Abstract
Background:
Depression is frequent among youth living with HIV (YLWH). Studies suggest that manualized treatment guided by symptom measurement is more efficacious than usual care.
Setting:
This study evaluated manualized, measurement-guided depression treatment among YLWH, ages 12-24 years at thirteen United States sites of the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT).
Methods:
Using restricted randomization, sites were assigned to either a 24-week, combination cognitive behavioral therapy and medication management algorithm (COMB-R) tailored for YLWH or to Enhanced Standard of Care (ESC), which provided standard psychotherapy and medication management. Eligibility included diagnosis of nonpsychotic depression and current depressive symptoms. Arm comparisons used t-tests on site-level means.
Results:
Thirteen sites enrolled 156 YLWH, with a median of 13 participants per site (range 2–16). At baseline there were no significant differences between arms on demographic factors, severity of depression, or HIV status. The average site-level participant characteristics were: mean age of 21 years; 45% male, 61% Black, and 53% acquired HIV through perinatal transmission. At Week 24, youth at COMB-R sites, compared to ESC sites, reported significantly fewer depressive symptoms on the Quick Inventory for Depression Symptomatology Self-Report (QIDS-SR score 6.7 vs. 10.6, p=0.01) and a greater proportion in remission (QIDS-SR score ≤ 5; 47.9% vs.17.0%, p=0.01). The site mean HIV viral load and CD4 T-cell level were not significantly different between arms at Week 24.
Conclusions:
A manualized, measurement-guided psychotherapy and medication management algorithm tailored for YLWH significantly reduced depressive symptoms compared to standard care at HIV clinics.
Keywords: Major Depressive Disorder (MDD), Cognitive Behavioral Therapy (CBT), Antidepressants, Adolescents, Human Immunodeficiency Virus (HIV)
INTRODUCTION
Depression is a common psychiatric comorbid condition among youth living with HIV (YLWH), with prevalence as high as 25% [1,2,3,4,5]. Depression reduces adherence to antiretroviral treatment (ART), increases caregiver burden, increases healthcare costs, and decreases quality of life [6,7,8]. Thus, treatment of depressive disorders is essential for improving both psychiatric and medical outcomes for YLWH, especially given that ART adherence dramatically increases life expectancy [3,5].
Prior reports indicate selective serotonin reuptake inhibitors (SSRIs) are generally safe and effective in the treatment of depression in adults with HIV [9,10], and that these treatments not only improve depression outcomes, but also lead to greater ART adherence and increased CD4 T-cell counts [5]. Several psychotherapies, including Cognitive Behavior Therapy (CBT), are effective in treating depression, but therapy needs to be tailored to the unique concerns of YLWH such as chronic illness, transition to adult care, interrelated psychosocial factors, cultural and sexual diversity, and internalized and enacted stigma [11–18]. Practice guidelines, including for those living with HIV suggest that a combination of medication management and an evidenced-based psychotherapy, such as CBT, show a more rapid reduction in depressive symptoms than a single treatment modality (i.e., either psychotropic medication or psychotherapy) [19–22]. CBT may be particularly helpful at improving treatment adherence. [15, 23]. Other studies show that measured-care treatment (care decisions guided by measures of symptomatology) is more effective than care not guided by measured assessments [24–26]. A prior study implemented a combined measured-care treatment approach, adapting and assessing the feasibility and acceptability of both CBT and a medication management algorithm (MMA) for use among YLWH. The combined approach was compared to usual care in a controlled trial that included 42 YLWH, with clinically confirmed depression, at four HIV medical care clinics [27]. Participants in combination treatment, compared to usual care, were significantly (p<0.001) less likely to report significant depressive symptoms at Week 24/End of Treatment (35% vs. 90%), and at Week 48/Final Follow-up (29% vs. 93%). The generalizability of these results and the assessment of treatment impact, was limited by participation of only four sites, and a small sample size. In addition, the combination intervention required a two-day in-person training for therapists and medication prescribers, with weekly therapist group supervision, audio recording of therapy sessions for quality control, and biweekly medication prescribers’ group supervision. IMPAACT 2002 was designed to address these limitations by enrolling more YLWH and depression and HIV care sites, and reducing the training and supervision burden for clinicians delivering the combination treatment.
Study Aims
This multisite, two-arm, cluster randomized trial tested a measured-care combined treatment strategy that included a MMA and manualized CBT for YLWH, diagnosed with nonpsychotic depression. Investigators predicted that, based on the previous research, participants in the combined treatment (COMB-R) arm would have fewer depressive symptoms, and improved biological measures of health (CD4+ T-cell count and HIV-1 RNA viral load (VL)) after 24 weeks compared to those in the Enhanced Standard of Care (ESC) arm, despite the HIV experience of ESC clinicians and their potential current use of SSRIs and CBT.
METHODS
Participants
YLWH were eligible if they were aged 12-24 years, engaged in care at a participating IMPAACT site, had documented HIV-1 confirmed by medical records, a diagnosis of nonpsychotic depression [either Major Depressive Disorder (MDD), Depression not otherwise specified, or Dysthymia as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV or V)], significant symptomatology at Entry (as defined by a Quick Inventory of Depressive Symptomatology-Clinician (QIDS-C) score ≥ 11), were aware of their HIV status as determined by site staff, were English-speaking, and were able and willing to provide written informed assent/consent and written parental or guardian permission (if required by State law and/or IRB policies). YLWH were excluded from the study if they had a history of any psychotic disorders and/or Bipolar I or II Disorder, had a diagnosis of a severe alcohol or substance dependence according to DSM-V or had moderate symptoms and were experiencing withdrawal or dependence symptoms within the month prior to enrollment, had depression and/or suicidal ideation, intended to relocate from the study site, were in therapy with a non-study therapist (unless willing to switch to a study-trained therapist), and if they were in imminent danger to themselves or others.
Sites and Randomization
Sites were eligible if they had a medication prescriber, a therapist, and the ability to recruit at least eight English-speaking participants. Participating sites were randomly assigned to either COMB-R or ESC using a restricted randomization procedure to minimize participant characteristics imbalance between treatment arms. Randomization at the site level was chosen to avoid loss of fidelity or cross-talk that could occur if therapists and medication prescribers were delivering both ESC and COMB-R. A pre-study survey of sites assessed clinic characteristics, including number of youth served, viral suppression status, route of HIV infection, initial levels of depression, sex at birth, and age. A computer program generated all possible site allocations to balance clinic characteristics, and one allocation was randomly chosen (see Text, Supplemental Digital Content 1, describing the randomization process). The study protocol was approved by Institutional Review Boards at all participating sites.
To prevent selection bias, sites created an initial list of potentially eligible participants arranged in random order by the IMPAACT Statistical and Data Management Center. Sites approached YLWH in order during the first year of accrual. The list of eligible participants was exhausted after a year and the random screening approach made it difficult to enroll YLWH, therefore the protocol was modified to allow sites to approach all potentially eligible YLWH in no pre-determined order.
The Combined Treatment (MMA and CBT) Intervention (COMB-R)
The MMA, developed for the earlier depression treatment feasibility study, was based on previously used algorithms [24–27]. The algorithm specifies the order, length of time, and doses of antidepressant medications at each stage, taking into consideration prior psychotropic medication history and measurement of current symptoms. It includes considerations such as drug-drug interactions and side effects. If measured symptoms indicate only partial or no improvement, doses may be increased, other medicines added, or treatment could progress to the next stage. The first two stages of the MMA are an SSRI antidepressant, and the third stage is a non-SSRI antidepressant. Staging and augmentation strategies are based on data from randomized trials and expert consensus [24–26].
CBT decreases negative mood and unhealthy cognitions, while enhancing strengths, positive experiences, and healthy cognitions [28]. CBT was adapted for YLWH to address factors such as medical symptoms, transition from pediatric to adult care, poverty, stigma, and alienation from families, and integrated motivational interviewing (MI) to engage patients in treatment [16–18, 29, 30]. The development and feasibility of the manual for YLWH was described previously [28, 30]. In this study, CBT is comprised of three stages of treatment: (1) Psychoeducation and Motivation for Treatment - addresses the psychosocial stresses of HIV infection, and treatment of current depressive symptoms; (2) Reducing Depressive Symptoms - teaches core skills of mood monitoring, behavioral activation, reducing negative thinking, and problem-solving; (3) Achieving and Maintaining Wellness - identifies strengths and techniques to continue wellness.
Training
All participating sites had a therapist and medication prescriber who participated in training prior to study initiation. COMB-R therapists received a four-hour group video conference training which included review of the CBT manual and skills training. Video-taped illustrative sessions were viewed and discussed, as were clinical vignettes. Medication prescribers received a two-hour group video training that included instructions, review of the MMA and discussion of strategies for integration of depression symptom assessments into medication management decisions. COMB-R therapists and medication prescribers had access to, but were not required to attend monthly group supervision calls to discuss cases with members of the core protocol team. The initial online trainings were recorded, and materials and videos preserved to train new staff. COMB-R therapists and medication prescribers were able to access the materials for refresher training, as needed.
ESC arm was “enhanced” as ESC site clinicians received a two-hour training on current principles for the use of medication developed by Graham Emslie, and on psychotherapy for depression developed by Betsy Kennard, before study participation. The webinar-based training, available as a refresher, did not provide details on the MMA or the CBT used in COMB-R treatment.
Measures
Assessments:
Throughout study follow-up, participants at both COMB-R and ESC sites completed assessments via an audio computer-assisted self-interview (ACASI) or paper at the required baseline and Weeks 1, 6, 12, 24, 36, and 48 study visits (data from Weeks 36 and 48 were not available at the time of these analyses). Study visits Weeks 1-24 included sessions with therapists and medication prescribers. The baseline assessment included demographic information. Clinical and virologic data, such as VL level, medication usage, and clinic visits, were abstracted from medical records.
Primary outcome measures (all assessed at Week 24).
Depressive Symptoms:
Participants completed the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR), a 17-item scale assessing nine depressive symptoms in the past seven days. QIDS-SR is a reliable and valid measure of depression in adults and adolescents [31, 32]. Total scores range from 0-27, whereby scores of 6-10 reflect mild symptoms, 11-15 moderate, and ≥16 severe. The primary efficacy outcome measure was the Week 24 QIDS-SR score. Two derived binary outcomes were also analyzed: response to treatment (>50% decrease from baseline), and remission from depression (QIDS-SR score of ≤5).
HIV-related laboratory measures:
Quantitative VL levels (copies/mL) and CD4 T-Cell Count (cells/uL) were abstracted from the medical records at baseline and Week 24, if obtained within the visit window (+/− 14 days), otherwise blood was obtained for these analyses. Viral suppression was defined as VL <40 copies/mL, generally the lower level of detection at participating laboratories. Stage 3 CD4 T-cell count was defined as <200 cells/uL.
Secondary outcome measures:
Current prescription of any antidepressant medication (and the specific medication) and the number of psychotherapy sessions attended were abstracted from medical or study records. Safety outcomes included new (post-entry) Grade 3 or higher signs and symptoms, diagnoses, suicide attempts, and psychiatric hospitalizations [33].
Power Analyses.
Sample size computations assumed a detectable difference between the COMB-R and ESC site-level mean QIDS-SR scores of 0.8 SD units and a standard deviation of five, which corresponds to a difference of four points and would generally detect a reduction to a lower depression category (e.g. from moderate to mild). With 14 sites, seven per arm, a two-sided 0.05 significance level, an intracluster correlation coefficient (ICC) range between 0.02 and 0.16, and an average of ten evaluable participants per site (N=10), the study would have at least 90% power if the ICC was less than 0.09 and at least 80% power if the ICC was between 0.09 and 0.15, even allowing for 10% non-evaluability. A planned interim analysis, conducted approximately 18 months after study accrual began, estimated the ICC based on the entry QIDS-SR to be 0.10, within the target range.
Data Analysis
The primary analyses to address the primary and safety objectives were site-level analyses. Summary measures were calculated for each site – the mean for continuous outcome measures (QIDS-SR, CD4 T-cell count) and the percentage for dichotomous outcomes (response to treatment, remission, undetectable plasma VL) and differences between arms assessed with a t-test at Week 24 (primary outcome timepoint) and at each study visit (“cross-sectional analysis”) [34]. To facilitate site-level analyses, several entry characteristics (e.g. depression, biological outcomes, and safety outcomes) were dichotomized. For nadir CD4 T-cell count, we applied the CDC specified age-based criteria shown in the 2014 revised surveillance case definition for HIV infection table [35]. Safety data assessed the occurrence of new grade 3 or higher signs and symptoms, diagnoses, and the occurrence of any pre-defined trigger event (psychiatric hospitalization or suicide attempt) which occurred at Week 24 or prior. Data were analyzed if collected within 30 days before or after the projected scheduled study week. In general, statistical tests were two-sided with alpha of 0.05. SAS software v9.4 (SAS Institute, Cary, NC) was used for analysis.
Because of the small number of clusters per arm, the Wilcoxon rank sum test, a non-parametric test that does not rely on a normality assumption, was performed to supplement the primary analysis [34]. Sensitivity analyses repeated the primary analysis after excluding the two lowest accruing sites. Primary QIDS-SR outcomes were re-analyzed after singly imputing missing data by two methods: (1) imputing missing values with a series of conservative values according to treatment group, assigning the “better” scores to the ESC group and the “worse” scores to the COMB-R group (“tipping point imputation”); and (2) using patterns of data at prior study visits to randomly select the imputed values (“past pattern imputation”).
RESULTS
Seventeen of 21 IMPAACT-funded U.S. sites applied for participation. Of these, 14 sites were chosen to participate (Figure 1). Six COMB-R and seven ESC sites enrolled 156 YLWH between March 2017 and March 2019 (one COMB-R site, concerned about recruiting sufficient participants, withdrew after randomization but prior to enrollment), with a median of 13 per site (range 2-16). Data for this final Week 24 analysis was retrieved on February 11, 2020.
Figure 1.
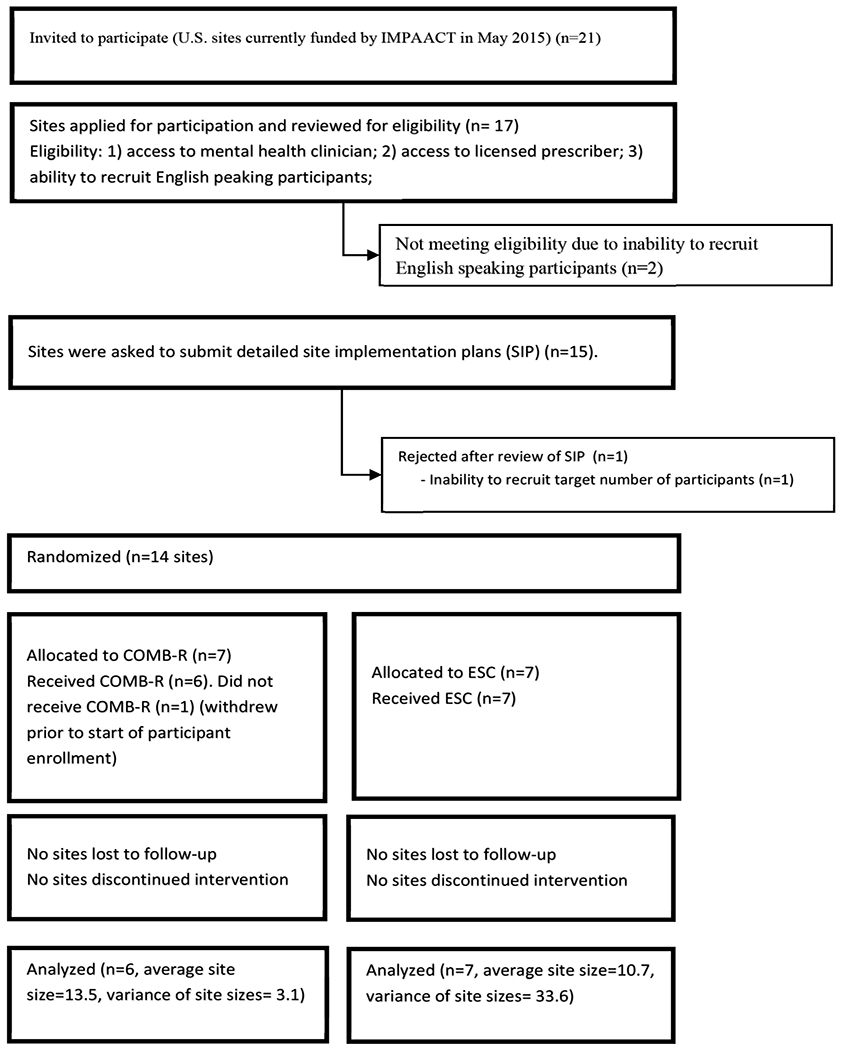
CONSORT Diagram for IMPAACT 2002
At baseline, site-level analysis showed no significant differences between arms on demographic factors, severity of depression, or HIV status (Table 1). The average of the mean participant age of all sites was 21.4 years (21.8% ≤ 18 years); 44.7 % were male, 60.7% were black, 52.9% had acquired HIV through perinatal transmission, 47.7% had severe depressive symptoms (QIDS-C ≥16), 24.5% were prescribed psychiatric medication, nearly all were prescribed antiretroviral treatment (92.5%), and 57.5% were virally suppressed.
Table 1:
Baseline Characteristics by Site-Level
| Outcome | COMB-R (N=6) |
ESC (N=7) |
P-value |
|---|---|---|---|
|
| |||
| Mean (s.d.) | Mean (s.d.) | ||
| % Male | 44.9 (24.4) | 44.6 (24.5) | 0.98 |
| Mean age (yrs) at entry | 21.5 (0.6) | 21.3 (1.8) | 0.74 |
| % Younger (12-18 yrs) | 17.1 (11.0) | 25.8 (28.3) | 0.50 |
| % Black | 65.1 (40.0) | 57.0 (32.6) | 0.69 |
| % with perinatal transmission | 49.1 (26.3) | 56.2 (22.1) | 0.61 |
| Mean QIDS-C | 16.6 (1.4) | 15.1 (2.0) | 0.15 |
| Mean ordinal QIDS-C | 3.7 (0.4) | 3.5 (0.3) | 0.25 |
| % with severe QIDS-C | 53.8 (21.9) | 42.6 (32.3) | 0.49 |
| Mean QIDS-SR | 16.2 (1.3) | 14.5 (3.2) | 0.26 |
| Mean ordinal QIDS-SR | 3.6 (0.3) | 3.3 (0.8) | 0.35 |
| % with severe QIDS-SR | 55.2 (16.5) | 39.3 (32.0) | 0.30 |
| Mean log10 RNA/ml | 2.2 (0.7) | 2.1 (0.3) | 0.82 |
| % with viral suppression (< 40 copies/ml) | 59.1 (27.3) | 56.2 (10.0) | 0.81 |
| Mean CD4 count/uL | 679.2 (150.7) | 704.7 (203.8) | 0.81 |
| % with stage 3 CD4 count (<200 cells/uL) | 9.8 (11.4) | 7.9 (9.9) | 0.76 |
| % with stage 3 nadir CD4 count | 28.3 (23.1) | 25.7 (21.1) | 0.83 |
| % with stage 3 CDC class | 20.3 (29.1) | 22.0 (21.5) | 0.91 |
| % on ARVs | 93.2 (6.7) | 92.0 (18.7) | 0.88 |
| % on integrase inhibitors | 73.8 (15.7) | 66.6 (32.1) | 0.63 |
| % on psychiatric medications | 28.0 (13.2) | 21.4 (19.0) | 0.49 |
| % on 2 or more psychiatric medications | 30.0 (20.0) | 44.8 (42.2) | 0.45 |
| % on anti-depressant medications | 25.2 (9.8) | 21.4 (19.0) | 0.67 |
| % on SSRI anti-depressant medications | 20.3 (10.5) | 15.8 (16.8) | 0.58 |
Note:
indicates p-value < 0.05, t-test with equal or unequal variances as appropriate based on F-test results
Note: For analysis of dichotomous variables, entries are means (s.d.) of site-specific percentages
Note: Quick Inventory for Depression Symptomatology Clinician Report (QIDS-C) and Self-Report (QIDS-SR) is scored from 0-27 with a higher score indicating greater symptom severity: 0-5 Not depressed, 6-10 Mild, 11-15 Moderate. 16-20 Severe, 21+ Very Severe
Note: Mean ordinal QIDS-C and QIDS-SR measures are ranked from 1-5 (none to very severe) and analyzed as a continuous measure
Note: Stage 3 nadir CD4 is computed across age ranges where Stage 3 was <750 cells (at < 1 year old), <500 cells at 1-5 yrs and < 200 cells at 6+ years
Note: Denominator for percent of participants on 2 or more psychiatric medications is the number on any psychiatric medication
Note: Denominator for percent of participants on INSTIs is the number on any ARV
Retention was high, 146 (93.6%) participants were on study at the Week 24 visit.
Depressive Symptoms
At study Week 24, QIDS-SR score was available for 66 of 81 (81.5%) participants in the COMB-R group and 67 of 75 (89.3%) participants in the ESC group, CD4 count was available for 137 of 156 (87.8%) participants, and VL was available for 140 of 156 (89.7%) participants. The site-specific average QIDS-SR was lower in the COMB-R sites compared to the ESC sites at Week 24 (mean QIDS-SR score 6.7 vs. 10.6, difference −3.9, 95% CI −6.8, −0.9, p=0.01, Table 2; Figure 2), similar to non-parametric and sensitivity analyses (data not shown). The averages of the site-specific proportions of participants with response and remission were higher in the COMB-R group than in the ESC group (Table 2; Figure 3). Comparing the means of COMB-R to ESC sites, approximately 44% more (62.3% vs. 17.9%, p<0.001) had a treatment response (change from Entry to Week 24 more than a 50% decrease in QID-SR). On average, about 30% more (47.9% vs.17.0%, p=0.01) reported a remission of symptoms (QIDS-SR ≤5) at Week 24.
Table 2:
Site-Level Efficacy Primary Analysis, T-Test Results
| COMB-R (N=6) | ESC (N=7) | Difference | |||
|---|---|---|---|---|---|
|
| |||||
| Outcome | Mean (95% CI) | Mean (95% CI) | Mean difference (95% CI) | T-statistic | P-value |
| Mean QIDS-SR | 6.70 (3.71, 9.70) | 10.57 (8.80, 12.34) | −3.86 (−6.79, −0.94) | −2.91 | 0.01 * |
| % with QIDS-SR Response | 62.3 (39.0, 85.5) | 17.9 (7.0, 28.9) | 44.3 (23.1, 65.5) | 4.61 | <0.001 * |
| % with QIDS-SR Remission | 47.9 (22.7, 73.0) | 17.0 (7.2, 26.8) | 30.9 (8.9, 52.9) | 3.09 | 0.01 * |
| Mean log10 RNA copies/ml | 2.23 (1.48, 2.97) | 2.06 (1.47, 2.65) | 0.17 (−0.65, 0.99) | 0.45 | 0.66 |
| % with viral suppression (< 40 copies/ml) | 57.2 (31.2, 83.3) | 61.7 (27.9, 95.4) | −4.4 (−43.3, 34.4) | −0.25 | 0.81 |
| Mean CD4 count (copies/uL) | 703 (466, 940) | 683 (524, 842) | 19 (−223, 262) | 0.18 | 0.86 |
| % with CD4 ≥ 200 copies/uL | 88.0 (64.5, 100.0) | 92.1 (84.2, 100.0) | −4.1 (−27.6, 19.3) | −0.43 + | 0.68 |
| % with CD4 < 200 copies/uL | 12.0 (0.0, 35.5) | 7.9 (0.0, 15.8) | 4.1 (−19.3, 27.6) | 0.43 + | 0.68 |
Note:
indicates p-value < 0.05
Note:
T-test uses unequal variance based on F-test results. Blank signifies equal variances
Note: For dichotomous variables, mean value is the mean of the site-specific percentages of participants with the given response
Note: Quick Inventory for Depression Symptomatology Self-Report (QIDS-SR) is scored from 0-27 with a higher score indicating greater symptom severity: 0-5 Not depressed, 6-10 Mild, 11-15 Moderate. 16-20 Severe, 21+ Very Severe. A participant had a QIDS-SR response if their score decreased by more than 50% from baseline. Remission was defined as a score <=5
Figure 2.

Site Mean QIDS-SR by Treatment and Week. Summary statistics are based on observations representing the mean of the individual QIDS-SR scores for each site. Shaded boxes represent COMB-R sites. Unshaded boxes represent ESC sites. Box plots represent medians (bar), 25th percentile (lower limit of box) and 75th percentile (upper limit of box). The mean of the site means are shown by a small square. Whiskers are drawn to the maximum (minimum) observation below (above) the upper (lower) fence, which is 1.5*Interquartile range (IQR) above (below) the 75th (25th) percentiles. A horizontal line drawn at the value of 5 represents QIDS-SR remission, which is a score of 5 or less.
Figure 3.

Site Percent with QIDS-SR Response by Treatment and Week. Summary statistics are based on observations representing the percent of individuals at each site with a QIDS-SR response, which is a decrease of over 50% from study entry to week 24. Shaded boxes represent COMB-R sites. Unshaded boxes represent ESC sites. Box plots represent medians (bar), 25th percentile (lower limit of box) and 75th percentile (upper limit of box). The mean of the site percentages are shown by a small square. Whiskers are drawn to the maximum (minimum) observation below (above) the upper (lower) fence, which is 1.5*Interquartile range (IQR) above (below) the 75th (25th) percentiles.
Cross-sectional analyses at each study visit (Figures 2 and 3), indicate that improvement in depression for the COMB-R sites is evident at Week 12 but the full impact is not seen until Week 24 (see Table, Supplemental Digital Content 2, parametric site-specific cross-sectional analysis results).
Missing data analyses show similar results as the primary analyses (see Tables, Supplemental Digital Content 3, which shows the results of the singly imputed tipping point analysis of QIDS-SR measures at Week 24; Supplemental Digital Content 4, which shows the cross-sectional results after singly imputing missing values based on prior patterns of observed data).
Biological Outcomes
The cluster-specific average log10 quantitative VL (COMB-R of 2.2 and ESC of 2.1) and the average proportion of participants at each site with viral suppression (COMB-R of 57.2% and ESC of 61.7%) were not significantly different between treatment groups at Week 24 in parametric (Table 2), non-parametric analyses, and sensitivity analyses (data not shown).
At Week 24, the cluster-specific average CD4 T-cell counts (COMB-R of 703 and ESC of 683) and the proportion with CD4 T-cell counts less than 200 (COMB-R of 12.0% and ESC of 7.9%) were not significantly different between treatment groups in the parametric (Table 2), non-parametric analyses, and sensitivity analyses (data not shown).
Treatment Characteristics
Site-level averages showed that COMB-R participants attended more sessions over the 24-week treatment period, although not statistically significant (11.5 vs. 8.9, p=0.31). At Week 24, a site-level average of 44.9% of youth at COMB-R sites were prescribed an antidepressant medication vs. 27.5% at ESC sites (p=0.06) and use of an SSRI was significantly greater (40.7% vs. 18.4%, p=0.02) than at ESC sites. There was no difference in site level averages in attendance at the required study visits (4.7 in both arms).
Safety Events
On average by site, between 8% and 10% of participants experienced a Grade 3 or higher signs, symptoms or diagnoses after baseline. There were no statistically significant differences in these site-level averages. Four participants in COMB-R and one participant in ESC experienced a psychiatric hospitalization or suicide attempt during the 24-week follow-up. The difference between the site-level averages (5.6% COMB-R vs. 1.2% ESC) was not statistically significant (See Table, Supplemental Digital Content 5, results of the safety analysis).
DISCUSSION
Combined CBT and a MMA delivered in HIV medical care sites by existing staff out-performed standard mental health treatment and resulted in a large reduction in depressive symptoms among YLWH. The average proportion in COMB-R whose symptoms remitted was nearly 50%, comparable to data in other clinical trials of combined CBT and medication management with non-medically ill youth (37-45% at Week 24) [36–39]. These results are significant given that the study was implemented with a medically-ill group and the comparison condition was active, specialized treatment. Youth at ESC sites had access to mental health treatment and psychopharmacological management by clinicians with experience with, and commitment to, YLWH, which is likely to be more effective than treatment available in non-specialized settings.
Several other design features contributed to the study’s internal validity and are worth noting. Unlike the prior study [27] with four sites and 44 individuals, this study included thirteen sites and 156 individuals with conservative site-level analyses. Due to the restricted randomization procedure, the intervention groups did not differ on important characteristics, such as gender and age, that otherwise could have obscured the intervention’s impact. In addition, the study attempted to equalize the perceived burden of participation by having only four required study visits with a therapist and a medication prescriber during the 24 weeks after enrollment. Additional psychotherapy and/or the use of medication were optional. This rigorously designed study with high retention demonstrates that combining manualized CBT and medication algorithms guided by symptom measurement is an extremely effective intervention for depression in YLWH, although its maximal impact is not evident until 24 weeks. Existing staff can deliver these interventions successfully after a short videoconference training and available monthly telephone supervision.
By Week 24, COMB-R was associated with a greater use of antidepressants and specifically with a greater use of SSRIs. Although medication use increased in COMB-R, it is notable that over half of the youth were not prescribed antidepressants at Week 24. Altogether, these data suggest that the tailored CBT had a role in motivating some youth to begin medication and assisted many others in attaining skills to address their depressive symptoms. The extent to which these gains can be maintained will be the focus for the analysis of the final 24 weeks of follow-up.
Despite nearly all youth being prescribed ART at baseline, less than two-thirds were virally suppressed. The suppression levels and CD4 T-cell counts did not change significantly over 24 weeks and were unrelated to the treatment condition. The lack of COMB-R’s impact on health indices may reflect the multitude of barriers to ART adherence for YLWH, of which, depressive symptoms are just one. Economic hardships, limited employment and occupational opportunities, racial and sexual stigma, and family stresses all influence ART adherence [8,40]. Amelioration of depressive symptoms are unlikely to immediately change these chronic issues. With more time, the CBT skills that effectively reduce depressive symptoms could possibly improve coping with other stresses, resulting in improved ART adherence. Structural changes may be needed (e.g. improved economics, biomedical agents such as long-acting ART) in conjunction with a reduction in depressive symptoms, to allow for increased ART adherence.
Despite the strengths of this study, there are a number of limitations. The sample was recruited from HIV clinical care sites, so results may not be generalizable to all YLWH. While the rigorous design of the cluster-randomized trial balanced participant demographic characteristics, other site-specific characteristics of participants and staff may have contributed to the outcomes, such as inherent differences in skill or impact of clinicians. Using a list of randomly ordered potentially eligible participants to approach for recruitment proved unworkable after the first year, possibly introducing bias.
This study found that a combined CBT and stepped-care MMA guided by symptom measures resulted in a significant reduction in depressive symptoms for YLWH. The combined intervention was delivered in the medical care setting by existing staff who participated in a brief video training and monthly consultation, suggesting that COMB-R, tailored for YLWH could be usefully disseminated to other medical care sites. Future research will determine how to sustain evidenced-based mental health interventions in medical settings and how to extend the skills for reducing depressive symptoms to improving ART adherence and reducing VL. The extension of efficacious interventions for depression such as COMB-R to centers outside of the U.S., where the burden of HIV is greater, is also imperative.
Supplementary Material
Supplemental Digital Content 1. Text that details restricted randomization methods. docx
Supplemental Digital Content 2. Table that shows cross-sectional efficacy analysis results for the QIDS-SR measures. docx
Supplemental Digital Content 3. Table that shows the results of the week 24 singly imputed tipping point missing data analysis for all QIDS-SR measures. docx
Supplemental Digital Content 4. Table that shows the results of cross-sectional missing data analyses, after singly imputing missing data based on prior patterns of observed data. docx.
Supplemental Digital Content 5. Table that shows the results of the safety analysis. docx
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of the site investigators and staff who conducted the IMPAACT 2002 study across the United States: BronxCare Health System, Bronx, NY: Luz Holguin, LMSW; Marvin Alvarado, MD; Martha Cavallo, CPNP; Mahboobullah Mirza Baig, MBBS. Jacobi Medical Center, Bronx, NY: Michael G. Rosenberg, MD, PhD; Marlene Burey, NP; Raphaelle Auguste, RN, BSN. University of Colorado School of Medicine, Children’s Hospital Colorado, Denver, CO: Daniel Reirden, MD; Kim Pierce DNP, RN, CPNP; Carrie Chambers BSN, RN; Christine Kwon, BS. University of California, San Diego, CA: Sharon Nichols, PhD; Veronica, Figueroa, M.S; Megan Loughran, B.A. Johns Hopkins University, Baltimore, MD: Mary Anne Knott-Grasso, CRNP; Aleisha Collinson-Streng, RN, BSN; Thuy Anderson, RN, BSN; Bonnie Addison, BA. David Geffen School of Medicine at the University of California, Los Angeles, CA: Jaime G. Deville, MD; Michele F. Carter, RN; Shellye Jones, LCSW; Patricia Tan, PhD. Rush University Cook County Hospital, Chicago, IL: Mariam Aziz, MD; Maureen McNichols RN, MS, CRC; Ixchell Ortiz Estes, NP; Katy Howe, LCSW. Children’s Diagnostic and Treatment Center, Fort. Lauderdale, FL: Lisa-Gaye Robinson, MD, MPH; Patricia A. Garvie, PhD; Kathleen Graham, PharmD; Hanna Major-Wilson, ARNP. Emory University School of Medicine, Atlanta, GA: Andres Camacho-Gonzalez, MD, MSc; Chanda Graves, PhD; LaTeshia Thomas-Seaton, MS, APRN; Nisha George, MPH. St. Jude Children’s Research Hospital, Memphis, TN: Megan L. Wilkins, PhD; Colin Quillivan, MS; Shelley Ost, MD; Sandra Jones, DNP. Texas Children’s Hospital/Baylor College of Medicine, Houston, TX: Mary Paul, MD; Chivon McMullen-Jackson, RN, BSN, CCRP; Kathy Pitts, PhD, APRN, CPNP, MPH; Terry Raburn, RN. Stony Brook Medicine, Stony Brook, NY: Sharon Nachman, MD; Allison Eliscu, MD; Melissa Shikora, LMSW; Barsha Chakraborty. Los Angeles County and University of Southern California Medical Center, Keck School of Medicine, Alhambra, CA: Yvonne Morales, LVN; LaShonda Spencer, MD; Allison Bearden, MD.
Financial Support:
Support for this project was provided by the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). Overall support for IMPAACT has been provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. Support for this project was also provided by the Providence / Boston Center for AIDS Research, a NIH-funded program (P30 AI042853). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Poster presented at the 23d International AIDS Conference, AIDS 2020 Virtual, July 2020
Potential conflicts of interest: None declared.
REFERENCES
- 1.Brown L, Whiteley L, Harper G, et al. Psychological symptoms among 2,032 youth living with HIV: a multisite study. AIDS Patient Care STDs. 2015;29:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bing EG, Burnam M, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 3.Gaughan DM, Hughes MD, Oleske JM, et al. Psychiatric hospitalizations among children and youths with human immunodeficiency virus infection. Pediatrics. 2004;113:e544–51. [DOI] [PubMed] [Google Scholar]
- 4.Gadow KD, Angelidou K, Chernoff M, et al. Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr. 2012;33(6):456–468. doi: 10.1097/DBP.0b013e31825b8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horberg MA, Silverberg SJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:384–390. [DOI] [PubMed] [Google Scholar]
- 6.Uthman OA, Magidson JF, Safren SA, et al. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc. 2017;20(Suppl 3):21497. doi: 10.7448/IAS.20.4.21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields EL, Bogart LM, Thurston IB, et al. Qualitative comparison of barriers to antiretroviral medication adherence among perinatally and behaviorally HIV-infected youth. Qual Health Res. 2017;27(8):1177–1189. doi: 10.1177/1049732317697674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero J, Nahata MC. Use of selective serotonin-reuptake inhibitors in the treatment of depression in adults with HIV. Ann Pharmacother. 2005;39:141–145. [DOI] [PubMed] [Google Scholar]
- 10.Elliott AJ, Russo J, Roy-Byrne PP. The effect of changes in depression on health-related quality of life (HRQoL) in HIV infection. Gen Hosp Psychiatry. 2002;24(1):43–47. [DOI] [PubMed] [Google Scholar]
- 11.Parsons JT, Golub SA, Rosof E, et al. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennard BD, Brown LK, Hawkins L, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract. 2014;21(2):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney M, Robins M, Ruberu M, et al. African-American and Latino families in TADS: Recruitment and treatment considerations. Cogn Behav Pract. 2005;2:221–229. [Google Scholar]
- 14.Castro-Couch M Review of cognitive-behavioral therapies with lesbian, gay, and bisexual clients. Arch Sex Behav. 2007;36:626–627. [Google Scholar]
- 15.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3(11):e529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiener LS, Kohrt B, Battles HB, et al. The HIV experience: youth identified barriers for transitioning from pediatric to adult care. J Pediatr Psychol. 2011;36(2):141–154. doi: 10.1093/jpepsy/jsp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliam PP, Ellen JM, Leonard L, et al. Transition of adolescents with HIV to adult care: characteristics and current practices of the adolescent trials network for HIV/AIDS interventions. JANAC. 2011;22(4):283–294. doi: 10.1016/j.jana.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryscavage P, Macharia T, Patel D, et al. Linkage to and retention in care following healthcare transition from pediatric to adult HIV care, AIDS Care. 2016;28:5, 561–565.doi: 10.1080/09540121.2015.1131967. [DOI] [PubMed] [Google Scholar]
- 19.Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. practice preparation, identification, assessment, and initial management. Pediatrics. 2018;141(3):e20174081. [DOI] [PubMed] [Google Scholar]
- 20.Richardson L, Ludman E, McCauley E, et al. Collaborative care for adolescents in primary care. JAMA. 2014;312:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association (APA). Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. 2010. [Google Scholar]
- 22.American Psychiatric Association (APA). Practice Guideline for the Treatment of Patients with HIV/AIDS. 2000.
- 23.Spoelstra SL, Schueller M, Hilton M, et al. Interventions combining motivational interviewing and cognitive behaviour to promote medication adherence: a literature review. J Clin Nurs. 2015;24:1163–1173. doi: 10.1111/jocn.12738. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. [DOI] [PubMed] [Google Scholar]
- 25.Emslie GJ, Mayes T, Porta G, et al. Treatment of resistant depression in adolescents (TORDIA): week 24 outcomes. Am J Psychiatry. 2010;167(7):782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes C, Emslie GJ, Crismon M, et al. Texas children’s medication algorithm project: update for Texas consensus conference panel on medication treatment of childhood major depressive disorder. J Am Acad Child and Adolesc Psychiatry. 2007;46:667–686. [DOI] [PubMed] [Google Scholar]
- 27.Brown L, Kennard B, Emslie G, et al. Effective treatment of depressive disorders in medical clinics for adolescents and young adults living with HIV: a controlled trial. J Acquir Immune Defic Syndr. 71:38–46, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennard B, Hayley C, Hughes J, et al. CBT Treatment Manual for ATN 080. 2010. [Google Scholar]
- 29.Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York: Guilford Press. 2011. [Google Scholar]
- 30.Kennard BD, Brown LK, Hawkins L, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract. 2014;21(2):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein IH, Rush AJ, Trivedi MH, et al. Psychometric properties of the quick inventory of depressive symptomatology in adolescents. Int J Methods Psychiatr Res. 2010;19(4):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore HK, Hughes CW, Mundt JC, et al. A pilot study of an electronic, adolescent version of the quick inventory of depressive symptomatology. J Clinl Psychiatry. 2007;68:1436–40. [DOI] [PubMed] [Google Scholar]
- 33.Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, Corrected Version 2.1. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases and Division of AIDS. July, 2017. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf [Google Scholar]
- 34.Hayes RJ, Moulton LH. Cluster Randomised Trials. Chapman and Hall/CRC. 2009. [Google Scholar]
- 35.Selik RM, Mokotoff ED, Branson B, et al. Revised surveillance case definition for HIV infection — United States, 2014. MMWR. 2014;63(3):1–10. [PubMed] [Google Scholar]
- 36.March JS, Silva S, Petrycki S, et al. The treatment for adolescents with depression study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007;64:1132–44. [DOI] [PubMed] [Google Scholar]
- 37.Goodyear I, Dubicka B, Wilkinson P, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomized controlled trial. BMJ . 2007;335:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brent D, Emslie G, Clarke G, et al. The treatment of adolescents with SSRI-resistant depression (TORDIA): a comparison of switch to venlafaxine or to another SSRI, with or without additional cognitive behavioral therapy. JAMA. 2008;299:901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–59. [DOI] [PubMed] [Google Scholar]
- 40.Kahana SY, Jenkins RA, Bruce D, et al. Structural determinants of antiretroviral therapy use, HIV care attendance, and viral suppression among adolescents and young adults living with HIV. PLOS ONE. 2016;11(4):e0151106. doi: 10.1371/journal.pone.0151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Text that details restricted randomization methods. docx
Supplemental Digital Content 2. Table that shows cross-sectional efficacy analysis results for the QIDS-SR measures. docx
Supplemental Digital Content 3. Table that shows the results of the week 24 singly imputed tipping point missing data analysis for all QIDS-SR measures. docx
Supplemental Digital Content 4. Table that shows the results of cross-sectional missing data analyses, after singly imputing missing data based on prior patterns of observed data. docx.
Supplemental Digital Content 5. Table that shows the results of the safety analysis. docx


