Abstract
The repair of articular cartilage defects is still challenging in the fields of orthopedics and maxillofacial surgery due to the avascular structure of articular cartilage and the limited regenerative capacity of mature chondrocytes. To provide viable treatment options, tremendous efforts have been made to develop various chondrogenically-functionalized biomaterials for cartilage tissue engineering. Peptides that are derived from and mimic the functions of chondroconductive cartilage extracellular matrix and chondroinductive growth factors, represent a unique group of bioactive agents for chondrogenic functionalization. Since they can be chemically synthesized, peptides bear better reproducibility, more stable efficacy, higher modifiability and yielding efficiency in comparison with naturally derived biomaterials and recombinant growth factors. In this review, we summarize the current knowledge in the designs of the chondroinductive/chondroconductive peptides, the underlying molecular mechanisms and their-functionalized biomaterials for cartilage tissue engineering. We also systematically compare their in-vitro and in-vivo efficacies in inducing chondrogenesis. Our vision is to stimulate the development of novel peptides and their-functionalized biomaterials for cartilage tissue engineering.
Keywords: Chondroinductive, Chondroconductive, Peptide, Biomaterial, Cartilage tissue engineering
Graphical abstract
Highlights
-
•
Chondroinductive/chondroconductive peptides and their-functionalized biomaterials are highly promising for cartilage tissue engineering.
-
•
In comparison with proteinous growth factors, peptides can be chemically synthesized with much higher efficiency, quantity and purity and can be easily modified to improve their functions and to conjugate to biomaterials.
-
•
Peptides can be divided into two categories: growth factor-derived and cell-cell adhesion molecule/ECM components-derived.
-
•
We summarize the current knowledge in the designs of the chondroinductive/chondroconductive peptides, the underlying molecular mechanisms and their-functionalized biomaterials for cartilage tissue engineering.
-
•
Further research may focus on the evaluation, design and development of more potent chondroinductive peptides and their-functionalized biomaterials. Meanwhile, more systematic studies should be performed to investigate and optimize the in-vitro and in-vivo efficacies of chondroinductive/chondroconductive peptide-based biomaterials for cartilage tissue engineering.
1. Introduction
1.1. Articular cartilage defects
Articular cartilage defects can be resulted from trauma, degeneration or systemic immune diseases, leading to the loss of articular structure and functions [1]. The avascular property of articular cartilage leads to a lack of classic healing cascade, such as coagulation, inflammation, blood invasion, and accumulation of multipotent mesenchymal stem cells (MSCs) [2], which largely limits the self-healing potential of adult articular cartilage. When defects further enlarge and affect subchondral bone tissues, the blood supplies from bone tissues may, to some extends, trigger classic healing pattern and enhance MSCs’ migration [3,4]. However, thereby-generated blood supply and migration of MSCs are too limited to facilitate the complete repair of osteochondral defects [5]. In addition, nearly no self-healing activities can be achieved from the adjacent cartilage tissue since mature chondrocytes have limited capacities of migration and proliferation [6]. Consequently, the repair of articular cartilage defects is highly challenging in the fields of orthopedics and oral and maxillofacial surgery.
In clinic, microfracture method triggers the self-healing capacity of subchondral bone tissue and bears inexpensive, short and simple nature as well as a quick recovery time, which makes it attractive for the repair of small articular cartilage defects (less than 2 cm2) [7]. However, its clinical efficacy can be potentially compromised by the formation of intralesional osteophetes and biomechanically-vulnerable fibrocartilage [8]. The small to medium defects (1–4 cm2) can be treated using mosaicplasty that harvests numerous small, cylindrical and full-thickness tissue blocks from a non-load-bearing donor site to fill cartilage defects [[9], [10], [11]]. However, such autologous osteochondral transplantation is also associated with a series of major drawbacks, e.g. the lack of available tissue, the donor site morbidity, and infections [12]. The inconsistent outcomes of microfracture prompted the development of autologous chondrocyte implantation (ACI), the gold-standard treatment for large-size cartilage defects (up to 12 cm2) or when microfracture fails [13]. This technique involves the harvest of chondrocytes from a low-weight-bearing region of the joint by biopsy punch in the first operation, in-vitro amplification of cell population, and transplantation into the debrided cartilage defects in the second operation. This treatment benefits the healing of articular cartilage defects by providing autologous and thus non-immunogenic chondrocytes with minimized complications in donor sites [8]. Clinical evidence has proved the long-term (more than 10 years) efficacy ACI in repairing large cartilage lesions [14,15]. Albeit so, ACI still bears intrinsic shortcomings, such as additional surgery and the risk of dedifferentiation and compromised chondrogenic capacity of chondrocytes during in-vitro expansion [9]. In the last decades, to provide alternative treatment options to these clinical techniques, enormous efforts have been made to develop cartilage tissue engineering techniques, which show promising application potential [16].
1.2. Cartilage tissue engineering
Cartilage tissue engineering is a promising technique that elaborately combines three major elements, such as biomaterial scaffolds, chondrogenic cells and bioactive agents [17]. Biomaterial scaffold is an indispensable element in tissue engineering. The scaffolds for cartilage tissue engineering should be biodegradable and biocompatible for biomedical application [18]. Furthermore, biomaterial scaffolds are also designed to mimic both compositions and architectures of articular cartilage extracellular matrix (ECM) so as to support the adhesion, migration, proliferation, and differentiation of chondrogenic cells [19]. The recent advances in the biomaterial scaffold for cartilage tissue engineering have already been extensively reviewed elsewhere [9,16,20]. However, the biomimetic property of biomaterial scaffolds is not sufficient yet to facilitate the complete repair of large cartilage or osteochondral defects, they still need to be functionalized by incorporating chondrogenic cells or bioactive agents or their combinations into the scaffolds [21].
Due to the lack of self-regenerative cells in the articular cartilage defects, chondrogenic cells are highly important for the repair of cartilage defects. Hitherto, a large variety of cells have been attempted for cartilage tissue engineering, such as fibroblasts, perichondrial cells, periosteal cells, genetically modified cells, chondrocytes and stem cells [22]. Bone marrow-derived MSCs (BMSCs) are commonly used for the repair of both osteochondral and chondral defects as they can release proliferative and regenerative factors directly into lesions and they are capable of differentiating into both cartilage and bone. A recent review has given a brief summary of MSCs for cartilage regeneration [23]. The authors point out that the major functions of MSCs (without chondroinductive growth factors) are to prevent chondrocyte apoptosis and delay cartilage deterioration, while they fail to induce cartilage regeneration. Consequently, various chondroinductive growth factors have been adopted to enhance the proliferation, differentiation and metabolic activity of MSCs, thereby facilitating cartilage regeneration [23].
Proteinous growth factors are also important elements for cartilage tissue engineering. The widely used growth factors include transforming growth factor-βs (TGF-βs), bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs) and insulin-like growth factors (IGFs), which have already been reviewed and discussed elsewhere [24,25]. The supplementation of growth factors can promote proliferation and differentiation of chondrogenic cells, as well as prevent hypertrophy, dedifferentiation, or transdifferentiation [26]. On the other hand, the use of these proteinous growth factors is also associated with various limitations, such as low production yield, high cost, and potential immunogenicity [27]. As promising alternatives to proteinous growth factors, peptides can be chemically synthesized, thus bearing higher yielding, lower cost and immunogenicity [25], which present an attractive group of bioactive agents to chondrogenically functionalize biomaterials for cartilage tissue engineering [23].
1.3. Peptides
Peptides are a unique class of bioactive agents to functionalize biomaterials for tissue engineering [28]. In human body, more than 7000 naturally occurring peptides have been identified as hormones, neurotransmitters, growth factors, ion channel ligands, or anti-infectives to regulate a large variety of physiological events [[29], [30], [31], [32]]. In contrast to proteinous growth factors, peptides can be chemically synthesized by solid phase peptide synthesis (SPPS), solution phase coupling, and ROP of α-amino acid NCAs, with much higher efficiency, quantity and purity [28,33]. Furthermore, peptides can be easily modified to improve their functions and to conjugate to biomaterials. These properties confer peptides very promising potential for pharmaceutical application [34]. Over the past two decades, nearly more than 60 peptide drugs have been approved worldwide [35]. A global industry analysis on peptide therapeutics in 2016 estimates the sales of peptide drugs more than 70 billion USD in 2019 with a compound annual growth rate (CAGR) of 9.1% to 2024 [36].
Peptides also show promising potential in the field of cartilage tissue engineering [37]. Through mimicking the actions of chondrogenesis-related ligands, cell-cell junction molecules and ECM compositions, a large variety of peptides have been designed to trigger desired cellular signaling pathways [[38], [39], [40], [41], [42], [43]]. These peptides are applied to functionalize biomaterial scaffolds so as to facilitate the adhesion, migration, proliferation and differentiation of chondrogenic cells [[38], [39], [40], [41], [42], [43]]. A recent review has given a brief summary of peptides for cartilage repair [21]. However, this review mainly focuses on the knowledge of cell-matrix interactions to inspire the design of new peptides. In our current review, we wish to systematically and comprehensively summarize the design, compare their in-vitro and in-vivo efficacies and analyze the underlying molecular mechanisms of the chondroinductive/chondroconductive peptides for cartilage tissue engineering.
2. The physiological properties of articular cartilage
Articular cartilage is a thin-layer, highly hydrated, aneural, avascular, and viscoelastic connective tissue, containing only one cell type — chondrocytes [9]. It covers on the epiphyseal surface of the articulating bones to provide a lubricated surface for articulation and transmit mechanical loading to underlying subchondral bone [44].
The ECM of articular cartilage is a specialized and viscoelastic connective tissue and is composed of three major macromolecules: fibers (collagen and elastin), proteoglycans, and glycoproteins. Collagen is the main fiber in ECM (75% of the dry weight) [45], being the most important constituent to provide tensile strength [44]. The classic fiber-forming collagens include collagen I, II, III, V, and XI, all of which comprise a right-handed triple helix of three α-chains, and a left-handed polyproline II-type helix of three parallel peptides [46]. Collagen I, containing a heterotrimer of two identical α1(I)-chains and one α2(I)-chain, is the most abundant collagen in bone tissue. Collagen II is the main collagen type in articular cartilage, forming a homotrimeric molecule with three α1 (II) chains, which can play an important role in regulating the mechanical transduction of chondrocytes [46]. Articular cartilage also contains collagen III, collagen IX, collagen XI, and collagen VI [47]. Collagen X is only present in the articular cartilage of the calcified layer [48]. Non-collagenous elements such as glycosaminoglycans (GAGs), proteoglycans and glycoproteins, contribute to two key mechanical properties: viscosity and compressive resistance of ECM [49,50]. The viscoelastic properties of the ECM depend on the relative ratios of elastic (e.g., elastin and fibrillin) and inelastic (e.g., collagen) elements [50]. Hyaluronan is a linear polysaccharide (a non-sulfated GAGs, or GAGs) as a part of the nascent cartilage ECM and influence neocartilage formation via its principal cell-surface receptor CD44 and CD168 [[51], [52], [53]]. Hyaluronate chain also functions as backbone to bind aggrecan molecules, forming high-molecular-weight aggregates [54], which contributes to compression resistance and shock absorption in the joint [55]. The interaction between aggrecan and hyaluronan is stabilized by a link protein, thereby generating a more stable aggregate structure [55]. Furthermore, the link protein 'locks' proteoglycans onto the hyaluronate chain that provides more protection to proteoglycans against proteolytic degradation, thus extending its useful life in matrix [54]. All these components of articular cartilage ECM form a complex and dynamic meshwork to support cell polarization, functions, tissue organization and homeostasis [50,56,57].
3. The biology of chondrogenesis
Chondrogenesis is an extremely complex physiological process with both morphological and biochemical changes of chondrocytes and ECM. This process is elaborately regulated by a set of cytokines, growth factors and signaling pathways [25].
3.1. Cellular changes during chondrogenesis
Chondrogenesis is divided into two main stages: condensation and differentiation [25]. Condensation begins with cell movement followed by an increase in cell-packing density. This process is related to enhanced cell-cell contact and interaction mediated by adhesion molecules, such as Ca2+-dependent N-cadherin, Ca2+-independent N-cadherin and gap junctions. During the condensation stage, the precartilaginous mesenchyme is divided into chondrogenic and non-chondrogenic domains [58]. The differentiation stage includes cell-matrix interactions. The increased cell proliferation and ECM remodeling are associated with the appearance of tenascin, thrombospondins (such as cartilage oligomer matrix protein, COMP) and the disappearance of collagen I, fibronectin and N-cadherin. These ECM conditions stimulate the transformation of the elongated and fibroblast-like chondroprogenitor cells into fully differentiated spherical chondrocytes, while the prechondrogenic clusters develop into the ECM components, including collagen II and aggrecan [59]. The most suitable markers of the stages in this process are the expression of various types of collagen. For example, collagen II, IX and XI specifically indicate the differentiation of chondroprogenitor cells; collagen VI indicates the proliferation of chondrocytes and collagen I, III and V reveal the condensation of MSCs [25,59].
3.2. Growth factors and signaling pathways to regulate chondrogenesis
The process of chondrogenesis is mainly regulated by growth factors and signaling pathways. The principal regulators of chondrogenesis are TGF-βs and BMPs, FGFs, IGFs and members of the Wingless-type (Wnt) signaling pathway.
The TGF-β superfamily consists of approximately 30–35 multifunctional molecules. TGF-β is the prototype of the TGF-β family of growth and differentiation factors, which is encoded by 33 genes in mammals and comprises homo- and heterodimers [60,61]. Human TGF-β superfamily peptides include three TGF-β isoforms (TGF-β1, -β2 and -β3), activins, nodal, BMPs, and growth and differentiation factors (GDFs) [62]. TGF-β1 is the first validated chondrogenic differentiation factor of MSCs and TGF-β2 and TGF-β3 are also proved to be effective in inducing chondrogenesis of MSCs [63]. TGF-β initiates its signaling by binding to the TGF-β receptor II and recruiting receptor I, which then phosphorylates its targets Smad2 and Smad3 [64]. The phosphorylated (p-) Smad2 and p-Smad3 form a complex with Smad4 and translocate to the nucleus where they interact with other actors to stimulate the transcription of chondrogenic genes, such as collagen II [65,66]. TGF-β1 also initiates Smad-independent signaling pathways, resulting in the activation of mitogen-activated protein kinase (MAPK) pathways such as the p38, c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) pathways [67].
BMPs are a group of proteinous growth factors in the TGF-β superfamily, consisting of dimerized monomers linked by disulfide bonds. [68]. In 1965, BMPs were discovered by Urist in the pioneering work, representing a landmark in the development of bone tissue engineering [69]. The classical role for BMPs is considered to be the induction of (ectopic) cartilage and bone formation [69,70]. BMPs play a pleiotropic role in promoting the differentiation of multipotent stem cells along different directions, e.g. osteogenesis [71], adipogenesis [72] and chondrogenesis [73]. Similar to other members of the TGF-β superfamily, BMPs bind to transmembrane serine/threonine kinase receptors on the cell membrane, thereby triggering specific intracellular signaling pathways to activate and affect gene transcription [74]. There are two types of BMP receptors: type I and type II. Each type includes three types. Type I receptors include activin receptor type-IA (ActR-IA or Alk-2), BMP receptor type-IA (BMPR-IA or Alk-3) and BMP receptor type-IB (BMPR-IB or Alk-6). The type II receptors include BMP receptor type-II (BMPR-II), activin receptor type IIA (ActR-IIA) and activin receptor type IIB (ActR-IIB) [74,75]. Receptors of both types are needed to form a functional complex to initiate further signaling events [76]. Upon ligand binding, type I receptor is phosphorylated by the constitutively active type II receptor at the GS box (glycine/serine-rich region), which enables the release of BMPR-IA-bond protein casein kinase II (CK2) to activate the smad 1/5/8 pathway [39]. P-smad1/5/8 (receptor-regulated Smads, R-Smads) assemble into a complex with Smad4 (common-partner Smad, Co-Smad) and translocate into the nucleus to regulate the transcription of target genes, such as osteogenic-related genes-inhibitor of DNA binding 1 (Id1), distal-less homeobox 5 (Dlx 5), runt-related transcription factor 2 (Runx2), osterix and chondrogenic-related genes-Sox9 (sex determining region Y box 9) [77]. In addition, the BMP also initiates Smad-independent signaling pathways [[78], [79], [80], [81]], at least partially, through BMP-induced receptors [76].
The Wnt signaling pathway is also a classic signaling pathway related to chondrogenic differentiation, which can be divided into canonical and noncanonical Wnt pathways [82]. Wnt ligand binds to the seven transmembrane receptors frizzled (Fzd) and the co-receptor lipoprotein-related proteins 5 and 6 (LRP-5/6) in the canonical Wnt pathway, whereas the noncanonical pathway does not require LRP-5 and -6 [82]. The activation of canonical Wnt signaling leads to the inactivation of GSK-3β that phosphorylates β-catenin and facilitates its degradation [83]. The inactivation of GSK-3β results in reduced degradation of β-catenin, which accumulates and transfers into the nucleus to trigger downstream transcription through the LEF-1/TCF complex [83,84]. The canonical Wnt signaling is known to promote osteogenesis by upregulating Runx2 expression [85] and to inhibit the chondrogenesis of hMSCs by repressing Sox9 transcription through Wnt-inducible Twist-1 [86,87].
The main transcription factor Sox9 is absolutely required for cell survival in precartilaginous condensations and for chondrocyte differentiation in cartilage primordia. Sox9 plays an important role in cell survival and controls cell morphology required for the transition of mesenchymal cells to chondrocytes [88]. The regulation of Sox9 expression by BMP signaling pathway plays an important role in the early stage of cartilage embryo development [89]. TGFβ/BMP activity directly controls the expression of Sox9 during cartilage formation, and controls downstream factors of the Sox9 enhancer, such as Smads and TAK1 (TGFβ activated kinase) [89,90]. In addition to Sox9, other members of the Sox family transcription factors (Sox5 and Sox6) are also involved in early chondrogenic differentiation and expression of ECM molecules [91].
4. Bioactive peptide to promote chondrogenesis
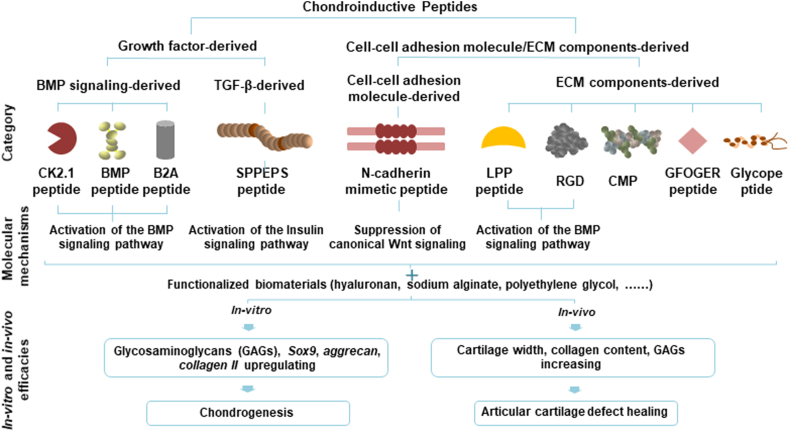
According to the original resources, the current chondroinductive/chondroconductive peptides can be divided into two large categories: 1) growth factor-derived peptides, 2) cell-cell adhesion molecule/ECM-components derived peptides (Fig. 1). BMP signaling-derived peptides include CK2.1, BMP peptide and B2A. SPPEPS is a common sequence of TGF-β3 and aggrecan. ECM-derived peptides include cell-cell adhesion molecule-derived peptides and ECM derived peptides. N-cadherin mimetic peptide is derived from a cell-cell adhesion molecule — N-cadherin. ECM components-derived peptides include RGD, collagen mimetic peptide (CMP), GFOGER peptide, glycopeptide and Link Protein N-terminal Peptide (LPP). In addition, there are a group of peptides that can self-assemble into hydrogels for tissue engineering. These peptides include RADA16 (AcN-(RADA)4-COHN2), KLD-12 (AcN-KLDLKLDLKLDL-CNH2), PS-b-PEO-Ada, palmitoyl-V3A3K3–Am and HSNGLPL [37,92,93]. The self-assembled peptides gelate through noncovalent self-assembly mechanisms, and these noncovalent interactions are useful for the attachment of bioactive groups that replicate the functions of proteins [37]. The self-assembling peptides mainly function as scaffolds and have been reviewed elsewhere [19]. On the other hand, another group of peptides can form thermosensitive hydrogels for cartilage tissue engineering. Polyalanine (PA), poly(alanine-co-phenylalanine) and poly(alanine-co-leucine) conjugated to PEG or poloxamer are thermosensitive copolymers. The strong hydrogen bonds or ionic interactions between peptide chains contribute to the gelation process [94,95]. A series of peptide-based thermosensitive hydrogels, such as PEG-b-PA, graphene (GO)/PEG-b-PA, reduced GO/PEG-b-PA, PEG-b-PA-b-poly(l-aspartate) (PD), poly(l-alanine-co-l-phenylalanine) (PAF)-b-PEG-b-PAF and PA-b-poloxamer (PLX)-b-PA [96,97]. Recently, Liu et al. show that BMSCs within the PAF-b-PEG-b-PAF produced more GAGs and collagen II and less collagen I than PA-PEG-PA and control after 12 weeks injection into rabbit cartilage defects in rats [96]. The peptide-based thermosensitive hydrogel has been reviewed elsewhere [97]. In this review, we mainly focus on the chondroinductive/chondroconductive peptides.
Fig. 1.
Schematic illustrating the summary of the category (according to the resources) and molecular mechanisms of chondroinductive/chondroconductive peptides as well as the parameters to evaluate the in-vitro and in-vivo efficacies. BMP: bone morphogenetic protein; TGF-β: transforming growth factor-β; ECM: extracellular matrix; CMP: collagen mimetic peptide; LPP: Link Protein N-terminal Peptide.
4.1. Growth factors-derived peptides
4.1.1. BMP signaling-derived peptides
BMPs, especially BMP-2, drive the development of cartilage and induces the differentiation of mesenchymal progenitor cells (MPCs) into chondrocytes [39,98]. Consequently, a series of peptides are designed to mimic the promoting effect of BMP-2 on chondrogenesis (Fig. 1).
4.1.1.1. CK2.1
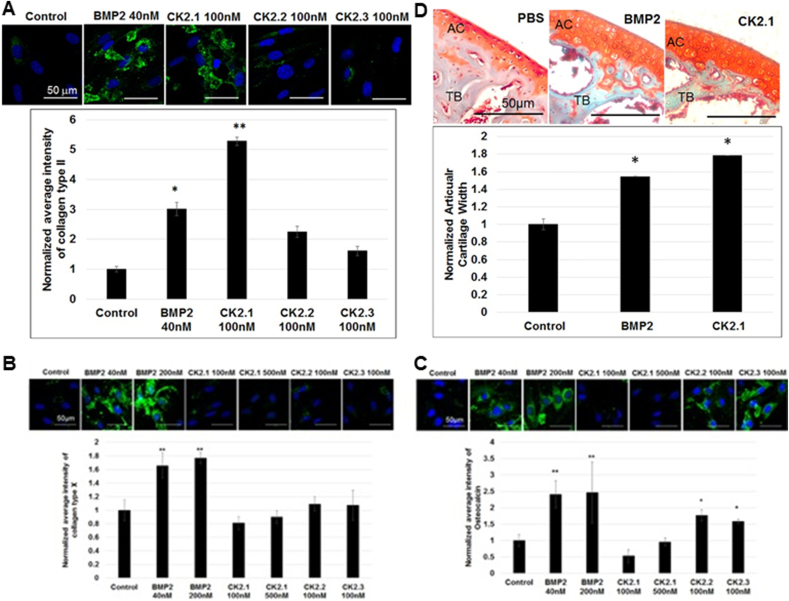
CK2 is a highly conserved and ubiquitously expressed enzyme with more than 300 substrates [39]. Three peptides CK2.1, CK2.2, and CK2.3 are designed to activate the BMP signaling pathway in the absence of BMP ligand through inhibiting the binding of CK2 to the corresponding binding sites on BMPR-IA [39]. Among these three peptides, CK2.1 is derived from CK2 phosphorylation sites generated by BMPR-IA at amino acids 466–469 (SYED) and has shown to be an effective inducer of cartilage formation [99] (Fig. 2). In the micromass chondrogenesis model of C3H10T1/2 cells, CK2.1 at its optimal concentration (100 nM) significantly enhances the Alcian blue staining and SBE (Smad binding element) luciferase activity by 3.7 folds and 3.2 folds, respectively, which is comparable with 40 nM BMP-2 for 3 weeks. Furthermore, the potency of CK2.1 (100 nM) in increasing the protein expression of collagen II (5.3 folds) is significantly higher than BMP-2 (3.0 folds). An advantageous property of CK2.1 over BMP-2 is that it does not lead to hypertrophy or mineralization, which is evidenced by no induction of collagen X and osteocalcin expression by CK2.1 [99,100]. When injected into the tail vein and knee cartilage defects of C57BL/6J mice [99,100], CK2.1 significantly increases cartilage width, collagen II and IX expression in femoral articular cartilage, but not collagen X or trabecular bone mineral density. In contrast, BMP-2 significantly enhances cartilage width, collagen X and trabecular bone mineral density, but not collagen II and IX expression [99] (Fig. 5).
Fig. 2.
Schematic illustrating the molecular mechanisms of bone morphogenetic protein (BMP) signaling-derived chondroinductive/chondroconductive peptides (CK2.1 peptide, BMP peptide and B2A peptide) through the activation of BMPs signaling to induce/promote the chondrogenesis of mesenchymal stem cells or chondrocytes.
Fig. 5.
(A) Bone morphogenetic protein (BMP)-signaling derived CK2.1 peptide induces the expression of collagen II in the micromass chondrogenesis model of C3H10T1/2 cells in an even higher efficacy than BMP-2, while CK2.2 and CK2.3 do not significantly enhance the expression of collagen II. BMP-2 but not CK2.1 significantly induce the expressions of (B) collagen X and (C) osteocalcin in C3H120T1/2 cells. In contrast, CK2.2 and CK2.3 significantly enhance the expression of (C) osteocalcin. (D) When injected into the tail vein of the mice, CK2.1 promotes the articular cartilage width in an even higher efficacy than BMP-2. © 2016 Reprinted with permission of John Wiley and Sons [99].
4.1.1.2. BMP peptide
BMP peptide is derived from residues 73–92 of the knuckle epitope of BMP-2 [101] and consists of 20 amino acids (KIPKASSVPTELSAISTLYL) (Fig. 2). It does not require additional processing such as disulfide bond formation or complex folding regimens like BMP-2 [102]. The BMP peptide is reported to have osteogenic activity in human BMSCs [40], progenitor bone marrow stromal (BMS) cells [103] and murine C3H10T1/2 cells [101]. Hitherto, most studies focus on its function of inducing osteogenesis and only one publication reports its effect on cartilage formation [104]. Renner et al. find that the BMP peptide shows significant activity in inducing chondrogenesis when its concentration reaches 100 μg/mL [104]. In the micromass chondrogenesis model of human BMSCs cells, the BMP peptide at 100 μg/mL can significantly enhance GAGs production by 1.8 folds, which is equivalent to the BMP-2. In contrast, the efficacy of BMP peptide to enhance total collagen content (1.7 folds) is significantly lower than that of BMP-2 (7.8 folds) at 3 weeks post-incubation. The BMP peptide stimulates the expression of Sox9, aggrecan and COMP at day 3 by are 1.2, 1.8, and 3.7 folds respectively. Furthermore, BMP peptide significantly increases ALP activity and collagen X by 3.8 folds, 9.2 folds respectively at 4 weeks post-incubation, which is much lower than BMP-2. These findings suggest that the BMP peptide promotes the production of GAGs without extensively upregulating hypertrophy as BMP-2 [104]. Furthermore, the BMP peptide is associated with a more homogeneous matrix molecular distribution than BMP-2. Hitherto, there is still a lack of in-vivo study to investigate the effect of BMP peptide on cartilage formation.
4.1.1.3. B2A
B2A is a designed and chemically synthesized peptide containing two major functional domains: BMP receptor-targeting domain and heparin-binding domain [38,105], which is linked by a hydrophobic spacer domain (KK-[NH(CH2)5CO]3 [38]) [106]. The heparin-binding sequence (RKRKLERIAR) is derived from c-jun/jun D, which conforms to the canonical XBBBXXBX heparin-binding motif, wherein B represents a hydrophilic amino acid with a basic charge, usually arginine or lysine, and X represents an uncharged or hydrophobic amino acid [107]. The BMP receptor-targeting domain (AISMLYLDENEKVVL) is derived from the amino acid residues 91–105 of mature human BMP-2 [108]. The hydrophobic spacer and heparin-binding domain form a linear backbone chain and the BMP receptor-targeting domain is grown off the backbone at the N-terminal lysine [38] (Fig. 2). B2A is designed with an original aim to promoting osteogenesis. As a co-factor of BMP-2, B2A significantly amplifies BMP-2-induced ALP activities in C3H10T1/2 cells and C2C12 cells by 5–40 folds with an optimal concentration of B2A at 5–10 μg/mL [38]. In contrast, the B2A peptide alone only marginally influences ALP activity in these cells [38]. Mechanistically, the B2A peptide is a positive modulator of recombinant BMP-2 receptor modulator, by binding to type I and type II receptors, preferentially BMPR-IB and ActR-II (as well as other isoforms in the following order: BMPR-IB = ActR-II » BMPR-IA = ActR-IIB > BMPR-II) [38]. Lin et al. show that B2A alone significantly enhances ERK1/2 activation (11.7 folds), while inhibits p-Smad1/5/8 in C2C12 cells. In contrast, the addition of BMP-2 turns off p-ERK activation and significantly enhance the p-Smad1/5/8 by 6.2 folds [38]. They also find that B2A shows a strong specificity to BMPs since it does not respond to other growth factors, such as FGF-2 (basic fibroblast growth factor-2), TGF-β1, VEGF (vascular endothelial growth factor) [38]. In clinic, B2A-coated ceramic granules are used as a bone substitute material to treat end-stage hindfoot arthritis [106].
B2A is also shown to enhance chondrogenesis. In the micromass chondrogenesis model of C3H10T1/2 cells, microarray analysis shows that B2A significantly enchances the mRNA expression of Sox9, collagen II, Fgf1, Fgfr1, Fgfr2, Twist1 and PDGF AA [109]. Consistently, B2A enhances protein expression of collagen II and PDGF AA in murine C3H10T1/2. In a rat mono-iodoacetate (MIA)-induced osteoarthritis (OA) model, B2A treatment significantly enhances cartilage GAGs and cartilaginous cells density [109].
4.1.2. TGF-β3-derived peptides
The SPPEPS peptide is a common sequence of two molecules TGF-β3 and aggrecan. In TGF-β3, the SPPEPS sequence is located in the latency-associated protein region [110] — a ligand for a number of integrins [111]. SPPEPS at its optimal concentration (100 ng/mL) significantly enhances the expression collagen II in rat BMSCs by 1.6 folds compared to the negative control group after 3 days [110]. Furthermore, at 7 days, KEGG analysis of the proteomics data shows that SPPEPS can activate “Insulin Signaling’’ pathways through an essential gene for cartilage development (i.e., GSK-3β). In addition, SPPEPS can also significantly upregulate the expression of collagen XIα1, which plays a critical role in cartilage formation [110]. Gene ontology analysis indicates that SPPEPS can also upregulate chondrogenesis-related genes such as ENPP1 and CLIC4 [110]. The pentenoate-functionalized hyaluronic acid (PHA) hydrogels with the functionalization by the conjugation of both SPPEPS and RGD can significantly enhance the expression of collagen II in rat BMSCs by about 300 folds than control group [110]. However, there is still a lack of data to investigate the in-vivo efficacy of SPPEPS.
4.2. Cell-cell adhesion molecule/ECM-derived peptides
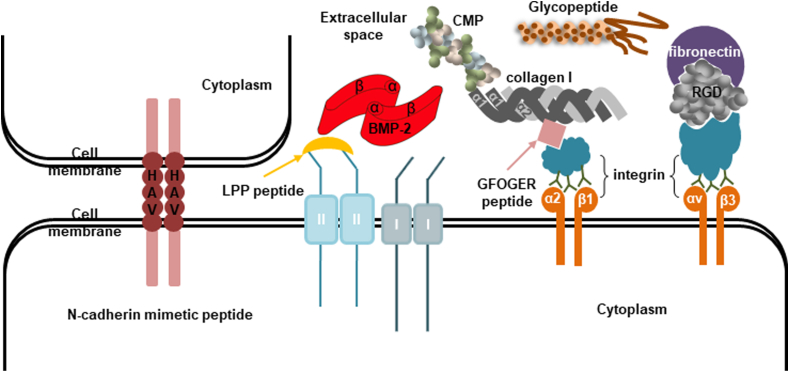
The coordinated relationship between cell-ECM and cell-cell adhesion has become a common theme in various development and regeneration processes. Cell-cell adhesion is typically mediated by a multiprotein complex that is made up of three general classes of proteins: ECM proteins, adhesion receptors and cytoplasmic plaque membrane proteins [[112], [113]]. ECM proteins are typically large glycoproteins, including the collagens, fibronectins, laminins, fibrin, vitronectin and proteoglycans that assemble into fibers or other complex macromolecular arrays. They can bind to adhesion receptors and also be tightly associated with the cell surface [[113], [114]]. Adhesion receptors are usually transmembrane glycoproteins that play a crucial role in mediating cell-cell and cell-ECM signaling. Adhesion receptors are divided into four categories: cadherins, integrins, selectins and immunoglobulins [[112], [113]]. They can recognize and interact with either other cell adhesion receptors on neighboring cells or with ECM proteins [113]. Cytoplasmic plaque membrane proteins are located on the intracellular surface of the plasma membrane, associating with cell adhesion receptors. They can link the adhesion systems to the cytoskeleton, regulate the functions of the adhesion molecules and transduce the signals that are initiated at the cell surface by the adhesion receptors [113]. Mimicking natural ECM is now one of the crucial strategies of cartilage tissue engineering. Peptides derived from ECM can induce cell proliferation, differentiation, and production of cartilage-like ECM [115]. The peptides derived from cadherins and integrins are mostly used for chondrogenic differentiation (Fig. 3).
Fig. 3.
Schematic illustrating the activation pattern of cell-cell adhesion molecule-derived peptide (N-cadherin mimetic) and extracellular matrix (ECM) components-derived peptides (Link Protein N-terminal peptide (LPP or link-N), GFOGER peptide, RGD, collagen mimetic peptide (CMP) and glycopeptide) to promote chondrogenesis.
4.2.1. Cell-cell adhesion molecule-derived peptide
Cadherins are Ca2+ dependent type-1 transmembrane glycoproteins that mainly mediate cell-cell adherence junctions and communicate with a group of connexins called catenins so as to mediate the functions of cadherins. The different types of N-, P-, R-, B- and E−cadherins all play important roles in tissue formation and signal cascade regulation [116,117]. N-cadherins play an essential role for mesenchymal cell condensation during chondrogenesis through mediating the aggregation of progenitor cells and promoting cell-to-cell interactions [118].
N-cadherin contains a conserved three-amino acid sequence, His-Ala-Val (HAV), which provides a homophilic cell adhesion recognition site and mediates cell-to-cell adhesion [118]. Optimized synthetic peptides (N-Ac-CHAVDC-NH2) containing the HAV domain have been shown to govern the specificity of cadherin binding and possess N-cadherin-like binding activity [119,120]. HAV is incorporated to HA hydrogel to form a functionalized HA hydrogel with N-cadherin mimetic peptide [41,53,121] (Fig. 3). Functionalization of HA hydrogels with N-cadherin mimetic peptides (Ac-HAVDIGGGC) increases GAGs and total collagen content by 1.5 folds and 1.8 folds in encapsulated hMSCs at 28 days compared to HA hydrogels functionalized with scrambled N-cadherin mimetic peptides (Ac-AGVGDHIGC) (by 0.9 folds and 1.3 folds). Furthermore, the mean intensities of chondroitin sulfate (CS) and collagen II staining are significantly higher for the N-cadherin mimetic peptides (1.39 folds and 1.63 folds) compared with the scrambled N-cadherin mimetic peptides. Implanting the hMSC-laden MeHA hydrogel disks in subcutaneous pockets of nude male mice for 28 days. GAGs and collagen content are increased by 1.8 folds and 1.3 folds in the group of N-cadherin mimetic peptides in comparison with the control group after 28 days of culture. Immunohistochemical staining also shows more intense and distributed collagen II and CS staining in the N-cadherin mimetic peptides by 1.62 folds and 1.59 folds compared with the scrambled N-cadherin mimetic peptides [53]. On the other hand, Li et al. show that N-cadherin mimetic peptide (Ac-HAVDIGGKLDLKLDLKLDL) significantly elevates the expression of collagen II, Aggrecan, Sox9 genes (1.4, 1.8 and 1.7 folds, respectively) on 3 days and GAGs content on 14 and 28 days of chondrogenic culture in hMSCs, compared with scrambled control peptide (Ac-AGVIDHGKLDLKLDLKLDL) [41]. The sequence of KLDLKLDLKLDL is a self-assembling peptide and functions as a 3D scaffold for encapsulation of chondrocytes [92]. Furthermore, the expression level of collagen II from N-cadherin mimetic peptide group is significantly lower (by 27.98%) and Runx2 and collagen X are not significantly different when compared with that of scrambled control peptide group on 14 days [41]. In a very recent study, Guo et al. have developed a bi-layered, modular hydrogel system to generate tissue-specific and cell-encapsulated hydrogel layers targeting the cartilage or bone. The system is fabricated by conjugating the polymeric hydrogel crosslinker, poly (glycolic acid)-poly (ethylene glycol)-poly (glycolic acid)-di(but-2-yne-1,4-dithiol) (PdBT), with chondrogenic N-cadherin mimetic peptide (GGGHAVDI) and osteogenic GHK peptide (GGGGHKSP), followed by mixing MSCs at physiological temperature. After a 12-week implantation in rabbit femoral condyle defects, the hydrogel system with N-cadherin mimetic peptide is associated with significantly higher histological measures of overall defect filling, cartilage surface regularity, GAGs/cell content in newly formed and adjacent cartilage compared to control [122]. Their results establish the utility of the functionalized bi-layered hydrogel system for the repair of the osteochondral defects.
N-cadherin peptide hydrogels suppress canonical Wnt signaling in hMSCs by increasing GSK-3β and GSK-3β-mediated degradation of β- catenin so as to decrease the nuclear translocation and of the associated transcriptional activity of β-catenin/LEF-1/TCF complex, thereby enhancing the chondrogenesis of hMSCs [41]. Specifically, the expression level of Wnt (Wnt-2, Wnt-6), Wnt-related genes (β-catenin, LEF1), Wnt receptor genes (LRP5, Fzd6, Fzd1), and non-chondrogenic marker genes (Runx2, Alp, collagen I) are down-regulated in the presence of N-cadherin peptide, while the expression level of Wnt inhibitory factor (WIF1, DKK1, GSK-3β) and chondrogenic marker genes (aggrecan, Sox9) are upregulated in the presence of N-cadherin mimetic peptide group compared with the scrambled control peptide group [41] (Fig. 4). After 3 days of chondrogenic culture, GSK-3β expression is significantly upregulated by 22.16% and the expression of p-GSK-3β, β-catenin and LEF-1 are decreased by 49.90%, 34.15% and 49.62% respectively in N-cadherin mimetic peptide group when compared to the scrambled control peptide group in hMSCs [41].
Fig. 4.
Schematic illustrating the molecular mechanism of the N-cadherin mimetic peptide to induce the chondrogenesis of mesenchymal stem cells or chondrocytes by inhibiting the Wnt signaling.
4.2.2. ECM components-derived peptides
Integrins mainly mediate cell-ECM adhesion and function as cell receptors for ECM proteins. The structure of each integrin consists of one α and one β subunit. There are at least 16 α subunits and 8 β subunits and different α and β subunits can form a complex. Both subunits are composed of three domains: an extracellular domain, a cytoplasmic region, and a transmembrane domain [123,124]. RGD and GFOGER are derived from specific ECM proteins that rely on integrins to promote chondrogenic differentiation.
4.2.2.1. RGD
RGD was first discovered in fibronectin in 1984 [125] and is later recognized as a common cell adhesion motif in adherent ECM, blood and cell surface proteins [126]. RGD binds to integrin on cell membrane to mediate cell adhesion, spreading and other cell activities [127] (Fig. 3). RGD is the minimal sequence and is normally combined with different amino acid linkers to form RGD peptide, such as GGGGRGDY [128,129], GCGYGRGDSPG [130,131], GRGDSP [132] and GGGGRGDSY [127]. RGD peptide must be combined with biomaterials to exert the above functions, since soluble RGD prevents cell spreading and proliferation in a dose-dependent manner [127]. In the field of cartilage tissue engineering, synthetic RGD-containing peptides have widely used to functionalize a large variety of biomaterials, such as microcavitary alginate hydrogel (MA), sodium alginate, agarose hydrogel, biodegradable polyethylene glycol (PEG), cellulose-binding domain (CBD) (come from the cellulobiohydrolase I (CBHI) of the modified fungus Trichoderma koningii) [42,[128], [129], [130], [131]]. RGD incorporation has been shown to significantly increase chondrogenesis as indicated by enhanced mRNA expression of Sox9, collagen II and aggrecan as well as the expression of aggrecan and collagen II. The presence of RGD in alginate does not directly enhance chondrogenic differentiation, instead, it dramatically amplifies TGF-β1-induced key chondrogenic signaling molecules, such as p-Smad2/3 (by 16 folds) and p-ERK1/2 is (by 3 folds), thereby promoting chondrogenesis [128].
On the other hand, the same integrin heterodimer can recognize several ECM proteins, and a particular ECM ligand may be recognized by more than one integrin [133]. Numerous integrin receptors including αvβ3 and α5β1 recognize and bind directly to the RGD motif [134]. Different integrin receptors mediate different effects. For example, αvβ3 plays distinct roles in adhesion and spreading while α5β1 is more important for differentiation [135]. And cell spreading can be significantly down-regulated by anti-αvβ3 antibody, but up-regulated by anti-β1 antibody and anti-α5 antibody [127,132]. These findings indicate that the receptors compete with each other for RGD ligands. It seems that αvβ3 integrin is particularly involved in cell spreading and cartilage formation [132].
4.2.2.2. Collagen mimetic peptide
Collagen mimetic peptide (CMP) with a specific amino acid sequence —(P(hydroxyl)PG)x —, exhibits strong affinity to both native and gelatinized collagen I under controlled thermal conditions and forms a triple helix conformation that resembles the native protein structure of natural collagens [136] (Fig. 3). The triple helix structure of CMP — (P(hydroxyl)PG)7 — can be further stabilized by attaching a T (tyrosine), thereby elevating its melting temperature [136]. The conjugation of ((P(hydroxyl)PG)7-T) to poly (ethylene oxide) diacrylate (PEODA) hydrogel can change the bioinertia and non-cell-adhesive property of PEODA, thereby creating a suitable bioactive microenvironment, and facilitating the efficient chondrogenic differentiation of MSCs [137,138]. After a 3-week culture, immunostaining analysis shows that the MSCs in CMP/PEODA hydrogels are associated with significantly enhanced expression of collagen II (1.8 folds) and significantly reduce expression of collagen X (0.4 folds) than those in PEODA [137,138]. Furthermore, the MSCs in CMP/PEODA hydrogels are also associated with significantly enhanced expression of GAGs (2.5 folds), total collagen content (2.0 folds), aggrecan (2.5 folds) and collagen II (2.0 folds) than those in PEODA hydrogels [137,138]. However, there is still a lack of data to identify the in-vivo efficacy of CMP.
4.2.2.3. GFOGER peptide
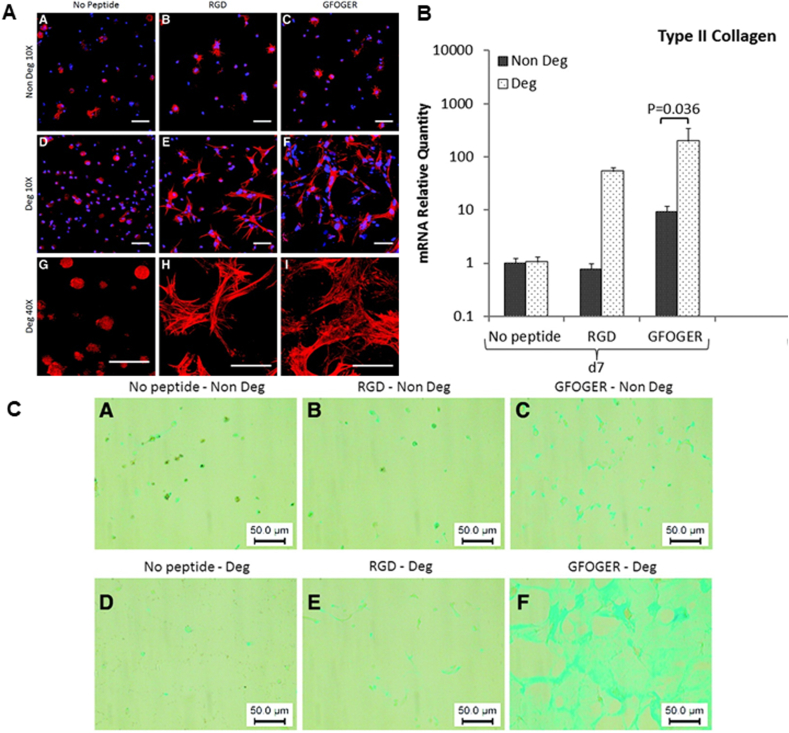
In 2000, the GFOGER peptide sequence was identified by Knight et al. as an integrin α2β1 (a major integrin collagen receptor) recognition site in residues 502–507 of the collagen I α1(I) chain [139] (Fig. 3). GFOGER-containing collagen mimetic peptide (sequence: (GPO)4GFOGER(GPO)4GCG, CMP) is chemically incorporated into PEG hydrogels to form GFOGER-modified hydrogels through Michael addition chemistry to functionalize PEG [43,140]. Michael addition chemistry does not require any catalysts or initiators and is used to form hydrogels from aqueous solutions in the presence of cells, which avoids the problems associated with UV exposure and toxic initiators in the photo polymerization process [43,141]. The hMSCs exhibit a highly spread morphology within the GFOGER-functionalized hydrogels, but a star-like morphology in RGD-functionalized hydrogels. Cell proliferation in GFOGER-functionalized hydrogel is 2.0 folds than that in RGD-functionalized hydrogel. Furthermore, in comparison with RGD-functionalized hydrogels, GFOGER-modified hydrogels are associated with a much higher expression of collagen II and aggrecan mRNA and GAGs content in hMSCs on 7 days (by 6.25, 10 and 1.2 folds, respectively) and 21 days (by 1.7, 1.3 and 1.3 folds, respectively) [140]. These findings suggest that the GFOGER peptide enhances cell spreading and proliferation in PEG hydrogels and provides a better chondrogenic microenvironment than the RGD peptide (Fig. 6).
Fig. 6.
(A) Fluorescent micrographs showing that after fixation and staining for nuclei (DAPI, blue) and actin filaments (phalloidin, red), cells in RGD containing gels display starlike morphologies with thick, individually discernible actin fibers, while GFOGER-modified gels induce a more homogenous spreading of cells with thinner and more dispersed actin filaments. (B) Figure showing that the gene expression of collagen II is highest in GFOGER-Deg when compared with No peptide and RGD-Deg after 7 days in culture. (C) GAG synthesis is confirmed qualitatively using Alcian blue staining. In GFOGER-Deg, the staining is strongly distributed throughout the whole sample compared with other groups. Deg: degradable gels; Non Deg: nondegradable gels. © 2014 Reprinted with permission of Mary Ann Liebert [140]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.2.2.4. Glycopeptide
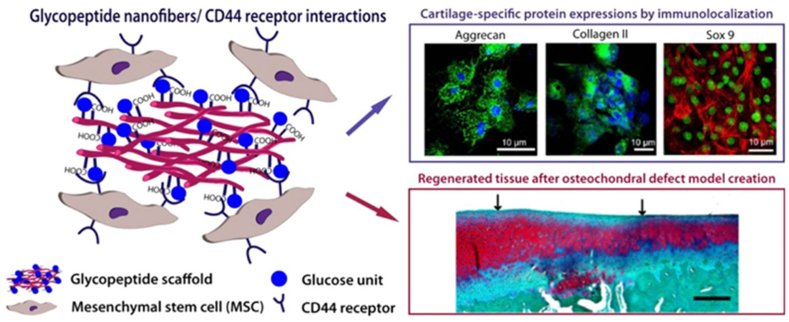
Glycopeptides are comprised of a polypeptide backbone with pendant carbohydrates [142] (Fig. 3). As simplified analogues of glycoproteins, glycopeptides are biodegradable and easy to prepare as well as maintain the ability to fold into well-defined secondary structures [142]. Yaylaci et al. synthesize Glc-PA, a self-assembled supramolecular GAG-like glycopeptide nanofiber by conjugating a Ser-linked β-d-glucose containing amphiphilic glycopeptide and a carboxylic acid bearing peptide amphiphile (PA) [143]. Positively charged Glc-PA is mixed with negatively charged E-PA to form 3D networks (Glc-PA/E-PA) as a result of interactions such as hydrogen bonding, van der Waals, and electrostatic forces. On 7 days, the mMSCs on Glc-PA/E-PA shows significantly higher amounts of collagen II, aggrecan and Sox9 gene expression in chondrogenic medium by 10, 14 and 38 folds, respectively in comparison with the control [143]. Furthermore, in a rabbit full thickness osteochondral defect treated with microfracture model, Glc-PA/E-PA shows that a strong and evenly distributed staining of collagen II and Safranin-O and relatively low staining of collagen I within a 12-week period [143] (Fig. 7). Ren et al. synthesize the glycopeptide PPLG-g-Man/HPPA hydrogels by conjugating poly(g-propargyl-l-glutamate) (PPLG) with azido-modified mannose and 3-(4-hydroxyphenyl) propanamide (HPPA) [144]. In comparison with the control, rabbit chondrocytes in the glycopeptide hydrogels are associated with significantly enhanced gene expression of aggrecan (4 folds) and collagen II (20 folds) as well as significantly decreased expression of collagen I (0.1 folds) at 14 days after incubation [144]. In line with the in-vitro data, after implanting the chondrocytes-laden glycopeptide hydrogel disks in subcutaneous pockets of nude male mice for 14 days, the glycopeptide hydrogel is associated with time-dependent increased chondrocyte density and GAGs biosynthesis as well as increased gene expressions of collagen II and aggrecan of chondrocytes in the glycopeptide hydrogels during the incubation period, while an obvious decrease in the gene expression of collagen I is detected after an incubation for 21 days [144].
Fig. 7.
Schematic illustrating that the supramolecular glycosaminoglycan (GAG)-like self-assembled glycopeptide nanofibers mimics hyaluronan and induces in-vitro chondrogenic markers, such as aggrecan, collagen II and Sox9 in mesenchymal stem cells (MSCs) as well as the cartilage regeneration in a microfracture-treated osteochondral defect model. © 2016 Reprinted with permission of American Chemical Society [143].
4.2.2.5. Link protein N-terminal peptide (LPP)
Link Protein N-terminal Peptide (LPP, also referred to as link-N) is a cleaved N-terminal 16-amino peptide (DHLSDNYTLDHDRAIH) from link protein [54,145]. Both native LPP and its biochemically synthesized form can stimulate the synthesis of aggrecan and collagen II in cartilage stem/progenitor cells (CSPCs) [[146], [147], [148]] and intervertebral disc (IVD) cells [149,150]. Wang et al. link LPP to RADA16 [151,152] to construct a functionalized-nanofiber hydrogel scaffold (LN-NS) [153]. The LN-NS significantly promotes the adhesion but not proliferation of BMSCs. Furthermore, compared with RADA16 hydrogel, LN-NS also significantly upregulates the expression of chondrocyte-related genes in BMSCs, such as collagen II and aggrecan on day 7 (increased by 1.8 folds and 1.5 folds, respectively) and on day 14 (increased by 2.8 folds, 2.0 folds, respectively) [153]. In primary chondrocytes, LPP directly binds to BMPR-II but not with BMPR-I through a direct peptide-protein association, thereby stimulating a production of endogenous BMPs, such as BMP-4 and BMP-7 [55] (Fig. 3). The thereby newly produced BMP-7 but not BMP-4 activates p-Smad1/5 and subsequent expression of chondrocyte-specific transcription factor Sox9 so as to stimulate downstream aggrecan and collagen II expression [55]. In contrast, PI3K/AKT inhibitor LY294002, p38 MAPK inhibitor SB203580, or ERK1/2 inhibitor U0126 fail to suppress LPP-induced production of aggrecan, collagen II, and Sox9 [55]. The levels of Runx2 and collagen X are not affected by LPP, which indicates that LPP has no effect on osteogenic induction [55,154]. LPP at its optimal concentration (50 ng/mL) stimulates the expression of Sox9, aggrecan and collagen II by 2.34, 1.58, and 1.32 folds, respectively at 7 days. Similarly, LPP significantly increases Sox9 and aggrecan in protein level, while collagen II is only enhanced in 100 and 500 ng/mL [154]. However, there is no in-vivo study to further investigate its efficacy.
5. Discussion: categorization, summary and prospective research direction
5.1. Categorization of peptides for cartilage tissue engineering
In the field of cartilage tissue engineering, the terms chondroinductive, chondroconductive and chondrogenic are frequently used to define, categorize and characterize the chondrogenesis-related bioactivities of biomaterials, cells and growth factors [9,23,25,155,156]. Similarly, these terms are also used to describe the bioactive properties of peptides for promoting or inducing chondrogenesis. However, similar as the terms, such as osteoinductive, osteoconductive and osteogenic in the field of bone tissue engineering, these terms are not always used correctly and consistently [157]. Therefore, it is highly important to clearly define these terms so as to correctly categorize the bioactive peptides for cartilage tissue engineering. By summarizing the most commonly usage of these terms, we give the following definitions of the terms as follows:
Chondroinductive: to describe a property to induce the differentiation of primitive, undifferentiated and pluripotent cells to develop into the cartilage-forming cell lineage. This term is mainly used to describe exogenous or endogenous growth factors (e.g. BMPs [[158], [159], [160]]) and other bioactive agents (such as Kartogenin [161]) and their-functionalized biomaterials. The most critical criterion for this term is that the bioactive agents or biomaterials can induce an in-vivo cartilage in ectopic (e.g. subcutaneous or intramuscular [53,144,162]) sites.
Chondroconductive: to describe a property to favor the adhesion, spreading, migration and differentiation of chondrogenic cells as well as ingrowth of cartilage tissue into a 3D porous scaffold. This term is mainly used to describe the property of a biomaterial scaffold (e.g. hydrogel [9,16]) and the bioactive agents to improve such a property of biomaterial scaffold.
Chondrogenic: to describe a property to form cartilage, such as producing ECM and zonal structure, etc. This term is mainly to describe the various types of cells (e.g. chondrocytes and MSCs [23,163]), which can develop the property of chondrocytes to form cartilage. This term may also be used to describe tissue engineered constructs to form cartilage.
In the following summary, we will briefly review the properties of the peptides and use the above terms to categorize them so as to systematically compare their efficacies in inducing in-vitro and in-vivo chondrogenesis (Table 1, Table 2, Table 3).
Table 1.
The origins, amino acid sequences and molecular mechanisms of chondroinductive/chondroconductive peptides for cartilage tissue engineering.
| Category | Sub-category | Name | Origin | Acid sequence | Molecular mechanisms | Refs. |
|---|---|---|---|---|---|---|
| Growth factor derived peptide | BMP signaling-derived peptide | CK2.1 | The amino acid residues 466–469 of BMPR-IA | SYED | Inhibition of the CK2-BMPR-IA interaction led to the activation of the Smad signaling pathway | [99,104] |
| BMP peptide | The amino acid residues 73–92 of the knuckle epitope of BMP-2 | KIPKASSVPTELSAISTLYL | Activation of the BMP signaling pathway through BMP type I and type II receptors | [101,104,165] | ||
| B2A | The amino acid residues 91–105 of BMP-2; heparin-binding motif | AISMLYLDENEKVVL; RKRKLERIAR | Activation of the ERK1/2, while inhibition p-Smad1/5/8 through binding to BMPR-IB and ActR-II | [38,109] | ||
| TGF-β derived peptide | SPPEPS | The latency-associated protein region in TGF-β3 | SPPEPS | Activation Insulin signaling pathways through GSK-3β | [110] | |
| Extracellular matrix (ECM)-derived peptide | Cell-cell adhesion molecule-derived peptide | N-cadherin mimetic peptide | N-cadherin contains a His-Ala-Val (HAV) sequence | Ac-HAVDIGGGC; Ac-HAVDIGGKLDLKLDLKLDL; GGGHAVDI | Suppression of canonical Wnt signaling by increasing GSK-3β, then GSK-3β-mediated degradation of β-catenin/LEF-1/TCF complex | [41,53,118,121,122] |
| ECM components-derived peptide | RGD | Integrin binding epitope in many ECM proteins | GGGGRGDY, GCGYGRGDSPG, GRGDSP, GGGGRGDSY | Activation of the Smad2 and ERK1/2 signaling pathways under the induction of TGF-β1 | [42,[127], [128], [129], [130],132] | |
| Collagen mimetic peptide (CMP) | ECM | (P(hydroxyl)PG)x | Lacking | [137,138] | ||
| GFOGER peptide | The amino acid residues 502–507 of the collagen I α1(I) chain | GFOGER | Lacking | [43,140,166] | ||
| Glycopeptides | ECM | – | Lacking | [143,144] | ||
| Link Protein N-terminal Peptide (LPP or link-N) | A cleaved N-terminal 16-amino peptide from link protein | DHLSDNYTLDHDRAIH | Activation of p-Smad1/5 through binding to BMPR-II and stimulating BMP-7 | [55,153,154] |
Table 2.
The optimal dosage and the efficacies of chondroinductive/chondroconductive peptides and their-functionalized biomaterials in inducing the in-vitro and in-vivo chondrogenesis.
| Name | Dosage | In-vitro efficacies | Functionalized material | In-vivo efficacies | Refs. |
|---|---|---|---|---|---|
| CK2.1 | 50 nM or 100 nM | At 3 weeks: Alcian blue staining 1.1 folds, pSBE (Smad binding element) luciferase activity 1.0 folds, collagen II 5.3 folds | Hyaluronan | The tail vein of C57BL/6J mice injection, C57BL/6J mice knee cartilage defects injection: increasing cartilage width, collagen II and IX expression | [99,104] |
| BMP peptide | 47 μM | At 3 weeks: glycosaminoglycans (GAGs) production 1.8 folds, total collagen content 1.7 folds, Sox9 1.2 folds, aggrecan 1.8 folds, COMP (cartilage oligomer matrix protein) 3.7 folds | Lacking | Lacking | [101,104,165] |
| B2A | 0.075–10 μg/mL | At 7 days: Sox9 1.3 folds, collagen II 1.6 folds | Lacking | Rat mono-iodoacetate (MIA)-induced osteoarthritis (OA) model injection: increasing cartilage GAGs and cartilaginous cells density | [38,109] |
| SPPEPS | 100 ng/mL | At 3 days: collagen II 1.6 folds | Pentenoate-functionalized hyaluronan (PHA) hydrogel | Lacking | [110] |
| N-cadherin mimetic peptide | 2 mM | At 3 days: collagen II 1.4 folds, aggrecan 1.8 folds, Sox9 1.7 folds; at 28 days: GAGs 1.5 folds, total collagen content 1.8 folds, chondroitin sulfate (CS) 1.39 folds, collagen II staining intensity 1.63 folds | MeHA hydrogels, KLD-12 hydrogel | Subcutaneous implantation in nude male mice: increasing GAGs and collagen content by 1.8 folds and 1.3 folds and more intense and distributed collagen II and CS staining by 1.62 folds and 1.59 folds; implantation in rabbit femoral condyle defects: promoting higher histological measures of overall defect filling, cartilage surface regularity, GAGs/cell content of neocartilage and adjacent cartilage | [41,53,118,121,122] |
| RGD | 1 mM or 1.97 mM | At 28 days: collagen II1.3 folds, aggrecan1.8 folds | Microcavitary alginate (MA) | Lacking | [42,[127], [128], [129], [130],132] |
| At 3 weeks: Sox910 folds, collagen IIA8.7 folds, collagen IIB6 folds | Sodium alginate | ||||
| At 3 days: Sox920 folds | Polyethylene glycol (PEG) | ||||
| At 7 days: Sox91.2 folds, collagen II1.9 folds, aggrecan3.1 folds | Cellulose-binding domain (CBD) | ||||
| Collagen mimetic peptide (CMP) | 250 μM or 500 μM | At 3 weeks: GAGs 2.5 folds, total collagen content 2.0 folds, aggrecan 2.5 folds and collagen II 2.0 folds | Poly (ethylene oxide) diacrylate (PEODA) hydrogel | Lacking | [137,138] |
| GFOGER peptide | 15 μM or 50 μM or 100 μM | At 7 days: collagen II 6.25 folds, aggrecan 10 folds, GAGs content 1.2 folds; at 21 days: collagen II 1.7 folds, aggrecan 1.3 folds, GAGs content 1.3 folds | PEG and CMP | Lacking | [43,140,166] |
| Glycopeptide | 10 mM | At 7 days: collagen II 10 folds, aggrecan 14 folds, Sox9 38 folds | Glc-PA/E-PA | Rabbit the full thickness osteochondral defect treated with microfracture model: distributing the staining of collagen II and Safranin-O and relatively low staining of collagen I | [143] |
| 0.6 mL, 6% (w/v) | At 14 days: aggrecan 4 folds and collagen II 20 folds | PPLG-g-Man/HPPA hydrogel | Subcutaneous implantation in nude male mice: increasing chondrocyte density and GAGs biosynthesis as well as increasing gene expression of collagen II and aggrecan | [144] | |
| Link Protein N-terminal Peptide (LPP or link-N) | 50 ng/mL | At 7 days: collagen II 0.9 folds, aggrecan 0.6 folds; at 14 days: collagen II 2.0 folds, aggrecan 1.1 folds | RADA16 hydrogel | Lacking | [55,153,154] |
Table 3.
The combination method of chondroinductive/chondroconductive peptides with biomaterials.
| Name | Biomaterial | Method | How | Ref. |
|---|---|---|---|---|
| CK2.1 | Hyaluronic acid (HA)-based hydrogel particles (HGPs) | Conjugate | Conjugation with the HGPs via Michael addition using cysteine-tagged peptide and acry-lated HGPs | [100] |
| SPPEPS | Pentanoate functionalized hyaluronic acid (PHA) | Conjugate | PHA is conjugated to GCGYGSPPEPS | [110] |
| N-cadherin mimetic peptide | KLD-12 hydrogels | Self-assembled | Not identified | [41] |
| MeHA hydrogels | Conjugate | N-cadherin mimic peptide (Ac-HAVDIGGGC) with a cysteine residue at the C-terminal end to permit Michael addition with MeHA hydrogels | [53] | |
| CMP | PEODA | Conjugate | PEODA conjugated with ACRL–PEG–CMP by photopolymerization of aqueous macromer solution | [138] |
| GFOGER | PEG and CMP | Conjugate | Conjugation with the PEG and CMP peptide via Michael addition | [43] |
| Glycopeptide | Glc-PA/E-PA | Mix | Glycopeptide or peptide amphiphiles are mixed to charged supramolecular gels | [143] |
| PPLG-g-Man/HPPA | Conjugate | Conjugation of PPLG with HPPA by enzyme-catalyzed crosslinking reaction | [144] | |
| RGD | Sodium alginate | Conjugate | GGGGRGDY peptide is conjugated to alginate via an amide bond between the terminal amine of the peptide and the carboxylate on sodium alginate | [128,129] |
| PEG | Conjugate | GCGYGRGDSPG peptide is conjugated to PEG via Cystein (C) introduced into the sequence using PEG-dithiol crosslinker | [130] | |
| Cellulose-binding domain (CBD) | Conjugate | The C-terminal of CBD is linked with proline–threonine (PT) to promote the exposure of GGGGRGDSY peptide to the outside structure of the chimeric protein. | [42] | |
| Agarose hydrogels | Conjugate | Using the heterobifunctional sulfo-SANPAH cross-linker to react the primary amines on the peptides with the NHS-ester group of sulfo-SANPAH to conjugate the GRGDSP peptide to agarose hydrogel | [132] | |
| LPP | RADA16 | Mix | RLN peptide powder mixed with RADA16 solution | [153] |
5.2. Summary of current research progress of bioactive peptides for cartilage tissue engineering
5.2.1. Growth factor-derived peptides
Chondroinductive growth factors (e.g. BMP-2) and their signaling pathways are one of the major sources to design and develop chondroinductive peptides for cartilage tissue engineering. BMP-2 is a highly chondroinductive, but it also bears a major disadvantage — inducing hypertrophy and mineralization of cartilage, finally leading to bone formation. Until now, there are three BMP-signaling derived peptides, such as BMP peptide, B2A, and CK2.1. The BMP peptide (KIPKASSVPTELSAISTLYL) is derived from residues 73–92 of the knuckle epitope of BMP-2 [101] (Fig. 2). Since the BMP peptide has been shown to induce ectopic bone formation [101], it can also be very promising chondroinductive peptide for cartilage tissue engineering. Unfortunately, there is, hitherto, only one publication reporting its effect on cartilage formation [104]. In comparison with BMP-2 protein, the efficacy of BMP peptide in inducing chondrogenic differentiation is much lower than BMP-2, whereas its efficacy in inducing hypertrophy/mineralization is also much lower than BMP-2. And there is a lack of data to show its capacity of inducing ectopic cartilage formation. The B2A peptide containing a BMP receptor-targeting domain (AISMLYLDENEKVVL) has been previously used only as a co-factor of BMP-2 to significantly amplify BMP-2-induced osteogenesis [38] (Fig. 2). For chondrogenesis, only one article shows that B2A alone enhances protein expression of collagen II and PDGF AA in murine C3H10T1/2 as well as enhance cartilage GAGs and cartilaginous cells density in a rat OA model [109]. There is still a lack of comparison with BMP-2 and the data of inducing ectopic cartilage formation. In contrast to BMP peptide and B2A, BMP signaling-derived peptide CK2.1 is designed to activate the BMP signaling pathway through inhibiting the binding of CK2 to the corresponding binding site (amino acids 466–469 (SYED)) on BMPR-IA [39], which is independent of BMP ligand (Fig. 2). 100 nM CK2.1 bears an even higher capacity of inducing chondrogenesis in both in-vitro (micromass model) and in-vivo (mice knee cartilage defects) in comparison with 40 nM BMP-2 [99]. Furthermore, the CK2.1 does not lead to hypertrophy or mineralization [99,100]. Interestingly, in comparison with BMP signaling-derived peptides, only one peptide SPPEPS has been found to adopt or develop peptides from another well-established chondroinductive growth factor TGF-β3. TGF-β3 can induce ectopic cartilage formation and is a major chondroinductive component in chondrogenic medium [162]. The SPPEPS peptide is derived from the LAP but not the critical binding domain of TGF-β3. Therefore, its potency in inducing chondrogenesis is very mild and only effective in inducing in-vitro collagen II.
In summary, BMP signaling-derived peptides have the following characters: 1) In comparison with BMP-2, CK2.1 bears a higher capacity of inducing chondrogenesis and does not induce any hypertrophy or mineralization of cartilage, which is very promising for cartilage tissue engineering; 2) BMP peptide is less effective in inducing chondrogenesis or hypertrophy than BMP-2; 3) However, there is still a lack of in-vivo data to prove their chondroinductivity and further experiments are highly needed to systematically evaluate their efficacies in cartilage tissue engineering; 4) B2A peptide and SPPEPS peptide seem more chondroconductive rather than chondroinductive.
5.2.2. Cell-cell adhesion molecule/ECM components-derived peptides
HAV is the conserved motif of N-cadherin and forms the key domain for various N-cadherin mimetic peptide (Fig. 3), such as Ac-HAVDIGGGC, N-Ac-CHAVDC-NH2, N-Ac-CHAVDIC-NH2 and N-Ac-CHAVDINC-NH2. N-cadherin mimetic peptide-conjugated materials only promote the in-vitro or in-vivo chondrogenesis in the presence of TGF-β3, which proves the chondroconductivity of N-cadherin mimetic peptides.
The bioactive domains on extracellular macromolecules of cartilage ECM are the main resource to design and develop bioactive peptides for cartilage tissue engineering. Similar as the structural and biochemical supporting function of ECM, most of ECM components-derived peptides are not chondroinductive. Instead, they are excellent candidates to modify and improve the chondroconductivity of biomaterials. For example, the soluble form of fibronectin-derived RGD motif inhibits cell adhesion and chondrogenesis, while various RGD peptides are active in promoting chondrogenesis when conjugated to biomaterials. RGD takes effect by binding integrin αvβ3 [134] (Fig. 3) and can dramatically amplifies TGF-β1-induced key chondrogenic signaling pathways, thereby promoting chondrogenesis [128]. In comparison, collagen type I-derived GFOGER peptide binds to integrin α2β1 (Fig. 3) and shows even higher activity in improving the chondroconductivity of hydrogels than RGD peptide [140].
Different from the integrin-binding peptides, CMP exhibits strong affinity to collagen I and forms a triple helix conformation that resembles the native protein structure of natural collagens [136] (Fig. 3). The co-conjugation of CMP and GFOGER to hydrogel significantly promotes chondrogenesis and reduces cartilage hypertrophy. Unfortunately, there is still a lack of data to show its in-vivo efficacy.
Among the ECM-derived peptides, LPP is the only one to activate BMP signaling so as to promote chondrogenesis. LPP binds to BMPR-II and stimulates a production of endogenous BMP-7 [55] (Fig. 3), thereby activating BMP signaling and chondrogenesis [55]. Furthermore, LPP has no effect on osteogenic induction [55,154]. However, there has been no study to show it in-vivo efficacy in promoting chondrogenesis.
Glycopeptide mimics natural glycoprotein in cartilage ECM, and has been shown to significantly enhance chondrogenesis when conjugated to biomaterials. However, there is a lack of data to clearly show its molecular mechanisms and its subcutaneous implantation does not induce de novo cartilage formation, which indicates its chondroconductive property.
In summary, cell-cell adhesion molecule/ECM components-derived peptides have the following characters: 1) RGD is the most investigated and well-established ECM-derived peptide to improve the chondroconductivity of biomaterials; 2) Another integrin-binding peptide GFOGER shows a better bioactivity for such an application; 3) Only one article shows the effect of co-conjugation CMP and GFOGER to biomaterial without the control with a single conjugation of either peptide, which makes it difficult to perform further comparison; 4) LPP can activate endogenous BMP synthesis to promote chondrogenesis without enhancing osteogenesis, while its effect hasn't yet been investigated in-vivo; 5) Glycopeptide is also a good candidate to promote chondroconductivity of biomaterials. However, further studies should be performed to elucidate its molecular mechanisms and the effect on osteogenesis.
5.3. The association between bioactive peptide and biomaterial
One advantage of peptide over proteinous growth factor is its modifiability, which allows an easier conjugation to materials. Most of the current chondroinductive/chondroconductive peptides, such as CK2.1, SPPEPS, N-cadherin mimetic peptide, GFOGER, glycopeptide and RGD are chemically conjugated to biomaterials for functionalization. The chemical conjugation methods include Michael addition and the use of various crosslinkers with temperature changes or UV irradiation. On the other hand, LPP and glycopeptides are mixed with biomaterials, while N-Cadherin and biomaterials are self-assembled (Table 3).
5.4. Advantages/disadvantages of chondroinductive/chondroconductive peptide and their-functionalized biomaterials
Growth factors, such as BMP-2 and TGF-β3 have been proven to be potently chondroinductive [24,25]. In comparison with the proteinous growth factors, the use of chondroinductive/chondroconductive peptides are associated with various advantages, such as lower cost, higher yield, lower immunogenicity, more stable structure, more modifiable and easier conjugation to materials [25]. Some peptides, such as CK2.1 bears an even higher efficacy in inducing chondrogenesis in comparison with BMP-2. Furthermore, the peptides induce no or much less hypertrophy and mineralization [27], which is highly important to maintain the hemostasis of newly formed cartilage. In comparison with naturally derived materials, peptide-functionalized synthetic materials bear more reproducibility, controllable immunogenicity, desirable complex structures and predictable biological functions. On the other hand, most of these peptides are chondroconductive rather than chondroinductive. For most of the peptides, there is still a lack of studies to reveal their mechanisms and systematically optimize their in-vitro and in-vivo efficacies, which hinders their clinical application.
5.5. Prospective research direction
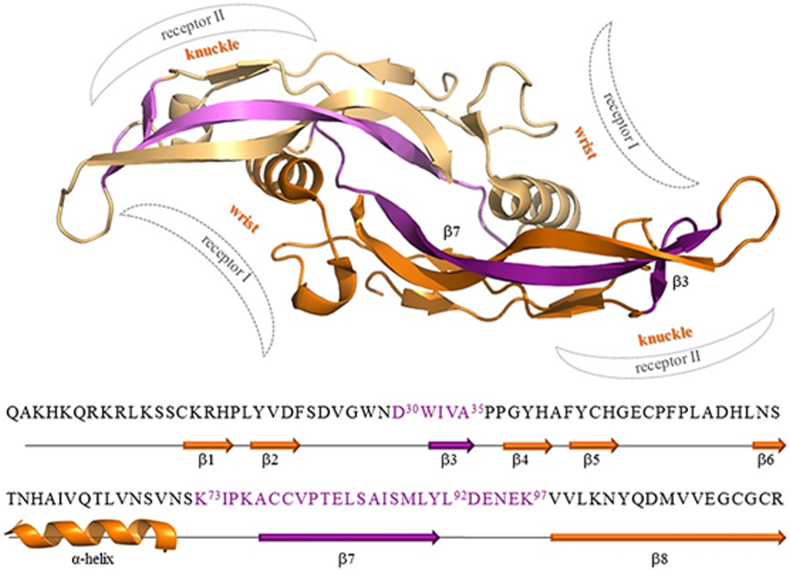
Chondroinductive peptide-functionalized biomaterials are supposed to induce de novo cartilage formation, which is highly important for cartilage tissue engineering. In fact, there are also many other BMP peptides, such as BMP97 peptide (KIPKASSVPTELSAISMLYLDENEK) and BMPchim peptide (KIPKASSVPTELSAISMLYLGPGGDWIVA) (Fig. 8), whose chondroinductive potentials haven't been investigated. The BMPchim peptide is much more potent in inducing osteoblastogenesis and activating BMP signaling than the BMP peptide (KIPKASSVPTELSAISTLYL) [164]. Of course, such a property may also raise the concern of inducing hypertrophy and mineralization of cartilage. Further studies are highly needed to evaluate the application potential of these BMP peptides in cartilage tissue engineering.
Fig. 8.
Ribbon representation of the BMP-2 dimer (pdbcode 2h62, chains A and B), with the two monomers colored in gold and orange, respectively. The wrist and knuckle regions, responsible for the binding to the type I and II receptors, are schematically indicated. At the bottom, both the primary and secondary structures of the BMP-2 monomer are reported. The purple-colored BMP-2 fragments are selected to design various BMP-derived peptides, such as the BMP peptide: K73IPKASSVPTELSAISMLYL92; BMP97 peptide: K73IPKASSVPTELSAISMLYLDENEK97; BMPchim peptide: K73IPKASSVPTELSAISMLYL92GPGGD30WIVA. © 2015 Reprinted with permission of John Wiley and Sons [164]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
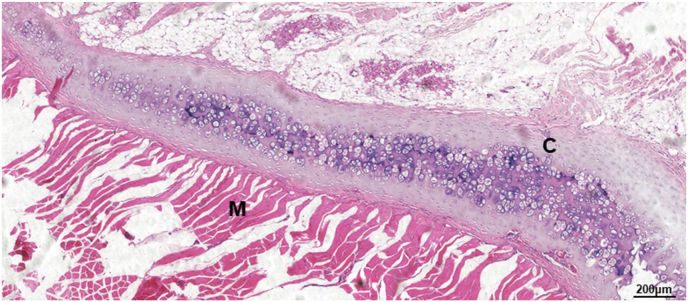
On the other hand, designing and developing novel and potent chondroinductive peptide is always a major research direction. As above mentioned, there is still no peptide mimicking the critical binding domain of TGF-β3. In our ongoing study, by mimicking the critical binding domain of TGF-β3 with its type II receptor, we have designed in total 10 peptides, one of which with collagen membrane as carrier can induce ectopic cartilage formation after implantation in erector spinae of Sprague-Dawley (SD) rats (Fig. 9). We are now performing in-vitro cellular experiments and in-vivo study to systematically characterize its chondroinductive effects of this peptide and hope to present the data in our near-future publication. In addition, FGFs and IGFs can also be the source to design chondroinductive/chondroconductive peptides.
Fig. 9.
Light micrographs of hematoxylin-eosin (HE) stained cross-sections of a TGF-β3-derived peptide-containing collagen membrane that was implanted in erector spinae of Sprague-Dawley (SD) rats for 21 days. Tremendous amount of de novo cartilage was induced by the TGF-β3-derived peptide. (C: cartilage; M: muscle). Scale bar = 200 μm.
To promote the clinical application of peptide-based biomaterials, the selection of biomaterial is also important. It is much more advantageous in the regulatory aspect to adopt the clinically available and chondroconductive biomaterials, such as collagen, gelatin, etc than novel biomaterials. Furthermore, extensive studies should be performed to systematically investigate and optimize in-vitro and in-vivo efficacies of peptide-based biomaterials to promote their clinical application.
6. Conclusions
Cartilage tissue engineering techniques are promising alternatives to current clinical treatment options. In comparison with proteinous growth factors, the application of chondroinductive/chondroconductive peptides are associated with various advantages, such as higher production yield, lower cost, lower immunogenicity, more stable structure, more modifiability and easier conjugation to materials. Hitherto, there two large categories chondroinductive/chondroconductive peptides: 1) growth factor-derived peptides and 2) cell-cell adhesion molecule/ECM components-derived peptides. In comparison with BMP-2, CK2.1 bears an even higher efficacy in inducing chondrogenesis and does not induces any hypertrophy or mineralization of cartilage, which is very promising for cartilage tissue engineering. Most of the other bioactive peptides are chondroconductive, which can be used to improve the chondroconductivity of biomaterials. According to the limited data, GFOGER seems more effective in improving the chondroconductivity of biomaterials than RGD. For some peptides, such as glycopeptide, the molecular mechanisms for their biological effects are not revealed. Further research may focus on the evaluation, design and development of more potent chondroinductive peptides and their-functionalized biomaterials. More systematic studies should be performed to investigate and optimize the in-vitro and in-vivo efficacies of chondroinductive/chondroconductive peptide-based biomaterials for cartilage tissue engineering.
CRediT authorship contribution statement
Mingjing Zhu: Conceptualization, Writing – original draft, Writing – review & editing. Wenchao Zhong: Writing – original draft, Figure editing. Wei Cao: Writing – original draft, Data curation. Qingbin Zhang: Conceptualization, Writing – original draft, Supervision. Gang Wu: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study is supported by the grants from Key Research and Development Plan of Zhejiang Province (No. 2021C04013).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.07.004.
Contributor Information
Qingbin Zhang, Email: doctorqingbin@hotmail.com.
Gang Wu, Email: g.wu@acta.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chiang H., Jiang C.-C. Repair of articular cartilage defects: review and perspectives. J. Formos. Med. Assoc. 2009;108(2):87–101. doi: 10.1016/S0929-6646(09)60039-5. [DOI] [PubMed] [Google Scholar]
- 2.Newman A.P. Articular cartilage repair. Am. J. Sports Med. 1998;26(2):309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 3.Mankin H.J. The response of articular cartilage to mechanical injury, the Journal of bone and joint surgery. American volume. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 4.Buckwalter J.J.C.o., research r. vol. 402. 2002. Articular cartilage injuries; pp. 21–37. [DOI] [PubMed] [Google Scholar]
- 5.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., Mandelbaum B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee an evidence-based systematic analysis. Am. J. Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 6.Redman S.N., Oldfield S.F., Archer C.W. Current strategies for articular cartilage repair. Eur. Cell. Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. discussion 23-32. [DOI] [PubMed] [Google Scholar]
- 7.Bae D.K., Yoon K.H., Song S.J. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy-the Journal of Arthroscopic and Related Surgery. 2006;22(4):367–374. doi: 10.1016/j.arthro.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Hangody L., Kish G., Karpati Z., Udvarhelyi I., Szigeti I., Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998;21(7):751–756. doi: 10.3928/0147-7447-19980701-04. [DOI] [PubMed] [Google Scholar]
- 11.Solheim E., Hegna J., Oyen J., Austgulen O.K., Harlem T., Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17(1):84–87. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Daher R.J., Chahine N.O., Greenberg A.S., Sgaglione N.A., Grande D.A. New methods to diagnose and treat cartilage degeneration. Nat. Rev. Rheumatol. 2009;5(11):599–607. doi: 10.1038/nrrheum.2009.204. [DOI] [PubMed] [Google Scholar]
- 13.Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin. Orthop. Relat. Res. 2001;391(Suppl):S349–S361. doi: 10.1097/00003086-200110001-00032. [DOI] [PubMed] [Google Scholar]
- 14.Peterson L., Vasiliadis H.S., Brittberg M., Lindahl A. Autologous chondrocyte implantation A long-term follow-up. Am. J. Sports Med. 2010;38(6):1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 15.Minas T., Von Keudell A., Bryant T., Gomoll A.H. The John insall award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin. Orthop. Relat. Res. 2014;472(1):41–51. doi: 10.1007/s11999-013-3146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinatier C., Guicheux J. Cartilage tissue engineering: from biomaterials and stem cells to osteoarthritis treatments, Ann. Phys. Rehabil. Med. 2016;59(3):139–144. doi: 10.1016/j.rehab.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Daly A.C., Freeman F.E., Gonzalez-Fernandez T., Critchley S.E., Nulty J., Kelly D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthcare Mater. 2017;6(22):20. doi: 10.1002/adhm.201700298. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Hu J., Athanasiou K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009;37(1–2):1–57. doi: 10.1615/critrevbiomedeng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai V., Dilisio M.F., Dietz N.E., Agrawal D.K. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J. Biomed. Mater. Res. 2017;105(8):2343–2354. doi: 10.1002/jbm.a.36087. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y.B., Liu X.C., Zeng L.D., Zhang J., Zuo J.L., Zou J., Ding J.X., Chen X.S. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29(36):20. [Google Scholar]
- 21.Mahzoon S., Detamore M.S. Chondroinductive peptides: drawing inspirations from cell-matrix interactions. Tissue Eng. B Rev. 2019;25(3):249–257. doi: 10.1089/ten.TEB.2018.0003. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone B., Stoddart M.J., Im G.-I. Multi-disciplinary approaches for cell-based cartilage regeneration. J. Orthop. Res. 2020;38(3):463–472. doi: 10.1002/jor.24458. [DOI] [PubMed] [Google Scholar]
- 23.Le H., Xu W., Zhuang X., Chang F., Wang Y., Ding J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420943839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puetzer J.L., Petitte J.N., Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng. B Rev. 2010;16(4):435–444. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 25.Augustyniak E., Trzeciak T., Richter M., Kaczmarczyk J., Suchorska W. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int. Orthop. 2015;39(5):995–1003. doi: 10.1007/s00264-014-2619-0. [DOI] [PubMed] [Google Scholar]
- 26.Tan A.R., Hung C.T. Concise review: mesenchymal stem cells for functional cartilage tissue engineering: taking cues from chondrocyte-based constructs. Stem Cells Trans. Med. 2017;6(4):1295–1303. doi: 10.1002/sctm.16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X.H., Biedrzycki A.H., Khalil A.S., Hess D., Umhoefer J.M., Markel M.D., Murphy W.L. Nanostructured mineral coatings stabilize proteins for therapeutic delivery. Adv. Mater. 2017;29(33):9. doi: 10.1002/adma.201701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreejalekshmi K.G., Nair P.D. Biomimeticity in tissue engineering scaffolds through synthetic peptide modifications-Altering chemistry for enhanced biological response. J. Biomed. Mater. Res. 2011;96A(2):477–491. doi: 10.1002/jbm.a.32980. [DOI] [PubMed] [Google Scholar]
- 29.Padhi A., Sengupta M., Sengupta S., Roehm K.H., Sonawane A. Antimicrobial peptides and proteins in mycobacterial therapy: current status and future prospects. Tuberculosis. 2014;94(4):363–373. doi: 10.1016/j.tube.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Buchwald H., Dorman R.B., Rasmus N.F., Michalek V.N., Landvik N.M., Ikramuddin S. Effects on GLP-1, PYY, and leptin by direct stimulation of terminal ileum and cecum in humans: implications for ileal transposition. Surg. Obes. Relat. Dis. 2014;10(5):780–786. doi: 10.1016/j.soard.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano C., Marchio M., Timofeeva E., Biagini G. Neuroactive peptides as putative mediators of antiepileptic ketogenic diets. Front. Neurol. 2014;5:14. doi: 10.3389/fneur.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson S.D., Safavi-Hemami H., McIntosh L.D., Purcell A.W., Norton R.S., Papenfuss A.T. Diversity of conotoxin gene superfamilies in the venomous snail, Conus victoriae. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0087648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Li D., Ding J.X., Chen X.S. Controlled synthesis of polypeptides. Chin. Chem. Lett. 2020;31(12):3001–3014. [Google Scholar]
- 34.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Lee A.C.L., Harris J.L., Khanna K.K., Hong J.H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019;20(10):21. doi: 10.3390/ijms20102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biz M. 2016. Global IndustryAnalysis, Size, Share, Growth, Trends and Forecast, Transparency Market Research; pp. 122–313. [Google Scholar]
- 37.Hastar N., Arslan E., Guler M.O., Tekinay A.B. Peptide-based materials for cartilage tissue regeneration. Adv. Exp. Med. Biol. 2017;1030:155–166. doi: 10.1007/978-3-319-66095-0_7. [DOI] [PubMed] [Google Scholar]
- 38.Lin X., Zamora P.O., Albright S., Glass J.D., Pena L.A. Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro. J. Bone Miner. Res. 2005;20(4):693–703. doi: 10.1359/JBMR.041104. [DOI] [PubMed] [Google Scholar]
- 39.Bragdon B., Thinakaran S., Moseychuk O., King D., Young K., Litchfield D.W., Petersen N.O., Nohe A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys. J. 2010;99(3):897–904. doi: 10.1016/j.bpj.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore N.M., Lin N.J., Gallant N.D., Becker M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011;7(5):2091–2100. doi: 10.1016/j.actbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Li R., Xu J.B., Wong D.S.H., Li J.M., Zhao P.C., Bian L.M. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/beta-catenin signaling. Biomaterials. 2017;145:33–43. doi: 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Chang J.-C., Hsu S.-h., Chen D.C. The promotion of chondrogenesis in adipose-derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomaterials. 2009;30(31):6265–6275. doi: 10.1016/j.biomaterials.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 43.Liu S.Q., Tian Q., Hedrick J.L., Hui J.H.P., Ee P.L.R., Yang Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31(28):7298–7307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., Lopez-Puertas J.M., Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng. B Rev. 2010;16(6):617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 45.Carballo C.B., Nakagawa Y., Sekiya I., Rodeo S.A. Basic science of articular cartilage. Clin. Sports Med. 2017;36(3):413–+. doi: 10.1016/j.csm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Gelse K., Poschl E., Aigner T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Zelenski N.A., Leddy H.A., Sanchez-Adams J., Zhang J.Z., Bonaldo P., Liedtke W., Guilak F. Type VI collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage. Arthritis & Rheumatology. 2015;67(5):1286–1294. doi: 10.1002/art.39034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eyre D.R., Weis M.A., Wu J.-J. Articular cartilage collagen: an irreplaceable framework? Eur. Cell. Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 49.Culav E.M., Clark C.H., Merrilees M.J. Connective tissues: matrix composition and its relevance to physical therapy. Phys. Ther. 1999;79(3):308–319. [PubMed] [Google Scholar]
- 50.B.J. Dzamba, D.W. DeSimone, Extracellular matrix (ECM) and the sculpting of embryonic tissues, in: E.S. Litscher, P.M. Wassarman (Eds.), Extracellular Matrix and Egg Coats2018, pp. 245-274. [DOI] [PubMed]
- 51.Knudson C.B. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly, Birth defects research. Part C, Embryo today : reviews. 2003;69(2):174–196. doi: 10.1002/bdrc.10013. [DOI] [PubMed] [Google Scholar]
- 52.Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 53.Bian L.M., Guvendiren M., Mauck R.L., Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. U.S.A. 2013;110(25):10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardingham T.E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem. J. 1979;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z., Weitzmann M.N., Sangadala S., Hutton W.C., Yoon S.T. Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J. Biol. Chem. 2013;288(39):28243–28253. doi: 10.1074/jbc.M113.451948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freedman B.R., Bade N.D., Riggin C.N., Zhang S., Haines P.G., Ong K.L., Janmey P.A. The (dys)functional extracellular matrix. Biochim. Biophys. Acta Mol. Cell Res. 2015;1853(11):3153–3164. doi: 10.1016/j.bbamcr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rozario T., DeSimone D.W. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 2010;341(1):126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuan R.S. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2, the Journal of bone and joint surgery. American. 2003;85–(A Suppl 2):137–141. doi: 10.2106/00004623-200300002-00019. [DOI] [PubMed] [Google Scholar]
- 59.Goldring M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012;4(4):269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morikawa M., Derynck R., Miyazono K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 2016;8(5):24. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patil A.S., Sable R.B., Kothari R.M. An update on transforming growth factor-beta (TGF-beta): sources, types, functions and clinical applicability for cartilage/bone healing. J. Cell. Physiol. 2011;226(12):3094–3103. doi: 10.1002/jcp.22698. [DOI] [PubMed] [Google Scholar]
- 62.Clark D.A., Coker R. Transforming growth factor-beta (TGF-beta) Int. J. Biochem. Cell Biol. 1998;30(3):293–298. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 63.Barry F., Boynton R.E., Liu B., Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 64.Wrana J.L., Attisano L., Wieser R., Ventura F., Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 65.Souchelnytskyi S., Tamaki K., Engstrom U., Wernstedt C., ten Dijke P., Heldin C.H. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-beta signaling. J. Biol. Chem. 1997;272(44):28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- 66.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe H., de Caestecker M.P., Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 68.Ducy P., Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57(6):2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 69.Urist M.R. Bone: formation by autoinduction. Science (New York, N.Y.). 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 70.Wang E.A., Rosen V., Cordes P., Hewick R.M., Kriz M.J., Luxenberg D.P., Sibley B.S., Wozney J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. U.S.A. 1988;85(24):9484–9488. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levi B., Hyun J.S., Nelson E.R., Li S., Montoro D.T., Wan D.C., Jia F.J., Glotzbach J.C., James A.W., Lee M., Huang M., Quarto N., Gurtner G.C., Wu J.C., Longaker M.T. Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cell. 2011;29(12):2018–2029. doi: 10.1002/stem.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tseng Y.H., Kokkotou E., Schulz T.J., Huang T.L., Winnay J.N., Taniguchi C.M., Tran T.T., Suzuki R., Espinoza D.O., Yamamoto Y., Ahrens M.J., Dudley A.T., Norris A.W., Kulkarni R.N., Kahn C.R. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000. doi: 10.1038/nature07221. U44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H.J., Im G.I. Combination of transforming growth factor-beta(2) and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng. 2009;15(7):1543–1551. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- 74.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 75.Sieber C., Kopf J., Hiepen C., Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20(5–6):343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Nohe A., Hassel S., Ehrlich M., Neubauer F., Sebald W., Henis Y.I., Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002;277(7):5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 77.Miyazono K. Signal transduction by bone morphogenetic protein receptors: functional roles of Smad proteins. Bone. 1999;25(1):91–93. doi: 10.1016/s8756-3282(99)00113-1. [DOI] [PubMed] [Google Scholar]
- 78.Xiao G., Gopalakrishnan R., Jiang D., Reith E., Benson M.D., Franceschi R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 2002;17(1):101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 79.Guicheux J., Lemonnier J., Ghayor C., Suzuki A., Palmer G., Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J. Bone Miner. Res. 2003;18(11):2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- 80.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17(24):2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 81.Hoffmann A., Preobrazhenska O., Wodarczyk C., Medler Y., Winkel A., Shahab S., Huylebroeck D., Gross G., Verschueren K. Transforming growth factor-beta-activated kinase-1 (TAK1), a MAP3K, interacts with Smad proteins and interferes with osteogenesis in murine mesenchymal progenitors. J. Biol. Chem. 2005;280(29):27271–27283. doi: 10.1074/jbc.M503368200. [DOI] [PubMed] [Google Scholar]
- 82.Chun J.-S., Oh H., Yang S., Park M. Wnt signaling in cartilage development and degeneration. Bmb Reports. 2008;41(7):485–494. doi: 10.5483/bmbrep.2008.41.7.485. [DOI] [PubMed] [Google Scholar]
- 83.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 84.Shimizu T., Kagawa T., Inoue T., Nonaka A., Takada S., Aburatani H., Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the notch/RBP-J kappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell Biol. 2008;28(24):7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaur T., Lengner C.J., Hovhannisyan H., Bhat R.A., Bodine P.V.N., Komm B.S., Javed A., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 86.Akiyama H., Lyons J.P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J.M., Taketo M.M., Nakamura T., Behringer R.R., McCrea P.D., de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reinhold M.I., Kapadia R.M., Liao Z.X., Naski M.C. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J. Biol. Chem. 2006;281(3):1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- 88.Lefebvre V., Angelozzi M., Haseeb A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019;61:39–47. doi: 10.1016/j.ceb.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zehentner B.K., Dony C., Burtscher H. The transcription factor Sox9 is involved in BMP-2 signaling. J. Bone Miner. Res. : the official journal of the American Society for Bone and Mineral Research. 1999;14(10):1734–1741. doi: 10.1359/jbmr.1999.14.10.1734. [DOI] [PubMed] [Google Scholar]
- 90.Gao L., Sheu T.J., Dong Y.F., Hoak D.M., Zuscik M.J., Schwarz E.M., Hilton M.J., O'Keefe R.J., Jonason J.H. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J. Cell Sci. 2013;126(24):5704–5713. doi: 10.1242/jcs.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smits P., Li P., Mandel J., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B., Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell. 2001;1(2):277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 92.Kisiday J., Jin M., Kurz B., Hung H., Semino C., Zhang S., Grodzinsky A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl. Acad. Sci. U.S.A. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camarero-Espinosa S., Cooper-White J.J. Combinatorial presentation of cartilage-inspired peptides on nanopatterned surfaces enables directed differentiation of human mesenchymal stem cells towards distinct articular chondrogenic phenotypes. Biomaterials. 2019;210:105–115. doi: 10.1016/j.biomaterials.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Patel M., Lee H., Park S., Kim Y., Jeong B.J.B. vol. 159. 2018. Injectable Thermogel for 3D Culture of Stem Cells; pp. 91–107. [DOI] [PubMed] [Google Scholar]
- 95.Singh N., Lee D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Contr. Release. 2014;193:214–227. doi: 10.1016/j.jconrel.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 96.Liu H., Cheng Y.L., Chen J.J., Chang F., Wang J.C., Ding J.X., Chen X.S. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi: 10.1016/j.actbio.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y.B., Yu J.K., Ren K.X., Zuo J.L., Ding J.X., Chen X.S. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules. 2019;20(4):1478–1492. doi: 10.1021/acs.biomac.9b00043. [DOI] [PubMed] [Google Scholar]
- 98.Senta H., Park H., Bergeron E., Drevelle O., Fong D., Leblanc E., Cabana F., Roux S., Grenier G., Faucheux N. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20(3):213–222. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Akkiraju H., Bonor J., Nohe A. CK2.1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2017;35(4):876–885. doi: 10.1002/jor.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akkiraju H., Srinivasan P.P., Xu X., Jia X.Q., Safran C.B.K., Nohe A. CK2.1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Res. Ther. 2017;8:11. doi: 10.1186/s13287-017-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saito A., Suzuki Y., Ogata S.i., Ohtsuki C., Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim. Biophys. Acta. 2003;1651(1–2):60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 102.Saito A., Suzuki Y., Kitamura M., Ogata S.I., Yoshihara Y., Masuda S., Ohtsuki C., Tanihara M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J. Biomed. Mater. Res. 2006;77A(4):700–706. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 103.He X.Z., Ma J.Y., Jabbari E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir. 2008;24(21):12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 104.Renner J.N., Kim Y., Liu J.C. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2012;18(23–24):2581–2589. doi: 10.1089/ten.TEA.2011.0400. [DOI] [PubMed] [Google Scholar]
- 105.Lin X., Elliot J.J., Carnes D.L., Fox W.C., Pena L.A., Campion S.L., Takahashi K., Atkinson B.L., Zamora P.O. Augmentation of osseous phenotypes in vivo with a synthetic peptide. J. Orthop. Res. 2007;25(4):531–539. doi: 10.1002/jor.20303. [DOI] [PubMed] [Google Scholar]
- 106.Glazebrook M., Younger A., Zamora P., Lalonde K.A. B2A Peptide Bone Graft Substitute: Literature Review, Biology and Use for Hindfoot Fusions. Curr. Orthop. Practice. 2013;24(5):476. [Google Scholar]
- 107.Verrecchio A., Germann M.W., Schick B.P., Kung B., Twardowski T., San Antonio J.D. Design of peptides with high affinities for heparin and endothelial cell proteoglycans. J. Biol. Chem. 2000;275(11):7701–7707. doi: 10.1074/jbc.275.11.7701. [DOI] [PubMed] [Google Scholar]
- 108.Buku A., Eggena P., Wyssbrod H.R., Schwartz I.L., Gazis D., Somoza L.I., Glass J.D. 1-Desamino, 7-lysine, 8-arginine vasotocin: attachment of reporter groups and affinity ligands through the lysine side chain. J. Med. Chem. 1987;30(8):1526–1529. doi: 10.1021/jm00391a045. [DOI] [PubMed] [Google Scholar]
- 109.Lin X., Shanmugasundaram S., Liu Y., Derrien A., Nurminskaya M., Zamora P.O. B2A peptide induces chondrogenic differentiation in vitro and enhances cartilage repair in rats. J. Orthop. Res. 2012;30(8):1221–1228. doi: 10.1002/jor.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahzoon S., Townsend J.M., Lam T.N., Sjoelund V., Detamore M.S. Effects of a bioactive SPPEPS peptide on chondrogenic differentiation of mesenchymal stem cells. Ann. Biomed. Eng. 2019;47(11):2308–2321. doi: 10.1007/s10439-019-02306-0. [DOI] [PubMed] [Google Scholar]
- 111.Ludbrook S.B., Barry S.T., Delves C.J., Horgan C.M.T. The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem. J. 2003;369(Pt 2):311–318. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aplin A., Howe A., Alahari S., Juliano R.J.P.r. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules. and selectins. 1998;50(2):197–263. [PubMed] [Google Scholar]
- 113.Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 114.Ruoslahti E., Pierschbacher M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 115.Arslan E., Garip I.C., Gulseren G., Tekinay A.B., Guler M.O. Bioactive supramolecular peptide nanofi bers for regenerative medicine. Advanced Healthcare Materials. 2014;3(9):1357–1376. doi: 10.1002/adhm.201300491. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki S.T. Protocadherins and diversity of the cadherin superfamily. J. Cell Sci. 1996;109(Pt 11):2609–2611. doi: 10.1242/jcs.109.11.2609. [DOI] [PubMed] [Google Scholar]
- 117.Hynes R.O. Specificity of cell adhesion in development: the cadherin superfamily. Curr. Opin. Genet. Dev. 1992;2(4):621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- 118.Cimenci C.E., Kurtulus G.U., Caliskan O.S., Guler M.O., Tekinay A.B. N-cadherin mimetic peptide nanofiber system induces chondrogenic differentiation of mesenchymal stem cells. Bioconjugate Chem. 2019;30(9):2417–2426. doi: 10.1021/acs.bioconjchem.9b00514. [DOI] [PubMed] [Google Scholar]
- 119.Blaschuk O.W., Sullivan R., David S., Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev. Biol. 1990;139(1):227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 120.Williams E., Williams G., Gour B.J., Blaschuk O.W., Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J. Biol. Chem. 2000;275(6):4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- 121.Kwon M.Y., Vega S.L., Gramlich W.M., Kim M., Mauck R.L., Burdick J.A. Dose and timing of N-cadherin mimetic peptides regulate MSC chondrogenesis within hydrogels. Advanced Healthcare Materials. 2018;7(9):10. doi: 10.1002/adhm.201701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo J.L., Kim Y.S., Koons G.L., Lam J., Navara A.M., Barrios S., Xie V.Y., Watson E., Smith B.T., Pearce H.A., Orchard E.A., van den Beucken J.J.J.P., Jansen J.A., Wong M.E., Mikos A.G. Bilayered, peptide-biofunctionalized hydrogels for in vivo osteochondral tissue repair. Acta Biomater. 2021;128:120–129. doi: 10.1016/j.actbio.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hynes R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 124.Ruoslahti E. Integrins. J. Clin. Invest. 1991;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pierschbacher M.D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 126.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 127.Connelly J.T., Garcia A.J., Levenston M.E. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28(6):1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 128.Re'em T., Tsur-Gang O., Cohen S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGF beta 1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials. 2010;31(26):6746–6755. doi: 10.1016/j.biomaterials.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Yao Y., Zeng L., Huang Y. The enhancement of chondrogenesis of ATDC5 cells in RGD-immobilized microcavitary alginate hydrogels. J. Biomater. Appl. 2016;31(1):92–101. doi: 10.1177/0885328216640397. [DOI] [PubMed] [Google Scholar]
- 130.Liu S.Q., Tian Q.A., Wang L., Hedrick J.L., Hui J.H.P., Yang Y.Y., Ee P.L.R. Injectable biodegradable poly(ethylene glycol)/RGD peptide hybrid hydrogels for in vitro chondrogenesis of human mesenchymal stem cells. Macromol. Rapid Commun. 2010;31(13):1148–1154. doi: 10.1002/marc.200900818. [DOI] [PubMed] [Google Scholar]
- 131.Teng B., Zhang S., Pan J., Zeng Z., Chen Y., Hei Y., Fu X., Li Q., Ma M., Sui Y., Wei S.J.A.b. 2021. A Chondrogenesis Induction System Based on a Functionalized Hyaluronic Acid Hydrogel Sequentially Promoting hMSC Proliferation, Condensation, Differentiation, and Matrix Deposition. [DOI] [PubMed] [Google Scholar]
- 132.Connelly J.T., Garcia A.J., Levenston M.E. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J. Cell. Physiol. 2008;217(1):145–154. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- 133.Jockusch B.M., Bubeck P., Giehl K., Kroemker M., Moschner J., Rothkegel M., Rudiger M., Schluter K., Stanke G., Winkler J. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 134.Redick S.D., Settles D.L., Briscoe G., Erickson H.P. Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J. Cell Biol. 2000;149(2):521–527. doi: 10.1083/jcb.149.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keselowsky B.G., Collard D.M., Garcia A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang A.Y., Mo X., Chen C.S., Yu S.M. Facile modification of collagen directed by collagen mimetic peptides. J. Am. Chem. Soc. 2005;127(12):4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 137.Lee H.J., Lee J.S., Chansakul T., Yu C., Elisseeff J.H., Yu S.M. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27(30):5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Lee H.J., Yu C., Chansakul T., Hwang N.S., Varghese S., Yu S.M., Elisseeff J.H. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng. 2008;14(11):1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 139.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., Barnes M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 140.Mhanna R., Ozturk E., Vallmajo-Martin Q., Millan C., Muller M., Zenobi-Wong M. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2014;20(7–8):1165–1174. doi: 10.1089/ten.tea.2013.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U.S.A. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonduelle C., Lecommandoux S. Synthetic glycopolypeptides as biomimetic analogues of natural glycoproteins. Biomacromolecules. 2013;14(9):2973–2983. doi: 10.1021/bm4008088. [DOI] [PubMed] [Google Scholar]
- 143.Yaylaci S.U., Ekiz M.S., Arslan E., Can N., Kilic E., Ozkan H., Orujalipoor I., Ide S., Tekinay A.B., Guler M.O. Supramolecular GAG-like self-assembled glycopeptide nanofibers induce chondrogenesis and cartilage regeneration. Biomacromolecules. 2016;17(2):679–689. doi: 10.1021/acs.biomac.5b01669. [DOI] [PubMed] [Google Scholar]
- 144.Ren K.X., He C.L., Xiao C.S., Li G., Chen X.S. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials. 2015;51:238–249. doi: 10.1016/j.biomaterials.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 145.Seyfried N.T., McVey G.F., Almond A., Mahoney D.J., Dudhia J., Day A.J. Expression and purification of functionally active hyaluronan-binding domains from human cartilage link protein, aggrecan and versican: formation of ternary complexes with defined hyaluronan oligosaccharides. J. Biol. Chem. 2005;280(7):5435–5448. doi: 10.1074/jbc.M411297200. [DOI] [PubMed] [Google Scholar]
- 146.Liu H., McKenna L.A., Dean M.F. An N-terminal peptide from link protein can stimulate biosynthesis of collagen by human articular cartilage. Arch. Biochem. Biophys. 2000;378(1):116–122. doi: 10.1006/abbi.2000.1758. [DOI] [PubMed] [Google Scholar]
- 147.Liu H., McKenna L.A., Dean M. An N-terminal peptide from link protein stimulates synthesis of cartilage proteoglycans. Biochem. Soc. Trans. 1997;25(3):427S. doi: 10.1042/bst025427s. 427S. [DOI] [PubMed] [Google Scholar]
- 148.Liu H., McKenna L.A., Dean M.F. The macromolecular characteristics of cartilage proteoglycans do not change when synthesis is up-regulated by link protein peptide. Biochim. Biophys. Acta. 1999;1428(2–3):191–200. doi: 10.1016/s0304-4165(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 149.Petit A., Yao G., Al Rowas S., Gawri R., Epure L., Antoniou J., Mwale F. Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng. 2011;17(7–8):899–904. doi: 10.1089/ten.TEA.2010.0494. [DOI] [PubMed] [Google Scholar]
- 150.Mwale F., Demers C.N., Petit A., Roughley P., Poole A.R., Steffen T., Aebi M., Antoniou J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J. Cell. Biochem. 2003;88(6):1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 151.Ma K., Wu Y., Wang B., Yang S., Wei Y., Shao Z. Effect of a synthetic link N peptide nanofiber scaffold on the matrix deposition of aggrecan and type II collagen in rabbit notochordal cells. J. Mater. Sci. Mater. Med. 2013;24(2):405–415. doi: 10.1007/s10856-012-4811-3. [DOI] [PubMed] [Google Scholar]
- 152.Wang B.C., Wu Y.C., Shao Z.W., Yang S.H., Che B., Sun C.X., Ma Z.L., Zhang Y.N. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. J. Biomed. Mater. Res. 2012;100A(3):646–653. doi: 10.1002/jbm.a.33300. [DOI] [PubMed] [Google Scholar]
- 153.Wang B., Sun C., Shao Z., Yang S., Che B., Wu Q., Liu J. Designer self-assembling peptide nanofiber scaffolds containing link protein N-terminal peptide induce chondrogenesis of rabbit bone marrow stem cells. BioMed Res. Int. 2014:2014. doi: 10.1155/2014/421954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He R.J., Wang B.C., Cui M., Xiong Z.K., Lin H., Zhao L., Li Z.L., Wang Z., Peggrem S., Xia Z.D., Shao Z.W. Link protein N-terminal peptide as a potential stimulating factor for stem cell-based cartilage regeneration. Stem Cell. Int. 2018;2018:11. doi: 10.1155/2018/3217895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Campos Y., Almirall A., Fuentes G., Bloem H.L., Kaijzel E.L., Cruz L.J. Tissue engineering: an alternative to repair cartilage. Tissue Eng. B Rev. 2019;25(4):357–373. doi: 10.1089/ten.TEB.2018.0330. [DOI] [PubMed] [Google Scholar]
- 156.Mustapich T., Schwartz J., Palacios P., Liang H.X., Sgaglione N., Grande D.A. A novel strategy to enhance microfracture treatment with stromal cell-derived factor-1 in a rat model. Front. Cell. Dev. Biol. 2021;8:9. doi: 10.3389/fcell.2020.595932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Albrektsson T., Johansson C. Osteoinduction, osteoconduction and osseointegration, European spine journal : official publication of the European Spine Society. Eur. Spinal Defor. Soc. Eur. Sec. Cervical Spine Res. Soc. 2001;10(Suppl 2):S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou S.H., Mizuno S., Glowacki J. Wnt pathway regulation by demineralized bone is approximated by both BMP-2 and TGF-beta 1 signaling. J. Orthop. Res. 2013;31(4):554–560. doi: 10.1002/jor.22244. [DOI] [PubMed] [Google Scholar]
- 159.Izzo M.W., Pucci B., Tuan R.S., Hall D.J. Gene expression profiling following BMP-2 induction of mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage. 2002;10(1):23–33. doi: 10.1053/joca.2001.0478. [DOI] [PubMed] [Google Scholar]
- 160.Glowacki J., Zhou S.H., Mizuno S. Mechanisms of osteoinduction/chondroinduction by demineralized bone. J. Craniofac. Surg. 2009;20:634–638. doi: 10.1097/SCS.0b013e3181928077. [DOI] [PubMed] [Google Scholar]
- 161.Jing H., Zhang X.Y., Gao M.C., Luo K., Fu W., Yin M., Wang W., Zhu Z.Q., Zheng J.H., He X.M. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and beta-catenin-related pathways. Faseb. J. 2019;33(4):5641–5653. doi: 10.1096/fj.201802137RRR. [DOI] [PubMed] [Google Scholar]
- 162.Han B., Jones I.A., Yang Z., Fang W., Vangsness C.T. Repair of rotator cuff tendon defects in aged rats using a growth factor injectable gel scaffold. Arthroscopy J. Arthr. Related Surg. 2020;36(3):629–637. doi: 10.1016/j.arthro.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 163.Liau L.L., bin Hassan M.N.F., Tang Y.L., Ng M.H., Law J.X. Feasibility of human platelet lysate as an alternative to foetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021;22(3):17. doi: 10.3390/ijms22031269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Falcigno L., D'Auria G., Calvanese L., Marasco D., Iacobelli R., Scognamiglio P.L., Brun P., Danesin R., Pasqualin M., Castagliuolo I., Dettin M. Osteogenic properties of a short BMP-2 chimera peptide. J. Pept. Sci. 2015;21(9):700–709. doi: 10.1002/psc.2793. [DOI] [PubMed] [Google Scholar]
- 165.Renner J.N., Liu J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnol. Prog. 2013;29(6):1550–1557. doi: 10.1002/btpr.1808. [DOI] [PubMed] [Google Scholar]
- 166.Connelly J.T., Petrie T.A., Garcia A.J., Levenston M.E. FIBRONECTIN- and collagen-mimetic ligands regulate bone marrow stromal cell chondrogenesis IN three-dimensional hydrogels. Eur. Cell. Mater. 2011;22:168–177. doi: 10.22203/ecm.v022a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.