Abstract
We identified nine patients from four unrelated families harboring three biallelic variants in SCN1B (NM_001037.5: c.136C>T; p.[Arg46Cys], c.178C>T; p.[Arg60Cys], and c.472G>A; p.[Val158Met]). All subjects presented with early infantile epileptic encephalopathy 52 (EIEE52), a rare, severe developmental and epileptic encephalopathy featuring infantile onset refractory seizures followed by developmental stagnation or regression. Because SCN1B influences neuronal excitability through modulation of voltage-gated sodium (NaV) channel function, we examined the effects of human SCN1BR46C (β1R46C), SCN1BR60C (β1R60C), and SCN1BV158M (β1V158M) on the three predominant brain NaV channel subtypes NaV1.1 (SCN1A), NaV1.2 (SCN2A), and NaV1.6 (SCN8A). We observed a shift toward more depolarizing potentials of conductance–voltage relationships (NaV1.2/β1R46C, NaV1.2/β1R60C, NaV1.6/β1R46C, NaV1.6/β1R60C, and NaV1.6/β1V158M) and channel availability (NaV1.1/β1R46C, NaV1.1/β1V158M, NaV1.2/β1R46C, NaV1.2/β1R60C, and NaV1.6/β1V158M), and detected a slower recovery from fast inactivation for NaV1.1/β1V158M. Combined with modeling data indicating perturbation-induced structural changes in β1, these results suggest that the SCN1B variants reported here can disrupt normal NaV channel function in the brain, which may contribute to EIEE52.
Keywords: developmental and epileptic encephalopathy, early infantile epileptic encephalopathy 52, EIEE52, SCN1B, voltage-gated sodium channel
1 ∣. INTRODUCTION
In humans, inherited heterozygous SCN1B variants have been associated with mild-to-moderate epileptic disorders within the genetic epilepsy with febrile seizures plus (GEFS+) spectrum. In contrast, biallelic variants cause early infantile epileptic encephalopathy 52 (EIEE52; Online Mendelian Inheritance in Man database #617350), characterized by infantile onset refractory seizures followed by cognitive decline and neurological features such as hypotonia, spasticity, and ataxia.1-5 Only a few individuals with EIEE52 have been reported so far, and a disease-causing mechanism remains unclear. SCN1B encodes for β1, an immunoglobulinlike molecule that modulates the function of voltage-gated sodium (NaV) channels, a family of nine membrane proteins responsible for initiating and propagating action potentials.5,6 As such, mutations in β1, as well as NaV channels, have been linked to epilepsy syndromes.5,7 We report nine EIEE52 patients from four unrelated Pakistani families that harbor three SCN1B variants identified by whole exome sequencing. We examined the effects of these mutations on the biophysical properties of the ubiquitous brain NaV channel subtypes NaV1.1 (SCN1A), NaV1.2 (SCN2A), and NaV1.6 (SCN8A). Our results reveal perturbation-induced alterations in multiple channel gating parameters that can be correlated to structural changes in β1. Altogether, these data lay the foundation for further studies into a putative relationship between aberrant SCN1B function and EIEE52.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Patient ascertainment
The study was approved by the University College London Institutional Review Board. Written informed consent was obtained from parents. Nine subjects from four unrelated consanguineous families presenting with similar epileptic encephalopathies were investigated using exome sequencing.
2.2 ∣. Exome sequencing and analysis
Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DNA Blood Midi Kit as previously described.8 Priority was given to rare biallelic functional variants with an allele frequency less than .001% in public databases (gnomAD, GME Variome, Iranome, and our in-house database of 15 500 exomes), according to a plausible recessive mode of inheritance. Sanger sequencing was performed for variant validation and parental segregation.
2.3 ∣. Channel electrophysiology
Human (h) hNaV1.1 (NM_001165963.1, SCN1A), hNaV1.2 (NM_021007.2, SCN2A), hNaV1.6 (NM_014191.3, SCN8A), and hβ1 (NM_001037.5) were expressed and tested as previously described.9 Significance of all data was analyzed using two-way analysis of variance with post hoc Bonferroni correction. Individual time point values were analyzed using two-way Student t-test. Obtained values reflect the mean, and error bars reflect SEM. Additional information on data acquisition can be found in Appendix S1.
3 ∣. RESULTS
3.1 ∣. Epileptic phenotype
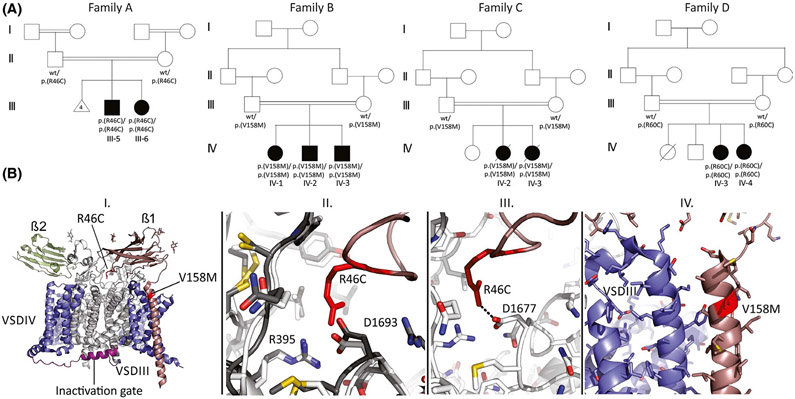
We identified nine patients from four unrelated consanguineous families of Pakistani ancestry (Families A–D; Figure 1A, Table S1). Family A consists of two affected siblings, a 9-year-old male (Patient 1) and a 5-year-old female (Patient 2), born to healthy parents. After normal psychomotor development in the first 6 months after birth, both patients started to suffer from recurrent myoclonic and generalized tonic–clonic seizures (GTCS). Subsequently, they showed developmental stagnation and regression by the age of 3 years, lacking speech and social interaction. Physical examination revealed generalized spasticity and hyperreflexia. Seizures were refractory to antiepileptic drugs (AEDs), including carbamazepine and clonazepam. Electroencephalographic (EEG) recordings at age 3 years revealed multifocal epileptic abnormalities within diffusely slowed and dysregulated cerebral activity. Family B consists of three affected children: a 6-year-old female (Patient 3) and two males (Patients 4 and 5) aged 5 and 1.5 years with healthy parents. In addition to developmental delay after birth, the patients started to suffer from GTCS by the age of 5 months followed by developmental stagnation. They were nonverbal, microcephaly was present in two subjects (Patients 4 and 5, range = −2.17 to −3.92 SD), and they all had hyperreflexia. Brain magnetic resonance imaging (MRI) was unremarkable. Their EEG recordings showed low-voltage cerebral activity intermixed with suppression–burst patterns from age 6 months up to 4 years (Figure S1). In all individuals, seizures were extremely refractory despite the use of several AEDs used alone or in combination. Family C consists of two affected females (Patients 6 and 7), deceased at the ages of 2 years and 7 months, born to healthy parents. After a regular neonatal course, both children were diagnosed with psychomotor delay and started to suffer from seizures. Subsequently, they showed developmental stagnation and regression. Seizures were resistant to AEDs. Both patients prematurely died due to unspecified epilepsy-related complications. Family D (Figure 1A, Table S1) consists of two affected females, aged 3 years (Patient 8) and 9 months (Patient 9), with healthy parents. A healthy brother and sister were deceased at 11 months due to unknown causes. Both patients suffered from focal motor seizures and were diagnosed with global developmental delay. Seizures were refractory to multidrug treatment. Neurological examination revealed hyperreflexia in both cases, and a brain MRI showed hydrocephalus in Patient IV:3. Their EEG at age 12 months revealed globally slowed cerebral activity and theta polymorphic activity intermixed with slow waves over the posterior regions (Figure S2).
FIGURE 1.
Pedigrees of the studied families and SCN1B variant modeling. (A) multigeneration pedigrees of families A–D showing parental consanguinity and history of recurrent Family A miscarriages. (B) I. Three-dimensional model of NaV1.7 channel encompassing β1 (brown) and β2 (green) subunits. The location of the R46C and V158M mutations in SCN1B (NM_001037.5) is indicated. II. Superposition of NaV1.2 (dark) and NaV1.7 (light) at the contact area for Arg46 in β1, showing that the interface is conserved. Arg46 makes multiple contacts with the channel. III. Dotted line represents an ionic interaction between Arg46 in β1 and Asp1677 in NaV1.7 (corresponding to Asp1693 in NaV1.2). IV. Structural details around the β1 residue V158 are shown
3.2 ∣. Exome sequencing results
After exome sequencing, three different missense variants in homozygosity in SCN1B were deemed the most plausible causative factor in the studied families: NM_001037.5: c.136C>T; p.(Arg46Cys) in Family A, NM_001037.5: c.472G>A; p.(Val158Met) in Families B and C, and c.178C>T; p.(Arg60Cys) in Family D. These variants are rare in public databases and affect conserved residues within or close to the immunoglobulin domain (Figure 1B, Genomic Evolutionary Rate Profiling score = 3.82–4.09). They are predicted to be pathogenic by in silico tools (Table S2). Familial segregation analysis confirmed biparental inheritance.
3.3 ∣. Effects of SCN1B variants on hNaV1.1, hNaV1.2, and hNaV1.6 channels
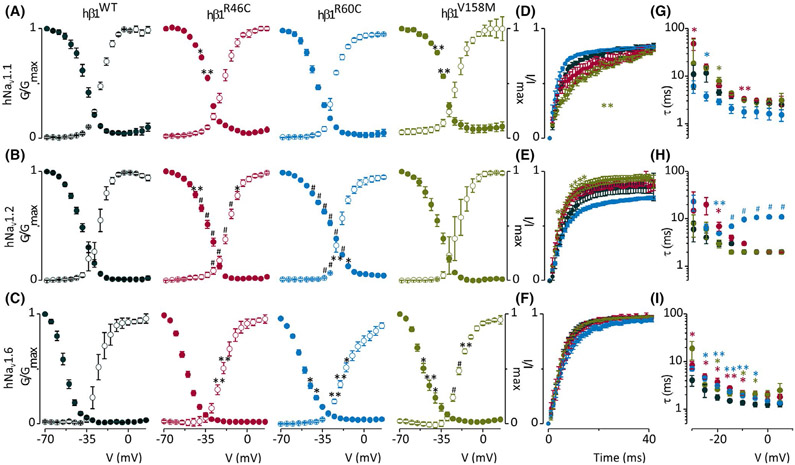
We examined the effects of all three β1-subunit mutations on NaV1.1, NaV1.2, and NaV1.6, which are predominantly expressed in the central nervous system.6,10 We recorded and compared gating parameters such as the conductance–voltage (G-V) relationship, channel availability, fast inactivation time constant (τ), and recovery from fast inactivation (RFI). We found all β1-subunits trafficked to the membrane (Figure S3) and that the G-V relationships (Figure 2, Figure S4, Table S3) of NaV1.2/β1R46C, NaV1.2/β1R60C, NaV1.6/β1R46C, NaV1.6/β1R60C, and NaV1.6/β1V158M are shifted to more depolarized potentials (±9 mV, p < .01; ±5 mV, p < .05; ±5 mV, p < .01; ±10 mV, p < .001; and ±5.5 mV, p < .001, respectively) compared to β1WT. Also, channel availability of NaV1.1/β1R46C, NaV1.1/β1V158M, NaV1.2/β1R46C, NaV1.2/β1R60C, and NaV1.6/β1V158M is shifted to more positive potentials (±3 mV, p < .05; ±3 mV, p < .05; ±6 mV, p < .01; ±8 mV, p < .01; and ±3 mV, p < .05, respectively) compared to β1WT. RFI measurements (Figure 2) of NaV1.1/β1V158M show a slower channel recovery (±4 ms, p < .01). The RFI results of NaV1.2/β1V158M show few individual time points that have a significantly faster recovery compared to β1WT, but the half-life time of the fit was not significantly different from β1WT. For τ (Figure 2), we observed several individual significant data points, although the half-life time of the fit of both SCN1B variants was not significantly different from β1WT. Surprisingly, τ values for NaV1.2/β1R60C showed a consistent U-shaped voltage dependence, with the fastest inactivation observed around −20 mV.
FIGURE 2.
Functional characterization of SCN1B missense variants. (A–C) Normalized conductance-voltage (G-V; open circles) and channel availability relationship (I-V; filled circles) of β1WT (black), β1R46C (red), β1R60C (blue), or β1V158 M (green) coexpressed with human (h) NaV1.1, NaV1.2 or NaV1.6. G-V and I-V V1/2 values are reported in Table S3. (D–F) Normalized recovery from fast inactivation using the same color scheme measured over a 40-ms timeframe using a double-pulse protocol to the maximum current of the I-V in A–C. Half-life recovery time points (ms) are presented in Table S3. Error bars are SEM with n = 5–11; *p < .05, **p < .01, #p < .001. (G–I) Rate (τ) of channel fast inactivation. Half-life time points (ms) are presented in Table S3. Error bars reflect SEM with n = 5–6; *p < .05, **p < .01, #p < .001
We then mapped SCN1B R46C/V158M mutations on available NaV channel cryogenic electron microscopic structures including NaV1.211 and NaV1.712, of which the latter is in complex with β1 (Figure 1B). A superposition of NaV1.2 and NaV1.7 shows that channel residues involved in binding β1 are conserved, allowing us to make a hybrid model of NaV1.2 with β1 bound. This shows that Arg46 is a critical residue, making ionic interactions with an Asp in NaV1.2 (D1693, corresponding to D1677 in NaV1.7). The R46C sequence variant is thus predicted to significantly weaken these interactions. Val158 is located at the top of the transmembrane helix of β1. As this residue does not directly contact the NaV channel (Figure 1B), the impact of the V158M sequence variant may involve altered membrane interactions.
4 ∣. DISCUSSION
Although the role of heterozygous SCN1B variants in temporal epilepsy and GEFS+ is well established, the scenario in subjects harboring biallelic variants is more complex. In rare cases, Dravet syndrome has been reported in these individuals2,3,8; however, a distinct association between homozygous SCN1B variants and a severe epileptic encephalopathy is now emerging. Nine individuals from seven unrelated families have been reported (Table S1), including a subject whose Dravet syndrome was reclassified to early infantile epileptic encephalopathy given the severe epileptic phenotype, cognitive decline, and premature death.2-4,8 Recurrent clinical features are early infantile onset seizures followed by psychomotor stagnation or regression, microcephaly, axial hypotonia, appendicular spasticity, and nonspecific brain atrophy. Seizure semiology and EEG features in EIEE52 are variable (Table S1). The response to AEDs is generally poor, and epilepsy remains refractory in most cases.2-4,8 Similar to Aeby et al.,1 developmental delay was present before seizure onset in Families B, C, and D. Together with the identification of neuronal pathfinding deficits in SCN1b-null mice before epilepsy onset,13 these observations are consistent with preexisting brain dysfunction and support the idea that SCN1B-related encephalopathy should be more comprehensively considered as a developmental epileptic encephalopathy. Hence, effective seizure control might exert a less dramatic impact on the overall cognitive performances in comparison to other, similar conditions.
Electrophysiological analysis of SCN1BR46C, SCN1BR60C, and SCN1BV158M showed a complex effect on neuronal NaV channel gating (Figure 2). Although there is still much to unravel about the underlying causal relationship, most SCN1B variants leading to changes in NaV channel gating or kinetics are predicted to increase neuron excitability (see Aeby et al.,1 Patino et al.,3 Chen et al.,14 and supplementary references 4-10, Appendix S1). In concert, previous studies on NaV channel variants linked to epilepsy syndromes assumed hyperexcitability as the underlying mechanism.15,16 However, recent work uncovered epilepsy-linked NaV channel mutants causing a depolarizing shift in voltage dependence of channel activation and inactivation, with or without delayed recovery from inactivation, thereby illustrating the complexities associated with defining causal connections.15,17,18 Assuming that the effects of SCN1BR46C, SCN1BR60C, and SCN1BV158M remain the same in vivo, it is not unreasonable to postulate that action potentials are also modulated by these SCN1B perturbations and contribute to EIEE52.5,7,14,17 Further studies at a neuronal network level in the brain are needed to get a grasp on the link between loss or gain of function and pathologies.
In conclusion, we show an abnormal gating of human NaV channels as a possible result of β1 perturbations, supporting the role of SCN1B variants in epileptogenesis. Furthermore, we refined the molecular and phenotypic spectrum of the severe encephalopathy caused by biallelic SCN1B variants. The results of this study also suggest that the response to AEDs in SCN1B-related disorders is challenging to predict due to the complex effects of β-subunits on NaV channel function. Additional cases will help to establish the best therapeutic approaches in SCN1B mutant patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients' families for their support and consent to the publication of this study. This research was conducted as part of the Queen Square Genomics group at University College London, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. We also thank Lisa Huckriede for help with electrophysiology experiments.
Funding information
This study was funded by the Medical Research Council (MR/S01165X/1, MR/S005021/1, G0601943), National Institute for Health Research University College London Hospitals Biomedical Research Centre, Rosetree Trust, Ataxia UK, MSA Trust, Brain Research UK, Sparks GOSH Charity, Muscular Dystrophy UK, Muscular Dystrophy Association (USA), Canadian Institutes of Health Research (PJT-148632; FVP), and a National Institutes of Health (USA) grant (R01NS091352) to F.B.
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Aeby A, Sculier C, Bouza AA, Askar B, Lederer D, Schoonjans AS, et al. SCN1B-linked early infantile developmental and epileptic encephalopathy. Ann Clin Transl Neur. 2019;6(12):2354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogiwara I, Nakayama T, Yamagata T, Ohtani H, Mazaki E, Tsuchiya S, et al. A homozygous mutation of voltage-gated sodium channel beta(I) gene SCN1B in a patient with Dravet syndrome. Epilepsia. 2012;53:e200–3. [DOI] [PubMed] [Google Scholar]
- 3.Patino GA, Claes LRF, Lopez-Santiago LF, Slat EA, Dondeti RSR, Chen CL, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;26(29):10764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramadan W, Patel N, Anazi S, Kentab AY, Bashiri FA, Hamad MH, et al. Confirming the recessive inheritance of SCN1B mutations in developmental epileptic encephalopathy. Clin Genet. 2017;92:327–31. [DOI] [PubMed] [Google Scholar]
- 5.O'Malley HA, Isom LL. Sodium channel beta subunits: emerging targets in channelopathies. Annu Rev Physiol. 2015;77:481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahern CA, Payandeh J, Bosmans F, Chanda B. The hitchhiker's guide to the voltage-gated sodium channel galaxy. J Gen Physiol. 2016;147:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan DI, Isom LL, Petrou S. Role of sodium channels in epilepsy. Cold Spring Harbor Perspect Med. 2016;6(6):a022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YP, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;17(369):1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist J, Dutton S, Diaz-Bustamante M, McPherson A, Olivares N, Kalia J, et al. Nav1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents. ACS Chem Biol. 2014; 16(9):1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitaker WR, Clare JJ, Powell AJ, Chen YH, Faull RL, Emson PC. Distribution of voltage-gated sodium channel alpha-subunit and beta-subunit mRNAs in human hippocampal formation, cor-tex, and cerebellum. J Comp Neurol. 2000;19(422):123–39. [DOI] [PubMed] [Google Scholar]
- 11.Pan XJ, Li ZQ, Huang XS, Huang GXY, Gao S, Shen HZ, et al. Molecular basis for pore blockade of human Na+ channel Nav1.2 by the mu-conotoxin KIIIA. Science. 2019;22(363):1309. [DOI] [PubMed] [Google Scholar]
- 12.Shen H, Liu D, Wu K, Lei J, Yan N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science. 2019;22(363):1303–8. [DOI] [PubMed] [Google Scholar]
- 13.Brackenbury WJ, Yuan YK, O'Malley HA, Parent JM, Isom LL. Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proc Natl Acad Sci U S A. 2013;110(3):1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Dickendesher TL, Oyama F, Miyazaki H, Nukina N, Isom LL. Floxed allele for conditional inactivation of the voltage-gated sodium channel beta 1 subunit Scn1b. Genesis. 2007;45:547–53. [DOI] [PubMed] [Google Scholar]
- 15.Begemann A, Acuna MA, Zweier M, Vincent M, Steindl K, Bachmann-Gagescu R, et al. Further corroboration of distinct functional features in SCN2A variants causing intellectual disability or epileptic phenotypes. Mol Med. 2019;25(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauxmann S, Verbeek NE, Liu YY, Zaichuk M, Muller S, Lemke JR, et al. Relationship of electrophysiological dysfunction and clinical severity in SCN2A-related epilepsies. Hum Mutat. 2018;39:1942–56. [DOI] [PubMed] [Google Scholar]
- 17.Barela AJ, Waddy SP, Lickfett JG, Hunter J, Anido A, Helmers SL, et al. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J Neurosci. 2006;8(26):2714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaman T, Abou Tayoun A, Goldberg EM. A single-center SCN8A-related epilepsy cohort: clinical, genetic, and physiologic characterization. Ann Clin Transl Neur. 2019;6:1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.