Abstract
Pregnancy and vascular endothelial growth factor (VEGF) stimulate uterine artery (UA) endothelial cell (UAEC) hydrogen sulfide (H2S) production via selectively upregulating cystathionine β-synthase (CBS) but not cystathionine γ-lyase (CSE) expression. This study was conducted to determine the mechanisms by which VEGF utilizes to stimulate pregnancy dependent upregulation of CBS and H2S production in human UAEC. The proximal human CBS promoter contains 4 specific protein 1 (Sp1a/b/c/d) sites and one YY1 site; luciferase assays using reporter genes driven by human CBS promoter with a series of 5’-deletions identified a promoter sequence (−574 to −394) containing Sp1d and the YY1 sites critical for basal and VEGF-stimulated CBS promoter activation. VEGF stimulated pregnancy-dependent recruitment of Sp1 to Sp1d and YY1 to YY1, and also recruited YY1 to Sp1c and increased Sp1/YY1 association in pregnant hUAEC, suggesting formation of a Sp1/YY1 complex at Sp1c site. Endothelial Sp1 and YY1 proteins were significantly greater in pregnant than nonpregnant human UA. VEGF stimulated pregnancy dependent Sp1 and YY1 protein expression in vitro. Treatment with Sp1 and YY1 siRNAs completely blocked Sp1/YY1-mediated pregnancy dependent CBS protein upregulation and H2S production by VEGF in hUAEC. VEGF did not trans-activate CSE promoter nor increase CSE expression and Sp1/YY1 knockdown did not affect CSE expression in hUAEC. Thus, pregnancy augments EC Sp1 and YY1 expression and promotes the recruitment of Sp1/YY1 to their DNA binding sequences in proximal human CBS promoter to upregulate CBS transcription, underlying a novel mechanism to mediate VEGF-stimulated pregnancy dependent endothelial H2S production in human UA.
Keywords: H2S biosynthesis, CBS transcription, Sp1/YY1, UAEC, VEGF, Pregnancy
Summary
Pregnancy augments human uterine artery cell (endothelium vs. smooth muscle)-specific expressions of Sp1 and YY1 and promotes their interactions with the proximal human CBS promoter in response to VEGF stimulation. This pathway provides a transcriptional mechanism responsible for VEGF-stimulated pregnancy dependent CBS expression and H2S production that accounts, in part, for uterine vasodilation during pregnancy.
INTRODUCITON
Once conceived, the maternal cardiovascular system undergoes dynamic changes including mild decreases in systemic blood pressure and vascular resistance and significant increases in blood volume and total cardiac output 1; these changes result in profound rise in uterine blood flow (UtBF) to facilitate maternal-fetal exchanges of respiratory gases (O2 and CO2), to exhaust metabolic wastes, and to provide the sole source of nutrients for fetal development and survival 2. Constrained UtBF is a denominator of many pregnancy disorders including preeclampsia with or without intrauterine fetal growth restriction, affecting the well-beings of the mother and fetus during pregnancy and predisposing both to a higher risk in metabolic diseases such as diabetes and cardiovascular diseases later in life 3. Local uterine artery (UA) production of orchestrated vasodilators, especially nitric oxide (NO) produced by UA endothelial cells (UAEC) 4, 5, has been long recognized to be critical for pregnancy-associated rise in UtBF. However, local UA NO blockade only modestly inhibits baseline UtBF in pregnant (P) sheep 6, clearly demonstrating that other mechanisms exist to mediate uterine hemodynamics during pregnancy.
Hydrogen sulfide (H2S) is a potent proangiogenic vasodilator that belongs to the “gasotransmitter” family after NO and carbon monoxide 7. Endogenous H2S is mainly produced via metabolizing L-cysteine by two enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) 8. UA H2S production is augmented via selective endothelium and smooth muscle CBS upregulation in ovine and human pregnancy in vivo 9, 10. H2S donors relax pressurized rat and human UA ex vivo 9, 11, linked to activation of the large conductance Ca2+-activated voltage-dependent potassium (BKCa) channels 11 that are crucial for pregnancy-associated rise in UtBF 12, 13. Thus, H2S serves as new vasodilator to mediate pregnancy-associated uterine vasodilation.
Vascular endothelial growth factor (VEGF) expression is augmented, and maternal circulating VEGF levels increase during pregnancy 14, 15. Dysregulated VEGF signaling by increased soluble fms-like tyrosine kinase-1 (sFlt1) leads to preeclampsia-like conditions in animals 16. Prenatal adenoviral VEGF gene delivery to UA reverse undernutrition-induced fetal growth restriction via upregulating UtBF 17, 18, implicating a crucial role of VEGF signaling in pregnancy-associated rise in UtBF. VEGF augments pregnancy-dependent H2S production via selectively upregulating CBS in primary human UAEC (hUAEC) in vitro 19. Endogenous H2S serves as an autocrine and paracrine factor for placental and endometrial angiogenesis 20, 21. H2S donors can partially rescue sFlt1-induced preeclampsia in rats, involving upregulating VEGF and angiogenesis 22, 23. Hence, interactions between VEGF and H2S are central for the two key mechanisms, i.e., the expansion and vasodilation of uterine and placental vasculatures, for upregulating pregnancy-associated rise in UtBF 24.
The mechanisms by which VEGF utilizes to stimulate pregnancy dependent UAEC H2S production via selective CBS expression are currently unknown. Simultaneous increases in UA endothelium CBS mRNA and protein in vivo during pregnancy 9, 10 and upon VEGF stimulation in primary hUAEC in vitro 19 suggest that transcriptional mechanisms are involved. The human CBS gene promoter is GC-rich and lacks the classic TATA box but contains various putative cis-regulatory elements for binding transcription factors including specificity proteins (Sp1) and Yin Yang 1 (YY1) 25. Sp1 is known to function as a trans-activator to mediate growth factor-regulated CBS expression 26 and the ubiquitously expressed YY1 regulates the expression of genes important for cell invasion and EC angiogenesis 27, 28. Sp1 and YY1 cooperate to regulate gene expression in vascular endothelium 29. This study was conducted to determine the transcriptional mechanisms controlling VEGF-stimulated pregnancy-dependent CBS upregulation in human UA. We concluded that enhanced expression of Sp1/YY1 and their coordinated interactions with the Sp1 and YY1 sites in the CBS promoter mediate VEGF-stimulated pregnancy-dependent CBS transcription for regulating H2S biosynthesis in human UAEC.
METHODS
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Detailed materials and methods are available in the Data Supplement.
Human subjects, cell isolation, and culture
Primary UAEC from the main UAs of nonpregnant (NP) and P women (n = 3/group), termed NP and P hUAEC, respectively, were isolated by collagenase digestion as previously described 19. The UAs were collected from patients undergoing hysterectomy with written consent at the University of California Irvine Medical Center, with ethical approval (HS #2013-9763) by the Institutional Review Board for Human Research. The NP subjects were recruited from women aged 30-45 years, without steroid treatment, and elective hysterectomy due to fibroids. The P subjects, aged 30 to 45 years, were recruited with suspected placental accrete; UAs were collected immediately after Cesarean hysterectomy between 35-36 weeks’ gestation. After purification and EC determination, cells were stored in liquid N2 at passage 2 (P2). Frozen UAEC aliquots were thawed and seeded in endothelial cell medium (ECM, ScienCell, La Jolla, CA) containing 5% fetal bovine serum (FBS), endothelial growth supplements, and 1% penicillin/streptomycin for experimental use within 5 passages. Cells at ~70% confluence were cultured in M199 medium containing 0.1% bovine serum albumin (BSA), 0.5% FBS, 1% antibiotics, and 25 mM hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES) overnight, and then treated with or without VEGF165 (VEGF) in fresh medium.
Statistical analysis
Each experiment was repeated at least three times with cells derived from different NP or P subjects. Data are presented as means ± SEM and analyzed by one-way or two-way analysis of variance (ANOVA), followed by the Newman Keuls test for multiple comparisons using SigmaPlot 14.5 (Systat Software Inc.). Student t-test was used for comparison of data between two groups. Significance was defined as p < 0.05 and higher statistical power was indicated in figure legends.
RESULTS
VEGF stimulates pregnancy-dependent trans-activation of human CBS promoter
Following treatment with 10 ng/ml VEGF, CBS mRNA began to increase and reached its maximum (2.99 ± 0.20 fold vs control, P < 0.001) at 24 h, declined but remained significantly greater than baseline at 48 h (Fig. 1A). In the presence of actinomycin D, the half-life of CBS mRNA levels in VEGF-treated cells (9.1 ± 0.4 h) did not differ from that (8.8 ± 0.4 h) in control cells (Fig. 1B). Basal activity of wild-type (WT) human CBS promoter was greater in P vs. NP hUAEC. Treatment with 10 ng/ml VEGF for 24 h increased WT human CBS promoter activity by 1.98 ± 0.06-fold (p<0.05) in P hUAEC but failed to activate CBS promoter in NP hUAEC. The stimulatory effects of VEGF on CBS promoter activation were concentration-dependent in P hUAEC; VEGF significantly increase CBS promoter activity at 1 ng/ml and reached its maximum at 10-100 ng/ml. Basal human CSE promoter activity did not differ in NP and P hUAEC; VEGF at all concentrations did not activate CSE promoter in NP and P hUAEC (Fig 1 C&D). Because VEGF stimulate H2S production in association with increased CBS but not CSE protein expression in P but not NP hUAEC 19, VEGF stimulation of pregnancy-dependent UAEC H2S production is largely mediated by selective upregulating CBS transcription.
Fig. 1: VEGF stimulates pregnancy dependent human uterine artery endothelial CBS expression.

A: Primary human uterine artery endothelial cells (hUAEC) were treated with 10 ng/mL vascular endothelial growth factor (VEGF) for up to 2 days to assess cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) mRNA. Bars with different letters differ significantly (p<0.05). Data (means ± SEM) were collected from different hUAEC preparations from different nonpregnnat (NP) and pregnant (P) women. B: P hUAEC were treated with or without actinomycin D (5 μg/ml). Total RNA samples were extracted at 0, 3, 6, 9 and 12 h post actinomycin D. CBS and L19 were analyzed using real time quantitative reverse transcription-PCR (RT-qPCR). Data (means ± SEM, n=3) were presented as a percentage of relative CBS expression at time 0. C: Both non-pregnant (NP) and P hUAEC were transfected with luciferase constructs driven by wild-type human CBS promoter (−753/+161) or human CSE promoter (−942/+98) and co-transfected with the thymidine kinase-Renilla luciferase vector. After VEGF (10 ng/ml) treatment for 24 h, trans-activation of CBS or CSE promoter was measured by luciferase reporter gene expressions as a ratio of firefly/Renilla luciferase activities. Data (mean ± SEM. N=3) were presented as fold of controls. * p<0.05 and ** p<0.01. D: P hUAEC were transfected with luciferase constructs driven by wild-type human CBS promoter or CSE promoter and co-transfected with the thymidine kinase-Renilla luciferase vector, treated with increasing doses (0-100 ng/ml) of VEGF for 24 h. Data were summarized as mean±SEM from three independent experiments; Bars with different letters differ significantly (p < 0.05).
Sp1 and YY1 are important for basal CBS promoter activity and its activation by VEGF
By using Length-Aware Site Alignment Guided by Nucleotide Association (LASAGNA) motif search tool (https://biogridlasagna.engr.uconn.edu/lasagna_search/index.php), we found 4 putative Sp1 binding sites (i.e., Sp1d, Sp1c, Sp1b, and Sp1a sites) and a putative YY1 binding site between Sp1c and Sp1d sites, in the full-length 914 bp proximal human CBS promoter (Fig. 2A). When Luc-constructs driven by the CBS promoter with a series of 5’ deletions were transfected in P hUAEC, baseline full-length CBS promoter activity began to significantly decrease from position −753 to −486 bp. The VEGF-stimulated full-length promoter activation was significantly dropped at position −574 to −486 bp when Sp1d was deleted. Deletion to position −394 containing the YY1 site further decreased basal CBS promoter activity and completely abrogated VEGF-stimulated CBS promoter activity (Fig. 2B). A deletion from position −259 to −95 bp containing the Sp1c further decreased basal CBS promoter activity but further 5’ deletions to delete the Sp1b and Sp1a sites did not reduce basal CBS promoter activity (Fig. 2B). Thus, the region (−574 to −394 bp) containing Sp1d (−532 to −523 bp) and YY1 (−483 to −467 bp) sites is critical for baseline and VEGF-stimulated human CBS promoter activity.
Fig. 2: Sp1/YY1 in VEGF-stimulated CBS transcription.
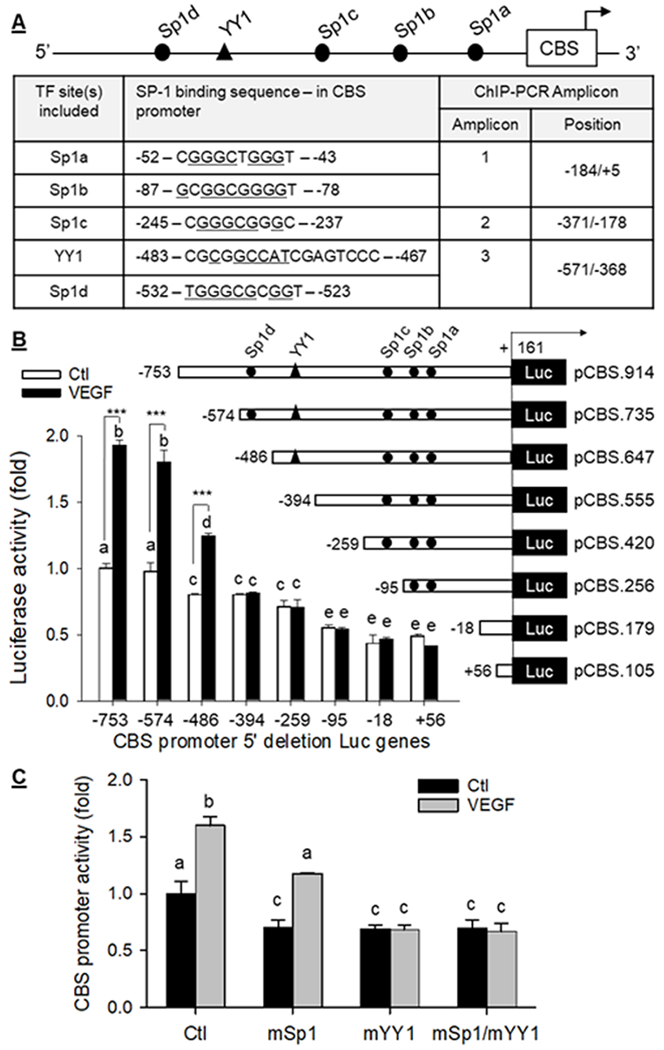
A: Putative Sp1 and YY1 binding sites in human CBS promoter and primers designed to amply amplicons containing specific Sp1/YY1 sites for chromatin immunoprecipitation-PCR. P hUAEC were transfected with luciferase reporter constructs driven by wild-type human CBS promoter (−753/+161) or its 5’-deletions (B), and wild type (WT) pCBS(−574).Luc construct or its mutations in the Sp1d, YY1, or both sites (C), and with thymidine kinase-Renilla luciferase vector as internal control. After treatment with VEGF (10 ng/ml) for 24 h, trans-activation of CBS promoter was measured. Data (means ± SEM, n=3) were expressed as fold of baseline WT CBS promoter activity. Bars with different letters differ significantly (p < 0.05). ***, p<0.001 vs controls.
To verify the importance of Sp1d and YY1 sites in human CBS transcription, we compared baseline and VEGF-stimulated full-length human CBS promoter activity with its mutants containing mutations in either Sp1d, YY1, or both sites. Mutations in either Sp1d or YY1 site alone resulted in ~33% (p < 0.05) decrease in basal human CBS promoter activity, which was not enhanced by mutations of both sites. VEGF stimulated WT human CBS promoter activity (1.60 ± 0.08-fold over baseline, p < 0.001) did not differ from that (1.67±0.01-fold over baseline, p<0.001) of its Sp1d mutant. However, YY1 mutation (mYY1) not only decreased basal CBS promoter activity but also completely abrogated VEGF-stimulated promoter activation. Mutations in both Sp1d and YY1 (mSp1/YY1) resulted in decreased basal CBS promoter activity similarly to that containing mYY1 and also completely abrogated VEGF-stimulated promoter activation (Fig. 2C).
Sp1 and YY1 interactions with human CBS promoter
In resting NP hUAEC, Sp1 was only recruited to Sp1d site (Amplicon 3) in human CBS promoter. In P hUAEC, in addition to Sp1d, Sp1 was also recruited to Sp1a/b site. Pregnancy increased Sp1 binding to Sp1a/b by 2.50 ± 0.26 (p<0.05) and Sp1d by 1.89 ± 0.17 (p<0.05) fold. Baseline Sp1 binding to Sp1c site (amplicon 2) was barely detectable in both NP and P cells. Treatment with 10 ng/ml VEGF for 24 h significantly increased Sp1 binding to Sp1a/b and Sp1d sites but did not affect Sp1 binding to Sp1c site in NP hUAEC. in P hUAEC, VEGF increased Sp1 binding to Sp1c by 2.04 ± 0.30 (p<0.05) and to Sp1d by 2.04 ± 0.30 (p<0.05) fold, but not to Sp1a/b. Baseline YY1 binding was only detected in amplicon 3 as expected since human CBS promoter only contains one YY1 site to be amplified in amplicon 3 (Fig. 3A). Baseline YY1 binding to the YY1 site did not differ between NP and P hUAEC; however, treatment with VEGF increased YY1 binding to the YY1 site by 2.44 ± 0.30 (p<0.05) fold in P hUAEC and modestly (p> 0.05) in NP hUAEC. Moreover, VEGF also increased YY1 binding to the Sp1c site by 4.05±0.25 (P<0.05) fold in P hUAEC (Fig. 3A), suggesting that VEGF recruited YY1 to the Sp1c site. We then preformed Co-IP studies to determine whether VEGF promotes Sp1 association with YY1. Sp1 antibody pulled down YY1 and YY1 antibody also pulled down Sp1 in untreated P hUAEC; treatment with 10 ng/ml VEGF for 30 min increased levels of Sp1-bound YY1 by 1.60 ± 0.02 (p<0.05) fold and YY1-bound Sp1 by 2.34±0.06 (p<0.05) fold (Fig. 3B). Thus, Sp1 and YY1 are associated with each other in resting hUAEC and the interaction can be stimulated by VEGF only in P hUAEC.
Fig. 3: Sp1/YY1 interactions with CBS promoter.

A: Binding of Sp1 and YY1 to human CBS promoter. Nonpregnant (NP) and pregnant (P) hUAEC were treated with or without VEGF (10 ng/ml, 24 h). Sheared DNA from the cells were subjected to chromatin immunoprecipitation (ChIP) using Sp1 or YY1 antibodies; IgG was used as negative controls. Sp1 and YY1 binding with specific sites were amplified by PCR using primers as designed in Fig. 2A. Data (means ± SEM) were summarized as fold of control from three experiments using cells from different women. *, p< 0.05 vs control. B. Sp1 and YY1 association. P hUAEC were treated with or without VEGF (10 ng/ml, 30 min). Proteins (1 mg/sample) were used immunoprecipitation (IP) with Sp1 or YY1 antibodies and IgG was used as control. The IP samples were immunoblotted with Sp1 or YY1. Levels of Sp1 bound YY1 and YY1 bound Sp1 were summarized as means ± SEM from NP or P hUAEC from three different women. *, p<0.05 vs control.
Sp1 and YY1 proteins in human UA in vivo and their expressions to VEGF in in hUAEC in vitro
Sp1 and YY1 proteins were immunolocalized in both EC and SMC of NP and P human UAs, with pregnancy-dependent cell (EC vs. SMC) specific expression patterns. Baseline Sp1 protein was detectable in EC but was only 50% of that in SMC (p <0.05) in NP UA. Pregnancy upregulated Sp1 protein by 2.89 ± 0.65 (p<0.01) fold in EC and significantly more by 4.52 ± 0.16 (p < 0.001) fold in SMC in human UA. Baseline YY1 protein was detectable in EC and was 5.06 ± 1.30 (p < 0.001) fold greater than that in SMC in NP UA. Pregnancy increased YY1 protein levels by 3.93 ± 0.68 (p<0.01) fold in EC and significantly more by 7.42 ± 2.02 (p<0.05) fold in SMC in human UA (Fig 4A). The pregnancy-dependent upregulated EC Sp1 and YY1 proteins were retained in cultured hUAEC, with 2.44 ± 0.50 (p<0.05) in Sp1 and 3.83 ± 0.25 (p<0.001) fold in P vs. NP hUAEC (Fig. 4B). Treatment with 10 ng/ml VEGF stimulated Sp1 and YY1 protein expressions in P hUAEC in a time-dependent manner; VEGF began to increase Sp1 and YY1 proteins at 24 h; both reached peak levels at 48 h (Sp1: 1.97 ± 0.16 fold, p<0.05; YY1: 2.29 ± 0.38 fold, p<0.05), and returned to baseline at 72 h. Treatment with increasing concentrations (0.1-100 ng/ml) of VEGF at 48 h also stimulated Sp1 and YY1 protein expressions in a dose-dependent manner. VEGF at 0.1 ng/ml effectively increased Sp1 and YY1 proteins; the responses peaked with 10 ng/ml VEGF stimulating Sp1 protein by 2.52 ± 0.24 (p<0.05) and YY1 by 2.59 ± 0.42 (p<0.05) fold. VEGF at 100 ng/ml did not stimulate Sp1 and YY1 protein expression (Fig. 4C).
Fig. 4: Sp1 and YY1 proteins in human uterine artery and effects of VEGF on hUAEC Sp1/YY1 expresison.
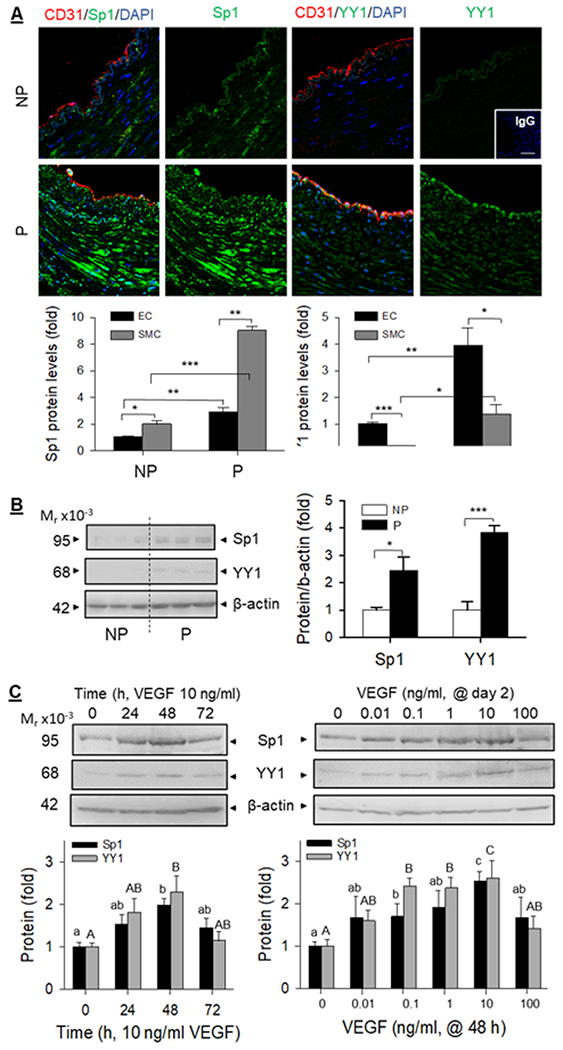
A: Paraffin-embedded human uterine artery rings were immunolabled by specific Sp1 or YY1 antibodies and CD31 antibody for labeling endothelial cells. Sections were mounted with DAPI to label nuclei and examined under confocal microscopy. Images were taken to determine Sp1 and YY1 proteins (relative green fluorescence intensity) in endothelium (EC) and smooth muscle (SM) using Image J and summarized in graphs. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs NP controls. B: Proteins (20 μg/lane) from NP an P hUAEC (n=3/group) were used for immunoblotting with Sp1 and YY1 antibodies and β-actin was measured as loading control. Data were summarized as means ± SEM and presented as fold of NP. *, p<0.05, ***, p<0.01 vs NP. C: P hUAEC were treated with VEGF (10 ng/ml, 3 days) or with increasing concentrations (0–100 ng/ml) of VEGF for 2 days to assess Sp1 and YY1 proteins by immunoblotting. Data were obtained from three experiments using cells from different women and calculated as means ± SEM (fold of baseline). Small and capital letter on bars represent differences in Sp1 and YY1, respectively, and bars with different letter differ significantly (p <0.05).
Sp1 and YY1 in baseline and VEGF-stimulated CBS expression and H2S production in hUAEC
Treatment with 10 ng/ml VEGF for 48h stimulated Sp1, YY1, and CBS, but not CSE proteins in P hUAEC. Treatment with specific Sp1 siRNA downregulated Sp1 by ~50% (p<0.01) and treatment with YY1 siRNA downregulated YY1 by ~53% (p<0.01) in P hUAEC. Sp1 knockdown did not alter baseline CBS protein; YY1 knockdown decreased baseline CBS protein by 49% (P<0.05). VEGF-stimulated CBS protein expression was unchanged by knockdown of Sp1 or YY1 alone but completely blocked by knockdown of both Sp1 and YY1. Knockdown of Sp1 or YY1 alone and both did not alter baseline and VEGF-stimulated CSE protein expression in P hUAEC (Fig. 5). Baseline H2S production in P hUAEC (0.31 pmol/cell/h) was significantly greater than that in NP hUAEC (0.06 pmol/cell/h), consistent with our previous report 19. VEGF stimulated H2S production in both NP and P hUAEC. Knockdown of either Sp1 or YY1 alone or their combination did not alter baseline H2S production but abrogated VEGF-stimulated H2S production in both NP and P hUAEC (Fig. 6).
Fig. 5: Sp1/YY1 knockdown on VEGF-stimulated CBS/CSE expression.
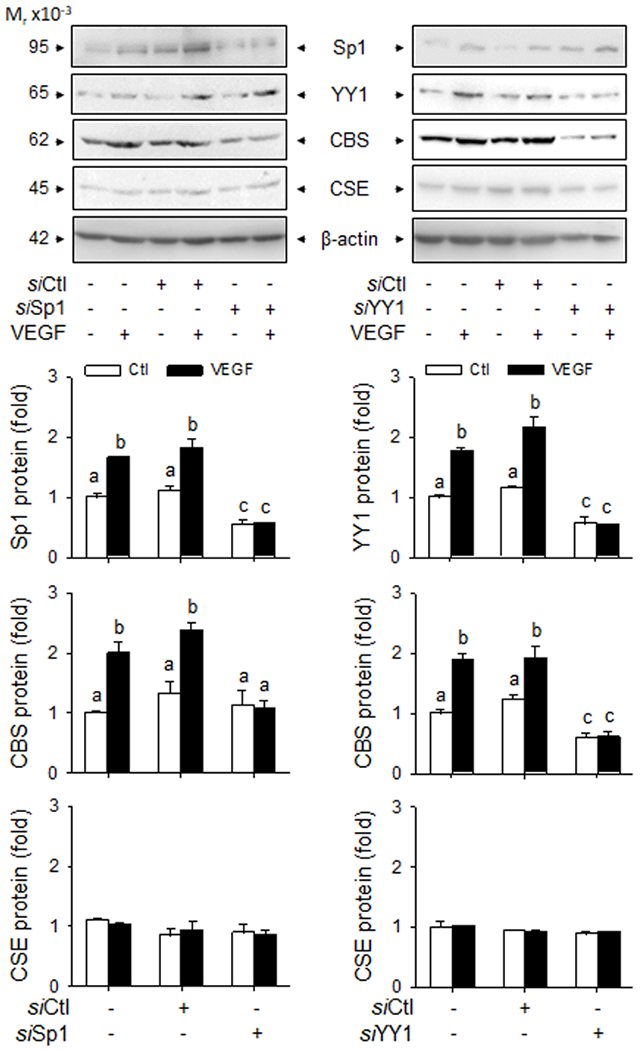
P hUAEC were infected with or without lentiviruses (10 MOI) of Sp1 (siSp1) or YY1 (siYY1) siRNAs for 72 h; lentivirus of scramble siRNA (siCtl) as control. The cells were then treated with or without VEGF (10 ng/ml) for 48 h. Cell lysates (20 μg/lane) were immunoblotted for Sp1, YY1, CBS, and CSE; β-actin was measured as loading control. Data were obtained from three experiments using cells from different women and calculated as means ± SEM (fold of baseline). Bars with different letters differ significantly (p< 0.05).
Fig. 6: Sp1/YY1 knockdown on VEGF-stimulated H2S production.
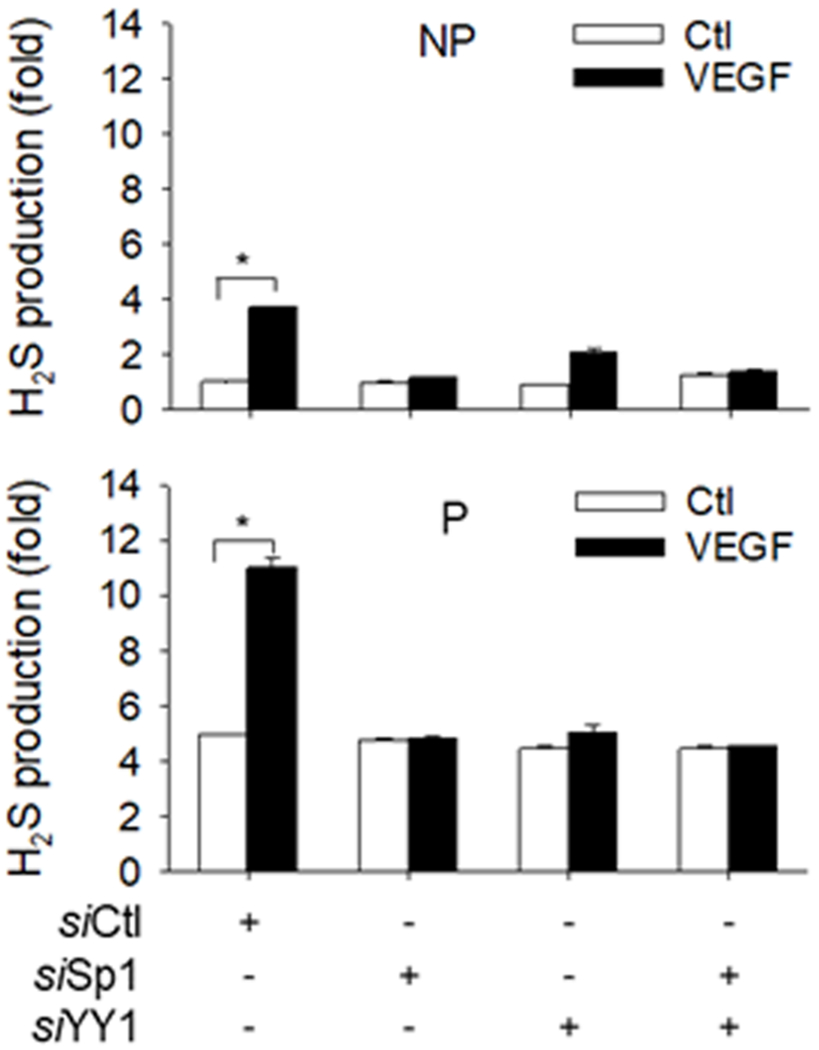
NP and P hUAEC were infected with or without lentiviruses (10 MOI) of Sp1 (siSp1) or YY1 (siYY1) siRNAs for 72 h; lentivirus of scramble siRNA (siCtl) as control. The cells were then treated with or without VEGF (10 ng/ml) for 48 h. Protein extracts (1 × 106 cells) were used to determine hydrogen sulfide (H2S) production by methylene blue assay. Data (means ± SEM) were collected from NP or P hUAEC (n=3/group) from different women. ***, p < 0.001 vs control.
DISCUSSION
Our recent studies have shown that enhanced local EC and smooth muscle (SMC) cell H2S production contributes to UA relaxation in pregnancy 9–11 and that endometrial stroma and trophoblast derived H2S functions as a paracrine factor to stimulate endometrial and placental angiogenesis 9, 20, 21. We have also reported that VEGF stimulates pregnancy dependent H2S production via selectively upregulating CBS protein expression in hUAEC 19. Therefore, augmented CBS/H2S signaling mediates the proangiogenic and vasodilatory effects of VEGF to upregulate UtBF during pregnancy. However, how VEGF-stimulates pregnancy-dependent uterine artery CBS/H2S signaling is yet to be determined. Herein we show that VEGF stimulates pregnancy-dependent CBS mRNA expression via upregulating CBS transcription via enhanced Sp1 and YY1 expression and their interactions with the corresponding binding sites in the proximal CBS promoter, without altering mRNA stability. Sp1 and YY1 knockdown completely abolished VEGF-stimulated CBS protein expression and H2S production in hUAEC. VEGF does not activate CSE transcription and Sp1 and YY1 knockdown also does not affect CSE expression in hUAEC. Thus, VEGF stimulation of pregnancy dependent UAEC H2S production primarily occurs via selective upregulation of CBS transcription involving enhanced expression of Sp1 and YY1 and their interactions with the CBS promoter.
The human CBS gene contains five transcription starting sites designated as −1a, −1b, −1c, −1d and −1e, respectively 25, leading to multiple transcripts with different 5’-untranslated regions, and the ones containing exon −1a and −1b are most abundant 25. Serum or fibroblasts growth factor stimulate human CBS transcription via the −1b promoter 25, suggesting that VEGF may activate this promoter. In the present study, we have first used luciferase reporter constructs driven by a series of 5’ deletion CBS promoter to determine the VEGF-responsive regions of the human CBS −1b promoter and found that the promoter sequence (−574 to −394) is primarily responsible for trans-activating CBS promoter by VEGF in hUAEC. Next, by using the LASAGNA motif search tool we found that the human CBS −1b promoter contains four Sp1 (a, b, c, and d) sites and one YY1 site adjacent to Sp1d. Interestingly, the Sp1d and YY1 sites are present in the promoter sequence (−574 to −394) critical for VEGF-stimulated trans-activation of CBS-1b promoter. We have therefore further determined the role of Sp1d and YY1 in VEGF-stimulated trans-activation of CBS promoter by luciferase reporter expression studies using full-length CBS promoter in which Sp1d and YY1 sites are mutated. The promoter with mutated Sp1d is with lower baseline activity but still can be trans-activated by VEGF. The promoter with mutated YY1 is not only with lower baseline activity but also a complete loss of response to VEGF stimulation and so does the one with Sp1/YY1 double mutations. Thus, Sp1 is needed to maintain baseline CBS-1b promoter activity and YY1 is critically important for VEGF-stimulated CBS transcription in hUAEC.
Herein we have also performed ChIP-PCR studies to further determine Sp1 and YY1 interactions with the human CBS promoter. In resting NP hUAEC, only one specific DNA sequence (amplicon 3) is amplified from the ChIP samples obtained by using Sp1 or YY1 antibodies, suggesting that recruitment of Sp1 to Sp1d site and YY1 to YY1 site is required for basal CBS expression in NP state. Of note, both sites are pocketed in the promoter sequence (−574 to −394) needed for basal and VEGF-stimulated trans-activation of CBS-1b promoter, as determined by promoter mapping luciferase reporter gene expression studies. In untreated P hUAEC, Sp1 is recruited to the Sp1d site with increased binding activity compared to that in NP cells and also to the Sp1a/b site. In addition to be recruited to the YY1 site with increasing binding activity, YY1 is also recruited to the Sp1c site, suggesting formation of a Sp1/YY1 complex at this site since Sp1c does not contain the core YY1 binding sequence. Thus, in addition to increasing interactions of Sp1 with Sp1d and YY1 with YY1, recruitments of Sp1 to Sp1a/b and YY1 to Sp1c seem to be needed for pregnancy-augmented EC CBS expression in human UA in vivo as we recently reported 9, 11.
In P hUAEC, VEGF stimulates the recruitment of Sp1 to Sp1d site and YY1 to YY1 site. Interestingly, VEGF also recruits Sp1 and YY1 to the Sp1c site. Recruitment of YY1 to Sp1c suggests formation of a YY1/Sp1 complex, which is supported by our Co-IP studies showing that VEGF increases Sp1 association with YY1 in P hUAEC. Thus, increased binding of Sp1 to Sp1d/c, YY1/YY1, and formation of a Sp1/YY1 complex on Sp1c, are important for VEGF-stimulated pregnancy dependent CBS transcription. In NP hUAEC, VEGF increases Sp1 recruitment to Sp1d site; however, without increased YY1 binding to YY1 and Sp1c sites in NP cells, this does not suffice to initiate CBS transcription by VEGF. VEGF also increases Sp1 binding to Sp1a/b site; however, this seems not be functionally important due to the inability of the promoter sequence (> −184) that contains Sp1a/b to initiate CBS transcription as determined by luciferase assay.
In human UA, Sp1 and YY1 proteins express in a pregnancy dependent cell (EC vs. SMC) specific manner. In vitro, VEGF stimulates Sp1 and YY1 protein expressions in P hUAEC. Increased EC Sp1 and YY1 expression adds another layer to the regulatory mechanism via Sp1/YY1-mediated upregulation of CBS transcription for explaining pregnancy-augmented VEGF stimulation of CBS expression in human UA. Both Sp1 and YY1 are also upregulated even more in SMC then EC in human UA. This study does not explore the functional sequalae of increased SMC Sp1 and YY1 in human UA. Sp1 promotes a contractile to synthetic phenotypic switch of vascular SMC 30 and YY1 inhibits vascular SMC differentiation 31. Thus, increased SMC Sp1 and YY1 may play a role in pregnancy-induced UA SMC remodeling involving a phenotypic switch that favors vasodilation.
Lastly, we used lentiviral delivery of specific siRNAs to knock down Sp1 or YY1 for demonstrating a role of Sp1/YY1 in CBS expression and H2S production in hUAEC. Knockdown of YY1 but not Sp1 decreases baseline CBS protein and knockdown of both Sp1 and YY1 but not alone blocks VEGF-stimulated CBS protein expression, without altering CSE protein expression. Consistent with our previous report 19, basal H2S production is lower in NP vs. P cells and VEGF stimulates H2S production in both NP and P hUAEC. The reason that VEGF stimulates H2S production in NP cells without affecting CBS and CSE protein expression can be attributed to posttranslational modifications of both enzymes such as phosphorylation of CBS 8 and CSE 32and CSE interaction with Ca2+/calmodulin 33, complexing the mechanism of H2S biosynthesis. Nonetheless, knockdown of either Sp1 or YY1 completely attenuates VEGF-stimulated H2S production in NP and P hUAEC, demonstrating a critical role of Sp1 or YY1 in basal and VEGF-stimulated H2S production in NP and P hUAEC.
In summary, our current study demonstrates that pregnancy augments EC Sp1 and YY1 expression in human UA and promotes the recruitment of Sp1/YY1 to their DNA binding sequences in proximal human CBS promoter; this pathway underlies a novel mechanism by which VEGF utilizes to stimulate endothelial CBS upregulation for producing H2S in human UA during pregnancy.
Perspectives
Our recent work has shown that enhanced H2S production via selective CBS upregulation contributes to pregnancy-associated uterine vasodilation and endometrial/placental angiogenesis 9, 11, 20, 21, as well as immune tolerance at the maternal-fetal interface 34, and that pregnancy augments VEGF-stimulated human artery endothelial cell H2S production via selective CBS upregulation 19. Adenoviral delivery of VEGF gene abelites undernutrition-induced fetal growth restriction via upregulating UtBF 17, 18. Administration of H2S donors rescue animal models of preeclampsia induced by sFlt1, involving upregulating VEGF 22, 23. Thus, the CBS/H2S and VEGF systems interact to play a key role in uterine hemodynamic regulation in pregnancy. Our current study further elaborates that upregulated EC Sp1 and YY1 and enhanced interactions of these transcription factors with their DNA binding sequences in the CBS promoter, underling the specific pathways by which VEGF utilizes to trans-activate CBS transcription for mediating VEGF-stimulated human UAEC H2S production in pregnancy. This pathway provides a novel strategy for potential therapeutic development for hypertension-related pregnancy disorders such as preeclampsia with fetal growth restriction.
Supplementary Material
Novelty and Significance.
What Is New?
Vascular endothelial growth factor (VEGF) stimulates pregnancy-dependent mRNA expression of the hydrogen sulfide (H2S) synthesizing enzyme cystathionine β-synthase (CBS) via upregulating CBS transcription without altering its mRNA stability. Pregnancy augments Sp1 and YY1 expressions in a cell (endothelial vs. smooth muscle)-specific manner in human uterine artery in vivo. VEGF stimulates pregnancy dependent Sp1 and YY1 expression in human uterine artery endothelial cells (hUAEC) in vitro. Enhanced interactions of Sp1 and YY1 with their corresponding binding sites and formation of a Sp1/YY1 complex in a Sp1 site in human CBS promoter are required for VEGF-stimulated pregnancy dependent CBS transcription in hUAEC. Furthermore, Sp1 or YY1 inhibition completely attenuates VEGF-stimulated pregnancy dependent CBS expression and H2S production in hUAEC.
What Is Relevant?
Interactions between VEGF signaling and CBS/H2S pathway play a critical role in the two key mechanisms (i.e., vasodilation and angiogenesis) for upregulating uterine blood flow that is critical for pregnancy health. The present study demonstrates that VEGF stimulates pregnancy dependent H2S production via upregulating CBS transcription by enhanced expressions of Sp1 and YY1 and coordinated interactions of Sp1 and YY1 with the proximal CBS promoter in human uterine artery endothelial cells. These findings provide a novel mechanism for controlling uterine artery H2S production pertaining to uterine hemodynamic regulation during pregnancy.
Acknowledgments:
The authors wish to thank all the volunteers for donating the tissue samples and Drs. Joshua Makhoul, Afshan Hameed, and Carole Major, as well as the attending obstetricians at the University of California Irvine Medical Center for their assistance in tissue collection.
Sources of Funding:
The present study was supported in part by the National Institutes of Health (NIH) grants R01 HD105699, RO1 HL70562, and R21 HD097498 (to D-b C). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.0090292. [DOI] [PubMed] [Google Scholar]
- 2.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121–5. doi: 10.1016/0002-9378(90)90834-t. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. Ii. Pregnancy effects on NO synthase expression. Am J Physiol. 1997;272:H1730–1740. doi: 10.1152/ajpheart.1997.272.4.H1730 [DOI] [PubMed] [Google Scholar]
- 5.Nelson SH, Steinsland OS, Suresh MS, Lee NM. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum Reprod. 1998;13:1361–1367 [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld CR, Roy T. Prolonged uterine artery nitric oxide synthase inhibition modestly alters basal uteroplacental vasodilation in the last third of ovine pregnancy. Am J Physiol Heart Circ Physiol. 2014;307:H1196–1203. doi: 10.1152/ajpheart.00996.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011 [DOI] [PubMed] [Google Scholar]
- 8.Filipovic MR, Zivanovic J, Alvarez B, Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev. 2018;118:1253–1337. doi: 10.1021/acs.chemrev.7b00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheibani L, Lechuga TJ, Zhang HH, Hameed A, Wing DA, Kumar S, Rosenfeld CR, Chen DB. Augmented H2S production via cbs upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod. 2017;96:664–672. doi: 10.1095/biolreprod.116.143834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechuga TJ, Qi QR, Magness RR, Chen DB. Ovine uterine artery hydrogen sulfide biosynthesis in vivo: Effects of ovarian cycle and pregnancy. Biol Reprod. 2019;100:1630–1636. doi: 10.1093/biolre/ioz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Bai J, Yang YH, Hoshi N, Chen DB. Hydrogen sulfide relaxes human uterine artery via activating smooth muscle BKCa channels. Antioxidants. 2020;9:274–279. doi: 10.3390/antiox9111127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281:H422–431. doi: 10.1152/ajpheart.2001.281.1.H422 [DOI] [PubMed] [Google Scholar]
- 13.Hu XQ, Dasgupta C, Chen M, Xiao D, Huang X, Han L, Yang S, Xu Z, Zhang L. Pregnancy reprograms large-conductance Ca2+-activated K+ channel in uterine arteries: Roles of ten-eleven translocation methylcytosine dioxygenase 1-mediated active demethylation. Hypertension. 2017;69:1181–1191. doi: 10.1161/HYPERTENSIONAHA.117.09059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Y, May V, Braas K, Osol G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Physiol. 1997;273:H938–944. doi: 10.1152/ajpheart.1997.273.2.H938 [DOI] [PubMed] [Google Scholar]
- 15.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884 [DOI] [PubMed] [Google Scholar]
- 16.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr DJ, Wallace JM, Aitken RP, Milne JS, Mehta V, Martin JF, Zachary IC, Peebles DM, David AL. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther. 2014;25:375–384. doi: 10.1089/hum.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, Peebles DM, David AL. Peri- and postnatal effects of prenatal adenoviral vegf gene therapy in growth-restricted sheep. Biol Reprod. 2016;94:142. doi: 10.1095/biolreprod.115.133744 [DOI] [PubMed] [Google Scholar]
- 19.Zhang HH, Chen JC, Sheibani L, Lechuga TJ, Chen DB. Pregnancy augments vegf-stimulated in vitro angiogenesis and vasodilator (NO and H2S) production in human uterine artery endothelial cells. J Clin Endocrinol Metab. 2017;102:2382–2393. doi: 10.1210/jc.2017-00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen DB, Feng L, Hodges JK, Lechuga TJ, Zhang H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol Reprod. 2017;97:478–489. doi: 10.1093/biolre/iox105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi QR, Lechuga TJ, Patel B, Nguyen NA, Yang YH, Li Y, Sarnthiyakul S, Zhang QW, Bai J, Makhoul J, Chen DB. Enhanced stromal cell CBS-H2S production promotes estrogen-stimulated human endometrial angiogenesis. Endocrinology. 2020;161:bqaa176. doi: 10.1210/endocr/bqaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacos E, Buhimschi IA, Buhimschi CS, Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631 [DOI] [PubMed] [Google Scholar]
- 23.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen sulfide attenuates sflt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol. 2014;25:717–725. doi: 10.1681/ASN.2013030291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Bazer FW (eds) Endocrinology of Pregnancy. Contemporary Endocrinology, Vol 9. 1998;Humana Press, Totowa, NJ.:pp 507–539. doi: 10.1007/978-1-4612-1804-3_18 [DOI] [Google Scholar]
- 25.Kraus JP, Oliveriusova J, Sokolova J, Kraus E, Vlcek C, de Franchis R, Maclean KN, Bao L, Bukovsk, Patterson D, Paces V, Ansorge W, Kozich V. The human cystathionine beta-synthase (cbs) gene: Complete sequence, alternative splicing, and polymorphisms. Genomics. 1998;52:312–324. doi: 10.1006/geno.1998.5437 [DOI] [PubMed] [Google Scholar]
- 26.Maclean KN, Kraus E, Kraus JP. The dominant role of Sp1 in regulating the cystathionine beta-synthase −1a and −1b promoters facilitates potential tissue-specific regulation by kruppel-like factors. J Biol Chem. 2004;279:8558–8566. doi: 10.1074/jbc.M310211200 [DOI] [PubMed] [Google Scholar]
- 27.Natesan S, Gilman MZ. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes & Dev. 1993;7:2497–2509. doi: 10.1101/gad.7.12b.2497 [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Kim JY, Xu S, Liu H, Yin M, Koroleva M, Guo J, Pei X, Jin ZG. Endothelial-specific YY1 governs sprouting angiogenesis through directly interacting with RBPJ. Proc Natl Acad Sci U S A. 2020;117:4792–4801. doi: 10.1073/pnas.1916198117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sade H, Holloway K, Romero IA, Male D. Transcriptional control of occludin expression in vascular endothelia: Regulation by Sp3 and YY1. Biochim Biophys Acta. 2009;1789:175–184. doi: 10.1016/j.bbagrm.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Yu S, Liu Y, Zhang J, Han L, Xu Z. MicroRNA-124 controls human vascular smooth muscle cell phenotypic switch via Sp1. Am J Physiol Heart Circ Physiol. 2017;313:H641–H649. doi: 10.1152/ajpheart.00660.2016. [DOI] [PubMed] [Google Scholar]
- 31.Jin M, Wu Y, Wang Y, Yu D, Yang M, Yang F, Feng C, Chen T. MicroRNA-29a promotes smooth muscle cell differentiation from stem cells by targeting YY1. Stem Cell Res. 2016;17:277–284. doi: 10.1016/j.scr.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Yan Q, Liu X, Li P, Li X, Chen Y, Simoncini T, Liu J, Zhu D, Fu X. 17beta-estradiol nongenomically induces vascular endothelial H2S release by promoting phosphorylation of cystathionine gamma-lyase. J Biol Chem. 2019;294:15577–15592. doi: 10.1074/jbc.RA119.008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Xu T, Li Y, Wang W, Lyu C, Luo D, Yang Q, Ning N, Chen ZJ, Yan J, Chen DB, Li J. Trophoblast H2S maintains early pregnancy via regulating maternal-fetal interface immune hemostasis. J Clin Endocrin & Metab. 105:e4275–e4289. 10.1210/clinem/dgaa357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


