Abstract
While there is emerging evidence of peripheral microvascular dysfunction in patients with heart failure with preserved ejection fraction that may be related to systemic inflammation and redox imbalance, disease-related changes in locomotor muscle microvascular responsiveness has not been determined. This study combined passive leg movement and biomarker assessments of inflammation and oxidative damage to determine the magnitude and mechanisms of lower limb microvascular function in patients with heart failure with preserved ejection fraction (71±1yrs; n=44) compared to healthy, similarly-aged controls (68±2yrs; n=39). Leg blood flow, heart rate, mean arterial pressure, and stroke volume were assessed, and plasma biomarkers of inflammation and oxidative damage were also determined. A significantly attenuated passive leg movement-induced peak change in leg blood flow (263±25 vs. 371±31 mL/min, heart failure with preserved ejection fraction vs. control) and leg vascular conductance (2.99±0.32 vs. 3.88±0.34 mL/min/mmHg, heart failure with preserved ejection fraction vs. control) was observed in patients compared to controls. Similarly, the total hyperemic response to passive leg movement, expressed as leg blood flowAUC and leg vascular conductanceAUC, was ~40% less in patients with heart failure with preserved ejection fraction vs. control. Significantly greater C-reactive protein, interleukin 6, and malondialdehyde were observed in patients with heart failure with preserved ejection fraction, but were not correlated with passive leg movement responses. These data provide new evidence of a decline in lower limb microvascular function within a milieu of vascular inflammation that may contribute to locomotor muscle dysfunction in patients with heart failure with preserved ejection fraction.
Keywords: HFpEF, hyperemia, vascular function, nitric oxide, inflammation
Journal Subject Codes: Heart Failure, Peripheral Vascular Disease, Endothelium, Hemodynamics, Biomarkers
INTRODUCTION
It is predicted that heart failure (HF) and other forms of cardiovascular disease will exceed 40% of the U.S. population by 2030 [1], making HF a burgeoning public health crisis. Just over half of HF patients have a preserved left ventricular ejection fraction (HFpEF) [2,3], and the prevalence of this form of HF relative to HF with reduced ejection fraction (HFrEF) is increasing 1% annually [4]. While HFpEF and HFrEF share a similar clinical presentation that is characterized by exertional dyspnea and exercise intolerance [2,3,5–8], the numerous proven therapies for patients with HFrEF [9] have been unsuccessful in the treatment of patients with HFpEF [10]. This lack of success with standard therapies highlights the truly unique nature of this clinical syndrome and demonstrates the need for a more comprehensive understanding of HFpEF pathophysiology in this phenotypically distinct patient group.
One of the most prominent unifying theories concerning the pathophysiology of HFpEF is the role of non-cardiac comorbidities (i.e. obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease) that accompany this clinical syndrome and result in systemic inflammation [11]. Indeed, circulating inflammatory biomarkers appear to be distinctly elevated in patients with HFpEF [12], and this inflammation has been associated with myocardial remodeling and dysfunction [11] through inflammatory activation of the coronary microvascular endothelium, oxidative stress, and impaired endothelial-cardiomyocyte nitric oxide (NO) signaling [13]. How this systemic inflammation impacts peripheral vascular function in patients with HFpEF remains unclear.
To date, a small number of studies have sought to evaluate peripheral vascular function using traditional, non-invasive testing (i.e. arterial flow-mediated dilation; FMD) in patients with HFpEF, and findings have been equivocal [14–18]. Our group recently documented a distinct pattern of upper limb peripheral vascular dysfunction in HFpEF patients specific to the microvasculature [19], which parallels a growing body of literature demonstrating microvascular dysfunction in this patient group [13,16,20,21]. However, the exact vasodilatory pathways involved in HFpEF-specific peripheral microvascular dysfunction remain somewhat unclear [22,23].
The peripheral hemodynamic response to passive leg movement (PLM) fills this void by providing an index of predominantly NO-mediated (~80%) microvascular function in the lower extremities [24]. Unlike FMD and RH measurements in the upper extremity, the PLM test involves continuous movement of the knee joint through a 90-degree range of motion, eliciting a transient increase in limb blood flow which is somewhat correlated with FMD testing [25]. Thus, the assessment of lower limb microvascular function using the PLM methodology represents a unique approach for assessment of NO bioavailability in the HFpEF patient population. The PLM test has been used extensively to assess and differentiate lower limb microvascular responses in healthy individuals [24,26,27], the elderly [28,29], and various disease states [30–32], making it a, potentially, clinically relevant non-invasive test of lower limb peripheral vascular function in patients with HFpEF.
Therefore, the overall goal of this investigation was to combine biomarkers of inflammation and oxidative stress with a novel assessment of primarily NO-dependent vasodilation to more fully characterize disease-specific adaptations in the lower extremity microvasculature of patients with HFpEF. We hypothesized that biomarkers of inflammation and oxidative stress would be elevated, and that PLM-induced hyperemia would be attenuated, in patients with HFpEF compared to healthy, similarly aged controls.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
All experimental procedures were approved by the University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Boards. All subjects provided oral and written informed consent prior to participation in the study, as set forth by the Declaration of Helsinki.
Subjects
A total of 83 subjects (n = 44 HFpEF patients, n = 39 healthy, similarly aged controls) participated in the current study. Subject characteristics for all individuals and disease specific characteristics of the patient group are documented in Table 1 and 2, respectively. Details regarding inclusion/exclusion criteria are provided in the online data supplement.
Table 1.
Subject characteristics
| Subject Characteristics | Controls | HFpEF | P value |
|---|---|---|---|
| Subjects, N (males/females) | 39 (20/19) | 43 (18/25) | --- |
| Age, yrs | 68 ± 2 | 71 ± 1 | 0.19 |
| Body mass, kg | 77 ± 3 | 98 ± 4* | <0.001 |
| Height, cm | 170 ± 2 | 169 ± 2 | 0.82 |
| Body mass index, kg/m2 | 26 ± 1 | 34 ± 1* | <0.001 |
| Body surface area, | 1.91 ± 0.04 | 2.00 ± 0.05* | <0.001 |
| Resting Heart rate, beats/min | 59 ± 1 | 65 ± 2* | 0.003 |
| Mean arterial blood pressure, mmHg | 100 ± 3 | 92 ± 3* | 0.04 |
| Glucose, mg/dL | 84 ± 3 | 97 ± 3* | <0.001 |
| Cholesterol, mg/dL | 207 ± 8 | 170 ± 9* | <0.001 |
| High-density lipoprotein, mg/dL | 55 ± 3 | 47 ± 3* | 0.015 |
| Low-density lipoprotein, mg/dL | 132 ± 5 | 96 ± 8* | <0.001 |
| Triglycerides, mg/dL | 126 ± 15 | 173 ± 17* | 0.04 |
Data are mean ± SEM.
Significantly different vs. Controls
Table 2.
HFpEF-specific characteristics.
| Clinical Characteristics | HFpEF |
|---|---|
| Echocardiography | |
| Ejection fraction (%) | 61 ± 2 |
| LV IVSd (cm) | 1.17 ± 0.04 |
| LV PWd (cm) | 1.22 ± 0.11 |
| LV ID diastole (cm) | 4.52 ± 0.12 |
| LD ID systole (cm) | 4.58 ± 0.07 |
| Peak E wave (cm/s) | 98 ± 5 |
| Peak A wave (cm/s) | 92 ± 7 |
| E/A ratio | 1.36 ± 0.17 |
| E’ lateral wall (cm/s) | 8 ± 1 |
| E/E’ ratio | 22 ± 8 |
| Disease-specific Biomarkers | |
| BNP (pg/ml) | 150 ± 26 |
| NT-pro BNP (pg/ml) | 620 ± 165 |
| ST2 (ng/ml) | 45 ± 6 |
| Galectin-3 (ng/ml) | 15 ± 1 |
| Cystatin C (ng/ml) | 17 ± 1 |
| Comorbidities | |
| COPD (%) | 7 |
| Diabetes (%) | 33 |
| CAD (%) | 29 |
| Hypertension (%) | 71 |
| Medications | |
| Beta receptor blocker (%) | 45 |
| ACEi or ARB (%) | 60 |
| Loop diuretics (%) | 81 |
| Diuretic (%) | 69 |
| Statin (%) | 67 |
| Calcium channel blockers (%) | 19 |
LV IVSD, left ventricle interventricular septum; LV PWd, left ventricle posterior wall diameter; LV ID, left ventricle internal diameter; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; angiotensin converting enzyme inhibitor; angiotensin receptor blocker; BNP, B-type natriuretic peptide; NT-proBNP, NT-pro B-type natriuretic peptide; ST2, suppression of tumorigenicity 2. Data are mean ± SEM or %.
Passive Leg Movement Protocol
The PLM procedure has been described in detail elsewhere [33]. After 20 minutes of supine rest and prior to leg movement, baseline measures were assessed for 1 minute. PLM was then performed by a trained investigator who articulated the subject’s knee joint through a 90° range of motion (full leg extension (180°) to flexion (90°)) at 1Hz for 1 minute. PLM has been previously shown to have good reliability as a non-invasive index of microvascular function [34]
Measurements
Common femoral arterial blood velocity and vessel diameter were assessed using ultrasound Doppler (GE a Logiq 7). Heart rate (HR) was monitored continuously from a standard three-lead ECG, and arterial blood pressure was measured using finger photoplethysmography (Finometer). Stroke volume (SV) was estimated using the Modelflow method [35], which, in combination with HR, was used to estimate cardiac output (CO). Plasma biomarkers of inflammation and oxidative stress were determined using standard quantitative methods. Additional methodological details are provided in the online data supplement.
Statistical Approach
Statistical analysis was performed using commercially available software (SigmaStat 3.10, Systat Software, Point Richmond, CA). Physical characteristics, resting cardiovascular measures, PLM-induced peak and total blood flow (AUC) responses and plasma biomarkers were compared between groups using Student’s unpaired t-tests to identify any group differences. A two-way ANOVA was used to compare differences between groups across time during PLM. When a significant main effect was observed a Holm-Sidak post hoc analysis was performed. The Significance was set at P < 0.05 and data are presented as group mean ± SEM. Exact P-values are given unless otherwise noted.
RESULTS
Characteristics of HFpEF Patients and healthy controls.
Patient and control characteristics are presented in Table 1. Despite being well-matched for age and height, patients with HFpEF had higher BMI, BSA, resting HR, and lower resting MAP (All P < 0.05; Table 1). Additionally, patients with HFpEF also had higher resting blood glucose levels despite having lower overall cholesterol, HDL, and LDL levels (Table 1). Disease-specific characteristics and pharmacological intervention information for patients with HFpEF patients are presented in Table 2. All 43 of the patients with HFpEF were currently taking one or more blood pressure lowering and/or cholesterol altering medications (Table 2).
Peripheral Responses to Passive Leg Movement.
Resting LBF and vascular conductance were not different between the two cohorts (both P > 0.05). At the onset of PLM, LBF and leg vascular conductance increased immediately above baseline in both groups (Figure 1A and 2A). Peak LBF during PLM was ~10% lower in patients with HFpEF (593 ± 34 mL/min) compared to controls (651 ± 35 mL/min), yet the difference did not reach statistical significance (P = 0.24). Similarly, peak conductance was not different between HFpEF patients (6.89 ± 0.54 mL/min/mmHg) and controls (6.77 ± 0.41 mL/min/mmHg) (P = 0.89). However, in HFpEF patients compared to controls the peak change in LBF (263 ± 25 mL/min and 2.99 ± 0.32 mL/min/mmHg, respectively, P = 0.008, Figure 1B) and leg vascular conductance (371 ± 31 mL/min and 3.88 ± 0.34 mL/min/mmHg, respectively, P = 0.049, Figure 2B) was significantly blunted. Notably, these differences in LBF and vascular conductance occurred in the absence of any differences in the MAP response, or peak change in MAP, during PLM between groups.
FIGURE 1:
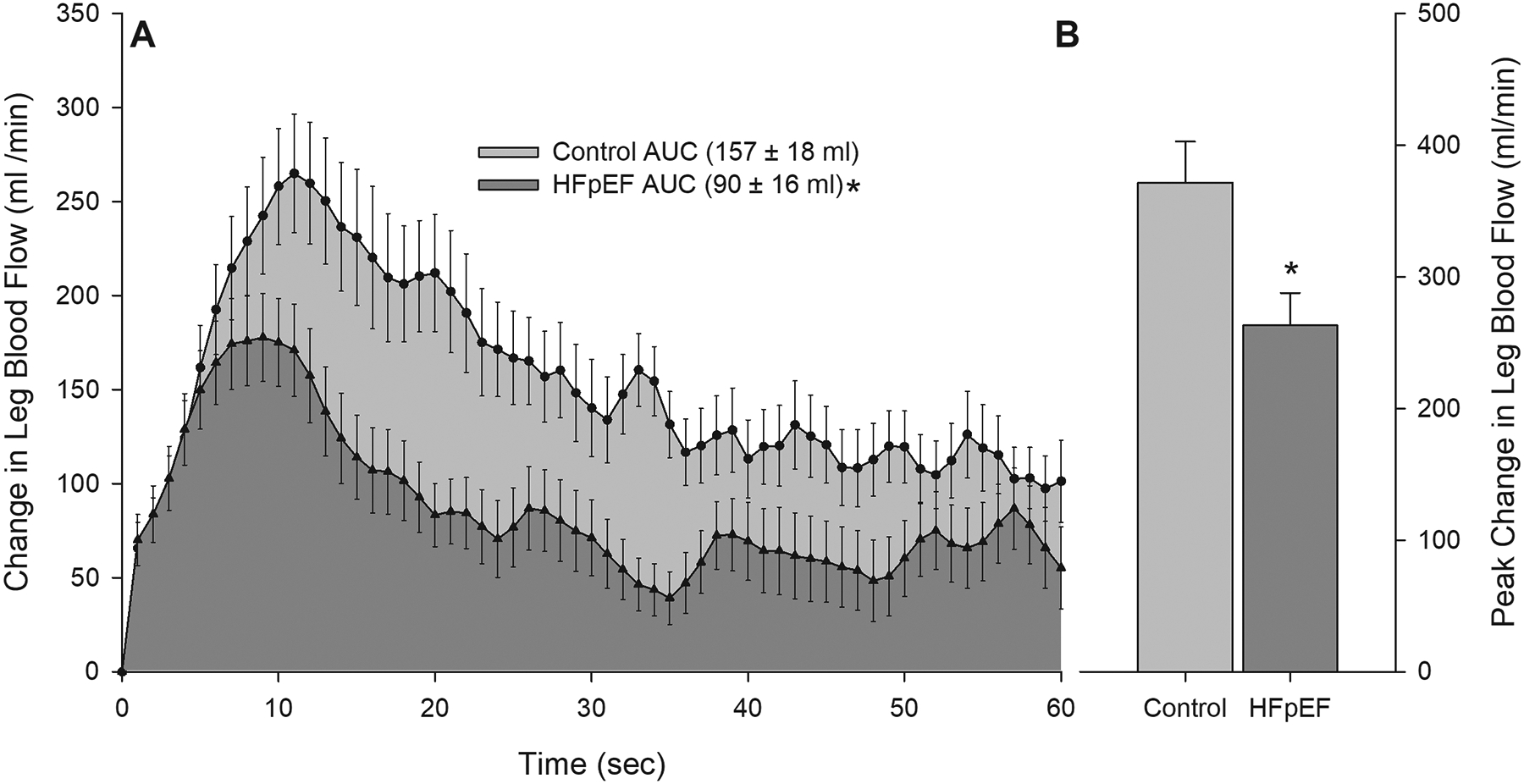
Panel A: Changes in leg blood flow during 1 minute of passive leg movement in controls and patients with HFpEF. Area under the curve (AUC) was calculated as the summed second-by-second values during passive movement. Panel B: Peak change in leg blood flow achieved during 1 minute of passive leg movement in controls and patients with HFpEF. *Significantly different than control, P < 0.05. HFpEF n = 43; Control n = 39. Data are mean ± SEM
FIGURE 2:
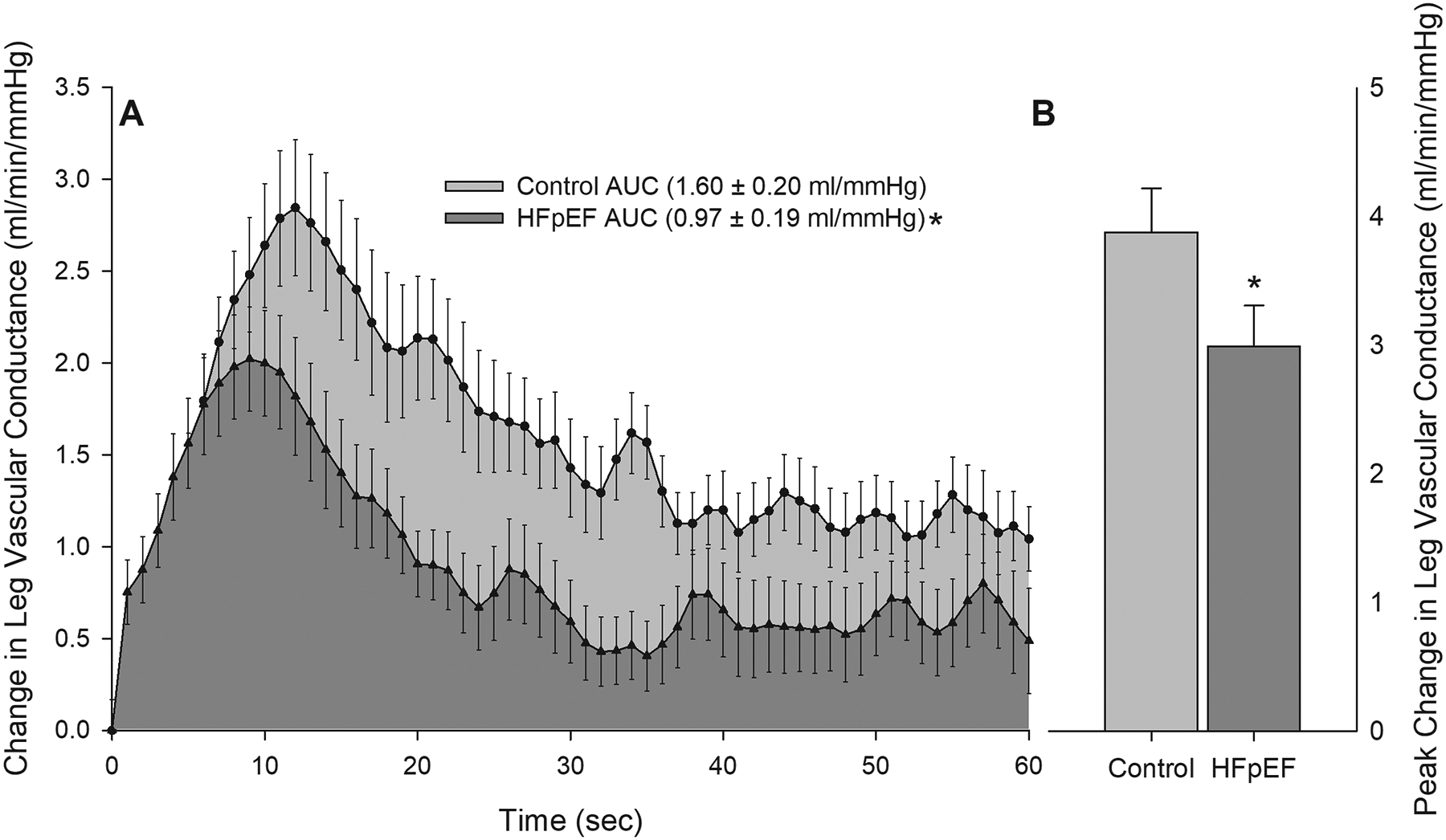
Panel A: Changes in leg vascular conductance during 1 minute of passive leg movement in controls and patients with HFpEF. Area under the curve (AUC) was calculated as the summed second-by-second values during passive movement. Panel B: Peak change in leg vascular conductance achieved during 1 minute of passive leg movement in controls and patients with HFpEF. *Significantly different than control, P < 0.05. HFpEF n = 40; Control n = 35. Data are mean ± SEM
Although both groups exhibited the typical transient increases in LBF and conductance in response to PLM, LBF and leg vascular conductance not only increased to a greater extent in controls compared to patients with HFpEF, but remained elevated relative to the patients with HFpEF throughout PLM (LBF P = 0.012; vascular conductance P = 0.008; Figure 1A and 2A, respectively) Furthermore, when PLM-induced changes in LBF and vascular conductance were assessed as AUC, the differences between groups were even greater (Figure 1A and 2A), with patients with HFpEF having a ~ 40% lower LBFAUC and vascular conductanceAUC (P = 0.009 and P = 0.024, respectively).
Central Hemodynamic Responses to Passive Leg Movement.
Immediately prior to PLM, resting HR (P = 0.03), SV (P < 0.01), and CO (P < 0.001) were all higher in patients with HFpEF (65 ± 2 bpm, 107 ± 5 ml, 6.8 ± 0.3 ml/min, respectively) compared to controls (59 ± 1 bpm, 87 ± 5 ml, 4.9 ± 0.3 ml/min, respectively). Which may be due to the higher BMIs observed in HFpEF patients and the added demand for perfusion of excess adipose tissue (Table 1). There were no differences in peak change in HR (9 ± 2 vs. 7 ± 1 bpm, HFpEF vs. Control, P = 0.28) or peak change in CO (0.8 ± 0.1 vs. 1.0 ± 0.2 ml/min, HFpEF vs. Control, P = 0.37) during PLM. However, peak change in stroke volume was higher in patients with HFpEF compared to controls (11 ± 1 ml vs. 9 ± 1ml, HFpEF vs. Control, P = 0.01).
Inflammation, Antioxidant Capacity and Oxidative Stress.
Despite no group differences for the pro-inflammatory cytokine TNFα (2.08 ± 0.25 vs. 1.85 ± 0.15 pg ml−1, HFpEF vs. Control; Figure 3A), patients with HFpEF had significantly greater plasma IL-6 concentrations (5.09 ± 0.87 vs. 2.7 ± 0.37 pg ml−1, HFpEF vs. Control; Figure 3C). Additionally, systemic inflammation, as assessed by CRP was greater in patients with HFpEF compared to controls (4637 ± 601 and 1020 ± 113 ng ml−1, respectively; Figure 3A). Protein oxidation, assessed by plasma protein carbonyl, was not different between groups (0.13 ± 0.01 vs. 0.13 ± 0.02 nmol mg−1, HFpEF vs. control; Figure 4A). Conversely, lipid peroxidation, as assessed by plasma malondialdehyde, was elevated in patients with HFpEF compared to controls (4.06 ± 0.57 vs. 1.30 ± 0.14 μM, HFpEF vs. control; Figure 4B). No notable correlations between any biomarkers of inflammation and oxidative stress and PLM responses were observed.
FIGURE 3:
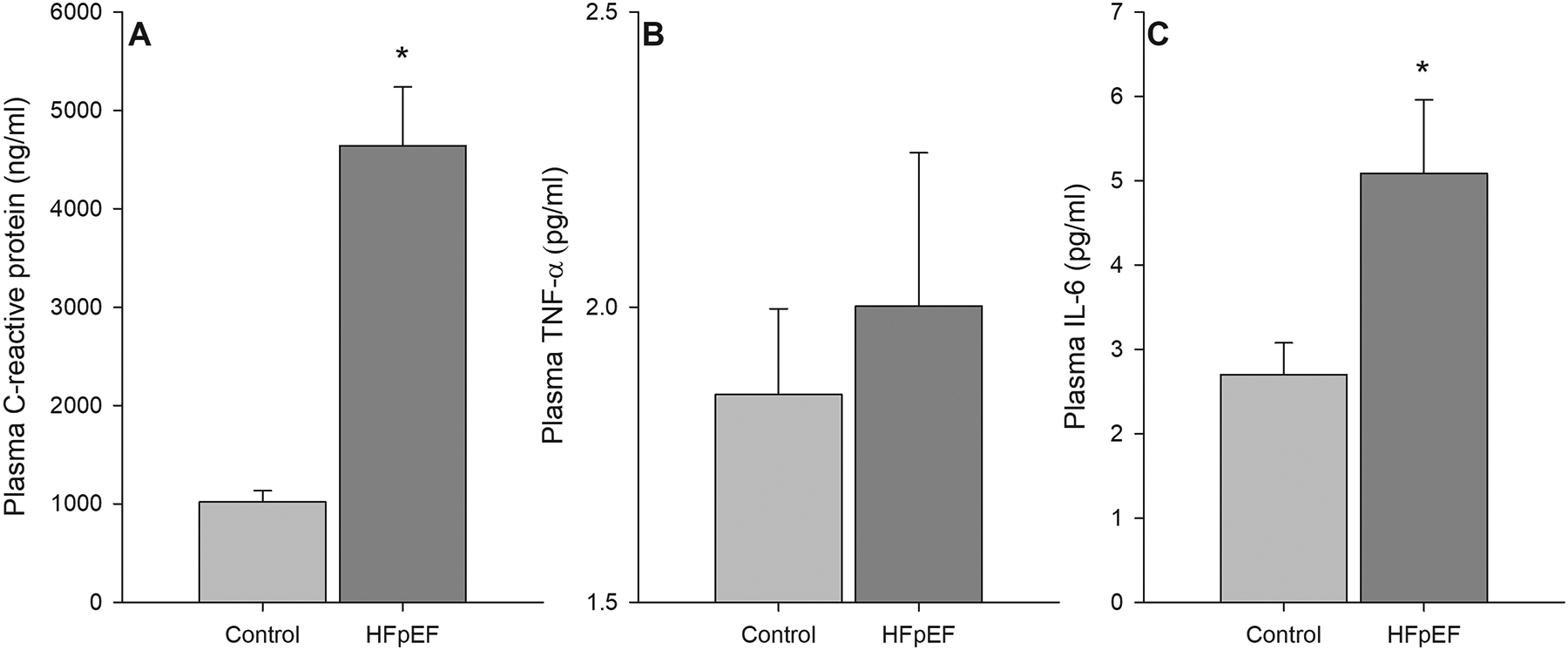
Serum biomarkers of inflammation, as assessed by C-reactive protein (panel A), Tumor Nercrosis Factor-α (panel B), and Interleukin-6 (panel C) in controls and patients with HFpEF. * Significantly different than control, P < 0.05. HFpEF n = 27; Control n = 35. Data are mean ± SEM
FIGURE 4:
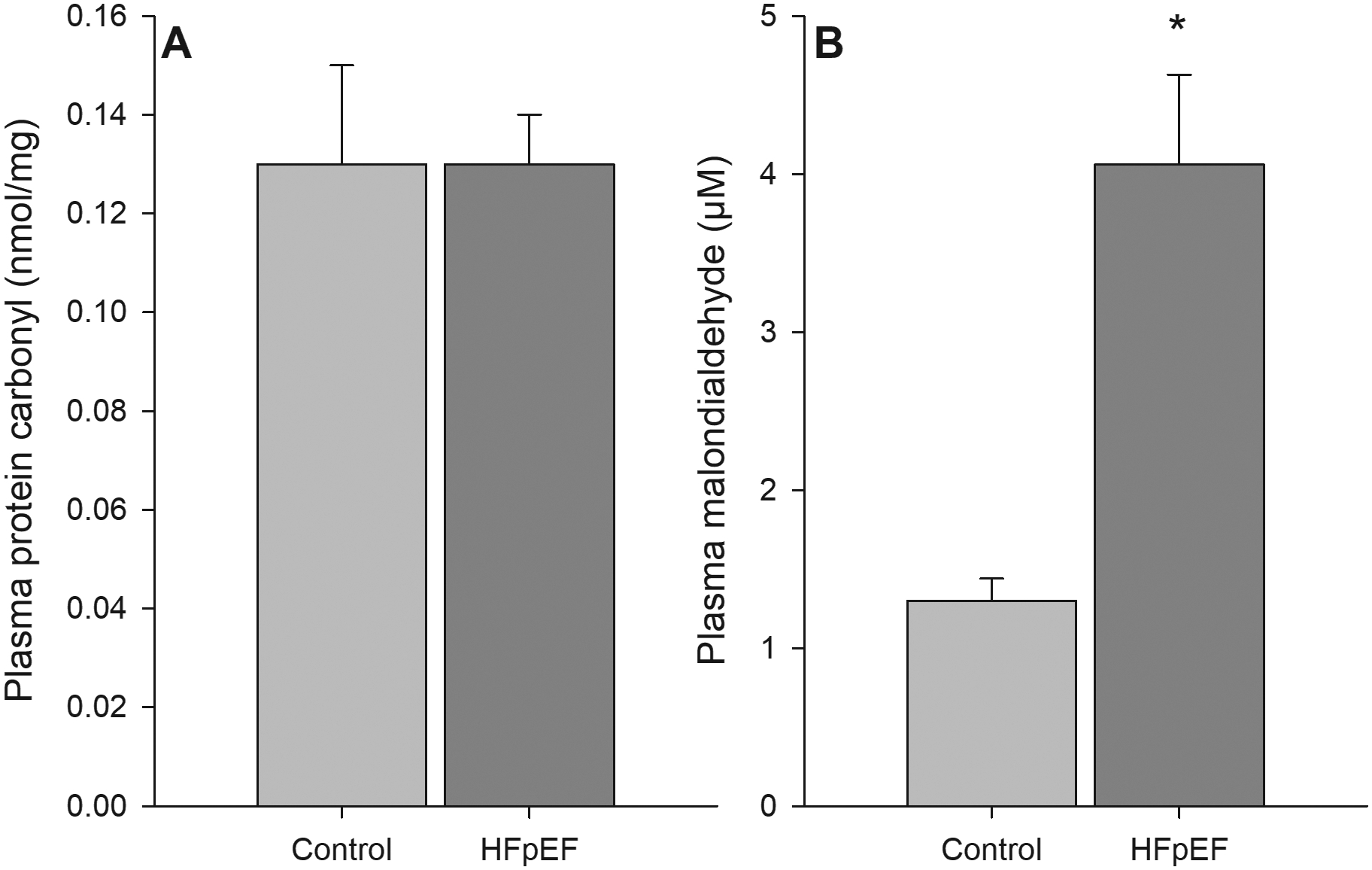
Serum biomarkers of oxidative damage, as assessed by protein carbonyl (panel A, HFpEF n = 26; Control n = 33) and malondialdehyde (panel B, HFpEF n = 27; Control n = 35) in controls and patients with HFpEF. * Significantly different than control, P < 0.05. Data are mean ± SEM
DISCUSSION
This study sought to characterize lower limb microvascular function in patients with HFpEF compared to healthy, similarly aged controls and to characterize the relationship between plasma biomarkers of inflammation, oxidative damage, and the blood flow response to PLM. We observed a significantly diminished PLM-induced hyperemic response, whether quantified as the peak change in LBF or LBFAUC, in patients with HFpEF compared to healthy, aged matched controls. In light of previous studies that have identified NO as the predominant factor governing the increase in LBF during PLM, these findings build upon an emerging line of evidence for a disease-related decline in NO bioavailability in this patient population. Patients with HFpEF also exhibited elevated biomarkers for both inflammation and oxidative damage, though a significant relationship was not observed between these biomarkers and PLM responses. Taken together, these findings provide new evidence for a decline in NO-mediated lower limb microvascular function within a milieu of systemic inflammation and redox imbalance that likely contribute to locomotor muscle microvascular function in patients with HFpEF.
Peripheral Vascular Response to PLM in HFpEF.
In contrast to HFrEF, where peripheral vascular dysfunction has been well documented, few studies have sought to evaluate peripheral vascular function using traditional testing (i.e. FMD) in patients with HFpEF, and the findings have been equivocal [14–18]. Despite this, several investigations have recently demonstrated impaired microvascular function, assessed by peripheral arterial tonometry (PAT), in patients with HFpEF [16,20,21]. Our group has previously documented attenuated upper limb vascular function in patients with HFpEF at the level of the microcirculation, assessed by RH, despite an apparent preservation of conduit artery FMD when corrected for shear stimulus [19]. These findings are in agreement with a growing body of literature supporting the importance of microvascular dysfunction and NO bioavailability to HFpEF pathophysiology [13,16,20,21]. However, recent studies have questioned the NO-dependent nature of both FMD [36–38] and RH [22] tests. Based in part on this lack of specificity for the NO pathway, our group has developed the PLM paradigm as an additional assessment of NO-dependent microvascular function in the leg.
PLM is unique in that this test directly interrogates the leg, which plays a major role in human locomotion and exercise capacity, and thus this mode of assessment is relevant to the physical challenges faced by patients with HFpEF. We have previously documented that the PLM hyperemic response is largely NO-mediated [24], and this has been corroborated in subsequent studies [39,40]. Therefore, the current study sought to utilize this novel vascular assessment to better characterize microvascular dysfunction and NO bioavailability in patients with HFpEF.
Our current finding is that PLM-induced hyperemia, as quantified by LBF and leg vascular conductanceAUC, was ~40% attenuated in patients with HFpEF, despite a preserved MAP response (Figure 1A and 2A). Additionally, we observed a ~ 25% lower peak change in LBF and vascular conductance (Figure 1B and 2B) during PLM in patients with HFpEF compared to controls. Together, these responses to PLM testing indicate a clear decrement in microvascular function in patients with HFpEF and suggest that disease-related maladaptations in the peripheral vasculature of the lower limbs may contribute to the severe exercise intolerance in this patient population [41]. This finding of impaired microvascular responsiveness in an ambulatory muscle group is particularly relevant in the context of previous studies from our group [42,43] and others [44,45] that have collectively identified a decline in exercising muscle blood flow in patients with HFpEF. Although it is important to acknowledge that exercising limb blood flow is governed by a complex combination of regulatory processes [46], it is tempting to speculate that the disease-related decline in microvascular reactivity observed in the present study is an important contributor to the overall decrement in muscle blood flow and exercise tolerance in this patient group [47]. Thus, data from the present study extends this previous work identifying microvascular dysfunction in patients with HFpEF [42–45], and provides new evidence of this phenomenon in the microvasculature of the locomotor muscle of this population.
The observed attenuation in the hyperemic response to PLM in patients with HFpEF also offers important new insight in terms of NO bioavailability in their peripheral microvasculature. Previous work from our group [24,39] and others [40] has indicated that the hyperemic response to PLM in young, healthy individuals is ~80% NO-dependent and, as such, provides a reliable non-invasive biomarker of NO bioavailability. While this may be indicative of attenuated NO bioavailability in patients with HFpEF, it is also important to note that healthy aging has, likewise, been documented to limit the NO-mediated component of the PLM-induced hyperemic response [48]. Despite this, NO still plays a small role in PLM-mediated vasodilation in healthy aged humans that might become further diminished in highly sedentary or diseased populations. Indeed, the magnitude of attenuation in the overall vasodilatory response (vascular conductance AUC) in patients with HFpEF compared to controls (Figure 2A) was similar to that previously observed in the elderly following NO synthase inhibition [48]. This indicates, albeit indirectly, that the attenuated PLM-induced hyperemic response in patients with HFpEF may be due to attenuated bioavailable NO that is distinct from that lost as a consequence of aging per se. With this in mind, our current findings highlight the NO signaling pathway as an important target for therapies to improve symptoms, and potentially outcomes, for patients with HFpEF.
Inflammation and Oxidative Stress.
In HFrEF, systemic inflammation and oxidative stress have been implicated as major contributors to the reduction in NO bioavailability [49,50] and the development of peripheral vascular dysfunction [31,51–54]. Oxidative stress can negatively impact NO bioavailability by uncoupling endothelial NOS [55] and increasing NO degradation from existing NO pools [56], which, in turn, is further exacerbated by the production of superoxide. Studies aimed at attenuating oxidative stress through antioxidant supplementation have been successful in improving endothelial function in both HFrEF and HFpEF [54,57] and have highlighted the deleterious effects of a redox imbalance [58] on endothelial function.
In the pathogenesis of patients with HFpEF, current convention holds that aging as well non-cardiac comorbidities [11] (i.e. obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease) initiate chronic systemic inflammation, as evidenced from elevated plasma biomarkers of inflammation [12] in patients with HFpEF. This systemic inflammation may then affect myocardial remodeling and dysfunction through the retardation of the coronary microvasculature and subsequent redox imbalance. Indeed, myocardial biopsies from patients with HFpEF have revealed a clear association with microvascular endothelial inflammatory activation, oxidative stress, and attenuated NO-dependent signaling from endothelial cells to cardiomyocytes [13]. Despite this evidence for cardiac involvement, the impact of inflammation and associated oxidative stress on peripheral vascular function is not well characterized in patients with HFpEF. Thus, the present study sought to explore potential mechanisms responsible for the observed decrement in PLM-induced hyperemia in patients with HFpEF by assessing plasma biomarkers of systemic inflammation and oxidative damage.
This study documented that CRP concentrations were elevated in patients with HFpEF compared to controls (Figure 3A), which is consistent with previous findings in this patient group [57,59]. Additionally, in patients with HFpEF the pro-inflammatory cytokine IL-6 concentration (Figure 3B) was significantly elevated, while TNFα concentrations (Figure 3C) tended to be higher compared to controls, which are also consistent with previous findings in these patients [57]. An elevation in these biomarkers is particularly compelling, given that aging itself is associated with increased expression of CRP, IL-6, and TNFα [60], and antihypertensive pharmacological interventions used to treat HFpEF patients are recognized to attenuate circulating pro-inflammatory mediators [61]. Despite the robust inflammation exhibited by the patients with HFpEF, compared to similarly aged control subjects, correlation analysis failed to reveal any significant association with the PLM-induced LBF responses. Regardless, these findings highlight a pro-inflammatory vascular milieu that may result in decreased NO bioavailability and/or oxidative damage. Indeed, the concentration of malondialdehyde, an indicator of lipid peroxidation, was elevated in patients with HFpEF (Figure 4B), while there was no difference in protein oxidation (assessed by plasma protein carbonyl) between the two groups (Figure 4A). The measures of oxidative damage in this study likely represent some degree of ongoing redox imbalance in patients with HFpEF. Inflammation and the resultant oxidative stress from either an increased production of reactive oxygen species or attenuated antioxidant capacity have been documented to negatively impact NO-bioavailability and endothelial function in the vasculature [54,57,58,62], suggesting that this axis of inflammation, oxidative stress, and NO may represent a potential target for therapeutic interventions. Indeed, we have recently identified the efficacy of interventions targeting oxidative stress to improve vascular outcomes in a variety of patient groups [62–64], including HFpEF [57]. However, whether interventional studies aimed at attenuating inflammation and oxidative stress could improve lower limb microvascular function in patients with HFpEF has yet to be examined, and therefore represents an area that is certainly worthy of further study.
Experimental Considerations
Given that the current study is the first to utilize PLM testing in HFpEF to examine lower limb microvascular function, the a priori decision was made to enroll healthy older controls based on the desire to establish that any observed microvascular dysfunction in patients with HFpEF was not a function of aging per se [48]. However, we acknowledge that matching patients and controls in this manner precludes consideration of how comorbidities common to the HFpEF phenotype may differentially affect microvascular function. By design, no prescribed medications were withheld on study days, which enabled the opportunity to study these patients in a “real‐world” setting. While we cannot exclude the potential for commonly prescribed HFpEF medications to influence the observed vascular responses, the persistence of marked microvascular dysfunction in the face of pharmacotherapeutics known to improve vascular outcomes [65,66] supports the relevance of our findings in further characterizing HFpEF pathophysiology. We also acknowledge that the lack of a statistically significant correlation between blood flow responses to PLM and biomarkers of inflammation and oxidative stress limits some aspects of data interpretation, which is likely due to a relatively small sample size. Finally, we recognize that systemic inflammation commonly associated with the HFpEF phenotype may impact multiple organ systems, including skeletal muscle, which may in turn influence vascular function. Inflammatory activation of locomotor muscle protein degradation and suppression of protein synthesis remains an understudied aspect of HFpEF pathophysiology that may be a root cause of muscle dysfunction and exercise intolerance in this patient population [67]. As such, therapies aimed at modifying catabolic processes and post translational modifications downstream of inflammation and oxidative stress may also improve exercise tolerance in patients with HFpEF.
Perspectives
The present study has identified a marked attenuation in PLM-induced hyperemia in patients with HFpEF compared to healthy similarly aged controls, a finding that contributes to the growing body of literature highlighting the importance of peripheral microvascular dysfunction to HFpEF pathophysiology. Additionally, the current study extends prior findings of microvascular function in the forearm [19] to the lower limb, which provides new insight into functional limitations related to activities of daily living in these patients. The observation that biomarkers of inflammation and oxidative damage are elevated in patients with HFpEF, combined with evidence that PLM-induced hyperemia is largely NO mediated [24], highlights the potential of the inflammation-oxidative stress-NO axis as a potential target for therapies that may favorably affect microvascular health and, ultimately, improve clinical status in this patient group. Indeed, the NO pathway has been targeted in several recent clinical trials in HFpEF, primarily using NO precursors that utilize the non-enzymatic nitrate-nitrite-NO pathway [68]. Unfortunately, this approach of supplementing the “alternative pool” of bioactive NO does not address the underlying mechanisms contributing to the disease-related dysfunction in endogenous, enzymatic NO signaling in patients with HFpEF. Thus, it is conceivable that pharmacologic interventions aimed at modulating specific steps in the NO-GC-CGMP signaling cascade, including administration of NO substrate (i.e. L-Citrulline), eNOS cofactors (i.e. tetrahydrobiopterin, BH4) or sGC stimulators, will prove more efficacious in patients with HFpEF.
Supplementary Material
NOVELTY and SIGNIFICANCE.
What is New?
This study has demonstrated, for the first time, a clear decrement in lower limb microvascular dysfunction in the presence of elevated markers of inflammation and oxidative stress in patients with heart failure with preserved ejection fraction (HFpEF).
What is Relevant?
The passive leg movement test is a non-invasive assessment of microvascular function that is predominantly nitric oxide (NO)-mediated. Therefore, the observed microvascular dysfunction in HFpEF patients may be due to decreased bioavailable NO that is distinct from that lost as a consequence of aging per se.
Summary.
This study provides new evidence for a decline in NO-mediated lower limb microvascular function within a milieu of systemic inflammation and redox imbalance that likely contribute to locomotor muscle microvascular function in patients with HFpEF.
Acknowledgements
The authors thank all subjects for their cheerful participation in the study.
Funding Sources
This study was funded in part by the National Institutes of Health (R01 HL118313, D.W.W.; R56 AG057584, R.S.R; T32 HL139451, K.B.) and the U.S. Department of Veterans Affairs (RX001311 and CX002152, D.W.W.; E6910-R, E1697-R, E1433-P, E9275-L, and E1572-P R.S.R).
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011; 123:933–944. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355:260–269. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. J Am Med Assoc 2006; 296:2209–2216. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N Engl J Med 2006; 355:251–259. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Gregory Hundley W, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. J Am Med Assoc 2002; 288:2144–2150. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006; 47:76–84. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure. A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007; 50:768–777. [DOI] [PubMed] [Google Scholar]
- 8.Fukuta H, Little WC. Contribution of Systolic and Diastolic Abnormalities to Heart Failure With a Normal and a Reduced Ejection Fraction. Prog Cardiovasc Dis 2007; 49:229–240. [DOI] [PubMed] [Google Scholar]
- 9.De Keulenaer G, Brutsaert DL. Response to Borlaug and Redfield. Circulation 2011; 123:2014. [Google Scholar]
- 10.Bhuiyan T, Maurer MS. Heart Failure with Preserved Ejection Fraction: Persistent Diagnosis, Therapeutic Enigma. Curr Cardiovasc Risk Rep 2011; 5:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62:263–271. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ, Kitzman DW, Borlaug BA, Van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation 2016; 134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Hear Fail 2016; 4:312–324. [DOI] [PubMed] [Google Scholar]
- 14.Farrero M, Blanco I, Batlle M, Santiago E, Cardona M, Vidal B, et al. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Hear Fail 2014; 7:791–798. [DOI] [PubMed] [Google Scholar]
- 15.Kishimoto S, Kajikawa M, Maruhashi T, Iwamoto Y, Matsumoto T, Iwamoto A, et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol 2017; 231:181–187. [DOI] [PubMed] [Google Scholar]
- 16.Maréchaux S, Samson R, Van Belle E, Breyne J, De Monte J, Dédrie C, et al. Vascular and Microvascular Endothelial Function in Heart Failure with Preserved Ejection Fraction. J Card Fail 2016; 22:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. Journals Gerontol - Ser A Biol Sci Med Sci 2013; 68:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol - Hear Circ Physiol 2007; 292. doi: 10.1152/ajpheart.00567.2006 [DOI] [PubMed] [Google Scholar]
- 19.Lee JF, Barrett-O’Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 2016; 102:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010; 56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y, et al. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol 2013; 168:36–40. [DOI] [PubMed] [Google Scholar]
- 22.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 2013; 113:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, et al. Contribution of Nitric Oxide to Reactive Hyperemia. Hypertension 1998; 32:9–15. [DOI] [PubMed] [Google Scholar]
- 24.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, et al. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 2012; 590:1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossman MJ, Groot HJ, Garten RS, Witman MAH, Richardson RS. Vascular function assessed by passive leg movement and flow-mediated dilation: initial evidence of construct validity. Am J Physiol Hear Circ Physiol 2016; 311:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: Partitioning the contributors. J Physiol 2005; 565:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mcdaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 2010; 108:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, et al. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 2010; 588:4507–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonathan Groot H, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: Evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol - Hear Circ Physiol 2013; 304:H610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, et al. Understanding exercise-induced hyperemia: Central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol - Hear Circ Physiol 2010; 299:H1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witman MAH, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: Partitioning the impact of central and peripheral dysfunction. Int J Cardiol 2015; 178:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS, et al. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol 2016; 120:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gifford JR, Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol 2017; 123:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lew L, Liu K, Pyke K. Reliability of the hyperaemic response to passive leg movement in young, healthy women. Exp Physiol Published Online First: 27 July 2021. doi: 10.1113/ep089629 [DOI] [PubMed] [Google Scholar]
- 35.Bogert LWJ, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 2005; 90:437–446. [DOI] [PubMed] [Google Scholar]
- 36.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, Driscoll GO, et al. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. 2010; :119–126. [DOI] [PubMed] [Google Scholar]
- 37.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol - Hear Circ Physiol 2011; 301:H1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wray DW, Witman M a H, Ives SJ, McDaniel J, Trinity JD, Conklin JD, et al. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 2013; 62:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, et al. The role of nitric oxide in passive leg movement-induced vasodilatation with age: Insight from alterations in femoral perfusion pressure. J Physiol 2015; 593:3917–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: A new tool to evaluate endothelial nitric oxide function. J Physiol 2012; 590:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: More than a heart problem. J. Geriatr. Cardiol 2015; 12:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JF, Barrett-O Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, et al. Impaired Skeletal Muscle Vasodilation during Exercise in Heart Failure with Preserved Ejection Fraction. doi: 10.1016/j.ijcard.2016.02.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratchford SM, Clifton HL, la Salle DT, Broxterman RM, Lee JF, Ryan JJ, et al. Cardiovascular responses to rhythmic handgrip exercise in heart failure with preserved ejection fraction. J Appl Physiol 2020; 129:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Lewis;, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Published Online First: 2006. doi: 10.1161/CIRCULATIONAHA.106.632745 [DOI] [PubMed] [Google Scholar]
- 45.Puntawangkoon C, Kitzman DW, Kritchevsky SB, Hamilton CA, Nicklas B, Leng X, et al. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. J Cardiovasc Magn Reson 2009; 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyner MJ, Casey DP. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol Rev 2015; 95:549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole DC, Behnke BJ, Musch TI, Poole DC. The role of vascular function on exercise capacity in health and disease Graphical Abstract HHS Public Access. J Physiol 2021; 599:889–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinity JD, Jonathan Groot H, Layec G, Rossman MJ, Ives SJ, Morgan DE, et al. Passive leg movement and nitric oxide-mediated vascular function: The impact of age. Am J Physiol - Hear Circ Physiol 2015; 308:H672–H679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Searles CD. The Nitric Oxide Pathway and Oxidative Stress in Heart Failure. Congest Hear Fail 2002; 8:142–155. [DOI] [PubMed] [Google Scholar]
- 50.Wray DW, Amann M, Richardson RS. Peripheral vascular function, oxygen delivery and utilization: the impact of oxidative stress in aging and heart failure with reduced ejection fraction. Heart Fail Rev 2017; 22:149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 2005; 26:65–69. [DOI] [PubMed] [Google Scholar]
- 52.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 1992; 19:918–925. [DOI] [PubMed] [Google Scholar]
- 53.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 1991; 84:1589–1596. [DOI] [PubMed] [Google Scholar]
- 54.Witman MAH, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O’Keefe Z, et al. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension 2012; 60:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003; 111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol 2009; 4:161–177. [DOI] [PubMed] [Google Scholar]
- 57.Ratchford SM, Clifton HL, Gifford JR, LaSalle DT, Thurston TS, Bunsawat K, et al. Impact of acute antioxidant administration on inflammation and vascular function in heart failure with preserved ejection fraction. Am J Physiol Integr Comp Physiol 2019; 317:R607–R614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ives SJ, Harris RA, Witman MAH, Fjeldstad AS, Garten RS, Mcdaniel J, et al. The role of redox balance. Hypertension 2014; 63:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michowitz Y, Arbel Y, Wexler D, Sheps D, Rogowski O, Shapira I, et al. Predictive value of high sensitivity CRP in patients with diastolic heart failure. Published Online First: 2007. doi: 10.1016/j.ijcard.2007.02.037 [DOI] [PubMed] [Google Scholar]
- 60.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 2008; 7:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nobrega O, Toledo J, Moraes C, Souza V, Tonet-Furioso A, Afonso L, et al. Tailored antihypertensive drug therapy prescribed to older women attenuates circulating levels of interleukin-6 and tumor necrosis factor-α Clin Interv Aging 2015; 10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, et al. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012; 59:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Witman HMA, McDaniel J, Fjeldstad AS, Ives SJ, Zhao J, Nativi JN, et al. A differing role of oxidative stress in the regulation of central and peripheral hemodynamics during exercise in heart failure. Am J Physiol Hear Circ Physiol 2012; 303:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossman MJ, Trinity JD, Garten RS, Ives SJ, Conklin JD, Barrett-O’keefe Z, et al. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am J Physiol - Hear Circ Physiol 2015; 309:H977–H985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.S W, J KS, L JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol 2003; 23:729–736. [DOI] [PubMed] [Google Scholar]
- 66.V A, G L, T S. Effects of antihypertensive treatment on endothelial function. Curr Hypertens Rep 2011; 13:276–281. [DOI] [PubMed] [Google Scholar]
- 67.T WJ, H MJ, S Y, S E, F DE. Impaired Exercise Tolerance in Heart Failure: Role of Skeletal Muscle Morphology and Function. Curr Heart Fail Rep 2018; 15:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh P, Vijayakumar S, Kalogeroupoulos A, Butler J. Multiple Avenues of Modulating the Nitric Oxide Pathway in Heart Failure Clinical Trials. Curr. Heart Fail. Rep 2018; 15:44–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


