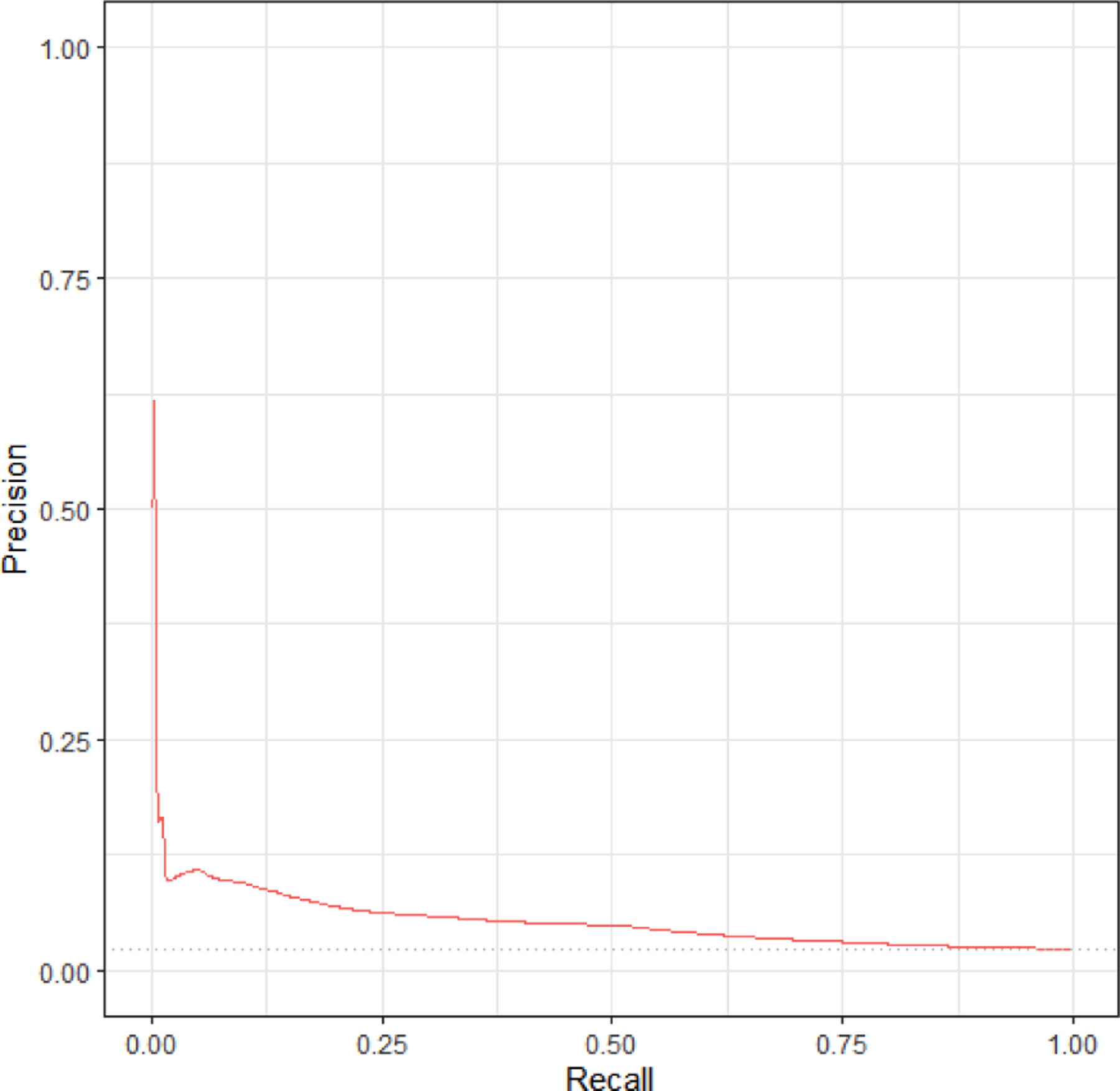
Precision recall (PR) curve for expanded comorbidity index in predicting SMM excluding transfusion-only cases (PR-ROC=0.054). Assessed using validation set of births, n = 23,525. Baseline of 0.023 (proportion of women with SMM.)
Figure 2.
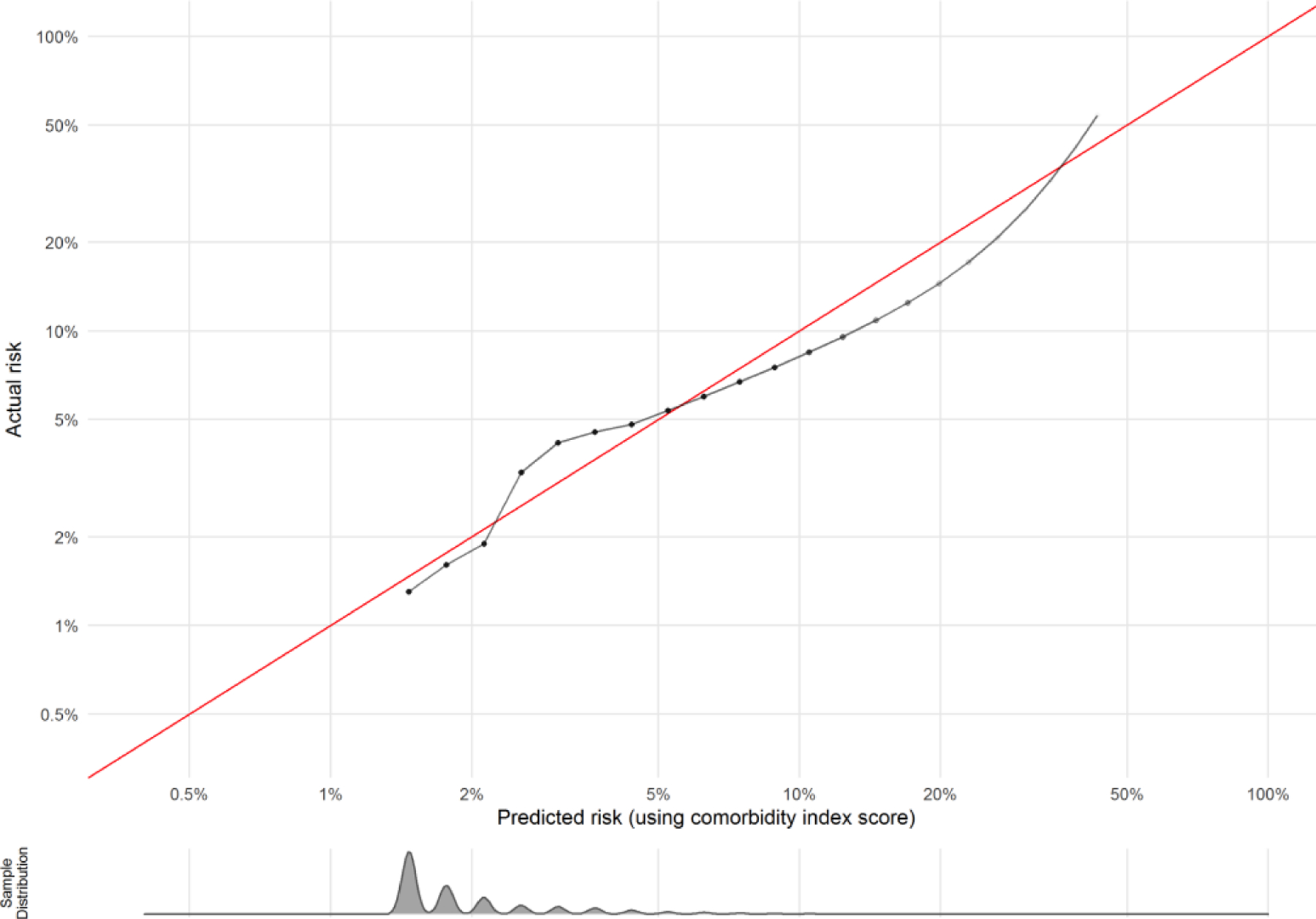
Calibration plot of preconception comorbidity index in predicting SMM excluding transfusion-only cases. Assessed using validation set of births, n = 23,525. Density plot below calibration curve shows distribution of the sample across predicted risks.
The risk prediction index was also applied to the secondary study outcome, SMM including cases in which blood transfusion was the only indicator. Risk scores are shown in Supplemental Table 4 and are overall similar. ROC and Precision-Recall curves for the secondary outcome of SMM including cases with blood transfusion as the only indicator are shown in Supplemental Figures 1a and 1b. The calibration plot in Supplemental Figure 2 depicts that the prediction of SMM including blood transfusion was less concordant with actual risk of these SMM events. The risk of blood transfusion is also significantly influenced by conditions that develop during pregnancy such as placenta accreta spectrum(33) that are not accounted for in our model, which includes only preconception comorbidities.
Conclusion
We present a novel preconception risk prediction index for SMM among infertile patients validated using commercial US claims data. In the absence of an absolute contraindication to pregnancy, preconception counseling to date has focused on risks conferred by individual comorbidities and is not tailored to the risks faced by infertile women. While it is known that infertile women are older and more likely to have co-morbid conditions than their fertile counterparts(6, 7, 12), the cumulative effect of individual comorbidities on the ultimate risk of SMM among infertile women is not known. Our index improves on existing comorbidity indices in two key aspects: 1) employing a preconception perspective to function as a physician counseling tool and 2) focusing exclusively on infertile women, who are at increased risk of SMM compared to the general obstetric population. (6, 12). In addition, our index spans the breadth of conditions that contribute to SMM risk among infertile women, ranging from pulmonary hypertension, a very high risk condition in pregnancy(34), to conditions such as uterine fibroids that confer less obstetric risk but are more prevalent among infertile women(35). A risk index of this nature used prenatally not only allows for counseling of infertile women, but may also allow for early and aggressive interventions that may mitigate the final risk for SMM. Other aspects to be evaluated include clinical impact such as utilization and interpretation of this tool by infertility physicians and economic impact of decreasing SMM, which is associated with a significant financial burden(36).
Scores for several of the preconception comorbidities merit discussion. Pulmonary hypertension, hematologic disorders, renal disease, chronic hypertension and cardiac disease have been consistently associated with SMM(21, 24, 37). The diagnosis of uterine fibroids, age 40 years or older, and prior uterine surgery, which are all more prevalent among infertile women than fertile women, were associated with moderate risk scores for SMM. Increased maternal age has been previously associated with increased SMM risk (18), however the moderate risk score assigned to age alone suggests that comorbidities that develop with age, such as chronic hypertension or cardiac disease, have a stronger influence on SMM risk than age alone. Endometriosis, polycystic ovarian syndrome, and thyroid disease, although appearing more frequently among infertile women than their fertile counterparts, had non-significant or inconsistent associations with SMM.
The risk prediction index had comparative performance with existing obstetric comorbidity indices(21, 24) with a ROC-AUC of 0.66. Calibration plots showed good concordance between predicted and actual risk of SMM. The predictive performance of our index underscores that antenatal conditions such as placental disorders also influence SMM risk. Because the goal of this study was to create a preconception counseling tool, we did not incorporate antenatal conditions that also modify SMM risk. Re-evaluation during SMM risk during pregnancy, at which time plurality and the development of antenatal conditions can be incorporated into the model, can further increase the accuracy of SMM risk prediction for an individual patient.
There are several limitations of this study. We chose SMM as the outcome to test our hypothesis because it is well-characterized, widely recognized as a measure to study obstetric outcomes, and readily accessed using administrative data. Although SMM is 100 times more common than maternal death, it remains a rare outcome and individual SMM indicators can be under- or over-reported as a result(20). In future studies we plan to expand the index to include less severe but more common adverse obstetric outcomes as well as neonatal outcomes. Utilizing insurance claims data has many advantages but also introduces selection bias of the study population as a whole because only insured patients are included, and due to the absence of linkage to a population-based registry, we are unable to distinguish subject misidentification or loss of continuity. For example, details regarding infertility diagnosis and treatment are not fully captured in insurance claims data and we do not have access to information on treatment that occurred outside of insurance coverage or why an individual patient changes or loses insurance coverage. In addition, the database may over or under-estimate risk based on the homogeneity of the population. Our group has, however, previously validated an obstetric risk prediction index using administrative claims data(24). Inclusion of women undergoing fertility treatment is an additional limitation of the analysis as this is a heterogenous group that may not be infertile and there may be subsets that are higher risk for SMM. Overall, however, we anticipate this would have resulted in an underestimate of SMM incidence. In future versions of this analysis, we also plan to include grouped claims to capture patients undergoing fertility treatment in states with mandated coverage. Finally, while the population at risk for SMM is women who carry a pregnancy beyond 20 weeks gestation, we recognize that not all women who seek preconception counseling go on to have a viable pregnancy, and the health outcomes of these women is an important area of research.
Categorization of comorbidities required us to group heterogeneous disorders, each of which are independently associated with adverse maternal outcomes. For example, the severity of endometriosis, the number and location of fibroids, and other medically relevant aspects of infertility related conditions cannot be determined from claims data and mild cases of these conditions can obscure effects caused by more serious disease. Infertility is also a heterogenous group of disorders, and SMM risk may vary by infertility diagnosis. In future studies, we aim to expand on heterogenous infertility diagnoses and comorbidities to understand the varying SMM risk associated with individual disorders or varying SMM risk based on the severity of individual comorbidities. Incorporating pharmacy information, such the number and type of medications used to treat a specific comorbid condition, may provide valuable information for both SMM risk stratification and patient counseling. These subgroup analyses are currently limited by the rare nature of SMM events but can be analyzed in larger datasets with more granularity. Granular information such as BMI are also difficult to capture using insurance claims data. In future versions of this index we hope to incorporate these additional covariates. The association between BMI and increased risk of SMM, however, has been attributed to comorbidities and cesarean birth that are represented independently in our model (27). Given the rare nature of SMM events, we were unable to individualize SMM risk among patient with an infertility diagnosis versus those who undergo fertility treatment. In future studies, we plan to utilize larger datasets to develop more individualized risk scores.
Strengths of the study include the novel application of an obstetric comorbidity index to an infertile population as a preconception counseling tool, the large size of our cohort which enables analysis of rare outcomes, and rigorous statistical methodology in the development and validation of the risk scores. We employed sophisticated causal inference statistical analyses and assessed discrimination of the model using ROC and P-R curves as recommended by the TRIPOD statement for prediction model development(22). We also assessed calibration to determine if the predictions generated by the model are accurate. The selection of preconception comorbidities built on our group’s experience with risk prediction. The frequencies of the most common comorbidities included similar to those reported elsewhere in literature(38–41). Finally, our goal for this project was to create an objective tool for decision-making, given the preponderance of evidence that unconscious bias based on race, ethnicity, class, etc. affect clinical care(42). Race has been consistently associated with maternal morbidity and mortality, with black women facing a severalfold higher risk of morbidity and mortality than any other race or ethnicity(43). Incorporating race as an independent variable in a predictive algorithm, however, has the potential to exacerbate racial inequities in care(44). To avoid the potential for race-based adjustments to deter clinicians from offering fertility treatments to women and amplifying disparities that already exist(45), we opted to exclude race as an independent variable in the model.
In conclusion, this SMM risk prediction index can help clinicians provide more accurate preconception counseling to infertile patients about their pregnancy risks and appropriate plan of care. Quantifying the risk of SMM not only provides objective information but also can facilitate the conversation between a physician and patient on treatment options that optimize her health and support delivery of a healthy pregnancy (46). This tool can assist both physicians and patients to navigate the complex decision-making process of fertility treatment and empower patients and physicians to identify modifiable risk factors prior to conception to decrease the risk of an adverse obstetric outcome.
Supplementary Material
Acknowledgments
Funding for this work was provided by a grant from the Stanford Women’s Health & Sex Differences in Medicine (WHSDM) Center and support from the Eunice Kennedy Shriver NICHD of the National Institutes of Health under award number 1K12HD103084.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Ruben Alvero is Scientific Advisor to Hannah Life Technologies and Orchid Bioscience. Dr. Deirdre Lyell is a consultant to Bloomlife.
Presented at the 76th annual meeting (virtual), American Society for Reproductive Medicine, October 17–21, 2020.
References
- 1.Prevention CfDCa. Severe Maternal Morbidity in the United States. In, 2020.
- 2.Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt) 2014;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright VC, Schieve LA, Reynolds MA, Jeng G. Assisted reproductive technology surveillance--United States, 2000. MMWR Surveill Summ 2003;52:1–16. [PubMed] [Google Scholar]
- 4.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstet Gynecol 2017;130:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol 2017;217:327 e1- e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke B, Brown MB, Wantman E, Baker VL, Doody KJ, Seifer DB et al. Risk of severe maternal morbidity by maternal fertility status: a US study in 8 states. Am J Obstet Gynecol 2019;220:195 e1- e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- 8.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014:1–21. [PubMed]
- 9.Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L et al. Assisted Reproductive Technology Surveillance - United States, 2015. MMWR Surveill Summ 2018;67:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Risk of cancer in infertile women: analysis of US claims data. Hum Reprod 2019;34:894–902. [DOI] [PubMed] [Google Scholar]
- 11.Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of incident chronic medical conditions in infertile women: analysis of US claims data. Am J Obstet Gynecol 2019;220:473 e1- e14. [DOI] [PubMed] [Google Scholar]
- 12.Murugappan G, Li S, Lathi RB, Baker VL, Luke B, Eisenberg ML. Increased risk of severe maternal morbidity among infertile women: analysis of US claims data. Am J Obstet Gynecol 2020;223:404 e1- e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin AS, Monsour M, Kissin DM, Jamieson DJ, Callaghan WM, Boulet SL. Trends in Severe Maternal Morbidity After Assisted Reproductive Technology in the United States, 2008–2012. Obstet Gynecol 2016;127:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol 2019;133:e78–e89. [DOI] [PubMed] [Google Scholar]
- 15.Coleman PK, Reardon DC, Calhoun BC. Reproductive history patterns and long-term mortality rates: a Danish, population-based record linkage study. Eur J Public Health 2013;23:569–74. [DOI] [PubMed] [Google Scholar]
- 16.Barclay K, Keenan K, Grundy E, Kolk M, Myrskyla M. Reproductive history and post-reproductive mortality: A sibling comparison analysis using Swedish register data. Soc Sci Med 2016;155:82–92. [DOI] [PubMed] [Google Scholar]
- 17.Sutton EF, Rogan SC, Lopa S, Sharbaugh D, Muldoon MF, Catov JM. Early Pregnancy Blood Pressure Elevations and Risk for Maternal and Neonatal Morbidity. Obstet Gynecol 2020;136:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisonkova S, Potts J, Muraca GM, Razaz N, Sabr Y, Chan WS et al. Maternal age and severe maternal morbidity: A population-based retrospective cohort study. PLoS Med 2017;14:e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol 2016;214:643 e1-e10. [DOI] [PubMed] [Google Scholar]
- 21.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol 2013;122:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernandez-Diaz S. Identifying pregnancies in insurance claims data: Methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf 2019;28:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard SA, Kennedy CJ, Carmichael SL, Lyell DJ, Main EK. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol 2020;136:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard SAKC, Carmichael SL, Lyell DJ and Main EK. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol In Press. [DOI] [PMC free article] [PubMed]
- 26.Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol 2019;33:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard SA, Carmichael SL, Main EK, Lyell DJ, Abrams B. Risk of severe maternal morbidity in relation to prepregnancy body mass index: Roles of maternal co-morbidities and caesarean birth. Paediatr Perinat Epidemiol 2020;34:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grobman WA, Bailit JL, Rice MM, Wapner RJ, Reddy UM, Varner MW et al. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol 2014;123:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J Matern Fetal Neonatal Med 2012;25:2529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easter SR, Bateman BT, Sweeney VH, Manganaro K, Lassey SC, Gagne JJ et al. A comorbidity-based screening tool to predict severe maternal morbidity at the time of delivery. Am J Obstet Gynecol 2019;221:271.e1-.e10. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 32.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One 2015;10:e0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gynecologists TACoOa. Obstetric Care Consensus: Placenta Accreta Spectrum. Obstet Gynecol 2018;132. [DOI] [PubMed] [Google Scholar]
- 34.European Society of G, Association for European Paediatric C, German Society for Gender M, Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–97. [DOI] [PubMed] [Google Scholar]
- 35.Zepiridis LI, Grimbizis GF, Tarlatzis BC. Infertility and uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2016;34:66–73. [DOI] [PubMed] [Google Scholar]
- 36.Vesco KK, Ferrante S, Chen Y, Rhodes T, Black CM, Allen-Ramey F. Costs of Severe Maternal Morbidity During Pregnancy in US Commercially Insured and Medicaid Populations: An Observational Study. Matern Child Health J 2020;24:30–8. [DOI] [PubMed] [Google Scholar]
- 37.Easter SR, Bateman BT, Sweeney VH, Manganaro K, Lassey SC, Gagne JJ et al. A comorbidity-based screening tool to predict severe maternal morbidity at the time of delivery. Am J Obstet Gynecol 2019;221:271 e1- e10. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization GHODRWHS. Prevalence of anemia among women of reproductive age (% of women age 15–49). The World Bank Data. [Google Scholar]
- 39.Verma I, Sood R, Juneja S, Kaur S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int J Appl Basic Med Res 2012;2:17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Practice Committee of the American Society for Reproductive M. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015;104:545–53. [DOI] [PubMed] [Google Scholar]
- 41.Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open 2019;2019:hoy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol 2014;210:435 e1–8. [DOI] [PubMed] [Google Scholar]
- 44.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight - Reconsidering the Use of Race Correction in Clinical Algorithms. N Engl J Med 2020;383:874–82. [DOI] [PubMed] [Google Scholar]
- 45.Quinn M, Fujimoto V. Racial and ethnic disparities in assisted reproductive technology access and outcomes. Fertil Steril 2016;105:1119–23. [DOI] [PubMed] [Google Scholar]
- 46.Dar S, Lazer T, Swanson S, Silverman J, Wasser C, Moskovtsev SI et al. Assisted reproduction involving gestational surrogacy: an analysis of the medical, psychosocial and legal issues: experience from a large surrogacy program. Hum Reprod 2015;30:345–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


