Abstract
Significance.
Dry eye is one of the leading causes for individuals to seek eye care, while the pathogenesis is poorly understood. One mechanism in which dry eye inflammation may ensue is by the release of damage associated molecular patterns (DAMPs) by damaged cells to stimulate the production of cytokines and matrix metalloproteinases. Examining DAMPs levels on the ocular surface during dry eye disease (DED) will increase our understanding of their potential involvement in the pathogenesis of DED.
Purpose.
This study aims to quantitate DAMPs, high-mobility group box 1 (HMGB1) and heat shock proteins (HSPs) on the ocular surface of normal and dry eye subjects; and to examine the impact of low humidity environment (LHE) on DAMPs and inflammation in dry eye subjects.
Methods.
Basal tears (10–20μl) and conjunctival impression cytology (CIC) samples were analyzed for HMGB1, HSP-27, −60, −70 and −90α by ELISA or Luminex assays in normal (n=15) and DED (n=15) subjects. In addition, a subset of DED subjects were exposed to LHE for 2hrs. The level of DAMPs in the tear film were evaluated by ELISA or Luminex assay. Interleukin (IL)-6, IL-8 or metalloproteinase (MMP)-9 mRNA were quantitated by real-time polymerase chain reaction (PCR) from CIC samples.
Results.
Compared to age-matched normals, HMGB1 was significantly elevated in the tear film of DED subjects (P=.031) while there was no significant difference in HSPs. CIC samples revealed no significant difference in intracellular DAMPs levels between both groups. Following LHE, there was increase in corneal staining (P=.005), HSP-60 levels in the tear film (P=.01) and MMP-9 mRNA (P=.001).
Conclusions.
Dry eye subjects had higher levels of HMGB1 in their tear film. Exposure to a LHE worsened corneal staining, increased conjunctival MMP-9 mRNA expression and increased tear film HSP-60 levels. Larger studies are needed to understand the involvement of DAMPs in stimulating dry eye inflammation.
Inflammation is a known component of dry eye, however the mechanisms that drive the chronic inflammatory response are not fully understood. In the US, dry eye affects about 14.4% of the population1 and a study in Canada reported that 25% of patients at ophthalmic clinics suffer from dry eye2, making it the leading reason for patients to seek ophthalmic care.1,3 It is well known the tear film in dry eye becomes hyperosmotic due to insufficient tear production or excessive tear evaporation.4 While the exact mechanism remains unknown, ocular surface desiccation stimulates the production of inflammatory cytokines and metalloproteinases5–7 which perpetuates the inflammatory cycle by disruption of cell-to-cell tight junctions8 of the ocular surface epithelium.4
Danger or damage associated molecular patterns also known as alarmins are released by activated or injured cells to signal the threat of tissue injury.9 We hypothesize, damage associated molecular patterns may play a vital role in dry eye by perpetuating inflammation once ocular surface homeostasis becomes compromised.10,11 In support of this, many alarmins are increased in systemic inflammatory diseases.12 Elevated blood levels of high-mobility group box 1 have been found in autoimmune diseases such as rheumatoid arthritis,13 lupus erythematosus13 and Sjögren syndrome which often present with secondary dry eye.14 Heat shock proteins are increased in response to cellular injury and necrosis15 and are elevated in several inflammatory disorders (e.g. atherosclerosis, rheumatoid arthritis and multiple sclerosis).16
As typical alarmins, the physiological roles of high-mobility group box 1 and heat shock proteins depend on their intracellular or extracellular localization. Intracellular high-mobility group box 1 is vital to maintain cellular homeostasis due of its chaperone functions related to DNA repair.17 Extracellular high-mobility group box 1 is known to modulate the inflammatory response of innate immune cells, such as dendritic cells18 and monocytes/macrophages.19 High-mobility group box 1 reaches the extracellular environment from passive release by dead, dying, or injured cells or from active secretion by activated immune cells. Then, depending on its oxidative state, high-mobility group box 1 can bind to different cellular receptors including C-X-C chemokine receptor type 4 , receptor of advanced glycation end-products, toll-like receptors −2, 4 and 9 to stimulate cytokine release,20,21 chemotaxis of immune inflammatory cells,20 and/or vascular cell adhesion protein 1 and intercellular adhesion molecule 1 expression.22
Highly conserved heat shock proteins play a critical role in the maintenance of cellular homeostasis. As molecular chaperones, heat shock proteins facilitate the folding and assembly of proteins and also assist in protein refolding and repair after environmental or metabolic stress.23 While intracellular heat shock proteins are not inflammatory, extracellular heat shock proteins can activation of toll-like receptor 2 and 4 to stimulate cytokine mRNA and protein production.24–26
As a key component of the innate immune system, toll-like receptors recognize specific pathogens associated molecular patterns to induce the release of inflammatory mediators and antimicrobial peptides during infection and inflammation. Toll-like receptors also recognize damage associated molecular patterns to initiate sterile inflammation in order to promote tissue repair, however excessive toll-like receptor activation has been associated with inflammatory and autoimmune diseases.12 Toll-like receptors involvement in dry eye has been studied by others27,28 and by our group.29–31 At the ocular surface several cell layers constitute not only a physical barrier to infection but also a key checkpoint of immune surveillance where toll-like receptors 1–10 are widely expressed by the corneal and conjunctival epithelia and stroma tissues.31
Dry eye modulates the expression of toll-like receptors on the ocular surface.29,30 Using an ex vivo corneal desiccation model we observed increased expression of toll-like receptor 430 whose ability to recognize several damage associated molecular patterns including high-mobility group box 1 has been documented.32–34 In particular, toll-like receptors 2 and toll-like receptors 4 have been shown to be activated by damage associated molecular patterns including heat shock proteins25,35, high-mobility group box 133,34 and calcium binding protein S100A.36 Elevated levels of S100A have been found in the tear film of dry eye subjects and was found to be positively correlated with dry eye severity.37,38 Therefore it is plausible that damage associated molecular patterns released from dying ocular surface cells may activate toll-like receptors to perpetuate the inflammatory response in dry eye (Figure 1). This study’s purpose was to comprehensively examine high-mobility group box 1 or heat shock proteins levels in tears and ocular surface cells in normal and dry eye subjects. In addition, the impact of desiccating environmental conditions on damage associated molecular patterns levels in dry eye subjects was further evaluated.
Figure 1.
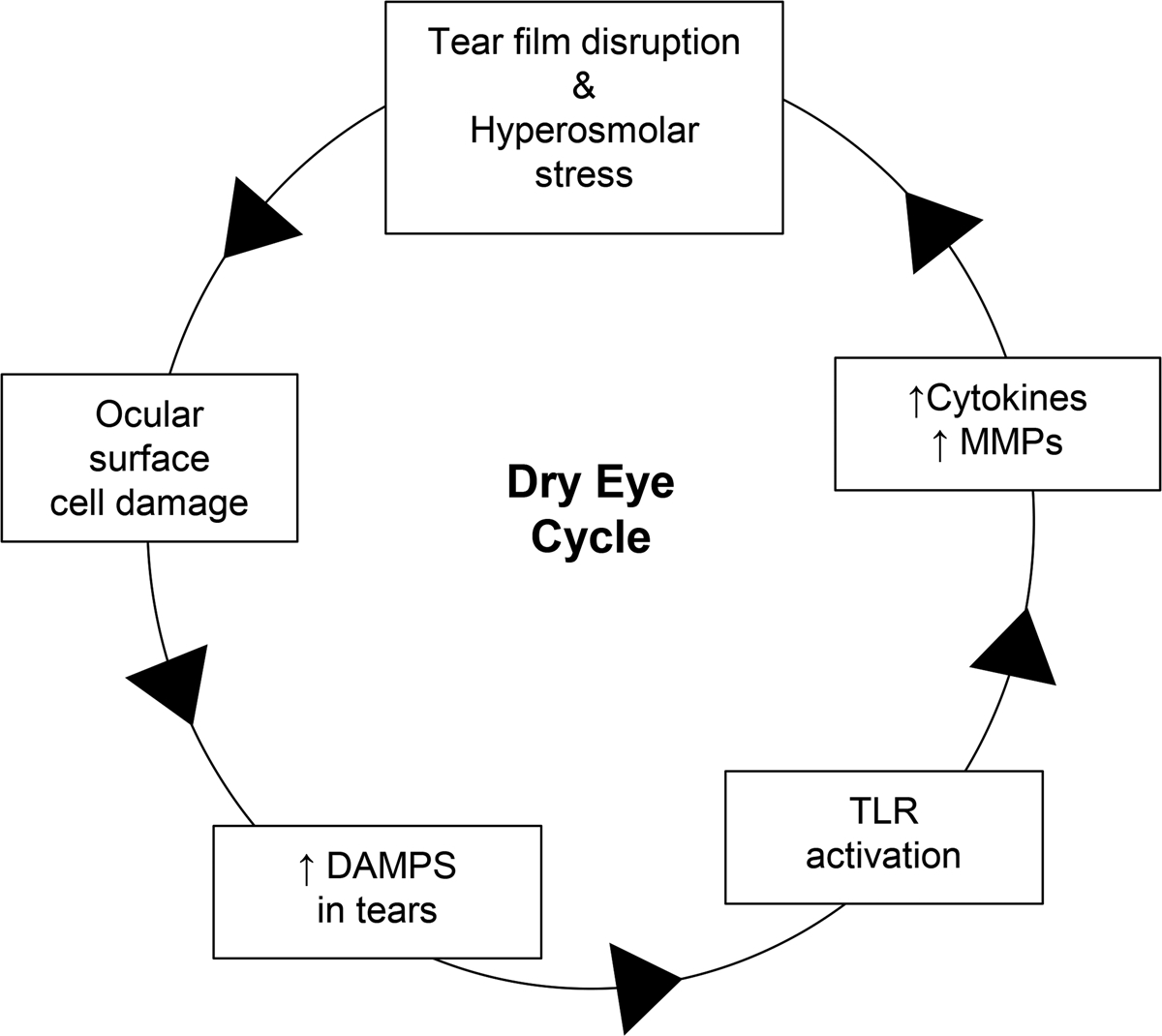
Damage associated molecular patterns (DAMPs) potential role in dry eye. In dry eye disease (DED), reduced tear production or increased tear evaporation causes tear film disruption and hyperosmolar stress leading to ocular surface damage and subsequent release of DAMPs (e.g. high mobility group box-1 and heat shock proteins). Excessive DAMPS in the tear film may activate toll-like receptors (TLRs) to release inflammatory cytokines and matrix metalloproteinases (MMPs), exacerbating epithelial cell damage and perpetuating the cycle of inflammation in DED.
METHODS
Subjects
This research was reviewed and approved by the University of Houston’s Institutional Review Board, and conforms with the principles and applicable guidelines for the protection of human subjects in biomedical research. All enrolled subjects reviewed and signed an informed consent prior to participation. Thirty normal and dry eye subjects (fifteen subjects per group) were enrolled in the study. A clinical examination was taken under normal building humidity (74°F and 40–45% humidity). Further, ten of the dry eye subjects were exposed to an enclosed dry environment to evaluate the impact of low humidity exposure. All subjects recruited were over the age of 18 and were currently not using any topical medications other than rewetting drops. Exclusion criteria for both groups included anyone pregnant or nursing, contact lens wear, any ocular surgeries within the previous six months, any active eye diseases including ocular allergies but excluding dry eye, anyone currently taking anti-inflammatory medication, and individuals with known allergy or sensitivity to fluorescein, lissamine green and topical anesthetics. All subjects were instructed not to instill artificial tears 2hrs prior to starting the study.
Normal and Dry Eye Classification
Dry eye subjects must have reported a symptom score greater or equal to 13 on the Ocular Surface Disease Index questionnaire and have one eye exceed the normal thresholds of three of the four following objective grading measures: tear production, corneal staining, conjunctival staining, and tear film stability. This classification scheme was based on a modified version of the classification schemes previously described.39
Clinical Signs
Normal and dry eye subjects were examined for tear film osmolarity, ocular surface integrity, tear production and tear film instability. For all clinical signs, a single measurement from each eye was obtained and the mean value was calculated. Tear film osmolarity was quantitated using the TearLab Osmolarity System (TearLab Corp, Escondido, CA). The system was calibrated following the manufacturer’s instructions at the beginning of each day of patient evaluation. For a subject to be classified as dry eye, a cutoff value >308 mOsM40 was applied. The phenol red thread test (ZONE-QUICK; Ayumi Pharmaceutical Corp, Tokyo, Japan) was performed to assess tear production. A sterile phenol red-impregnated cotton thread was inserted between the lower eyelid and eye surface and removed after 15sec for measurement of the length of the red portion of the thread. The cutoff value for dry eye was ≤10mm. Corneal and conjunctival epithelial integrity were evaluated using sodium fluorescein (Soft Glo, HUB Pharmaceuticals, Plymouth, MI) or Lissamine Green staining (Green Glo, HUB Pharmaceuticals, Rancho Cucamonga, CA), respectively. After instillation, grading was performed using a modified National Eye Institute’s staining scale of 0 to 3 in each of 4 bulbar conjunctiva quadrants (nasal, temporal, inferior, and superior) or 5 corneal areas (central, nasal, temporal, inferior, and superior). Staining ≥4 in the combined 5 quadrants was considered indicative of dry eye.40 Finally, tear film instability was examined by measuring the tear break up time using the Dry Eye Test-modified fluorescein strip (Akorn Pharmaceuticals, Chicago, IL) which has been specifically made narrower to deliver a small amount of fluorescein solution to the ocular surface. The cutoff value for dry eye was <10 secs.
Basal Tears Collection and Analysis
Prior to clinical examination, basal tears were collected using a 10μl microcapillary tube (BLAUBRAND intraMARK, Wertheim, Germany) which was placed in the lower temporal fornix, allowing tears from the lower meniscus to fill the tube via capillary action. For each subject, tears from both eyes were pooled into a single sample. All tear samples were immediately stored at −80°C after collection and thawed at the time of analysis. Tear samples were centrifuged at 2000rpm for 5min prior to Luminex and ELISA analysis to avoid cellular debris and for optimal sample recovery.
Conjunctival Impression Cytology
A single drop of 0.5% proparacaine hydrochloride anesthetic was instilled onto each eye. A sterile polyether sulfone membrane (Supor Membrane Disc Filters, 0.2μm pore size, 25mm, plain, PALL Corp. Port Washington, NY) was cut into 8 equal pieces (~60mm2/piece). Two conjunctival impression cytology samples from each eye were collected from the medial and lateral bulbar conjunctiva and stored at −80°C in either RLT lysis buffer (Qiagen, Germantown, MD) for RNA extraction and quantitative real-time polymerase chain reaction (qPCR); or in phosphate buffered saline (PBS) solution containing 0.2% Tween and a protease inhibitors cocktail (Complete Mini, Roche Diagnostics, Nutley, NJ) for protein analysis using Luminex or ELISA assays.
Quantitative Real-time Polymerase Chain Reaction
Total RNA from conjunctival impression cytology membrane samples was extracted using the RNeasy Micro Kit (Qiagen). Quantitative real-time polymerase chain reaction was used to quantitate relative mRNA expression of interleukin 6, interleukin 8, metalloproteinase 9 and high-mobility group box 1. Complementary DNA was generated from 250ng of total RNA using iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Samples containing no reverse transcriptase or water in place of RNA (no template control) served as negative controls. Ten nanograms of complementary DNA reactions were analyzed using a CFX96 Real-Time System (Bio-Rad). Real-time polymerase chain reaction amplification of complementary DNA was performed with SsoAdvanced SYBR Green Supermix (Bio-Rad) using PrimePCR SYBR Green primers for human interleukin-6 (unique assay ID: qHsaCID0021314), interleukin-8 (qHsaCED0046633), metalloproteinase-9 (qHsaCID0011597) and high-mobility group box 1 (qHsaCED0048035). For each gene, samples were processed in technical triplicates and amplified gene products were normalized to housekeeping RPL27 (qHsaCID0023846). The mean relative fold change interleukin-6, interleukin-8, metalloproteinase-9 and high-mobility group box 1 was calculated then by use of the CFX Manager software.
High-mobility Group Box 1 ELISA Assay
The levels of high-mobility group box 1 in tear samples and conjunctival impression cytology lysates from dry eye and normal subjects were determined using a commercially available ELISA assay (IBL International, Hamburg, Germany) as per manufacturer’s instructions. Each sample was diluted in phosphate-buffered saline and tested in duplicate (10μg total protein of sample per replicate). Optical density was measured at a wavelength of 450nm and 600nm as reference wavelength with a plate reader (FLUOstar Omega, BMG LABTECH, San Diego, CA). Tear concentration of high-mobility group box 1 was determined using a standard curve and each subject’s sample dilution factor.
Heat Shock Proteins Luminex Assay
The relative levels of four heat shock proteins 27, 60, 70 and 90α in tear samples and conjunctival impression cytology lysates were quantitated using a Luminex immunobead-based assay (EMD Millipore, San Diego, CA). Following manufacturer’s instructions each sample was diluted in phosphate-buffered saline and tested in duplicate (1μg total protein of sample per replicate) using the Luminex MAGPIX system and xPONENT software. The median fluorescence intensity for each individual analyte per sample was obtained as a readout. Final values were adjusted to account for each subject’s sample dilution factor.
Low Humidity Environment
Ten of the 15 dry eye subjects were recruited from the first study and exposed to desiccating conditions in our environmentally controlled room located at The Ocular Surface Institute, College of Optometry-University of Houston. This is a 19’ 8”×14’ 7” enclosed area that is dedicated to dry eye studies requiring environmental control. The room is equipped with built-in humidity sensors and a desiccant unit which is manually turned ON and OFF by a button placed at one of the room’s walls which allows for switching between the “Normal mode” and “Dry mode”. Once the system is activated, the relative humidity drops from 40% (normal humidity) to ~4% (low humidity) in about 90min and stays with minimal variation indefinitely, even if the door is opened and closed for 5min at a time. Temperature is kept at 75°F and illumination is motion activated. Room’s ventilation and air flow are provided by a wall-mounted fan (airflow range: 2660 to 3190 CFM) located about four feet above the floor. Room’s space is divided into two functional units: the seating area which mimics a small waiting room where subjects can seat and have access to TV and/or video games; and the clinical examination area which houses equipment for a comprehensive eye exam as well as a countertop fridge and lab materials for sample collection and short-term storage. A layout of the environmentally controlled room is shown in Figure 2. For the present study the following workflow was followed: subjects were first examined at normal humidity in a routine clinical examination room at The Ocular Surface Institute, they were then seated in the environmentally controlled room’s waiting area (74.3–74.8°F, 4.2–4.3% relative humidity) and watched a 2hr film of their choice on Netflix on a light-emitting diode TV screen. All participants were advised to not use artificial tears or sleep while remaining in the room. Following low humidity exposure, subjects were examined at the environmentally controlled room’s clinical examination area. Tear osmolarity, Phenol Red Thread test, tear break-up time, and corneal and conjunctival staining were measured. Prior to clinical evaluation, basal tears were collected using a microcapillary tube to compare damage associated molecular patterns levels before and after exposure to low-humidity environment. Conjunctival impression cytology samples were collected before and after exposure to low humidity environment in different eyes to avoid residual inflammation due to previous conjunctival impression cytology collection. Membranes were analyzed for mRNA expression of damage associated molecular patterns (high-mobility group box 1, heat shock proteins 27, 60, 70 and 90α) and inflammatory molecules (interleukins-6 and −8, and metalloproteinase-9) using quantitative real time polymerase chain reaction.
Figure 2.
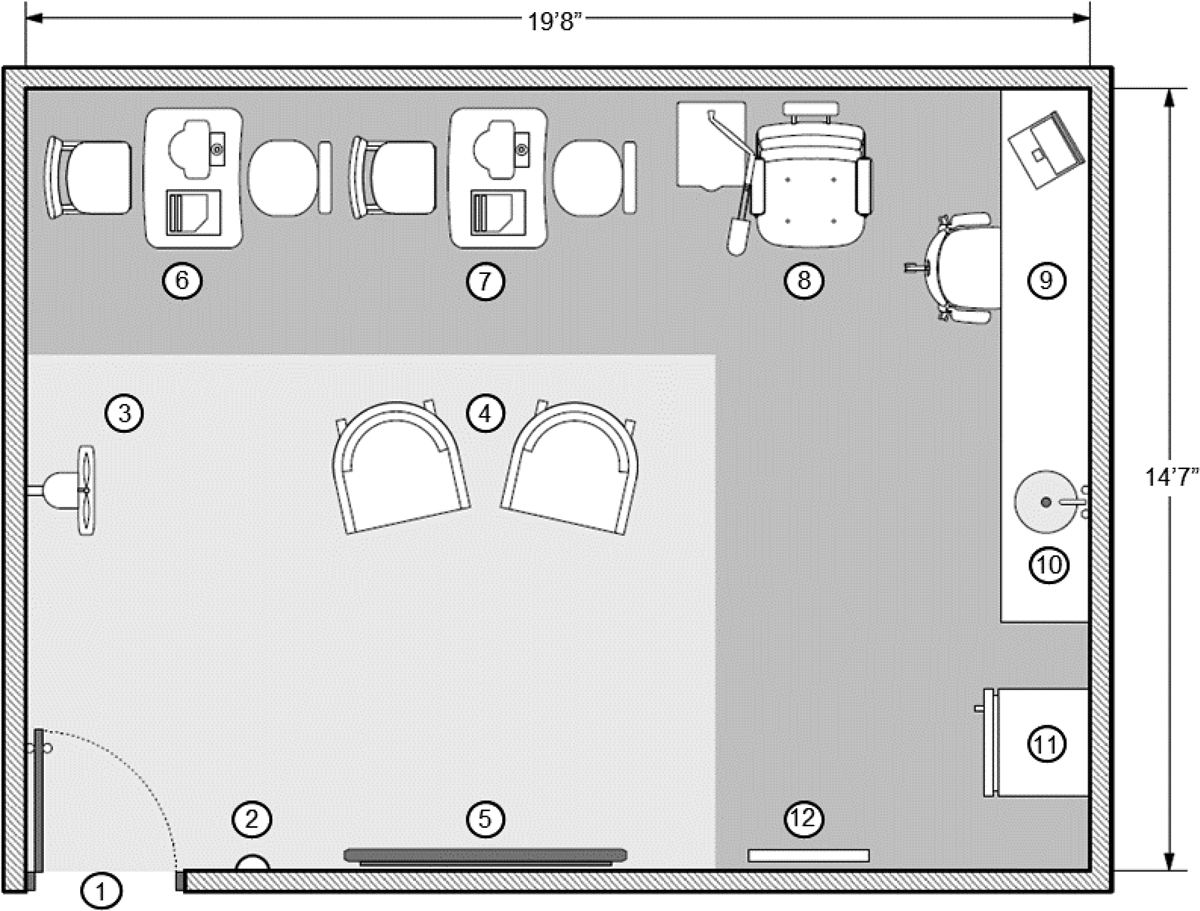
The Ocular Surface Institute (TOSI) environmentally controlled room. The enclosed area is divided into two functional units: the seating area (light gray) where subjects have access to TV and video games; and the clinical examination area that houses all equipment needed for a comprehensive eye exam (dark gray). 1. Entrance; 2. ON/OFF switch; 3. Wall-mounted fan; 4. Subjects sofas; 5.TV screen; 6.OCULUS Keratograph, 7. Tear lab, 8. Slit lamp; 9. Desk work area; 10. Sink; 11. Countertop refrigerator, 12. Visual acuity screen
Statistical Analysis
All data were analyzed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA). Since the quantity and variability of high mobility group box-1 and heat shock proteins have not been explored in the tear film, a sample size of 12 was selected based on a formerly published statistical approach.41 An additional three subjects were included in each group (total n=15) in the event some participants were excluded due to insufficient tear volume for biological analysis. Unpaired T-test was used for subject’s age and clinical signs/symptoms. To analyze levels of high-mobility group box 1 and heat shock proteins, normal and dry eye subjects were paired by age and outliers were removed after being identified by Grubb’s (alpha=0.05). Next, normality of the data was assessed by D’Agostino-Pearson omnibus K2 and statistical significance was determined by applying paired T-test or non-parametric Wilcoxon matched-pairs signed rank test accordingly. Pre-Post low-humidity environment data were analyzed using two-way ANOVA with Dunnett’s or Bonferroni’s correction for multiple comparisons.
RESULTS
Normal and Dry Eye Subjects
A total of 30 subjects were included in this study (15 normal and 15 with dry eye disease). Among normal and dry eye subjects, the mean age ± SEM was 56.5 ± 2.5 years old (range 29 to 64) and 56.8 ± 2.7 (range 35 to 73), respectively. In both groups, 60% of the study subjects were female. When comparing clinical scores for normal and dry eye groups, there was a significant difference for Ocular Surface Disease Index, tear breakup time, corneal staining and conjunctival staining (Table 1). Compared to normal age-matched subjects, the dry eye subjects had significantly higher Ocular Surface Disease Index scores (26.9 ± 3.6 vs. 14.3 ± 2.2; P=.005), corneal staining (5.1 ± 1.2 vs. 1.8 ± 0.6; P=.02), and conjunctival staining (7.6 ± 1.0 vs. 3.6 ± 0.7; P=.003). There was no significant difference in Phenol Red Thread test (PRTT) (25.8 ± 1.3 vs 23.8 ± 2.1; P =.43) or tears osmolarity (314.8 ± 2.5 vs. 311.2 ± 2.9; P=.364) between the normal and dry eye groups.
Table 1.
Clinical Signs and Symptoms in Normal and Dry Eye Disease (DED) Subjects.
| Normal (N=15) | DED (N=15) | P-value | |
|---|---|---|---|
| Age | 56.5 ± 2.5 | 56.8 ± 2.7 | .96 |
| Female (%) | 60 | 60 | N/A |
| OSDI | 14.3 ± 2.2 | 26.9 ± 3.6 | .005 |
| PRTT (mm) | 25.8 ± 1.3 | 23.8 ± 2.1 | .43 |
| TBUT (sec) | 6.3 ± 0.9 | 2.6 ± 0.6 | .002 |
| Corneal staining | 1.8 ± 0.6 | 5.1 ± 1.2 | .02 |
| Conjunctival staining | 3.6 ± 0.7 | 7.6 ± 1.0 | .003 |
| Tear osmolarity (mOsM) | 314.8 ± 2.5 | 311.2 ± 2.9 | .36 |
Data is presented as mean ± SEM and were analyzed using an independent Student’s t- test. OSDI = Ocular Surface Disease Index; PRTT = Phenol Red Thread Test; TBUT = Tear Film Break-Up Time
High-mobility Group Box 1 and Heat Shock Proteins in Normal and Dry Eye Subjects
To assess if damage associated molecular patterns were increased in the tear film of dry eye subjects compared to normal, the levels of high-mobility group box 1 and heat shock proteins were analyzed using ELISA and Luminex assays respectively. In addition, to determine whether damage associated molecular patterns production within conjunctival epithelial cells was upregulated, conjunctival impression cytology samples were taken from the nasal and temporal bulbar conjunctiva of each eye. High-mobility group box 1 was found to have significant higher levels in the tears of dry eye compared to normal subjects (9.5 ± 5.5 ng/ml vs 0.5 ± 0.3 ng/ml; P=.03) but no difference was observed in conjunctival cells (103.5 ±16.6 ng/ml vs. 121.7 ± 19.9 ng/ml) (Table 2). Tear film or conjunctiva levels of heat shock proteins 27, 60, 70 or 90α were not significantly different between dry eye and normal subjects (Table.2).
Table 2.
Tear Film and Conjunctival Damage Associated Molecular Patterns (DAMPs) Levels in Normal and Dry Eye (DED) Subjects.
| DAMP | Normal | DED | P-value |
|---|---|---|---|
| Tear film HMGB1 (ng/ml) | 0.5 ± 0.3 | 9.5 ± 5.5 | .03 |
| Conj. HMGB1 (ng/ml) | 121.7 ± 19.9 | 103.5 ± 16.6 | .54 |
| Tear film HSP-27 (MFI) | 24056.0 ± 7029.0 | 47922.0 ± 17612.0 | .22 |
| Conj. HSP-27 (MFI) | 56807.0 ± 8138.0 | 43204.0 ± 7429.0 | .28 |
| Tear film HSP-60 (MFI) | 390.0 ± 91.2 | 391.4 ± 102.8 | .99 |
| Conj. HSP-60 (MFI) | 9793.0 ± 977.9 | 9146.0 ± 1281 | .62 |
| Tear film HSP-70 (MFI) | 561.3 ± 65.4 | 567.6 ± 139.9 | .97 |
| Conj. HSP-70 (MFI) | 8225.0 ± 1059.0 | 6217.0 ± 1276.0 | .22 |
| Tear film HSP-90α (MFI) | 13533.0 ± 3320 | 17816 ± 6450 | .63 |
| Conj. HSP-90α (MFI) | 11600.0 ± 1611 | 9522.0 ± 1599.0 | .38 |
Tear film and conjunctival impression cytology samples were analyzed to quantitate high-mobility group box 1 (HMGB1, ng/ml) and determine the medium fluorescence intensity (MFI) for heat shock protein (HSP)-27,−60. −70, and −90α.
Effect of Low-humidity Environment on Ocular Surface Integrity and Damage Associated Molecular Patterns Expression
To determine if additional desiccating stress was able to increase the levels of damage associated molecular patterns at the ocular surface, a subset of 10 subjects from the dry eye group were re-recruited and exposed to low humidity environment for two hours. The mean ± SEM of the subjects’ age was 61.0 ± 3.0 years. Following low humidity exposure, corneal staining was significantly increased (2.2 ± 1.8 vs. 4.1 ± 2.1; P=.005). There was no significant difference found in phenol red thread test, tear breakup time, conjunctival staining or tear film osmolarity (Table 3). To assess the magnitude of change in damage associated molecular patterns levels induced by low humidity environment, the Post/Pre-low humidity environment ratio was calculated for each of the studied damage associated molecular patterns, only heat shock protein 60 showed approximately 8-fold increase (8.2 ± 3.9; P=.01) while the others remained practically unaltered (Figure 3A). Finally, to evaluate the impact of low humidity environment on ocular surface inflammation and integrity, mRNA expression of interleukins 6 and 8, and metalloproteinase 9 were assessed at pre- and post-low humidity environment instances. Environmental desiccating stress significantly upregulated metalloproteinase 9 (2.4 ± 0.4-fold; P=.001) mRNA expression. There was no significant change in interleukins 6 or 8 mRNA expression (Figure 3B).
Table 3.
Effect of Low Humidity Environment (LHE) on Ocular Surface Signs.
| Pre-LHE | Post-LHE | P-value | |
|---|---|---|---|
| PRTT (mm) | 20.9 ± 8.2 | 22.9 ± 6.8 | .41 |
| TBUT (sec) | 4.0 ± 2.6 | 3.4 ± 1.4 | .48 |
| Corneal Staining | 2.2 ± 1.8 | 4.1 ± 2.1 | .005 |
| Conjunctival staining | 3.2 ± 3.3 | 4.7 ± 4.3 | .05 |
| Osmolarity (mOsM) | 309.6 ± 5.4 | 306.9 ± 12.6 | .47 |
Data is presented as mean ± SEM were analyzed using an independent Student t-test. OSDI = Ocular Surface Disease Index; PRTT = Phenol Red Thread Test; TBUT = Tear Film Break-Up Time, MFI = Mean Fluorescense Intensity.
Figure 3.
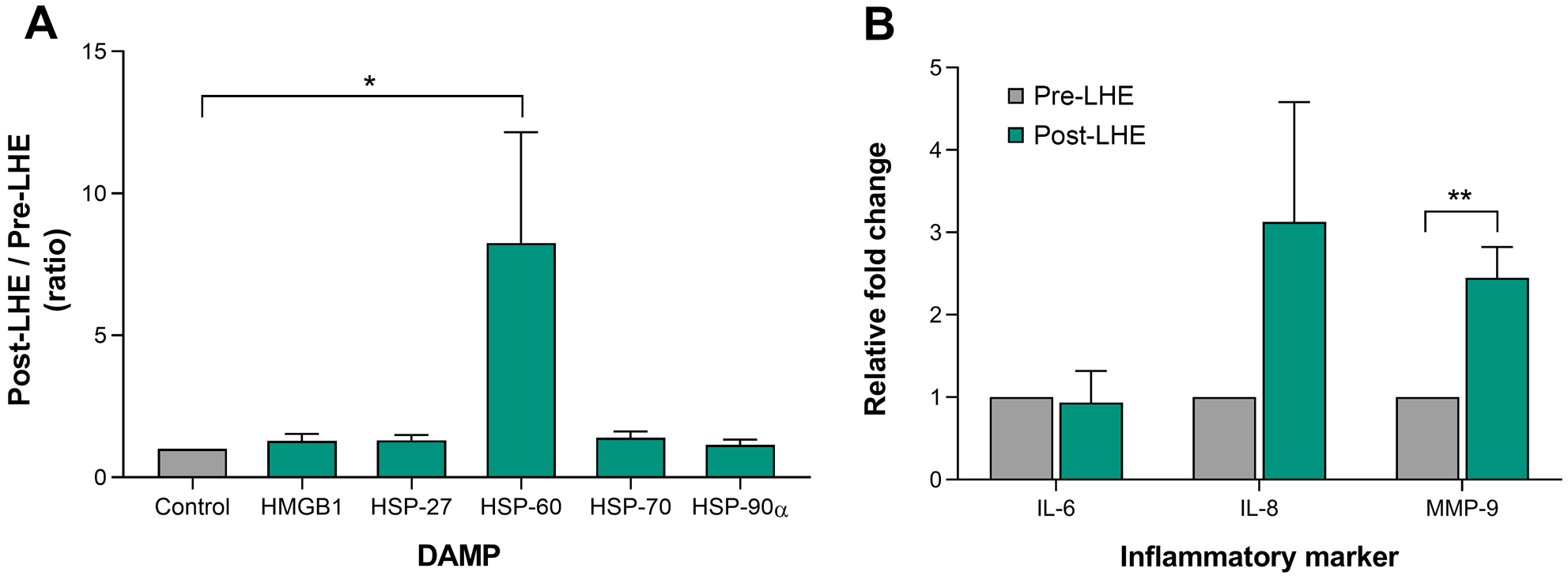
Effect of low humidity environment (LHE) on damage associated molecular patterns (DAMPs) and inflammatory cytokines. Post-LHE/ Pre-LHE ratio values of high-mobility group box 1 (HMGB1), heat shock protein (HSP)-27, HSP-60, HSP-70 and HSP-90α in tear film. (A). mRNA expression of interleukin (IL)-6, IL-8 and metalloproteinase (MMP)-9 in conjunctival impression cytology (CIC) samples (B). * P < .01; ** P < .001
DISCUSSION
In this study, high-mobility group box 1 was significantly increased in dry eye subjects compared to normal age-matched subjects. These findings support our previous study where high-mobility group box 1 expression was elevated in the corneal epithelium of mice with experimental dry eye mice and in human corneal epithelial cells treated with dry eye culture conditions (e.g. hyperosmolar stress and TNFα treatment).10 The increase of high-mobility group box 1 was observed only in tears but not in conjunctival impression cytology samples of dry eye subjects. This result is not surprising given high-mobility group box 1 is a ubiquitous nuclear protein and it is expected that intracellular levels will tend to remain unaltered and lower compared to secreted high-mobility group box 1 which will be modulated by external stimuli.
High levels of pro-inflammatory high-mobility group box 1 in the tear film of dry eye patients supports the evaluation of anti-HMGB1 agents to reduce inflammation in the ocular surface. In a recent clinical pilot study the efficacy of glycyrrhizin, a natural high-mobility group box 1 inhibitor isolated from the licorice root was reported to reduce corneal staining, increase tear break up time, and increase Schirmer’s scores in moderate dry eye patients after 28 days of twice daily use of glycyrrhizin 2.5% eye drops.11 However, caution needs to be taken since damage associated molecular patterns-triggered inflammation is not necessarily detrimental but regenerative and therefore low levels of damage associated molecular patterns are crucial to promote healthy tissue repair and healing through a physiological immune response.12 In agreement, a study found reduced levels of high-mobility group box 1 in the skin of diabetic human and mouse supporting a beneficial role for high-mobility group box 1 in wound repair.42
Other interesting topic is the potential value of high-mobility group box 1 as biomarker for dry eye diagnosis or disease severity. Although pro-inflammatory high-mobility group box 1 has been found in the tears of patients affected by other inflammatory disorders like conjunctivitis and blepharitis,43 its potential value as a potential marker for diagnosis and evaluation of dry eye patients cannot be disregarded given all the challenges that dry eye clinical management implies. Currently, S100A8, S100A9, lipocalin-1, secretory phospholipase A2 and metalloproteinase 944 as well as interleukins 1β and 645 have been proposed as dry eye biomarkers.
Among the objective signs evaluated, only corneal staining showed a minimal correlation when compared to tears high-mobility group box 1 (R2 ≤0.5, data not shown). Although weak, this trend might reflect hyperosmolar stress-mediated corneal epithelial necrosis (which presents clinically as positive staining) as a causative factor for passive release of high-mobility group box 1 extracellularly into the tear film. A large body of publications indicates that high-mobility group box 1 can be actively secreted by immune and non-immune cells as well as passively released under injury or stress.46,47 Besides its pro-inflammatory role, extracellular high-mobility group box 1 has been shown to act as a recruiter for stem cells in bone marrow and cardiac tissue and is necessary for epithelial regeneration.48–50 Thus, areas of epithelium damage, shown by corneal and conjunctival staining, might actively secrete high-mobility group box 1 as a chemoattractant for limbal corneal stem cells to promote healing. Finally, it is also possible that the correlation between high-mobility group box 1 and ocular surface staining results via receptor for advanced glycation endproducts activation. In fact, S100A proteins and high-mobility group box 1 have been shown to interact with receptor for advanced glycation endproducts to perpetuate cellular damage and failure of inflammatory resolution.51 In dry eye, high-mobility group box 1 bound to receptor for advanced glycation endproducts may increase the production of metalloproteinases leading to corneal and conjunctival damage. Further research is needed to determine if the increase in high-mobility group box 1 seen in dry eye is inflammatory, regenerative, or possibly both, in nature.
In this study, there was no significant change in heat shock proteins 27, 60, 70 or 90α in the tear film and conjunctiva levels between dry eye and normal subjects. Interestingly, the levels of all damage associated molecular patterns in conjunctival impression cytology samples were lower in dry eye subjects although statistical significance was not reached. Heat shock proteins 27, 60, 70 and 90α are induced by cellular stress and play a key role as cytoprotectants. Among them, intracellular heat shock protein 70 has been shown to reduce inflammatory cytokine production in dendritic cells52 and astrocytes.53 Therefore, low levels of protective intracellular heat shock protein 70 in dry eye may make the ocular surface more susceptible to inflammation. Additional studies are needed given the small sample size of the current study. Using the current data, we calculated the appropriate sample size for these molecules using G Power 3.1 software and standard power conditions (alpha=5% two tailed, power=80%). Our analysis revealed that our study was underpowered for heat shock protein 60 (tears and conjunctiva), heat shock protein 70 (tears) and heat shock protein 90 (tears) suggesting a larger sample size is needed for these molecules of interest.
To determine if acute desiccating stress could modulate the expression of damage associated molecular patterns, dry eye subjects were exposed to acute low humidity environment. A previous study that found matrix metalloproteinase-9 and inflammatory cytokines are increased after a two-hour exposure to 5% relative humidity suggesting a potential increase in damage associated molecular patterns as well.54 Two hours of low humidity exposure resulted in increased corneal staining and matrix metalloproteinase-9 mMRA expression in conjunctival samples; however, we did not detect an increase of high-mobility group box 1 in the tear film. Its plausible, additional time would be needed to create additional desiccating stress on the ocular surface. This explanation is based on our results from previous in vitro experiments where a gradient of hyperosmolar stress was cultured with human corneal epithelial cells over 6, 12 and 24hrs. The lowest level of hyperosmolar stress (400mOsM), did not show a significant increase in extracellular high-mobility group box 1 until after 12hrs of exposure, while higher osmolarity levels (450–500mOsM) began showing a significant increase in high-mobility group box 1 as early as 6hrs in our cell culture model.10
To look closer into the low humidity environment impact on dry eye subjects, the post/pre-exposure ratio was calculated for all the studied damage associated molecular patterns. Heat shock protein 60 showed approximately 8-fold increase while the others remained unaltered. Heat shock protein 60 is known to have very strong immunogenic properties and it has been reported to elicit autoimmune chronic conjunctival inflammation and scarring.55 Also, it has been shown to elicit a pro-inflammatory response in macrophages by acting as an endogenous ligand of toll-like receptor 4.56 Furthermore, heat shock protein 60 can bind to toll-like receptor 4 ligand, lipopolysaccharide to enhance their biological activity57,58 and exacerbate inflammation. Therefore, increased levels of heat shock protein 60 after 2hrs of acute low humidity environment might exacerbate of inflammation in dry eye subjects caused by desiccation stress. Interestingly, elevated levels of extracellular (released/secreted) heat shock protein 60 have been reported in saliva and serum from type 2 diabetes patients.59 Diabetics are known to be at a higher risk of developing gingivitis and periodontal disease; which could reflect a vicious tissue damage-chronic inflammation circle as it is observed in dry eye.
In conclusion, we demonstrate that damage associated molecular patterns are elevated on the ocular surface during dry eye. Data revealed elevated levels of high-mobility group box 1 in the tear film of patients with dry eye and increased cellular stress-related heat shock protein 60 after acute environmental desiccating stress. Interestingly, low humidity environment also increased the levels of damaging metalloproteinase 9, which is measured clinically when assessing the level of dry eye severity. Together our results suggest damage associated molecular patterns involvement in the etiology in dry eye disease.
ACKNOWLEDGMENTS
We are grateful to Ar. Raul Lema for schematic of the environmentally controlled room shown in Figure 1. This work was supported by National Institutes of Health Grant EY023628 (RLR), University of Houston College of Optometry Core Grant NIH/NEI P30 EY07551 and NIH/NEI T35 EY007088. These data were presented, in part, at the American Academy of Optometry Meeting.
REFERENCES
- 1.Moss SE, Klein R, Klein BE. Prevalence of and Risk Factors for Dry Eye Syndrome. Arch Ophthalmol 2000;118:1264–8. [DOI] [PubMed] [Google Scholar]
- 2.Doughty MJ, Fonn D, Richter D, et al. A patient Questionnaire Approach to Estimating the Prevalence of Dry Eye Symptoms in Patients Presenting to Optometric Practices Across Canada. Optom Vis Sci 1997;74:624–31. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien PD, Collum LM. Dry Eye: Diagnosis and Current Treatment Strategies. Curr Allergy Asthma Rep 2004;4:314–9. [DOI] [PubMed] [Google Scholar]
- 4.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II Pathophysiology Report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- 5.Li DQ, Luo L, Chen Z, Kim HS, et al. JNK and ERK MAP Kinases Mediate Induction of IL-1beta, TNF-alpha and IL-8 following Hyperosmolar Stress in Human Limbal Epithelial cells. Exp Eye Res 2006;82:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar Saline is a Proinflammatory Stress on the Mouse Ocular Surface. Eye Contact Lens 2005;31:186–93. [DOI] [PubMed] [Google Scholar]
- 7.Li DQ, Lokeshwar BL, Solomon A, Monroy D, et al. Regulation of MMP-9 Production by Human Corneal Epithelial Cells. Exp Eye Res 2001;73:449–59. [DOI] [PubMed] [Google Scholar]
- 8.Mauris J, Woodward AM, Cao Z, et al. Molecular Basis for MMP9 Induction and Disruption of Epithelial Cell-Cell Contacts by Galectin-3. J Cell Sci 2014;127:3141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono H, Rock KL. How Dying Cells Alert the Immune System to Danger. Nat Rev Immunol 2008;8:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lema C, Reins RY, Redfern RL. High-Mobility Group Box 1 in Dry Eye Inflammation. Invest Ophthalmol Vis Sci 2018;59:1741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burillon C, Chiambaretta F, Pisella PJ. Efficacy and Safety of Glycyrrhizin 2.5% Eye Drops in the Treatment of Moderate Dry Eye Disease: Results from a Prospective, Open-label Pilot Study. Clin Ophthalmol 2018;12:2629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccinini AM, Midwood KS. DAMPening Inflammation by Modulating TLR Signalling. Mediat Inflamm 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voll RE, Urbonaviciute V, Herrmann M, Kalden JR. High Mobility Group Box 1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Isr Med Assoc J 2008;10:26–8. [PubMed] [Google Scholar]
- 14.Dupire G, Nicaise C, Gangji V, Soyfoo MS. Increased Serum Levels of High-mobility Group Box 1 (HMGB1) in Primary Sjogren’s Syndrome. Scand J Rheumatol 2012;41:120–3. [DOI] [PubMed] [Google Scholar]
- 15.Gulke E, Gelderblom M, Magnus T. Danger Signals in Stroke and Their Role on Microglia Activation After Ischemia. Ther Adv Neurol Disord 2018;11:1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandia R, Munjal AK, Iqbal HM, Dhama K. Heat Shock Proteins: Therapeutic Perspectives in Inflammatory Disorders. Recent Pat Inflamm Allergy Drug Discov 2017;10:94–104. [DOI] [PubMed] [Google Scholar]
- 17.Lange SS, Mitchell DL, Vasquez KM. High Mobility Group Protein B1 Enhances DNA Repair and Chromatin Modification After DNA Damage. Proc Natl Acad Sci U S A 2008;105:10320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumitriu IE, Bianchi ME, Bacci M, et al. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 2007;81:84–91 [DOI] [PubMed] [Google Scholar]
- 19.Andersson U, Wang H, Palmblad K, et al. High Mobility Group 1 Protein (HMG-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J Exp Med 2000;192:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually Exclusive Redox Forms of HMGB1 Promote Cell Recruitment or Proinflammatory Cytokine Release. J Exp Med 2012;209:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Hreggvidsdottir HS, Palmblad K, et al. A Critical Cysteine is Required for HMGB1 Binding to Toll-like Receptor 4 and Activation of Macrophage Cytokine Release. Proc Natl Acad Sci U A 2010;107:11942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiuza C, Bustin M, Talwar S, Tropea M, et al. Inflammation-promoting Activity of HMGB1 on Human Microvascular Endothelial Cells. Blood 2003;101:2652–60. [DOI] [PubMed] [Google Scholar]
- 23.Parsell DA, Lindquist S. The Function of Heat-shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu Rev Genet 1993;27:437–96. [DOI] [PubMed] [Google Scholar]
- 24.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 Stimulates Cytokine Production Through a CD14-dependant Pathway, Demonstrating its Dual Role as a Chaperone and Cytokine. Nat Med 2000;6:435–42. [DOI] [PubMed] [Google Scholar]
- 25.Asea A, Rehli M, Kabingu E, et al. Novel Signal Transduction Pathway Utilized by Extracellular HSP70: Role of Toll-like Receptor (TLR) 2 and TLR4. J Biol Chem 2002;277:15028–34. [DOI] [PubMed] [Google Scholar]
- 26.De Maio A, Vazquez D. Extracellular Heat Shock Proteins: A New Location, A New Function. Shock 2013;40:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HS, Hattori T, Park EY, et al. Expression of Toll-Like Receptor 4 Contributes to Corneal Inflammation in Experimental Dry Eye Disease. Invest Ophthalmol Vis Sci 2012;53:5632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LWW MOVE TO TEXT as Barabino S, et al. IOVS 2006;47:ARVO E-Abstract 5594. [Google Scholar]
- 29.Redfern RL, Patel N, Hanlon S, et al. Toll-like Receptor Expression and Activation in Mice with Experimental Dry Eye. Invest Ophthalmol Vis Sci 2013;54:1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redfern RL, Barabino S, Baxter J, et al. Dry Eye Modulates the Expression of Toll-like Receptors on the Ocular Surface. Exp Eye Res 2015;134:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redfern RL, McDermott AM. Toll-like Receptors in Ocular Surface Disease. Exp Eye Res 2010;90:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JS, Svetkauskaite D, He Q, et al. Involvement of Toll-like Receptors 2 and 4 in Cellular Activation by High Mobility Group Box 1 Protein. J Biol Chem 2004;279:7370–7. [DOI] [PubMed] [Google Scholar]
- 33.Klune JR, Dhupar R, Cardinal J, et al. HMGB1: Endogenous Danger Signaling. Mol Med 2008;14:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Gamboni-Robertson F, He Q, et al. High Mobility Group Box 1 Protein Interacts with Multiple Toll-like receptors. Am J Physiol Cell Physiol 2006;290:C917–24. [DOI] [PubMed] [Google Scholar]
- 35.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. In: Beutler B, Wagner H, eds. Toll-Like Receptor Family Members and Their Ligands. Current Topics in Microbiology and Immunology. Heidelberg: Springer; 2002:169–84. [DOI] [PubMed] [Google Scholar]
- 36.Vogl T, Wolf M, Petersen B, et al. Human S100A8 and S100A9 Activate Phagocytes Via Toll-like receptor 4 Independent of RAGE. Cell Commun Signal 2009;7:A91. [Google Scholar]
- 37.Zhou L, Beuerman RW, Chan CM, et al. Identification of Tear Fluid Biomarkers in Dry Eye Syndrome Using iTRAQ Quantitative Proteomics. J Proteome Res 2009;8:4889–905. [DOI] [PubMed] [Google Scholar]
- 38.Grus FH, Podust VN, Bruns K, et al. SELDI-TOF-MS ProteinChip Array Profiling of Tears From Patients with Dry Eye. Invest Ophthalmol Vis Sci 2005;46:863–76. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan BD, Crews LA, Messmer EM, et al. Correlations Between Commonly Used Objective Signs and Symptoms for the Diagnosis of Dry Eye Disease: Clinical Implications. Acta Ophthalmol 2014;92:161–6. [DOI] [PubMed] [Google Scholar]
- 40.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology Report. Ocul Surf 2017;15:539–74. [DOI] [PubMed] [Google Scholar]
- 41.Julious SA. Sample Size of 12 Per Group Rule of Thumb for a Pilot Study. Pharm Stat 2005;4:287–91. [Google Scholar]
- 42.Straino S, Di Carlo A, Mangoni A, et al. High-mobility Group Box 1 Protein in Human and Murine Skin: Involvement in Wound Healing. J Invest Dermatol 2008;128:1545–53. [DOI] [PubMed] [Google Scholar]
- 43.Cavone L, Muzzi M, Mencucci R, et al. 18β-glycyrrhetic Acid Inhibits Immune Activation Triggered by HMGB1, a Pro-inflammatory Protein Found in the Tear fluid During Conjunctivitis and Blepharitis. Ocul Immunol Inflamm 2011;19:180–5. [DOI] [PubMed] [Google Scholar]
- 44.Enriquez-de-Salamanca A, Bonini S, Calonge M. Molecular and Cellular Biomarkers in Dry Eye Disease and Ocular Allergy. Curr Opin Allergy Clin Immunol 2012;12:523–33. [DOI] [PubMed] [Google Scholar]
- 45.Na KS, Mok JW, Kim JY, et al. Correlations Between Tear Cytokines, Chemokines, and Soluble Receptors and Clinical Severity of Dry Eye Disease. Invest Ophthalmol Vis Sci 2012;53:5443–50. [DOI] [PubMed] [Google Scholar]
- 46.Lotze MT, Tracey KJ. High-mobility Group Box 1 Protein (HMGB1): Nuclear Weapon in the Immune Arsenal. Nat Rev Immunol 2005;5:331–42. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi ME, Crippa MP, Manfredi AA, et al. High-mobility Group Box 1 Protein Orchestrates Responses to Tissue Damage via Inflammation, Innate and Adaptive Immunity, and Tissue Repair. Immunol Rev 2017;280:74–82. [DOI] [PubMed] [Google Scholar]
- 48.Tamai K, Yamazaki T, Chino T, et al. PDGFRalpha-positive Cells in Bone Marrow Are Mobilized by High Mobility Group Box 1 (HMGB1) to Regenerate Injured Epithelia. Proc Natl Acad Sci U A 2011;108:6609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palumbo R, Bianchi ME. High Mobility Group Box 1 Protein, a Cue for Stem Cell Recruitment. Biochem Pharmacol 2004;68:1165–70. [DOI] [PubMed] [Google Scholar]
- 50.Rossini A, Zacheo A, Mocini D, et al. HMGB1-stimulated Human Primary Cardiac Fibroblasts Exert a Paracrine Action on Human and Murine Cardiac Stem Cells. J Mol Cell Cardiol 2008;44:683–93. [DOI] [PubMed] [Google Scholar]
- 51.Ramasamy R, Yan SF, Schmidt AM. The Diverse Ligand Repertoire of the Receptor for Advanced Glycation Endproducts & Pathways to the Complications of Diabetes. Vascul Pharmacol 2012;57:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka T, Shibazaki A, Ono R, Kaisho T. HSP70 Mediates Degradation of the p65 Subunit of Nuclear Factor KappaB to Inhibit Inflammatory Signaling. Sci Signal 2014;7:ra119. [DOI] [PubMed] [Google Scholar]
- 53.Yu WW, Cao SN, Zang CX, et al. Heat Shock Protein 70 Suppresses Neuroinflammation Induced by Alpha-synuclein in Astrocytes. Mol Cell Neurosci 2018;86:58–64. [DOI] [PubMed] [Google Scholar]
- 54.López-Miguel A, Tesón M, Martín-Montañez V, et al. Clinical and Molecular Inflammatory Response in Sjögren Syndrome-Associated Dry Eye Patients Under Desiccating Stress. Am J Ophthalmol 2016;161:133–141.e1-2. [DOI] [PubMed] [Google Scholar]
- 55.Peeling RW, Bailey RL, Conway DJ, et al. Antibody Response to the 60-kDa Chlamydial Heat-shock Protein Is Associated with Scarring Trachoma. J Infect Dis 1998;177:256–9. [DOI] [PubMed] [Google Scholar]
- 56.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting Edge: Heat Shock Protein 60 Is a Putative Endogenous Ligand of the Toll-like Receptor-4 Complex. J Immunol 2000;164:558–61. [DOI] [PubMed] [Google Scholar]
- 57.Osterloh A, Kalinke U, Weiss S, et al. Synergistic and Differential Modulation of Immune Responses by Hsp60 and Lipopolysaccharide. J Biol Chem 2007;282:4669–80. [DOI] [PubMed] [Google Scholar]
- 58.Habich C, Kempe K, van der Zee R, et al. Heat Shock Protein 60: Specific Binding of Lipopolysaccharide. J Immunol 2005;174:1298–305. [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Dunn P, Martinus RD. Detection of Hsp60 in Saliva and Serum from Type 2 Diabetic and Non-diabetic Control Subjects. Cell Stress Chaperones 2011;16:689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


