Abstract
The so-called “resting state”, in which participants lie quietly with no particular inputs or outputs, represented a paradigm shift from conventional task-based studies in human neuroimaging. Our foray into rest was fruitful from both a scientific and methodological perspective, but at this point, how much more can we learn from rest on its own? While rest still dominates in many subfields, data from tasks have empirically demonstrated benefits, as well as the potential to provide insights about the mind in addition to the brain. I argue that we can accelerate progress in human neuroscience by deemphasizing rest in favor of more grounded experiments, including promising integrated designs that respect the prominence of self-generated activity while offering enhanced control and interpretability.
Keywords: resting state, task-based, functional connectivity, naturalistic tasks, brain-behavior prediction
From task, to rest, to a “third wave” in human neuroimaging
The phenomenon of so-called resting-state activity in the human brain has revolutionized how we acquire and analyze neuroimaging data and yielded breakthroughs in our understanding of brain function. Since structured activity in the absence of any explicit task was first described more than two decades ago, studying the brain “at rest” has elucidated principles of macroscale brain organization, shed light on the balance between spontaneous activity and evoked activity, and revealed the extent to which behaviorally relevant patterns of brain function are trait- versus state-like, among other discoveries. But how much more do we have to learn from rest alone?
Here, I argue that just as rest represented a paradigm shift from the first wave of traditional task-based approaches, the time is now ripe for another shift—this time toward a “third wave” of paradigms that marry the broadened perspective and methodological toolkit we gleaned from our foray into rest with the enhanced control and interpretability afforded by tasks. First, I critically re-examine common uses of rest and argue that moving toward task-based acquisitions (broadly defined) will accelerate progress in areas that have been dominated by rest in recent years, including characterizing brain functional organization and brain-behavior relationships. Next, I elaborate on another point in favor of task data, namely that these data can speak to brain-mind relationships in addition to, and in combination with, these two goals. Finally, I consider the most promising modern task paradigms, including integrated designs that blend the best of both the resting-state and task-based worlds.
Re-examining the supposed advantages of rest
After a somewhat slow start [1–3], resting-state acquisitions (see Glossary), in which subjects lie quietly with no externally imposed task or stimulation, have captured an impressive share of the human neuroimaging market. (An online search via dimensions.ai reveals that in 2008, the term “resting state” appeared in roughly 4 percent of all scholarly sources containing the term “fMRI” in the title or abstract [85/2,132], while in 2020 this was up to 33 percent [1,620/4,962].) Rest is convenient to collect and easy to share, making it an attractive option, especially in the age of large-scale data collection and discovery science. Compared to the highly constrained task paradigms that dominated the first wave of neuroimaging, rest has many touted benefits. But do these hold up to scrutiny?
Rest data are not unique in how they can be analyzed
First, it is important to acknowledge that a major benefit of the resting-state “second wave” was that it broke us out of the traditional analysis mode, which was dominated by task design and the general linear model, and spurred us to develop more powerful ways to find structure in high-dimensional data. Many would agree that the most popular family of approaches, functional connectivity, has revolutionized how we think about the brain [4]. As it turns out, evoked activity that is precisely locked to task timings is only the tip of the iceberg for the signals we record (Fig. 1). The explosion of methods for characterizing the remainder of the variance has opened up new fields within neuroscience and yielded fundamental insights into brain organization and the neural code.
Figure 1. From task, to rest, to a “third wave” in human neuroimaging.
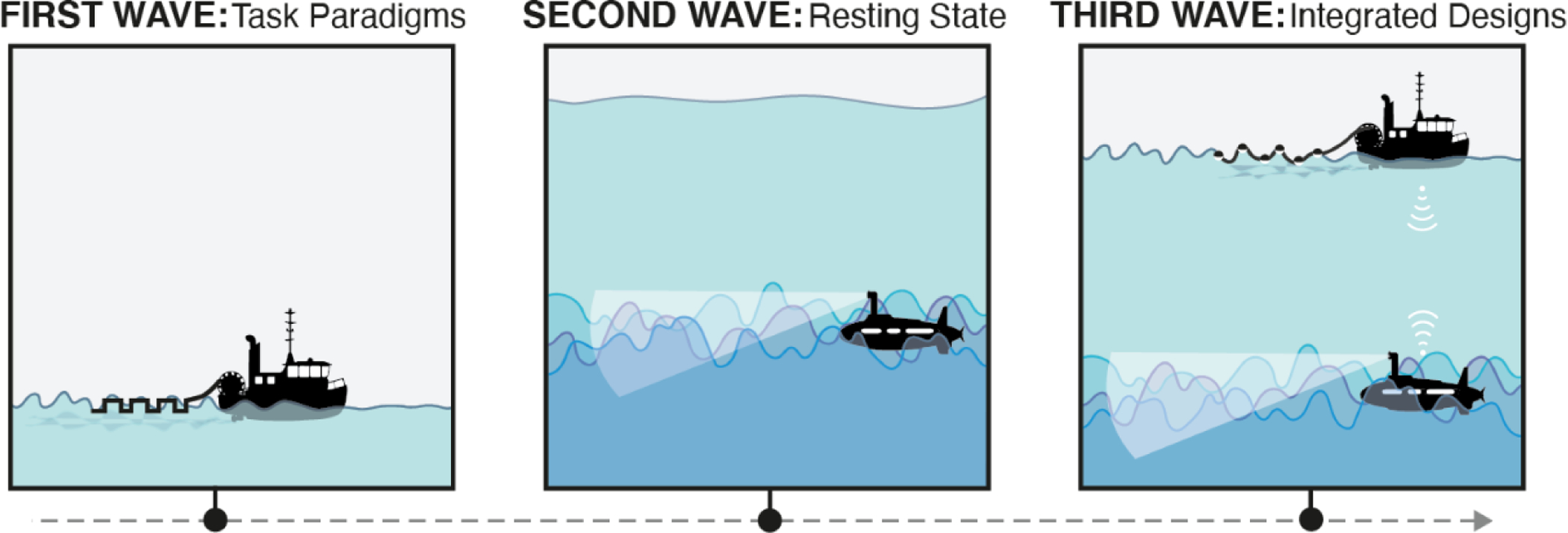
The first wave of functional neuroimaging consisted largely of investigators (represented by the boat) using highly controlled task paradigms (represented by the boxcar, denoting “task on”-“task off” periods) to attempt to isolate specific cognitive processes. While this approach could generate and localize small ripples in brain activity, ultimately, it ignored a large fraction of variance that arose from task-unrelated, seemingly spontaneous signals. In the second wave, investigators (represented by the submarine) dove headlong into the depths to characterize the spatiotemporal structure of this resting-state activity. But because we had little to anchor our observations to, it was difficult to interpret these signals in the context of ongoing cognition. The third wave calls for us to partially resurface and regain some experimental control, while still respecting the importance of self-generated activity in dynamic patterns of brain function.
But although it took the blank slate of rest to inspire these creative tools, rest is by no means the only type of data on which we can use them. Most functional connectivity techniques can be just as validly applied to data acquired during a task. In fact, task-based connectivity is in many cases more meaningful and informative than resting-state connectivity (see the following sections for specific examples, and Box 1 for considerations when using task data in functional connectivity analyses). While rest is sometimes considered uniquely valuable for multimodal and cross-species work, see Box 2 for a critical reexamination of this perspective.
Box 1. Functional connectivity on task-based data.
Some have raised concerns that task-induced coactivations lead to spurious correlations in functional connectivity analyses [115]. One proposed solution is to regress the task design (i.e., stimulus onsets) and calculate functional connectivity on the residuals of this regression [116,117]. Depending on the task, this approach might be more or less feasible; for example, it might be straightforward to model blocks in a finger-tapping task, but in a working memory task where the bulk of the processing happens between external cues (and likely with dynamics that are not well modeled by a simple boxcar), it is harder to see how one could plausibly remove all or even most task-related signal variance. Many tasks, even “simple” ones, have anticipatory, attentional, and learning and/or adaptation effects that are not captured by a typical design matrix, even if the shape of the hemodynamic response is allowed to vary across brain regions, trials, and individuals [115,118,119] (which is rarely done in practice). Despite all of this, empirically, it seems that using residualized versus non-residualized task connectivity often does not substantially alter the pattern of observed results [21,120,34]. If or how task-evoked signals are handled should depend on the ultimate goal of the study, but should not be used as a reason to avoid tasks in research using functional connectivity tools.
This issue may be another point in favor of naturalistic paradigms (e.g., movie watching, story listening). Thanks to a shared, time-locked stimulus across subjects, one can take the group-average timecourse as an index of task-evoked activity. This, being a full data-driven, largely model-free approach, likely captures much more of the task-related variance than any classic forward model (i.e., design matrix). Investigators concerned with removing as much of the task-evoked signal as possible could regress this average timecourse from each voxel or spatial region and use the residuals for functional connectivity analyses.
Box 2. Advantages of task for multi-modal and cross-species work.
For multimodal or high-resolution acquisitions, tasks provide a ground truth that enables averaging across runs or sessions, and helps parse signal into meaningful and non-meaningful components. Whereas combining rest acquisitions is nearly always limited to spatial summary statistics that collapse across time in some way (since time is not commensurate across rest sessions), task paradigms allow for combining data across sessions while retaining information in the temporal domain. Averaging can take place over individual trials in more traditional paradigms, or entire timecourses in naturalistic paradigms. In multimodal imaging (e.g., combinations of fMRI, EEG, MEG, intracranial recordings, etc.), this permits a pseudo-simultaneous acquisition when a truly simultaneous acquisition is not possible. In high-resolution imaging, which often suffers from low signal-to-noise ratio, task paradigms can boost precious signal and help tease out meaningful variability. For example, in layer-specific fMRI, where baseline differences in signal quality, hemodynamic response function, and blood volume between cortical laminae can muddy interpretations, seeing an effect of task manipulation on comparisons across layers—in other words, “differences of differences”— increases confidence that effects are neuronal and not artifactual in origin.
Should rest continue to play a role in cross-species comparisons? If resting-state activity is substantially influenced by ongoing cognition, and the mental contents of “rest” for humans and non-humans are very different, scanning at rest could obscure rather than reveal links across species. If resting-state activity is not strongly influenced by ongoing cognition, and instead mostly reflects basic physiology, then the choice of paradigm should not matter much (and would be more reason to take advantage of the increased quality of task data in humans). Despite the challenges of awake imaging in non-human primates, these animals can successfully perform many tasks inside and outside the scanner, including movie watching [121,122]. Just as in humans, functional organization is broadly similar between rest and naturalistic viewing [123], but the latter affords the opportunity to link observed activity patterns to a ground-truth stimulus, and opens the door to inter-species correlation [124]. (There is even some evidence that similar to humans, movie-watching reduces head motion relative to awake rest in non-human primates [125], so investigators might choose these paradigms for purely practical reasons.) Phenomena like replay that have been detected across species are good candidates for study at rest, but only in conjunction with task data to characterize sequence-related activity in the first place (e.g., [88]). Thus, overall, task data offer several advantages over rest for cross-species work.
Thus, a desire to use functional connectivity is no longer—and has never been—a good reason to scan at rest. It is unfortunate that terms like “resting-state networks” have become ingrained in our vocabulary when we have known from the beginning that these networks are just as present and identifiable during tasks, if not more so. Simply calling them “functional networks” would be more accurate and appropriate, and might help promote the collective acknowledgment that structured brain dynamics, and the tools used to characterize them, extend to almost any observable brain state.
Does rest provide unique insights into functional organization?
Rest data are commonly used to map the functional organization of the brain. Regions with coherent signal fluctuations are grouped into networks whose topographical properties, internal (within-network) dynamics, and external (between-network) dynamics can be further studied. Rest was critical to our initial discoveries of this macroscale network architecture, and many researchers continue to believe that rest is the best state in which to characterize functional organization. Is this true?
One assumption, which was especially common in earlier days of resting-state research but in some subfields persists, explicitly or implicitly, is that rest is a neutral backdrop, offering a view of brain functional organization that is unbiased by the influence of externally imposed tasks. (Here I refer to bias at the relatively abstract level of cognitive state; see Box 1 for a discussion of bias from task-evoked activity at the level of the signal.) But the idea that rest is a passive state has long been problematic [5–7]. In fact, rest is its own task that varies in unobserved and uncontrolled ways across sessions, individuals, and populations. Again, this is by no means a new argument [8,9]. But it bears repeating, especially with the rise of large-scale imaging efforts. From a theoretical perspective, then, rest is just as biased as any other task state, and it is biased in ways that may be less easily measured or mitigated.
Empirically, does rest outperform other acquisition states for characterizing functional organization? On one hand, functional organization shows a degree of stability such that major networks can be mapped in both groups and individuals across many distinct acquisition states [10–14]. On the other hand, network topography reconfigures to a substantial extent as subjects engage in various activities [12,15], including considerable individual-task interactions [10,16,17]. Thus, no single acquisition state can give us a full picture of brain functional organization. In theory, then, rest is no better or worse than any other task. Yet in practice, it is often easy to treat rest as though it reflects the “true” underlying functional topography (cf. how often the word “intrinsic” appears alongside descriptions of rest-derived activity patterns, whereas one rarely sees this term in conjunction with task-derived results). This assumption can muddy our interpretations and suggest a degree of stability and generalizability that is not there. A growing body of work finds that minute for minute, estimates of functional connectivity derived from one or a combination of task states are often more stable, generalizable, heritable, and meaningful (in the sense of relationships with behavior) than estimates derived from rest alone [18–21]. In one recent study, rest was a particularly poor proxy for latent (i.e., model-based average) functional organization compared with other tasks [19]. Thus, rest may in fact yield a more biased picture.
Perhaps we should flip the typical perspective—i.e., that rest gives intrinsic information, while tasks give state-level information—on its head: rest may be best for studying states rather than traits, while combining data across task paradigms may bring us closest to identifying a generalized functional architecture. But using rest acquisitions as a window into states is likely to be most useful if we combine rest acquisitions with other explanatory variables that we can measure or, ideally, manipulate; I discuss this further in the final section.
Is rest the best state to reveal brain-behavior relationships?
A second way that rest data are often used is to relate patterns of functional brain organization to phenotypic measures acquired outside the scanner (e.g., age [22,23], cognitive ability [11], personality [24,25], symptom scores [26]), and to build models that predict these measures from functional connectivity in new subjects. Most studies to date have focused on trait-like between-subject differences, though the approach can also be applied to within-subject changes [27]. While several papers that initially popularized this approach used rest data [11,22], recent work is converging on the fact that task data is, in many cases, much more sensitive and powerful for this purpose.
In one-to-one comparisons, tasks typically yield better predictions of behavior than rest [20,28–30]. The difference is striking: for predicting the same target behavior(s) in the same participants, using certain tasks can increase explained variance more than threefold [28,29]. Tasks increase sensitivity to meaningful between-subject variability not only in healthy young adults, but also in developmental [28,31], aging [32], and clinical populations [33–35]. While tailoring the task to the target behavior, akin to a “stress test”, may afford some of the best accuracy [31,36], even in-scanner tasks that are seemingly unrelated to the target behavior yield better predictions than does rest (for example, predicting fluid intelligence using data from a finger-tapping task). Tasks also enable evoked activity-based, rather than connectivity-based, predictions, which may be not only more accurate in some cases [37], but also more interpretable. Analogous to how functional organization is better estimated from several task states than from rest alone, behavior prediction models trained on data from a combination of tasks (sometimes including rest) consistently outperform models trained on a matched amount of rest data alone [19,20].
Why the advantage for task-acquired data? Better data quality may be a factor: people generally move less [38] and have an easier time staying awake during certain tasks; this effect may be even more pronounced for hard-to-scan populations (e.g., children [39,40], patients with psychiatric illness [38]). On a cognitive level, tasks also perturb ongoing neural activity in differential ways across individuals [41]. One interpretation is that tasks act as a sort of “lens” through which we can project subjects to constrain overall variability (i.e., reduce noise) while preserving meaningful differences. We do not yet fully understand how and why tasks enhance behaviorally relevant variability. But given the evidence to date, the practical view should favor task over rest for brain-behavior predictive modeling.
Can rest further our understanding of brain-mind relationships?
What has rest taught us about the mind? In contrast to the goals discussed above, many researchers may not consider understanding the mind to be the primary aim of resting-state work. However, whether or not this goal is explicitly stated, rest data are often interpreted in light of assumed mental processes (though see [42] for a counter-perspective). This is at best an inefficient and at worst a misleading way to understand how the brain gives rise to the mind.
Although they have separate origins, resting-state research and the concepts of the default mode and default-mode network are now tightly intertwined [43]. Though it took some time for “rest” to be widely accepted as a highly active state in its own right rather than a passive baseline [2,5–7], most researchers now recognize that resting-state signals reflect a mix of intrinsic functional organization and the mentation people do when left to their own devices, namely self-referential thinking: reflecting on the past, planning for the future, and ruminating on social relationships. But these activities are not inherently at odds with experimenter-imposed paradigms. In other words, default-mode regions are only less active during a task if you are not using the right task(s) [6]. Furthermore, is it meaningful to treat this collection of activities as a monolith? If we replace “rest” with “intrinsic activity” [44] (or “self-referential processes”, “mind wandering”, “daydreaming”, etc.), are we content to map that term onto a summary of resting activity patterns and leave it at that? Probably not—we would generally like to draw more specific links between brain and mind. But this requires a more fine-grained window into what the contents of the mind are at any given moment, which in turn requires some kind of experimental control, whether pre hoc (via experimental instructions, as in tasks), in the moment (via experience sampling), or post hoc (via retrospection).
If the primary goal of a given study is to map functional brain topography without directly linking features of that topography to cognition, rest does yield maps that are to some degree stable and sensitive to behavior (though perhaps less so than maps derived from more controlled task states, as discussed in the previous two sections). But there are two potential issues with using rest in this way. First, like any other acquisition state, rest can easily invite reverse inference: reasoning backward from a map of brain regions to infer the involvement of specific mental processes commonly ascribed to those regions, without directly manipulating those processes [45]. For example, aberrant resting-state connectivity in psychiatric patients in the default-mode network is often interpreted as mechanistically linked to hyper- or hypo-active self-referential processes, such as rumination or social cognition [46–48]; resting connectivity differences in the fronto-parietal network are sometimes interpreted as mechanistically linked to executive function [49,50]; and reduced connectivity between visual and prefrontal regions in dyslexia has been interpreted as mechanistically linked to failures of attention to visual stimuli [51]. But without directly manipulating or measuring these processes (along with behavior) during the experiment itself, such interpretations are not necessarily warranted. Just because an individual or group shows, for example, less coherent activity in a network at rest, does not mean they cannot coherently activate that network when cognition requires it. Furthermore, how do we know if differences at rest reflect intrinsic endophenotypes, or if certain people simply tend to engage in different mental activities while in the scanner? Either is interesting, but pure rest affords us no way to tease apart these possibilities.
Second, even resisting the temptation of reverse inference, as a field, what can we learn from topographic maps derived solely from rest data? What does it mean that certain networks occupy more, or different, real estate in some individuals than others? What are the consequences of these differences for the mind and its functions? Encouragingly, recent work has shown, for example, that resting-state spatial topography of cortical networks predicts individual cognitive and affective phenotypes [52], and that total cortical representation of the executive function network at rest is associated with both age and executive performance [53]. But while these studies reinforce the value of topographical approaches, they do not necessarily reinforce the value of rest per se: it is possible—perhaps even likely given the evidence outlined in the previous section—that applying the same tools to data acquired during task would have yielded just as robust, if not more robust, relationships with behavior. Furthermore, by relating brain measures derived from an unconstrained cognitive state (i.e., rest) to cognitive measures acquired outside the scanner, these studies can establish only very indirect associations between topography and cognitive phenotypes. They cannot, for example, inform theories of cognition by characterizing interactions between the spatial extent of a region or network and its functional properties during cognition itself (e.g., the strength of activations or multivariate representations), or suggest any compensatory mechanisms or alternative strategies that may be at play within or across individuals. To this end, other recent work has used dense sampling approaches that combine rest with hypothesis-driven task paradigms to probe the function of specific territories [14,54], finding evidence for individually distinct, topographically interdigitated networks that subserve dissociable cognitive functions (i.e., language [14], episodic projection and theory of mind [54]). These exciting studies are beginning to bridge brain and mind in individual subjects, although again, the specific value of rest for this work is unclear because these networks seem to be just as easily delineated during task (cf. [14]). In short, while these are promising avenues of inquiry, the same conclusions might have been reached (perhaps even more efficiently, or with stronger possible interpretations) by foregoing rest and using one or a combination of tasks instead. The trade-off between acquisition types will be increasingly consequential as we scale these paradigms to larger data collection efforts, balancing the need for more subjects with the need for adequate data per subject. These efforts will be necessary to understand the consequences of topographical differences for behavior within and across individuals.
The third wave: Integrated designs
The irony of calling for a pendulum swing back to task-based paradigms, the bread-and-butter of human functional neuroimaging since the early 1990s, is not lost on me. (The phrase “take to task” has never been so appropriate.) But we do not go back to tasks with the same set of narrow approaches geared toward isolating single functions and single regions; rather, we move forward into a “third wave” armed with a new suite of tools and a broader perspective on brain functional organization (Fig. 1). Third-wave designs can involve using rest-inspired analyses on data from traditional tasks to gain fresh insights into the systems-level architecture supporting those tasks, as described at the end of the preceding section. There also exist several promising avenues that blend the best of both the resting-state and task-based worlds into integrated experimental designs (Fig. 2). I outline some of these below.
Figure 2. Third-wave integrated designs that blend task- and rest-inspired approaches.
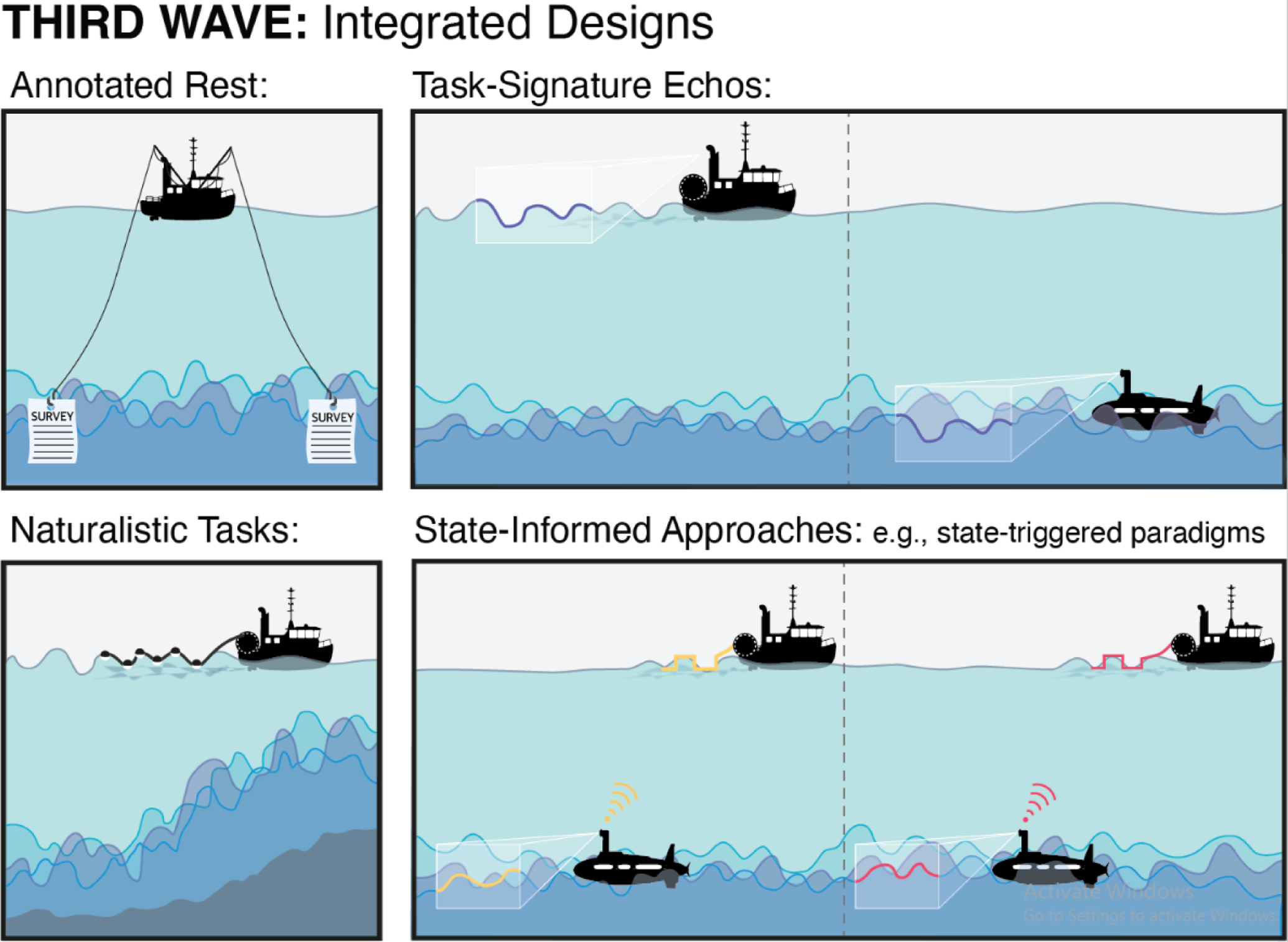
In the third wave, we have an opportunity to draw from the best of both worlds during acquisition and analysis. Four families of integrated designs are depicted. Annotated rest refers to acquiring introspection data—i.e., first-person reports of ongoing mental experiences during or after the scan—that can be linked to spatiotemporal features of brain activity. Naturalistic tasks (e.g., movie watching, story listening) are richer, more dynamic, and in some ways more ecologically valid than traditional tasks. These paradigms thus create stronger yet more organic ripples, bringing surface-level and deeper phenomena closer together. In task-signature echoes, activity patterns are characterized using data with a known task manipulation, and then rest data are mined to see if echoes of these signatures can be detected at rest, suggesting a kind of replay, reinstatement, or engagement in similar mental activities. Finally, in state-informed approaches, experimenters monitor brain state (either post hoc, or online using real-time neuroimaging), and deliver tasks at certain intervals to causally test the role of ongoing activity in shaping perception and behavior toward an external stimulus.
Introspection and “annotated rest”
One way to understand what people are doing during rest is simply to ask them [55–57]. Having participants provide first-person reports of their mental experiences, either retrospectively or in real-time, gives us anchor points for resting-state activity which increases the interpretability of within-subject dynamics and/or between-subject differences [58].
How frequently these reports should be made, and what format they should take, are open questions and depend on specific research goals (reviewed in [55]). While retrospective reports are less disruptive in the moment, they do not allow mapping between instantaneous thoughts and instantaneous brain activity patterns; however, they may still be useful for capturing broad differences (e.g., across development, in certain patient populations). In-the-moment thought probes, which sample experiences at regular, random, or adaptive intervals, allow for a more granular mapping between thoughts and brain activity [59]. In both cases, reports are “surface level” in the sense that subjects can only report experiences they are conscious of, and they depend on subjects’ capacity for accurate introspection, which may vary across individuals and populations (but can also be trained to some degree). We also risk altering the system by measuring the system. Yet even if the ultimate goal is to understand how trains of thought arise organically, injecting some declarative measures is likely the best way forward.
Movies and stories as drivers of cognition
So-called “naturalistic” paradigms, such as watching movies or listening to stories, are often much more engaging than traditional tasks, thereby driving brain activity in a way that is at once stronger and potentially closer to freeform cognition [60]. These stimuli possess features at every rung of the perceptual hierarchy, from low-level audiovisual (light and/or sound), to mid-level categories (faces, scenes), to high-level (language, affect), yielding multiplexed neural responses that are rich in both shared [61] and idiosyncratic [62,63] components.
Naturalistic paradigms may be a good replacement for rest when study goals are open-ended. Logistically, these paradigms are relatively easy to acquire: in their minimal form they require only basic presentation equipment and a way to synchronize onset time with the scanner; no complex task timing, logging, participant training, or behavior recording is needed. Thus, the barrier to entry is low for researchers and clinicians accustomed to acquiring rest and for scan centers participating in large-scale data collection efforts. (Of course, these paradigms do require researchers to choose a specific stimulus or set of stimuli, which should be informed by the research question(s), planned analyses, and population(s) under study; see [61,64,65] for theoretical and practical guidance on stimulus selection.) Data acquired during these paradigms are flexibly analyzed using tools developed for traditional task data (e.g., model-based regression) as well as those developed for rest (e.g., functional connectivity). As with traditional tasks, major functional networks and reliable brain states [66,67] are readily identified during naturalistic stimulation, if the goal is to interrogate systems-level organization. Individual differences in functional connectivity are often more stable during movie watching than during rest [68,69], and better predict behavior [30].
At the same time, the presence of a time-locked stimulus shared across participants opens the door to a unique set of tools, namely inter-subject correlation and related approaches [70,71]. These analyses are especially powerful because they require few assumptions about the structure of either the task (i.e., which features are important) or the neural response (i.e., the hemodynamic response function), and thus can recover the maximal stimulus-driven signal. Inter-subject correlation is a natural denoiser in that nearly all noise arises from artifacts within brains, so by looking across brains—i.e., using one brain as the model for another—the observed signals are almost guaranteed to be neuronal in origin [71–73]. Note that these approaches are not necessarily limited to capturing activity that is shared across all subjects; they can also capture activity patterns that are more stereotyped in certain subjects than others [63,74], which can, in turn, be related to state- or trait-related behaviors, including clinical phenotypes [75–81].
Echoes of task signatures at rest
Another type of integrated design involves using task paradigms to learn signatures of brain activity that correspond to particular mental states or activities and then searching for echoes of these same signatures in unstructured rest. As proof-of-concept with robustly observable phenomena, two recent studies learned signatures of body movements and arousal level, respectively, from task or multimodal imaging data, and found that recapitulations of these signatures could classify similar events or fluctuations during rest [82,83] (see also [84]). First characterizing, and then detecting echoes of, more nuanced cognitive states—e.g., what a participant is thinking about—is more challenging, but there has been some progress here too [85,86]. For example, in another recent study [87], researchers developed an unsupervised method to map dynamic functional connectivity onto distinct cognitive states, validated this method using task data with known state transitions, then applied this same method (but not necessarily the same states) to rest. The phenomena of replay and reactivation have been studied using this approach as well, with reports that fast, stereotyped patterns of neural activity in response to sequences are also detected at rest [88–91], where they support planning [92] and predict memory performance [93].
A directed thought may diverge in some ways from the same self-generated thought—in other words, being instructed to think of a positive memory may not be exactly the same, either phenomenologically or neurally, as having one come to mind spontaneously. Still, leveraging task paradigms with known ground truths to create a “dictionary” of states that can then serve as templates for rest may shed some initial light on the brain-mind relationship during unconstrained cognition. With this approach, we can draw on tools for large-scale meta-analysis (e.g., [94,95]) as well as reuse existing data from both task-based and rest designs, making it an efficient way to accelerate discovery and develop hypotheses for new experiments.
State-informed paradigms
The “echo detection” approaches outlined above go from task to rest. We can also flip the direction and go from rest to task, to ask how ongoing activity affects perception and behavior for an extrinsic stimulus.
There is a rich literature on how pre-stimulus brain activity predicts trial-wise accuracy and reaction time on sensory tasks [96–101]. More recent work has begun to use predefined signatures of arousal [82] in a prospective design to directly test their relevance for behavior and has reported slower reaction times and more frequent misses when stimuli are presented during a low arousal state [102]. Some studies have gone beyond unidimensional performance measures to explore not just how attentive someone is at a given moment, but what they are likely to be most attentive to, finding category-specific signatures that bias detection of, e.g., faces versus objects [103,104], or prime subsequent self-referential [105] or social [106] processing. A compelling next step is to search for ever more nuanced signatures of how upcoming stimuli will be perceived, even within categories. For example, can pre-stimulus brain activity predict whether an ambiguous social stimulus will be perceived as positive, neutral, or negative?
Another powerful extension is to close the loop by using real-time neuroimaging for state-triggered paradigms [107,108]. In these adaptive experiments, participants’ brain activity is monitored for when they enter a specific state, which then triggers a task event for which processing should be facilitated or inhibited (or otherwise affected) by that state. (This approach relies on similar technology as neurofeedback, but doesn’t necessarily involve neurofeedback that the participant is consciously aware of.) States could be predefined (i.e., supervised), learned bottom-up through initially random trial-and-error (i.e., unsupervised), or some combination, and they might be defined with respect to univariate activity, multivariate activity [109], and/or functional connectivity [110–112]. Along with brain stimulation techniques, these designs can get us closer to learning causal relationships between moment-to-moment “resting” brain activity and behavior.
Concluding Remarks
In certain circumstances, rest may still be an appropriate—or perhaps the only—choice. For example, in very young infants, comatose patients, or subjects in altered states of consciousness, resting-state acquisitions may be the most flexible and feasible. Still, experimenters should carefully examine their assumptions in choosing acquisition states, even for these special cases. For example, movies or spoken language can reveal time-locked brain activity in otherwise unresponsive individuals [113,114], potentially providing deeper clues into residual cognition than rest alone.
While rest likely still has a place, the choice to collect resting-state data should be deliberate in the same way that the choice of any other task is deliberate—driven by where it can add true value, and not just be an easy “default” (see Outstanding Questions). Big-data consortia should consider reducing the amount of rest they acquire in favor of task paradigms that are more sensitive to behavior; naturalistic paradigms may be particularly good candidates. Investigators designing smaller-scale experiments should question if and why rest suits their research goals, and if they do move forward with rest, collect additional data whenever possible (e.g., introspection) to boost explanatory power. If human neuroimaging is to continue to advance either basic science or translational tools, the next wave of progress will most likely come from a pivot toward more precise and interpretable designs.
Outstanding Questions.
How much does scan condition (i.e., task) matter for drawing out within- or between-subject variability? Can we, and should we, tailor stimulus content to a trait or state of interest? How specific is the task-behavior predictive relationship—in other words, does the best prediction performance for certain behaviors come from only a narrow range of tasks, and/or are there some tasks that are good global predictors of behavior within or across domains?
Why do tasks enhance brain-behavior relationships? How much of the improvement is due to basic data quality (e.g., increased arousal) versus more specific shifts in neural activity, connectivity, or both?
Can we design bespoke narrative stimuli that feel naturalistic to subjects, but have certain manipulations “baked in” that allow us to tap into and/or dissociate particular cognitive operations, moods, or other processes?
How can we balance the need to innovate on paradigms with the need for statistical rigor, which often requires larger sample sizes? The next wave of progress is most likely to come from novel paradigms that go beyond either rest or conventional tasks. The ongoing replication crisis in psychology and neuroscience has highlighted concerns over reproducibility and generalizability in small sample sizes. Yet investigators and funders planning large-scale data collection efforts are often reluctant to include new and untested paradigms, and tend to default to rest and a handful of well-characterized traditional tasks (which, while robust at the group level, are often less sensitive to meaningful within- and across-subject differences). How can we, individually and/or collectively, develop and convincingly validate new paradigms to include in more ambitious data-collection efforts?
How can we standardize and streamline integrated designs (e.g., naturalistic paradigms, annotated rest) to lower the barrier to entry for data collection, especially for large-scale consortium efforts?
Highlights.
Studying brain function at rest—i.e., absent any experimenter-imposed task—has grown immensely popular over the last decade(s) and now accounts for a large share of human neuroimaging research.
While resting-state research gave us new analysis methods and a broadened perspective on brain functional architecture, it also suffers from fundamental limitations that place a ceiling on the insights we can draw from it.
Data acquired during tasks have empirical benefits, including enhanced interpretability and sensitivity to brain-behavior relationships.
At this point, progress in human neuroscience is most likely to come from “third wave” paradigms that reintroduce task-like manipulations and/or pair imaging data with additional measurements, providing more anchor points for understanding the brain and mind.
Acknowledgements
E.S.F. is supported by NIH grant R00MH120257. The author thanks Nessa Bryce/Beyond Bounds Creative for figure conceptualization and design, and Nessa Bryce, Javier Gonzalez-Castillo, Meghan Meyer, and Monica Rosenberg for helpful discussion and comments on an earlier draft.
Glossary
- Default mode
Collection of internally driven mental states, commonly linked to concepts such as self-referential thinking, autobiographical memory, daydreaming, mind-wandering, and social cognition.
- Default-mode network
Functional neuroanatomy associated with the default mode, typically including posterior cingulate cortex/precuneus, medial prefrontal cortex, and lateral parietal cortex. Also called default network.
- Experience sampling
A technique that presents forced-choice or open-ended probes at various intervals that ask subjects to report their ongoing thoughts or experiences just prior to the probe.
- Functional connectivity
A family of approaches for characterizing statistical dependencies between activity timecourses from different spatial locations in the brain. Rather than the magnitude of activity in single regions (first-order), in functional connectivity, the measure of interest is relationships between regions (second-order), which capture the degree to which regions tend to fluctuate together.
- Inter-subject correlation
An analysis technique that leverages an identical time-locked stimulus across subjects to explore spatiotemporal signatures of shared processing. Typically calculated as the correlation between activity timecourses in single regions (e.g., region A’s timeseries in subject 1 to region A’s timeseries in subject 2), or the pattern of activity across regions within each timepoint (e.g., regions A-Z at timepoint 1 in subject 1, regions A-Z at timepoint 1 in subject 2). Related to inter-subject functional connectivity (e.g., region A’s timeseries in subject 1 to region B’s timeseries in subject 2).
- Reactivation
Re-expression of activity patterns that occurred during encoding, thought to support memory consolidation. Can occur in hippocampus and/or neocortex. Not necessarily sequential (unlike replay).
- Real-time neuroimaging
A brain-computer interface that continuously monitors a subject’s brain activity during scanning, and uses features of this activity for closed-loop paradigms such as brain-state-triggered task events.
- Replay
Re-occurrence of a sequence of neural activity that also occurred during behavior, but on a faster timescale. Typically associated with the hippocampus.
- Resting state
A passive acquisition state in which subjects lie still and are not instructed to think of anything in particular. Can be eyes-closed or eyes-open (in the latter case, subjects are often asked to maintain visual fixation on a central crosshair while blinking normally). Here, the term “pure rest” is used to describe an acquisition consisting purely of a neuroimaging timeseries, with no additional measurements of behavior, physiology, etc.
- Task
Any externally imposed paradigm that manipulates inputs to and/or outputs from subjects. Here, “task” generally refers to scan conditions in which subjects’ cognition is directed (or at least sampled) by the experimenter in some way. However, “task” in the common sense of “duty” can also describe pure rest, in that lying still and allowing one’s mind to wander requires a good deal of compliance and tends to encourage certain types of mental activities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biswal BB (2012) Resting state fMRI: A personal history. NeuroImage 62, 938–944 [DOI] [PubMed] [Google Scholar]
- 2.Buckner RL (2012) The serendipitous discovery of the brain’s default network. NeuroImage 62, 1137–1145 [DOI] [PubMed] [Google Scholar]
- 3.Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic resonance in medicine 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 4.McIntosh AR (2004) Contexts and catalysts. Neuroinform 2, 175–181 [DOI] [PubMed] [Google Scholar]
- 5.Andreasen NC et al. (1995) Remembering the past: Two facets of episodic memory explored with positron emission tomography. The American journal of psychiatry 152, 1576–85 [DOI] [PubMed] [Google Scholar]
- 6.Binder JR et al. (1999) Conceptual Processing during the Conscious Resting State: A Functional MRI Study. Journal of Cognitive Neuroscience 11, 80–93 [DOI] [PubMed] [Google Scholar]
- 7.Stark CEL and Squire LR (2001) When zero is not zero: The problem of ambiguous baseline conditions in fMRI. PNAS 98, 12760–12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL et al. (2013) Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16, 832–837 [DOI] [PubMed] [Google Scholar]
- 9.Morcom AM and Fletcher PC (2007) Does the brain have a baseline? Why we should be resisting a rest. NeuroImage 37, 1073–1082 [DOI] [PubMed] [Google Scholar]
- 10.Gratton C et al. (2018) Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn ES et al. (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18, 1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole MW et al. (2014) Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM et al. (2009) Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America 106, 13040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braga RM et al. (2020) Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. Journal of Neurophysiology 124, 1415–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krienen FM et al. (2014) Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society B: Biological Sciences 369, 20130526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi M et al. (2020) There is no single functional atlas even for a single individual: Functional parcel definitions change with task. NeuroImage 208, 116366. [DOI] [PubMed] [Google Scholar]
- 17.Finn ES et al. (2017) Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage 160, 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao H et al. (2021) Cross-paradigm connectivity: reliability, stability, and utility. Brain Imaging and Behavior 15, 614–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick EM et al. (2021) Latent functional connectivity underlying multiple brain states. bioRxiv DOI: 10.1101/2021.04.05.438534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott ML et al. (2019) General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. NeuroImage 189, 516–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho JW et al. (2021) Impact of concatenating fMRI data on reliability for functional connectomics. NeuroImage 226, 117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dosenbach NU et al. (2010) Prediction of individual brain maturity using fMRI. Science 329, 1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen AN et al. (2019) Evaluating the Prediction of Brain Maturity From Functional Connectivity After Motion Artifact Denoising. Cerebral Cortex 29, 2455–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois J et al. (2018) Resting-State Functional Brain Connectivity Best Predicts the Personality Dimension of Openness to Experience. Personality Neuroscience 1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu W-T et al. (2018) Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Social cognitive and affective neuroscience 13, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lake EMR et al. (2019) The Functional Brain Organization of an Individual Allows Prediction of Measures of Social Abilities Transdiagnostically in Autism and Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry 86, 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song H and Rosenberg MD (2021) Predicting attention across time and contexts with functional brain connectivity. Current Opinion in Behavioral Sciences 40, 33–44 [Google Scholar]
- 28.Greene AS et al. (2018) Task-induced brain state manipulation improves prediction of individual traits. Nature Communications 9, 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R et al. (2020) Task-induced brain connectivity promotes the detection of individual differences in brain-behavior relationships. NeuroImage 207, 116370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn ES and Bandettini PA (2021) Movie-watching outperforms rest for functional connectivity-based prediction of behavior. NeuroImage 235, 117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheinost D et al. (2021) Functional connectivity during frustration: a preliminary study of predictive modeling of irritability in youth. Neuropsychopharmacology DOI: 10.1038/s41386-020-00954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avelar-Pereira B et al. (2017) Age-Related Differences in Dynamic Interactions Among Default Mode, Frontoparietal Control, and Dorsal Attention Networks during Resting-State and Interference Resolution. Front. Aging Neurosci 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarpal DK et al. (2020) Context-specific abnormalities of the central executive network in first-episode psychosis: relationship with cognition. Psychological Medicine DOI: 10.1017/S0033291720004201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao H et al. (2018) Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun 9, 3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi S et al. (2021) Reward Processing in Novelty Seekers: A Transdiagnostic Psychiatric Imaging Biomarker. Biological Psychiatry DOI: 10.1016/j.biopsych.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfarb EV et al. (2020) Hippocampal seed connectome-based modeling predicts the feeling of stress. Nature Communications 11, 2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sripada C et al. (2020) Toward a “treadmill test” for cognition: Improved prediction of general cognitive ability from the task activated brain. Human Brain Mapping 41, 3186–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huijbers W et al. (2017) Less head motion during MRI under task than resting-state conditions. NeuroImage 147, 111–120 [DOI] [PubMed] [Google Scholar]
- 39.Greene DJ et al. (2018) Behavioral interventions for reducing head motion during MRI scans in children. NeuroImage 171, 234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderwal T et al. (2015) Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. NeuroImage 122, 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene AS et al. (2020) How Tasks Change Whole-Brain Functional Organization to Reveal Brain-Phenotype Relationships. Cell Reports 32, 108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laumann TO and Snyder AZ (2021) Brain activity is not only for thinking. Current Opinion in Behavioral Sciences 40, 130–136 [Google Scholar]
- 43.Callard F and Margulies DS (2011) The subject at rest: novel conceptualizations of self and brain from cognitive neuroscience’s study of the ‘resting state.’ Subjectivity 4, 227–257 [Google Scholar]
- 44.Raichle ME and Snyder AZ (2007) A default mode of brain function: A brief history of an evolving idea. NeuroImage 37, 1083–1090 [DOI] [PubMed] [Google Scholar]
- 45.Poldrack RA (2006) Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences 10, 59–63 [DOI] [PubMed] [Google Scholar]
- 46.Wise T et al. (2017) Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl Psychiatry 7, e1105–e1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilbach L et al. (2016) Transdiagnostic commonalities and differences in resting state functional connectivity of the default mode network in schizophrenia and major depression. NeuroImage: Clinical 10, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malhi GS et al. (2020) Default mode dysfunction underpins suicidal activity in mood disorders. Psychological Medicine 50, 1214–1223 [DOI] [PubMed] [Google Scholar]
- 49.Kupis L et al. (2021) Brain Dynamics Underlying Cognitive Flexibility Across the Lifespan. Cerebral Cortex DOI: 10.1093/cercor/bhab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jockwitz C et al. (2017) Influence of age and cognitive performance on resting-state brain networks of older adults in a population-based cohort. Cortex 89, 28–44 [DOI] [PubMed] [Google Scholar]
- 51.Finn ES et al. (2014) Disruption of Functional Networks in Dyslexia: A Whole-Brain, Data-Driven Analysis of Connectivity. Biological Psychiatry 76, 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong R et al. (2019) Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion. Cerebral cortex 29, 2533–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui Z et al. (2020) Individual Variation in Functional Topography of Association Networks in Youth. Neuron 106, 340–353.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiNicola LM et al. (2020) Parallel distributed networks dissociate episodic and social functions within the individual. Journal of Neurophysiology 123, 1144–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Castillo J et al. (2021) How to Interpret Resting-State fMRI: Ask Your Participants. J. Neurosci 41, 1130–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorgolewski KJ et al. (2014) A Correspondence between Individual Differences in the Brain’s Intrinsic Functional Architecture and the Content and Form of Self-Generated Thoughts. PLoS ONE 9, e97176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vatansever D et al. (2020) Distinct patterns of thought mediate the link between brain functional connectomes and well-being. Network Neuroscience 4, 637–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinon LM et al. (2019) The disentanglement of the neural and experiential complexity of self-generated thoughts: A users guide to combining experience sampling with neuroimaging data. NeuroImage 192, 15–25 [DOI] [PubMed] [Google Scholar]
- 59.Kam JWY et al. (2021) Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. PNAS 118, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonkusare S et al. (2019) Naturalistic Stimuli in Neuroscience: Critically Acclaimed. Trends in Cognitive Sciences 23, 699–714 [DOI] [PubMed] [Google Scholar]
- 61.Nastase SA et al. (2019) Measuring shared responses across subjects using intersubject correlation. Social Cognitive and Affective Neuroscience 14, 667–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang LJ et al. (2018) Endogenous variation in ventromedial prefrontal cortex state dynamics during naturalistic viewing reflects affective experience. bioRxiv DOI: 10.1101/487892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finn ES et al. (2020) Idiosynchrony: From shared responses to individual differences during naturalistic neuroimaging. NeuroImage 215, 116828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grall C and Finn ES The ‘naturalistic’ fallacy: Leveraging the power of media to drive cognition. . 27-July-(2021), PsyArXiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eickhoff SB et al. (2020) Towards clinical applications of movie fMRI. NeuroImage 217, 116860. [DOI] [PubMed] [Google Scholar]
- 66.van der Meer JN et al. (2020) Movie viewing elicits rich and reliable brain state dynamics. Nature Communications 11, 5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tseng J and Poppenk J (2020) Brain meta-state transitions demarcate thoughts across task contexts exposing the mental noise of trait neuroticism. Nature Communications 11, 3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanderwal T et al. (2017) Individual differences in functional connectivity during naturalistic viewing conditions. NeuroImage 157, 521–530 [DOI] [PubMed] [Google Scholar]
- 69.Wang J et al. (2017) Test–retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Human Brain Mapping 38, 2226–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasson U et al. (2004) Intersubject Synchronization of Cortical Activity During Natural Vision. Science 303, 1634–1640 [DOI] [PubMed] [Google Scholar]
- 71.Simony E et al. (2016) Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications 7, 12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simony E and Chang C (2020) Analysis of stimulus-induced brain dynamics during naturalistic paradigms. NeuroImage 216, 116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uddin LQ (2020) Bring the Noise: Reconceptualizing Spontaneous Neural Activity. Trends in Cognitive Sciences 24, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finn ES et al. (2018) Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nature Communications 9, 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasson U et al. (2009) Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Research 2, 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salmi J et al. (2013) The brains of high functioning autistic individuals do not synchronize with those of others. NeuroImage: Clinical 3, 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byrge L et al. (2015) Idiosyncratic Brain Activation Patterns Are Associated with Poor Social Comprehension in Autism. The Journal of Neuroscience 35, 5837–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolton TAW et al. (2018) Brain dynamics in ASD during movie-watching show idiosyncratic functional integration and segregation. Human Brain Mapping 39, 2391–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mäntylä T et al. (2018) Aberrant Cortical Integration in First-Episode Psychosis During Natural Audiovisual Processing. Biological Psychiatry 84, 655–664 [DOI] [PubMed] [Google Scholar]
- 80.Guo CC et al. (2015) Out-of-sync: disrupted neural activity in emotional circuitry during film viewing in melancholic depression. Scientific Reports 5, 11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Z et al. (2019) Individualized psychiatric imaging based on inter-subject neural synchronization in movie watching. NeuroImage DOI: 10.1016/j.neuroimage.2019.116227 [DOI] [PubMed] [Google Scholar]
- 82.Chang C et al. (2016) Tracking brain arousal fluctuations with fMRI. Proceedings of the National Academy of Sciences [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan FM et al. (2017) Decoding fMRI events in sensorimotor motor network using sparse paradigm free mapping and activation likelihood estimates. Human Brain Mapping 38, 5778–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C et al. (2016) Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. PNAS 113, 9653–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen RH et al. (2018) The Human Brain Traverses a Common Activation-Pattern State Space Across Task and Rest. Brain Connectivity 8, 429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim D et al. (2020) Spontaneously emerging patterns in human visual cortex and their functional connectivity are linked to the patterns evoked by visual stimuli. Journal of Neurophysiology 124, 1343–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Castillo J et al. (2019) Imaging the spontaneous flow of thought: Distinct periods of cognition contribute to dynamic functional connectivity during rest. NeuroImage 202, 116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higgins C et al. (2021) Replay bursts in humans coincide with activation of the default mode and parietal alpha networks. Neuron 109, 882–893.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y et al. (2019) Human Replay Spontaneously Reorganizes Experience. Cell 178, 640–652.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schuck NW and Niv Y (2019) Sequential replay of nonspatial task states in the human hippocampus. Science 364, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wittkuhn L and Schuck NW (2021) Dynamics of fMRI patterns reflect sub-second activation sequences and reveal replay in human visual cortex. Nature Communications 12, 1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Momennejad I et al. (2018) Offline replay supports planning in human reinforcement learning. eLife 7, e32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schapiro AC et al. (2018) Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nature Communications 9, 3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laird AR et al. (2013) Networks of task co-activations. NeuroImage 80, 505–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yarkoni T et al. (2011) Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baria AT et al. (2017) Initial-state-dependent, robust, transient neural dynamics encode conscious visual perception. PLOS Computational Biology 13, e1005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boly M et al. (2007) Baseline brain activity fluctuations predict somatosensory perception in humans. PNAS 104, 12187–12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hesselmann G et al. (2008) Ongoing Activity Fluctuations in hMT+ Bias the Perception of Coherent Visual Motion. J. Neurosci 28, 14481–14485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadaghiani S et al. (2009) Distributed and Antagonistic Contributions of Ongoing Activity Fluctuations to Auditory Stimulus Detection. J. Neurosci 29, 13410–13417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sadaghiani S et al. (2015) Ongoing dynamics in large-scale functional connectivity predict perception. PNAS 112, 8463–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson GJ et al. (2013) Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Human Brain Mapping 34, 3280–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodale SE et al. (2021) fMRI-based detection of alertness predicts behavioral response variability. eLife 10, e62376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hesselmann G et al. (2008) Spontaneous local variations in ongoing neural activity bias perceptual decisions. PNAS 105, 10984–10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Podvalny E et al. (2019) A dual role of prestimulus spontaneous neural activity in visual object recognition. Nature Communications 10, 3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer ML and Lieberman MD (2018) Why People Are Always Thinking about Themselves: Medial Prefrontal Cortex Activity during Rest Primes Self-referential Processing. Journal of Cognitive Neuroscience 30, 714–721 [DOI] [PubMed] [Google Scholar]
- 106.Spunt RP et al. (2015) The Default Mode of Human Brain Function Primes the Intentional Stance. Journal of Cognitive Neuroscience 27, 1116–1124 [DOI] [PubMed] [Google Scholar]
- 107.LaConte SM (2011) Decoding fMRI brain states in real-time. Neuroimage 56, 440–454 [DOI] [PubMed] [Google Scholar]
- 108.Lorenz R et al. (2016) The Automatic Neuroscientist: A framework for optimizing experimental design with closed-loop real-time fMRI. NeuroImage 129, 320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.LaConte SM et al. (2007) Real-time fMRI using brain-state classification. Human Brain Mapping 28, 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koush Y et al. (2013) Connectivity-based neurofeedback: Dynamic causal modeling for real-time fMRI. NeuroImage 81, 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monti RP et al. (2017) Real-time estimation of dynamic functional connectivity networks. Human Brain Mapping 38, 202–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheinost D et al. (2020) Connectome-based neurofeedback: A pilot study to improve sustained attention. NeuroImage 212, 116684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naci L et al. (2017) Detecting and interpreting conscious experiences in behaviorally non-responsive patients. NeuroImage 145, 304–313 [DOI] [PubMed] [Google Scholar]
- 114.Laforge G et al. (2020) Individualized assessment of residual cognition in patients with disorders of consciousness. NeuroImage: Clinical 28, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cole MW et al. (2019) Task activations produce spurious but systematic inflation of task functional connectivity estimates. NeuroImage 189, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Aidroos N et al. (2012) Top-down attention switches coupling between low-level and high-level areas of human visual cortex. PNAS 109, 14675–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fair DA et al. (2007) A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage 35, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez-Castillo J et al. (2012) Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci USA 109, 5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Handwerker DA et al. (2004) Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage 21, 1639–1651 [DOI] [PubMed] [Google Scholar]
- 120.Kraus BT et al. (2021) Network variants are similar between task and rest states. NeuroImage 229, 117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dubowitz DJ et al. (2001) Direct comparison of visual cortex activation in human and non-human primates using functional magnetic resonance imaging. Journal of Neuroscience Methods 107, 71–80 [DOI] [PubMed] [Google Scholar]
- 122.Russ BE and Leopold DA (2015) Functional MRI mapping of dynamic visual features during natural viewing in the macaque. NeuroImage 109, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu T et al. (2018) Delineating the Macroscale Areal Organization of the Macaque Cortex In Vivo. Cell Reports 23, 429–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mantini D et al. (2012) Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nature Methods 9, 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milham M et al. (2020) Accelerating the Evolution of Nonhuman Primate Neuroimaging. Neuron 105, 600–603 [DOI] [PMC free article] [PubMed] [Google Scholar]


