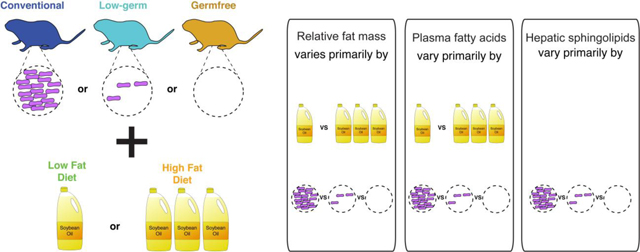
Abstract
Studies in mice using germfree animals as controls for microbial colonization have shown that the gut microbiome mediates diet-induced obesity. Such studies use diets rich in saturated fat, however, Western diets in the USA are enriched in soybean oil, composed of unsaturated fatty acids (FAs), either linoleic or oleic acid. Here, we addressed whether the microbiome is a variable in fat metabolism in mice on a soybean oil diet. We used conventionally-raised, low-germ, and germfree mice fed for 10 weeks diets either high (HF) or low (LF) in high-linoleic-acid soybean oil as the sole source of fat. Conventional and germfree gained relative fat weight and all mice consumed more calories on the HF versus LF soybean oil diet. Plasma fatty acid levels were generally dependent on diet, with microbial colonization status affecting iso-C18:0, C20:3n-6, C14:0, and C15:0 levels. Colonization status, but not diet, impacted levels of liver sphingolipids including ceramides, sphingomyelins, and sphinganine. Our results confirm that absorbed fatty acids are mainly a reflection of the diet and that microbial colonization influences liver sphingolipid pools regardless of diet.
Keywords: germfree, adiposity, linoleic acid, ceramide, sphingomyelin, sphinganine
Graphical abstract
1. Introduction
The microbiome has emerged as central to the health of an organism [1]. Germfree animals, animals devoid of a microbiome, often serve as controls to understand a variety of host phenotypes, including metabolism, the development of the immune system, the structure and function of gut cells, and animal behavior [2,3]. Early studies indicated that germfree mice are protected from diet induced obesity [4–7] and opened a large area of research into the interaction between the microbiome and lipid metabolism, catabolism and storage. Since those initial studies, the microbiome has been demonstrated to impact lipid processing across the body. Indeed, the gut microbiota has been shown to influence the types and quantities of fatty acids and sphingolipids circulating in plasma and in the liver in particular [6,8,9]. Circulating plasma lipids result directly from interactions between diet and the small intestinal microbiome [9]. Hepatic lipids result from multi-organ metabolism and are directly and indirectly influenced by gut microbes throughout the gastrointestinal tract [8,10].
How gut microbes influence host lipid metabolism has been shown to depend on diet. For instance, diet affects the lipids that are altered by gut microbes in circulation and in the liver [8,9,13]. Diet also interacts with the microbiome in lipid storage. While germfree mice on a lard or tallow-based diet are protected from diet-induced obesity, those on a palm or coconut oil diet are not [11,12]. These animal studies where the type of fatty acid is carefully controlled have demonstrated different lipid composition in the diet is a key factor in how the microbiome affects host lipid metabolism and storage. These findings may be of great interest in human health, particularly in the context of the obesity epidemic. However, the dominant oil consumed in Western societies has not been tested for interactions with the gut microbiome.
Soybean oil is the major oil consumed in the United States of America [14] and contains over 85% unsaturated omega-6 fatty acids (FAs). Unsaturated and saturated fats are observed to differentially impact host weight, metabolism, fat deposition, and immune system [13,15,16]. How the microbiome interacts with a high fat diet when it is predominately composed of unsaturated omega-6 fatty acids has not been ascertained. To this end, here we address the effect of matched high and low soybean oil diets on germfree, low-germ, and conventional mice with regards to weight, fat gain, circulating plasma lipids, and hepatic sphingolipids.
2. Materials and Methods
2.1. Mouse experiments
All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Usage Committee of Cornell University protocol 2010–0065. We used three sets of conventional male C57BL/6 mice (n=32, 35, 24) and two sets of germfree male C57BL/6 mice (n=36, 28) (Table 1). At weaning (3 weeks of age), littermates were split into cages with up to four mice/cage. Littermates were split to balance mouse weights within a cage and between the two diets. Conventional mice were maintained in the Accepted Pathogen Facility at Cornell University with filter top cages and the germfree mice in flexible, plastic (“bubble”) isolators [3] at Cornell University. All animals within a given germfree study were maintained within the same isolator at the same time. Animals in all studies were maintained under a 12-hour light cycle.
Table 1.
Mouse studies.
| Study | Microbial status | Genotype | N | Vendor | N for plasma FAs | N for hepatic sphingolipids | N for qPCR |
|---|---|---|---|---|---|---|---|
| CNV1 | Conventional (Murine Pathogen Free) | C57BL/6J | 32 | F2 generation* | 28 | 9 | 22 |
| CNV2 | Conventional (Murine Pathogen Free) | C57BL/6NTac | 35 | Taconic Biosciences (Hudson, NY, USA) | 34 | 12 | 10 |
| CNV3 | Conventional (Murine Pathogen Free) | C57BL/6NTac | 24 | Taconic Biosciences (Hudson, NY, USA) | 24 | 0 | 0 |
| GF (GF1) | Germfree | C57BL/6NTac | 36 | Taconic Biosciences (Hudson, NY, USA) | 35 | 12 | 28 |
| LG (GF2) | Low-germ (initially germfree) | C57BL/6NTac | 28 | Taconic Biosciences (Hudson, NY, USA) | 27 | 8 | 25 |
bred in the Accepted Pathogen Facility at Cornell University, originally from Jackson Laboratories (Bar Harbor, ME, USA)
The cages were supplied with either the LF (low fat, 16% kcal SBO) or HF (high fat, 44% kcal SBO) diet (Table 2) ad libitum. Diets were custom designed by Envigo (formerly Harlan Laboratories, Inc., Madison, WI, USA) and delivered pelleted, irradiated, and vacuum packed. For the conventional mice, we stored open, in-use diet bags at 4°C and unopened, bags at −20°C. For germfree mice, the in-use bags were stored within the germfree isolator where the animals were housed. We stocked cages with Pure-o-cel (The Andersons, Maumee, Ohio, USA), cotton nestlets, and plastic houses so to avoid the introduction of exogenous fat. For the germfree mice, all supplies were autoclaved at conditions to render the supplies germfree [3]. To bring the feed into the germfree isolator, the vacuum-packed feed bags were soaked in Clidox-S (Pharmacal Research Laboratories, CT, USA) or Exspor (Ecolab Inc., MN, USA) for least 30 minutes before moving the bags into the inner-port door where they were fumigated with Clidox-S or Exspor for a minimum of 2 hours. For all mice, feed pellets were placed in the cages and not on the wire racks to minimize loss and crumb buildup of the diets as the HF SBO diet does not maintain pelleted form well and turns to powder easily. Twice weekly, we completely replaced cages and feed. We weighed the amount of new feed provided. To obtain mouse weights, we weighed mice in plastic beakers at the same approximate time of day twice weekly. To weigh germfree mice, we used a metal scale that was autoclaved and brought into the isolator.
Table 2.
Ingredients and chemical composition of dietary treatments.
| Ingredient | 16% kcal SBO diet (TD.130215) (g/kg) | 44% kcal SBO diet (TD.130214) (g/kg) |
|---|---|---|
| Casein (ethanol washed), “Vitamin-Free” Test | 200.0 | 240.0 |
| L-Cystine | 3.0 | 3.6 |
| Corn Starch | 371.986 | 162.354 |
| Maltodextrin | 80.0 | 80.0 |
| Sucrose | 200.0 | 200.0 |
| Soybean Oil, high linoleic | 70.0 | 230.0 |
| Cellulose | 30.0 | 30.0 |
| Mineral Mix, AIN-93G-MX (94046) | 35.0 | 42.0 |
| Vitamin Mix, Teklad (40060) | 10.0 | 12.0 |
| TBHQ, antioxidant | 0.014 | 0.046 |
| % by weight, % kcal | % by weight, % kcal | |
| Protein | 18.3, 19.0 | 22, 18.7 |
| Carbohydrate | 62.3, 64.7 | 43.6, 37.2 |
| Fat | 7.0, 16.3 | 23, 44.1 |
| Kcal/g | 3.9 | 4.7 |
SBO: soybean oil; TBHQ: tertiary butylhydroquinone
We collected fresh fecal samples once weekly from the beakers into tubes on dry ice, which were later stored at −80°C. For the germfree mice, fecal samples were collected per cage and for the conventional mice, per mouse. The conventional mice were handled exclusively inside of a biosafety cabinet and we changed personal protective equipment and wiped all surfaces with a sterilant between cages to prevent cross-contamination.
Feed intake was determined twice weekly in half of the cages for the CNV1, CNV2, GF1, and LG (GF1, see below) studies. We filtered feed crumbs out of the used bedding using a large hole colander followed by a fine mesh sieve, weighed the recovered feed, and subtracted this amount from the known amount of feed provided.
After 10 weeks on the SBO diets, mice were gavaged orally with 6 mg per gram mouse weight phosphate buffered saline (PBS), LA, or ALA. Within a cage, we gavaged roughly half of the mice with a fatty acid (FA) and the other half with PBS, selecting which mouse received which gavage so to balance mouse weights between gavage groups. Following gavage, we moved mice to a fresh cage supplied with water but lacking feed. After 1.5 hours, we euthanized mice by decapitation. Epididymal fat pads were removed and promptly weighed. We collected trunk blood resulting from decapitation into EDTA coated tubes and stored them on ice within 1 hour. Tubes were spun at 900 rcf at 4°C for 10 minutes and plasma was collected and stored at - 80°C.
Final body weights of mice were compared to weights at weaning, and epididymal fat pads were compared to final body weights. Data for body weight relative to weaning weight, epididymal fat pads relative to body weight, and caloric consumption (derived from feed intake) were analyzed by ANOVAs (Analysis Of Variance) as a function of diet, microbial status, and their interaction. Tukey honest significant differences (HSD) were computed to obtain significance values for pairwise comparisons.
2.2. FA extraction and detection from plasma
We added 125 mg of heptadecanoic acid (C17:0, 99+% pure, Sigma Chemicals, St. Louis, MO, USA) to the plasma as an internal standard for absolute quantification of extracted FAs. The amount of plasma used in the extraction was 200 μl or the maximum of plasma acquired. We extracted lipids using the Bligh and Dyer method [17]. FAs were converted to FA methyl esters (FAMEs) and measured by gas chromatography (GC). FA methyl esters (FAMEs) were prepared using 14% BF3 in methanol, dried under N2, dissolved in hexane with butylated hydroxytouelene, and stored at −20°C. FAMEs were measured in triplicate on a Hewlett-Packard 5890 series II gas chromatograph with a flame ionization detector (GC-FID) using H2 as the carrier. Peak areas were measured using PeakSimple software (SRI Instruments, Menlo Park, CA, USA). These peak areas were corrected using an equal weight mixture of known FAs measured multiple times throughout the GC run. See Su et al. [18] for further details. To account for the variable plasma volume used, each corrected peak area was normalized by the average plasma volume used across all samples (140 μl). We converted these peak areas to quantitative amounts using the known amount of spiked C17:0 internal standard. All samples were run at random on the GC-FID.
To accurately determine structural identity of each peak in the GC spectrum, select plasma samples were analyzed by covalent-adduct chemical ionization on a Saturn 2000 ion trap mass spectrometer (Varian, Inc., Walnut Creek, CA, USA) [19]. A few peaks could not be resolved to a single FA due to co-migration: C18:1n-9/7/? and several of the identified conjugated linoleic (CLA) and alpha-linolenic acids (ClnA). Arbitrary designators (e.g. ClnA.7, ClnA.2) were used to name these unresolved FAs.
2.3. Sphingolipid extraction from liver tissue
Liver samples were defrosted on ice and homogenized in 1 mL of PBS in tubes containing 1 mm zirconium beads (OPS Diagnostics, Lebanon, NJ, USA) on a Mini Bead Beater homogenizer (BioSpec products, Bartlesville, OK, USA). Protein concentrations of the liver PBS homogenate were determined using the Lowry method (BioRad, Hercules, CA, USA) and 400 μg protein of liver homogenate was loaded into 96-deep well plates for lipid extractions. Lipids were extracted by adding 450 μL of 1:1 dichloromethane:methanol, 50 μL of 10% diethylamine in methanol, and 50 μL of the internal standard C12 ceramide (d18:1/12:0) (Avanti Polar Lipids, Alabaster, AL, USA) to samples with continuous shaking overnight (12 hours) on a plate shaker. The next day, 900 μL of 1:1 dichloromethane:methanol was added to each sample and gently mixed on a rotating shaker for an hour. Samples were then spun at 1500 x g for 15 minutes to pellet cellular debris. The supernatants were transferred to a new 96-deep well plate and stored at −20°C prior to analysis by high performance liquid chromatography-mass spectrometry (HPLC-MS). Sphingolipid abundances were measured using targeted mass spectrometry on an Agilent 1200 HPLC linked to an Agilent 6430 triple quadrupole mass spectrometer according to previous methods [10].
2.4. Quantitative real-time PCR
To assess the microbial load in the mice (see Table 1), we performed quantitative real-time PCR (qPCR) using primers targeting the 16S rRNA gene [20]. We extracted DNA from fecal pellets taken from mice at weeks 9 to 11 on the SBO diets using the PowerSoil DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA) following the manufacturer’s protocol, and each sample was eluted with 50 μl Solution C6. We ran 10 μl reactions using the LightCycler 480 platform and the SYBR Green I Master kit (Roche Diagnostics Corporation, Indianapolis, IN, USA): 2 μl of DNA, each qPCR primer at 500 nM, and 5 μl of SYBR Green I Master mix. Cycling conditions were 5 minutes at 95°C followed by 45 cycles consisting of 10 seconds at 95°C, 20 seconds at 56°C, and 30 seconds at 72°C after which fluorescence from SYBR Green was read. Cycle threshold (Ct) values were calculated using the absolute quantification/2nd derivative max function available on the LightCycler 480 software. All reactions were run in triplicate and the mean Ct values were used in subsequent calculations. One conventional mouse sample was run in all qPCR runs to serve as an internal standard and 16S rRNA gene copy numbers from a given run are reported relative to this sample in that run. This standard was within one Ct across the three qPCR runs in this study. Significance values among mice groups were determined by ANOVA with Tukey HSD.
2.5. Statistical analyses on FA and sphingolipid profiles
We utilized adonis (PERMANOVA) [21] to investigate how the full set of FAs in the conventional or germfree mouse studies were influenced by the technical and experimental variables. The technical variables included: normalized sum of FAs extracted (continuous variable), plasma volume used in extraction (continuous variable), mouse study (factor, see Table 1), FA extraction A date (Bligh-Dyer extraction part I, factor), FA extraction B date (Bligh-Dyer extraction part II, factor), GC run date (factor). The experimental variables were diet (factor), cage (factor), gavage (factor), and microbial status (conventional, low-germ, or germfree, factor). We determined the differences among samples by computing a distance matrix using the Bray-Curtis dissimilarity metric [22] using the phyloseq package [23]. As we were unable to model the distance matrix with all technical and experimental variables, we first modeled the data using the full set of technical variables: Bray-Curtis distance matrix ~ plasma volume * normalized sum of FAs * study * FA extraction date A * FA extraction date B * GC run date, where the multiplication sign indicates additive (e.g. GC run date + study) and interactive effects (e.g. GC run date:study) among the variables as implemented in R [24] using the vegan package [21] with 10,000 permutations. After running the full model, the non-significant terms were removed with p < 0.05 being considered significant. Then the experimental variables were added: + diet * gavage * microbial status * study* cage. This model was then reduced so to keep the residuals less than 0.05. Principal Coordinate Analysis plots were made using the phyloseq package [23] with the ordplot function and a t-distribution. For analysis of hepatic sphingolipids, we utilized the model Bray-Curtis distance matrix ~ diet * microbial status * study * cage.
To determine the specific FAs altered by different experimental conditions (diet, gavage, and microbial status), we first reduced the list of FAs to only those present in two of the three conventional studies and one of the two germfree studies. Then we utilized a linear mixed model to determine which of the FA amounts were significantly affected by the experimental conditions as compared to a null model. The null model was normalized FA mass ~ normalized sum of FAs + plasma volume + (1|cage) + (1|GC run date) + (1|FA extraction date A), where the terms cage, GC run date, FA extraction date A, and study were handled as random effects and all others as fixed effects. We excluded FA extraction date B as it is directly correlated with date A. For the experimental model, we added the terms diet*gavage*microbial status*(1|study). These models were run in R [24] using the lme4 package [25] with REML = FALSE and the control optimizer set to “bobyqa”. We used a likelihood ratio test to compare the experimental and null models for each FA, and p-values were corrected with a Bonferroni correction; corrected p-values < 0.05 were considered significant. For each FA found significant, we reduced the experimental terms (diet*gavage*microbial status*(1|study) so that the reduced model was not significantly different (p < 0.05) from the full experimental model as determined by a likelihood ratio test. The lsmeans function [26] was used to compute the reduced model estimates on a model lacking the terms (1|GC run date) + (1|FA extraction date A) to avoid overfitting the data. Estimated differences between groups (e.g. HF versus LF diets) were calculated with a Tukey’s correction for multiple testing (p<0.01 was used to call significance). For hepatic sphingolipids, the null model was sphingolipid data ~ (1|cage), and the experimental model added the following terms: diet*microbial status*(1|study). We corrected p-values using the Benjamini and Hochberg False Discovery Rate [27] instead of a Bonferroni correction with significance called at p<0.05 due to the lower power in these data compared to that for the plasma FAs. To investigate the composition of ceramide pools in the liver, we compared the abundance of each ceramide (Cer (d18:1/16:0), Cer (d18:1/18:0), Cer (d18:1/20:0), Cer (d18:1/22:0), Cer (d18:1/24:0), and Cer (d18:1/24:1)) relative to all ceramides in our sphingolipid model above. All statistical analyses were computed in R [24].
3. Results
3.1. Fat mass is affected by diet SBO content and microbial status, while microbial status dominates effects on body weight
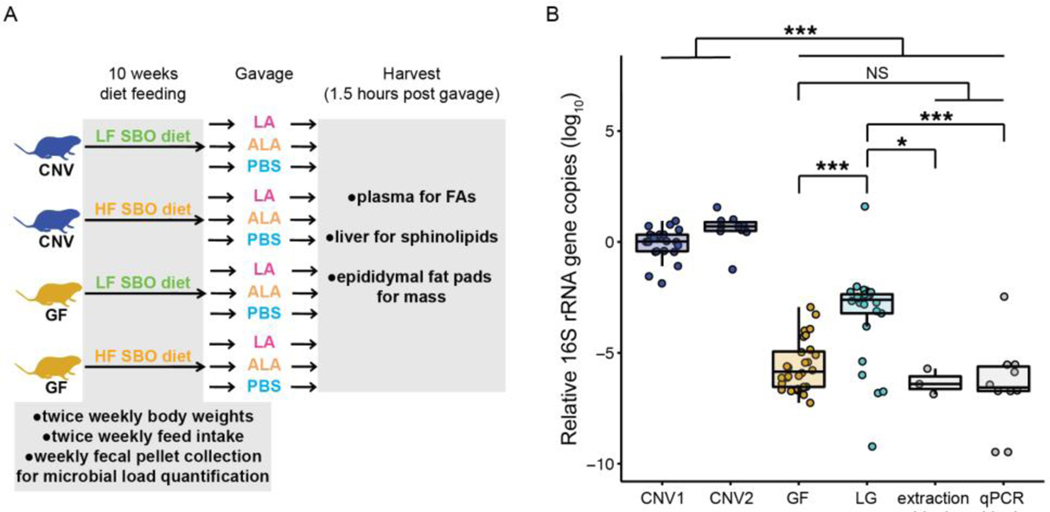
To assess the effect of the presence of microbes and soybean oil consumption on adipogenicity, lipid absorption and processing of a high linoleic diet, we fed conventional (CNV) and germfree (GF) mice one of two paired soybean oil diets. For both diets, fat derived from high linoleic acid soybean oil (SBO); the diets differed solely in calorie content from fat. Mice were fed one of the two diets for 10 weeks beginning at weaning (Fig 1A). To maintain the quality of the feed we did not autoclave diets as would normally be performed for feed being given to germfree animals. Germfree mice maintained for prolonged periods of time on diets that cannot be autoclaved will inevitably acquire a microbiota. Visually, GF1 and GF2 study mice appeared germfree, as evidenced by their grossly enlarged cecal size and over-abundance of bile following oil gavage [2]. Although the GF1 experiment mouse feces had 16S rRNA copies indistinguishable to that of the extraction and qPCR blanks (ANOVA, Tukey HSD, p>0.05, Fig 1B), values measured for the GF2 experiment were greater (ANOVA, Tukey HSD, p-values<0.05, Fig 1B). Assuming the blanks had no bacteria, GF2 fecal pellets are estimated to have 104 - 105 bacterial cells per extracted fecal pellet, three orders of magnitude lower than that in the conventional animals (ANOVA, Tukey HSD, p<0.0001, Fig 1B), which have an estimated ~108 bacterial cells. Hence, while the GF1 animals can be considered germfree (hereafter, GF), the GF2 animals are “low-germ” and hereafter labeled LG.
Figure 1. Contribution of diet and microbial load to body weight, fat pad mass, and fed intake.
A. Overview of SBO diet periment. Conventional and germfree mice were fed low or high fat SBO diets beginning at weaning. Body weights and feed take were monitored during this time and fecal pellets were collected. After 10 weeks, mice were gavaged with LA, ALA, or BS. One and a half hours post gavage, mice were sacrificed, blood plasma, liver, and epididymal fat pads were collected. B. icrobial load in conventional and germfree mouse experiments as determined by 16S rRNA gene copy number in fecal pellets. ata are relative to a single mouse from the CNV1 study. N= 3 to 28 per group. Note that GF refers to the GF1 study and LG is th F2 study. C. Body weight at euthanasia relative to body weight at weaning of animals by diet and microbial status. D. Epididym t mass relative to total body weight at euthanasia by diet and microbial status. E. Average calorie intake for each mouse by diet d microbial status. For C, D, and E boxplot lines indicate the 50% quartile, and the two thinner lines show the 25% and 75% uartiles. N= 12 to 52 per group. *, p<0.05; **, p<0.001; ***, p<0.0001. CNV: conventional; GF: germfree; LG: low-germ; SBO ybean oil; LA: linoleic acid; ALA: alpha-linolenic acid; PBS: phosphate buffered saline; FAs: fatty acids; rRNA: ribosomal NA; qPCR: quantitative polymerase chain reaction; NS: nonsignificant; HF: high fat; LF: low fat.
To analyze the effect of diet and microbial status (CNV, GF, or LG) on body weight and fat gain, we measured body weights over the course of the experiment (Supplementary Fig 1A and B, Supplementary Table 1) and epididymal fat pad mass at euthanasia (Supplementary Fig 1C, Supplementary Table 1). In performing an ANOVA on relative body weight as a function of diet, microbial status (MS), and their interaction, we observed that MS explained most of the variation (R2 = 0.5560 for MS compared to 0.0908 for diet, Table 3). In contrast, the same ANOVA model for relative epididymal fat showed near equal contributions of diet and MS (R2 = 0.2141 for diet, 0.2967 for MS, Table 3).
Table 3.
ANOVA on relative body weight, relative epididymal fat mass, and caloric intake.
| Relative body weight, multiple R2 = 0.5687 | |||
|---|---|---|---|
| Term | R2 (% variation) | F value | p-value |
| Diet | 0.0908 | 31.372 | 9.99e-08 |
| MS | 0.4263 | 73.640 | <2e-16 |
| Diet:MS | 0.0517 | 8.926 | 2.18e-4 |
| Relative epididymal fat mass, multiple R2 = 0.5207 | |||
| Term | R2 | F value | p-value |
| Diet | 0.2141 | 66.562 | 1.30e-13 |
| MS | 0.2967 | 46.123 | 2.56e-16 |
| Diet:MS | 0.0099 | 1.544 | 0.217 |
| Average kcal intake, multiple R2 = 0.7981 | |||
| Term | R2 | F value | p-value |
| Diet | 0.6058 | 291.10 | <2e-16 |
| MS | 0.1879 | 45.14 | 1.39e-14 |
| Diet:MS | 0.0044 | 1.06 | 0.350 |
MS: microbial status.
Furthermore, we observed that CNV mice on either diet had greater relative body weights and greater relative fat pad masses compared to their LG and GF counterparts (Fig 1C, Fig 1D, Supplementary Tables 2, 3, Tukey HSD on ANOVA, p-values <0.005). In comparing between the diets, CNV HF fed versus LF fed mice had both greater relative body weight and epididymal fat pad mass (Fig 1C, Fig 1D, Supplementary Tables 2, 3, Tukey HSD on ANOVA, p-values <0.0001). On the other hand, GF mice had only greater relative epididymal fat pad mass in comparing animals between the SBO diets (Fig 1D, Supplementary Table 2, Tukey HSD on ANOVA, p-value <0.001), while HF LG mice trended towards greater epididymal fat pad mass but were not significantly different for either relative epididymal fat pad or body weight (Fig 1C, Fig 1D, Supplementary Tables 2, 3, Tukey HSD on ANOVA, p-values >0.05).
We measured feed intake in two of the three conventional studies and both of the germfree studies (Fig 1E, Supplementary Fig 1D, Supplementary Table 1). Using the same ANOVA model as before, we observed that diet largely explains differences in fed intake (R2 = 0.6058 for diet, 0.1879 for MS, Table 3). In particular, mice on the HF diet consumed significantly more calories, compared to mice on the LF diet regardless of MS (Fig 1E, Supplementary Table 4, p-values <0.0001). Together, these results indicate that GF mice gained more relative fat mass, but not more relative body weight, with increased calorie intake on the HF SBO diet. Interestingly, LG mice experienced neither relative fat mass nor body mass gains despite increased caloric intake. We also observed that within the diet groups, CNV mice consumed more calories than LG or GF mice, except for LF CNV versus LF GF (Fig 1E, Supplementary Table 4, p-values<0.05), correlating with increased relative body and epididymal fat weights in CNV mice compared to GF or LG mice.
3.2. Microbial status is a minor effector on plasma FAs and a major effector on hepatic sphingolipids
Given the observed roles for SBO diet content and microbes in fat storage, next we investigated how these factors affect lipid absorption and processing. As circulating lipids contain lipids directly post absorption in the small intestine, we asked how the absorption of two of the unsaturated FAs present in SBO would be handled in mice acclimated to the HF or LF SBO diet. To this end, we measured plasma FAs 1.5 hours post gavage (oral delivery to the stomach) of a large bolus of linoleic acid (C18:2n-6, LA), alpha-linolenic acid (C18:3n-3, ALA), or PBS control (Fig 1A). We previously determined that 1.5 hours post gavage of these FAs, they can be observed in circulating plasma [28].
We first analyzed the entire set of measured FAs to determine if experimental design or sample handling influenced the data by using a Permutational Multivariate Analysis of Variance Using Distance Matrices (PERMANOVA) with the adonis function [21]. To do so, we created a distance matrix of the data using the Bray-Curtis dissimilarity metric [22], which computes the dissimilarity among samples by composition and quantity. After model simplification, we were able to explain 97.2% of the data with the model shown in Table 4. These models indicate that diet (~13% of the data), microbial status (~5%), and gavage (~3%) contribute to plasma FAs.
Table 4.
PERMANOVA on plasma FAs, residuals = 0.02842.
| Term | R2 (% variation) | F-statistic | p-value |
|---|---|---|---|
| Normalized sum of FAs | 0.42505 | 254.252 | 0.0001 |
| Gavage:Cage | 0.16746 | 0.919 | 0.6625 |
| Diet | 0.12976 | 77.617 | 0.0001 |
| CNV, LG, or GF | 0.05638 | 16.864 | 0.0001 |
| Gavage | 0.03417 | 10.220 | 0.0001 |
| FA extraction B date | 0.02908 | 17.395 | 0.0001 |
| Plasma volume used in extraction:Study | 0.02879 | 4.305 | 0.0006 |
| Study:GC run | 0.02678 | 3.203 | 0.0016 |
| FA extraction A date | 0.02597 | 15.534 | 0.0001 |
| Study | 0.02467 | 7.379 | 0.0002 |
| Plasma volume used in extraction | 0.0139 | 8.312 | 0.0008 |
| Normalized sum of FAs:Plasma volume used in extraction | 0.00958 | 5.730 | 0.0035 |
FA: fatty acid; CNV: conventional; LG: low-germ; GF: germfree; GC: gas chromatography.
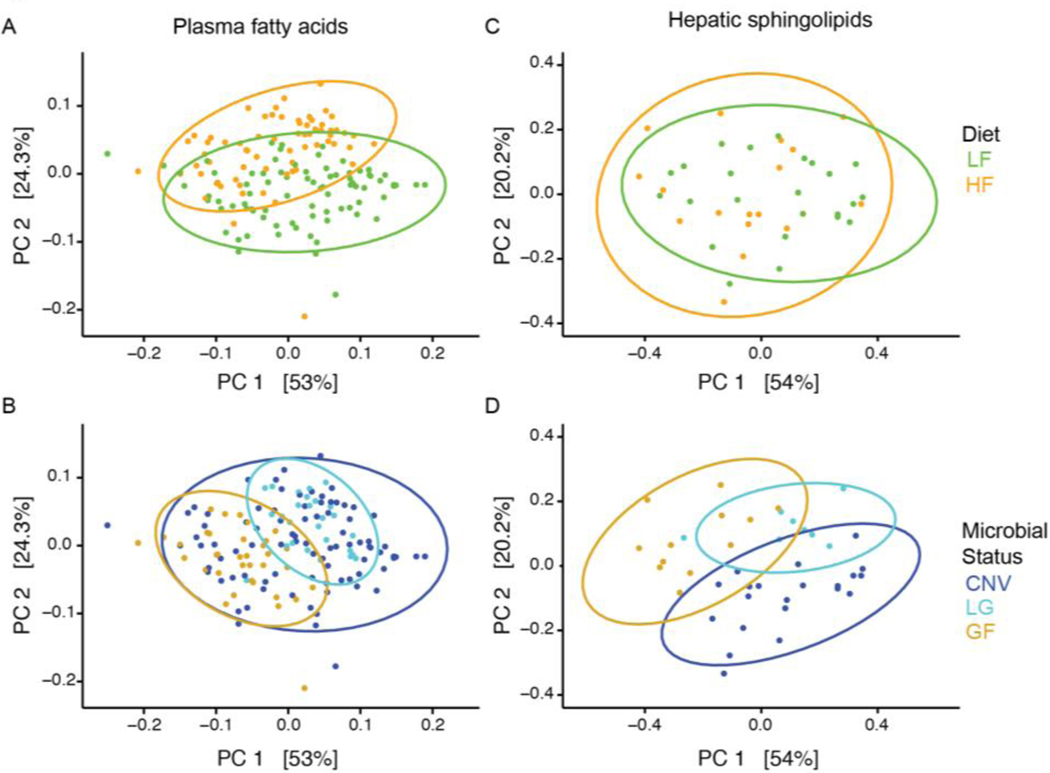
Principal Coordinate Analysis (PCoA) plots also indicate that diet contributes to the set of plasma FAs, and microbial status less so (Fig 2A and B). Furthermore, a linear regression of total extracted plasma FAs and microbial load measured by qPCR showed no relationship (Fig 3A). These observations indicate that microbial status has a minor effect on the overall plasma FA profile, whereas the animal’s diet has a greater effect.
Figure 2. Total plasma FA compositions cluster by diet and microbial status whereas hepatic sphingolipid compositions cluster primarily by microbial status.
PCoA plots from Bray-Curtis computed distance matrices for plasma FAs (A, B) and for hepatic sphingolipids (C, D), colored by diet (A, C) or microbial status (B, D). PCoA: principal coordinate analysis; PC: principal coordinate; SBO: soybean oil; LF: low fat; HF: high fat; CNV: conventional; LG: low-germ; GF: germfree.
Figure 3. Hepatic sphingolipids not plasma FAs are correlated with microbial load.
Estimated microbial load is derived from the average 16S rRNA copy number for all CNV mice, just GF1 mice (GF mice), or just GF2 mice (LG mice) as reported in Figure 1B. The data, A, plasma FAs, B, hepatic ceramides, C, hepatic sphingomyelins, or D, hepatic sphinganine, were fit using a linear regression. Only data from animals gavaged with PBS are shown to facilitate comparison between plasma FAs and hepatic sphingolipids. For the hepatic sphingolipids (B, C, and D), adjusted p-values are shown, which were corrected using the Benjamini and Hochberg False Discovery Rate across all classes of sphingolipids. Only sphingolipid classes with adjusted p<0.05 are shown. LF: low fat; HF: high fat; F-stat: F-statistic; rRNA: ribosomal RNA; CNV: conventional; GF: germfree; LG: low-germ; PBS: phosphate buffered saline; FAs: fatty acids.
Hepatic lipids, in contrast to plasma FAs, represent lipid processing influenced by multiple organs. They have been previously observed to be dependent on microbial colonization [8] and sphingolipids are known to be closely linked to metabolic diseases [29]. To determine if hepatic sphingolipids were affected by microbial colonization in our SBO fed mice, we measured sphingolipids in livers of mice gavaged with PBS only (see Table 1). Unlike what we observed for plasma FAs (Fig 3A), the total amounts of ceramides, sphingomyelins, and sphinganine were positively linearly related to microbial load (Fig 3B). In accord, whereas the plasma FA data clustered readily by diet in PCoA (Fig 2A), the sphingolipid data did not (Fig 2C), and instead clustered well by microbial status (Fig 2D). The PERMANOVA analysis supported this observation: microbial status explained more of the variance in the sphingolipid data (~37%) compared to diet (~3%) (Table 5).
Table 5.
PERMANOVA on hepatic sphingolipids, residuals = 0.01946.
| Term | R2 (% variation) | F-statistic | p-value |
|---|---|---|---|
| Cage | 0.53772 | 5.527 | 0.0001 |
| CNV, LG, or GF | 0.37423 | 57.701 | 0.0001 |
| Study | 0.03795 | 11.702 | 0.0006 |
| Diet | 0.03065 | 9.450 | 0.0011 |
CNV: conventional; LG: low-germ; GF: germfree.
3.3. Specific plasma FAs and hepatic sphingolipids affected by diet, gavage, and/or microbial status
To determine which circulating FAs were impacted by diet, gavage, and microbial status, and which specific sphingolipids were affected by diet and microbial status, we ran linear mixed models separately for FAs and sphingolipids. After removing FAs not present in two of the three conventional studies and one of the two of the germfree studies (LG and GF mice), we considered 38 distinct FA peaks. Five of these 38 represent more than one FA that could not be adequately separated.
We identified 17 FAs with p <0.05 after a Bonferroni correction significantly impacted by diet and/or gavage (Table 6). Nine of the FAs were affected by diet, with the HF diet reducing the amount of the FA except for LA and ALA (Table 6, Supplementary Table 5, and Supplementary Figures S2–S6), which were present in greater amounts in the high fat diet. As expected, the LA gavage increased plasma LA amounts (p-values<0.0001) and the amount of ALA was higher in animals gavaged with ALA (p-values<0.0001) (Table 5, Supplementary Table 5, and Supplementary Figures S4, S5). Several ClnAs were also higher when the animal had been gavaged with LA (p-values<0.001, Table 6, Supplementary Table 5, and Supplementary Figures S6, S7). The interaction of diet and gavage was important for three FAs. In particular, only for the LF diet was C20:5n-3 present in higher amounts in animals gavaged with PBS (p-values<0.0001), and there was less C16:0 iso in HF-ALA mice compared with LF-ALA mice (p-values<0.01) (Table 6, Supplementary Table 5, and Supplementary Figures S2, S7).
Table 6.
Plasma FAs significantly impacted by diet, gavage, and/or microbial status.
| FA | Diet | Gavage | MS | Diet:Gavage | Diet:MS | Gavage:MS | Diet:Gavage:MS |
|---|---|---|---|---|---|---|---|
| C14:0 | LF>1HF*** | PBS>ALA5* | NS2 | (HF)3GF>CNV*; (HF)GF>LG*; (CNV)LF>HF* | |||
| C15:0 | LA>ALA*; LA>PBS* | (HF)LA>PBS* | (HF,LA)GF>CNV**; (HF,GF)LA>ALA** | ||||
| C16:0 iso | NS | (ALA)LF>HF* | |||||
| C16:0 | LF>HF*** | ||||||
| C16:1n-7 | LF>HF*** | ||||||
| C18:0 iso | LF>HF*** | (CNV)LF>HF*** | |||||
| C18:1n-7/9/? | LF>HF*** | NS | |||||
| C18:2n-6 | HF>LF** | LA>ALA**; LA>PBS*** | LG>CNV** | ||||
| C18:3n-6 | PBS>LA5* | LG>CNV***LG>GF*** | |||||
| C18:3n-3 | HF>LF* | ALA>LA***ALA>PBS*** | (CNV)ALA>LA***(CNV)ALA>PBS***(LG)ALA>LA***(LG)ALA>PBS***(GF)ALA>LA* | ||||
| C20:3n-6 | PBS>LA5*** | CNV>GF** | |||||
| ClnA.7 | NS | GF>CNV**; GF>LG*** | (HF)GF>CNV**; (HF)GF>LG*** | (LA)GF>CNV*; (LA)GF>LG*** | |||
| C20:5n-3 | (LF)PBS>LA*** | ||||||
| ClnA.2 | LA>ALA***LA>PBS*** | GF>LG** | |||||
| ClnAs44 | LA>ALA**; LA>PBS*** | (LA)GF>CNV*; (LA)GF>LG***; (GF)LA>ALA*; (GF)LA>PBS*** | |||||
| C22:5n-6 | LF>HF*** | NS | |||||
| C24:1n-9 | ALA>LA5**; PBS>LA5*** |
FA is higher for condition on listed at center of “>”.
Term is required for the linear mixed model but term is not significant after multiple testing correction among contrasts.
Effect listed is only for the indicated diet. For example, (HF)GF>CNV means that only for the HF diet was more FA present in GF compared to CNV animals.
This peak represents two ClnAs.
Model appears incorrect for estimate, see Supplementary Figures.
, p<0.01;
, p<0.001;
, p<0.0001
FA: fatty acid; MS: microbial status; NS: non-significant; LF: low fat; HF: high fat; CNV: conventional; LG: low-germ; GF: germfree; LA: linoleic acid; ALA: alpha-linolenic acid; PBS: phosphate buffered saline.
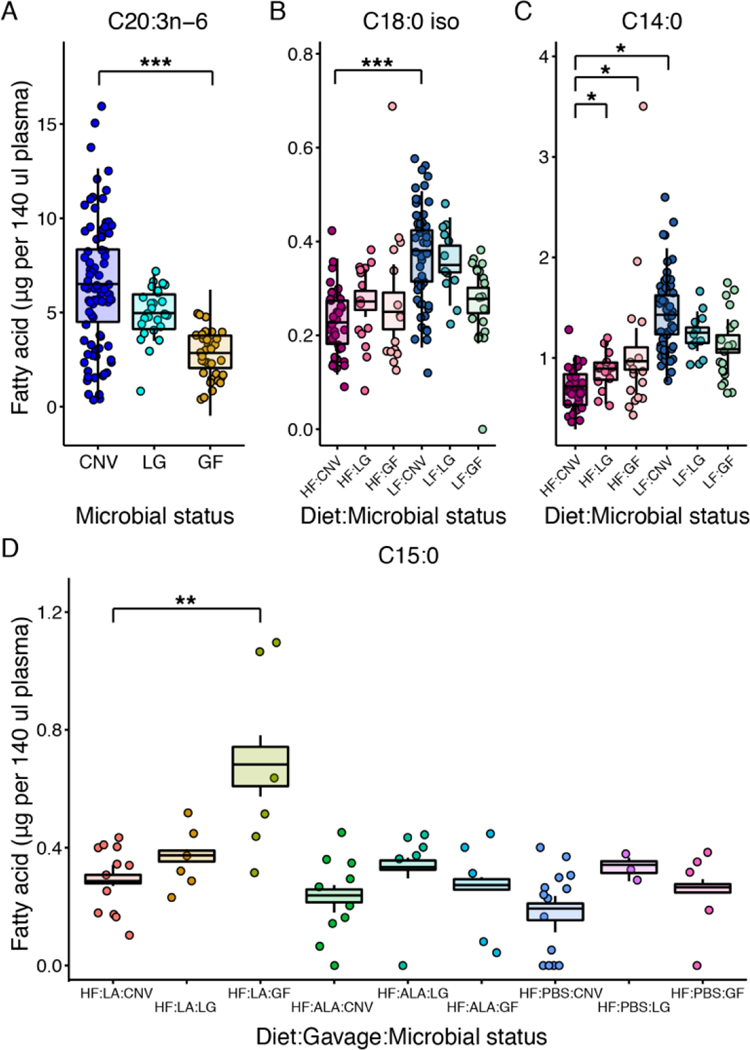
Microbial status affected the amount of 10 FAs (Table 6, Supplementary Table 5, and Supplementary Figures S2–S7). C20:3n-6 was the only FA solely affected by microbial status and it was higher in conventional mice (p-values<0.001, Figure 4, Table 6, Supplementary Table 5), suggesting it is produced by or its production in the host is stimulated by microbes. Similarly, C18:0 iso was higher in colonized mice, but only those on the LF diet (p-values<0.001, Figure 4, Table 6, Supplementary Table 5). Other FAs were higher in GF mice: two ClnAs (ClnA.7 and ClnAs4) in GF animals gavaged with LA compared to CNV or LG mice, (p-values<0.01, Table 6, Supplementary Table 5, Supplementary Figures S6, S7) and C14:0 and C15:0 in GF-HF diet animals (HF-LA animals for C15:0) (p-values<0.01, Figure 4, Table 6, Supplementary Table 5), suggesting microbes directly or indirectly promote the metabolism of these FAs.
Figure 4. Plasma FAs differing by microbial load and diet.
Plasma A, C20:3n-6, B, C18:0 iso, C, C14:0, and D, C15:0. Normalized data are shown as points and the boxplots show the covariate adjusted means from the least squares means estimates. N=3 to 148 per group. LF: low fat; HF: high fat; CNV: conventional; LG: low-germ; GF: germfree; LA: linoleic acid; ALA: alpha-linolenic acid; PBS: phosphate buffered saline.
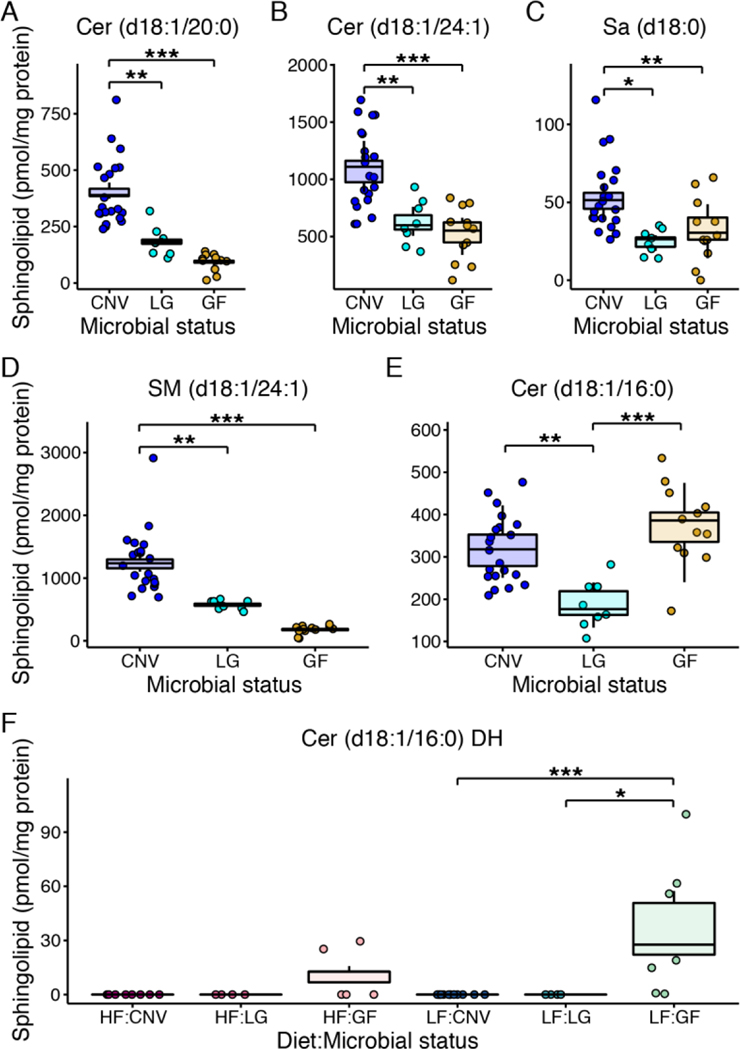
For sphingolipids, we observed six sphingolipids to vary by microbial status whereas diet contributed to only one sphingolipid in interaction with microbial status (Supplementary Table 6, and Figure 5). Cer (d18:1/20:0), Cer (d18:1/24:1), Sa (d18:1), and SM (d18:1/24:1) mirror the trend observed for total sphingolipid amounts, whereby sphingolipids levels increase as microbial load increases (p-values<0.05, Supplementary Table 6, and Figure 5A–D). Cer (d18:0/16:0) was lower in LG animals and Cer (d18:0/16:0) DH was higher in GF animals, but only those on the LF diet (p-values≤0.0001, Supplementary Table 6, and Figure 5E and F).
Figure 5. Hepatic sphingolipids differing by microbial load and diet.
Hepatic A, Cer (d18:1/20:0), B, Cer (d18:1/24:1), C, Sa (d18:0), D, SM (d18:1/24:1), E, Cer (d18:1/16:0), and F, Cer (d18:1/16:0) DH. Data are plotted as in Figure 4. N=4 to 21 per group. Cer: ceramide; dihydro (DH), sphinganine (Sa), sphingomyelin (SM), LF: low fat; HF: high fat; CNV: conventional; LG: low-germ; GF: germfree.
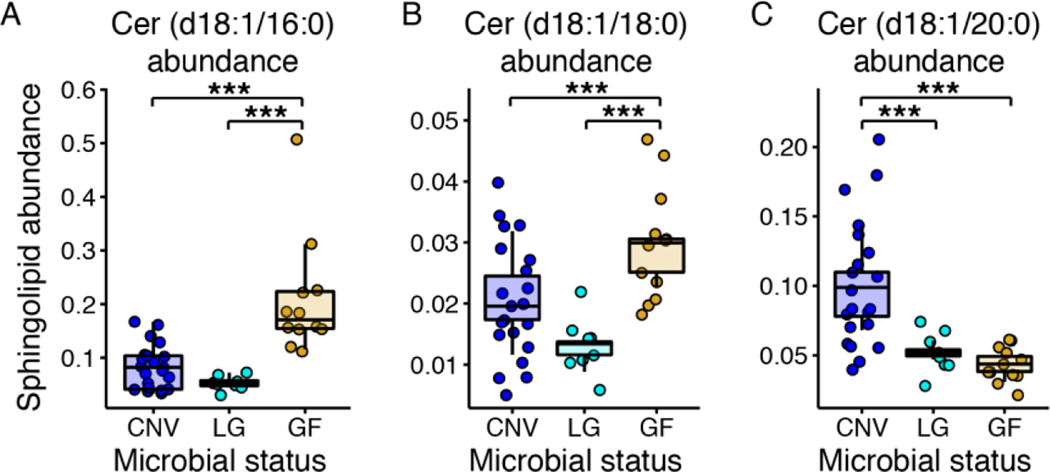
Ceramide fatty acyl chain length is an important indicator of metabolic function. The Cer (d18:1/16:0)/Cer (d18:1/24:0) ratio is an indicator of metabolic syndrome with higher Cer (d18:1/16:0) levels producing diet induced obesity and insulin resistance [30]. In our data, we observed no significant differences in this ratio across all mice (p>0.05, data not shown). To better understand the composition of ceramide pools, we computed the abundance of each ceramide relative to the sum of all measured ceramides and ran the same linear mixed model used on all sphingolipids on this reduced set. Cer (d18:1/16:0) and Cer (d18:1/18:0) relative abundances were highest in the germfree animals (p-values<0.05, Supplementary Table 7, and Figure 6A and B) and Cer (18:1/20:0) was highest in the conventional animals (p-values<0.05, Supplementary Table 7, and Figure 6C). These data illustrate that in SBO fed mice, hepatic ceramide abundance is altered by microbes, irrespective of the percentage of calories from soybean oil.
Figure 6. Hepatic ceramide abundances differing by microbial load.
Abundance of hepatic A, Cer (d18:1/16:0), B, Cer (d18:1/18:0), and C, Cer (d18:1/20:0). Data are plotted as in Figure 4. N=8 to 21 per each microbial status group. Cer: ceramide; CNV: conventional; LG: low-germ; GF: germfree.
4. Discussion
In the present study, we isolated the effects on body fat, plasma fatty acids, and hepatic sphingolipids by the dominant oil in the Western diet consumed in the USA, where SBO makes up roughly 7% of calories. Notably, we used SBO with >50% LA, exclusively available up to about 2010, in custom HF and LF diets with SBO as the sole source of fat. This design allowed us to quantify the contribution of dietary soybean oil content and microbial load on body weight and fat gain in mice. Our results show that fat gain is driven by microbial load as well as the fat content of the diet. Fat gain is associated with increased caloric intake, while body weight gain is primarily driven by microbial load. We further show that while many circulating FAs are dependent on dietary SBO content, specific circulating FAs are dependent on the gut microbiota. Finally, we observe that the microbial load in the gut of SBO-fed mice impacts levels of hepatic ceramides, sphingomyelins, and sphinganine, suggesting that the gut microbiota either supply additional sphingolipids or promote hepatic ceramide and sphingomyelin synthesis.
The microbiome has been implicated in fat storage in mice. In a landmark paper, Bäckhed and colleagues showed that germfree mice fed a high fat diet (with lard as a fat source) were protected from diet-induced obesity [5]. Since this early seminal observation, a more nuanced picture has emerged showing that the type of fat in the diet is an important factor in whether a germfree animal is resistant to diet-induced obesity. That is, germfree mice have been shown to acquire more fat mass on a high fat compared to a low fat diet [7,11,12]. Two of these studies [7,12] matched the fat source between the high and low fat diets, but used a mixture of several dietary fats, including saturated fat. Similar to previous observations, we observe that germfree mice acquire less adiposity and weight compared to conventional animals. However, our data demonstrate that germfree animals are not resistant to diet-induced fat gain on an SBO diet, though they are resistant to diet-induced body weight gain. Together these observations are in agreement with previous studies indicating that the microbiome enhances energy harvest and storage from the diet [4,5], and corroborate previous work showing that lean mass gain is impaired in germfree animals [31].
This study was carried out for 10 weeks to ensure diet-induced weight gain could be observed, which is challenging to do with germfree animals, especially when the diet cannot be autoclaved. One of the germfree experimental groups (GF2) was indeed contaminated at a low level, and rather than dispose of these animals, we included them in the study as “low-germ”. The retention of this group led to the observation that liver sphingolipid levels were linearly related to microbial biomass in the gut. The addition of the LA/ALA gavage treatment at the end of the study allowed us to further ask if plasma FAs were altered specifically in the context of an acute treatment of one of the FAs in SBO, and resulted in us observing microbial interactions dependent on the gavage for C15:0 and ClnAs.
Differences in hepatic and serum lipids have been previously observed to be diet and microbiota dependent [8,32–35] and plasma lipids are well known to be directly influenced by dietary lipids [36–39]. Similar to a previous study, we observed more differential lipid molecules between conventional and germfree mice in the liver compared to plasma [8]. In our study, we observe many plasma FAs that differ solely by diet and/or gavage, and fewer affected by microbes. In particular, our data indicate two plasma FAs that are positively affected by the presence of microbes: C18:0 iso and C20:3n-6. C18:0 iso may be a microbial product of a branched chain amino acid and a branched short chain fatty acid [40]. C20:3n-6 (dihomo-gamma-linolenic acid, DGLA) can be made from LA by the host [41] and is a precursor of the anti-inflammatory and anti-proliferative prostaglandin PGE1 [42–44]. Therefore, microbial modulation of DGLA may represent a mechanism by which the microbiome impacts inflammatory conditions and cancer development.
Four FAs were observed to be lower in colonized compared to germfree animals. Specifically, we observed lower C15:0 in colonized mice on a HF diet, gavaged with LA compared to similar germfree mice. C15:0 is generally thought to be produced by rumen microbes, found in dairy, could be carried over in the ethanol washed casein present in the mouse diet, and it has been observed in germfree rats, dependent on diet [45]. Our observation that C15:0 is lower in HF-fed and LA-gavaged colonized animals suggests two hypotheses: (i) that gut microbes chronically conditioned to a HF-diet environment with an acute LA exposure have the capacity to metabolize C15:0; or (ii) that the absorption of C15:0 is altered, perhaps due to differences in bile acid pools [46,47] between GF and colonized mice when gavaged with LA. C14:0 was also lower in HF-CNV but not dependent on LA gavage. One ClnA (ClnA.7) was higher in GF animals only on the HF diet and another (ClnAs4) was higher in GF animals when gavaged with LA. Conjugated ALAs (ClnAs) are well known to be produced by microbes from ALA [48,49], so we are uncertain as to why our data show ClnAs increasing following LA gavage and why their levels are higher in GF animals. We also did not observe an increase in the amount of C18:0 or C18:1 with ALA gavage, which are final metabolic products in CLA and ClnA metabolism [49].
It should be noted that we previously characterized the gut microbiota composition of CNV mice fed the HF or LF SBO diets, and observed minimal to no significant differences between the microbiota composition of HF and LF diet mice [28]. Hence, we hypothesize that any diet-dependent microbiome effects on lipid pools reflect functional differences in the microbiomes of mice on the HF and LF SBO diets.
While we observed diet and microbial-dependency on plasma FAs, we observed primarily microbial-dependency of hepatic sphingolipids. Surprisingly, hepatic sphingolipid levels were linearly related to microbial load in the cecum. In accord, the sphingolipid production capacity of the gut microbiome has recently been linked to sphingolipid levels in the liver [10]. Whether the gut microbiota act as a source of sphingolipids to the host or signal to alter host sphingolipid production and processing of sphingolipids remains to be elucidated.
The sphingoid backbone of sphingolipids is predominantly produced from palmitic acid (C16:0), which comprises 14% of the FAs in SBO and is the primary product of endogenous de novo fatty acid synthesis. Animals on high saturated fat diets (typically lard and/or milk fat, sometimes in combination with SBO) have increased hepatic ceramides, sphingomyelins, and sphingosine [50]. Perhaps because palmitic acid is a ubiquitous component of lipids, and endogenous synthesis was downregulated in our HF diet mice, we did not observe that animals consuming more SBO had more hepatic sphingolipids.
Instead, the presence of microbes increased the amounts of specific sphingolipids. Cer (d18:1/16:0) may be an exception to this statement: the increased levels of DH Cer (d18:0/16:0) (LF diet only) and increased abundance of Cer (d18:1/16:0) may indicate increased Cer (d18:1/16:0) pools in germfree mice. Caesar et al. also noted more hepatic Cer (d18:1/16:0) in lard-fed germfree compared to conventional mice [8]. In conventional animals, hepatic Cer (d18:1/16:0) elevation is causative of insulin resistance and steatosis [30,51,52] and plasma Cer (d18:1/16:0) elevation is associated with cardiovascular disease [53]. However, insulin resistance is prevented in germfree animals [6]. Germfree animals do have increased hepatic cholesterol levels and increased bile acids [3,6]. This dysregulation in cholesterol levels and bile acids may be related to elevated Cer (d18:1/16:0) levels and there is evidence of a relationship between bile acids and Cer (d18:1/16:0) [54]. It should be noted though that since the ratio of (d18:1/16:0)/Cer (d18:1/24:0) was not different in our germfree mice compared to conventional mice, these ceramide levels may not be pathogenic.
Differing from the observations of Caesar et al [8], our data show lower levels of Cer (d18:1/20:0) and Cer (d18:1/24:1) in germfree mice compared to conventional mice. There is some evidence of lower levels of these sphingolipids in mice on a lard containing HF diet [50] and thus it could be that these ceramides were lower in the conventional mice compared to the germfree mice of Caesar et al [8] by a diet dependent mechanism. Finally, increased levels of hepatic Sa (d18:0) have been associated with increased mitochondrial respiration and obesity [55]. As we observed greater hepatic Sa (d18:0) in conventional mice, these data may signal increased hepatic mitochondrial function and be linked to the greater absolute weight of conventional animals.
Our findings indicate that a diet high in SBO leads to fat gain in mice regardless of the presence of a microbiome, although colonized mice were able to accumulate more fat and body weight. The microbiome density in the gut has a greater effect than dietary fat content on the sphingolipids in the liver and also contributes to circulating plasma lipids. Collectively, these results suggest that the microbiome is involved in the alteration of host signaling for both lipid processing and lipid storage.
Supplementary Material
Supplementary Figure S1. Mouse weight, fat pad mass, and food consumption on soybean oil diets. A. Body weight of animals over time relative to body weight at weaning when placed on SBO diets. B. Body weight of animals at euthanasia relative to weight at weaning. C. Epididymal fat mass relative to body weight at euthanasia. D. Average calorie intake for each animal. Feed intake was measured per cage. For A and D, error bars indicate standard deviations across all animals in a study. For B and C, dark lines indicate the 50% quartile, and the two thinner lines show the 25% and 75% quartiles. See Supplementary Table 1 for mean values. LF: low fat; HF: high fat; CNV1: conventional 1; CNV2: conventional 2; CNV3: conventional 3; GF: germfree 1; LG: low-germ.
Supplementary Figure S2. Plasma FAs C14:0 and C16:0 iso differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S3. Plasma FAs C15:0, C16:0, and C16:1n-7 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S4. Plasma FAs C18:0 iso, C18:1n-7/9/?, and C18:2n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S5. Plasma FAs C18:3n-6, C18:3n-3, and C20:3n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S6. Plasma FAs ClnA.7 and C22:5n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S7. Plasma FAs C20:5n-3, C24:1n-9, ClnAs4, and ClnA.2 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Highlights:
Germfree, low-germ, and conventional mice gain adiposity on a high soybean oil diet
Plasma fatty acids are affected by diet and less so by microbial colonization
Hepatic sphingolipid levels are dependent on microbial colonization status
Acknowledgements/grant support
We thank members of the Ley lab, Jiyao Zhang, the staff of the Cornell Animal Facility, Jennifer Mosher, Sylvie Allen, and Lynn Marie Johnson for their assistance, helpful discussions, and insight. S.C.D.R. is an Eli & Edythe Broad Fellow of the Life Sciences Research Foundation. This work was funded by NIH grant DP2OD007444 and by the Max Planck Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ALA
alpha-linolenic acid
- ANOVA
Analysis Of Variance
- Cer
ceramide
- CLA
conjugated linoleic acid
- ClnA
conjugated linolenic acid
- CNV
conventional
- Ct
cycle threshold
- DGLA
dihomo-gamma-linolenic acid
- DH
dihydro
- EPA
eicosapentaenoic acid
- FA
fatty acid
- GC
gas chromatography
- GF
germfree
- HF
high fat
- HPLC-MS
high performance liquid chromatography-mass spectrometry
- HSD
honest significant difference
- LA
linoleic acid
- LF
low fat
- LG
low-germ
- MS
microbial status
- PBS
phosphate buffered saline
- PCoA
Principal Coordinate Analysis
- qPCR
quantitative polymerase chain reaction
- Sa
sphinganine
- SBO
soybean oil
- SM
sphingomyelin
- So
sphingosine
- TBHQ
tertiary butylhydroquinone
Footnotes
Declaration of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rojo D, Méndez-García C, Raczkowska BA, Bargiela R, Moya A, Ferrer M, et al. Exploring the human microbiome from multiple perspectives: factors altering its composition and function. FEMS Microbiology Reviews 2017. 10.1093/femsre/fuw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–20. 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- [3].Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology 2007;19:59–69. 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- [4].Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007;104:979–84. 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–23. 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010;24:4948–59. 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- [7].Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, et al. High-Fat Diet: Bacteria Interactions Promote Intestinal Inflammation Which Precedes and Correlates with Obesity and Insulin Resistance in Mouse. PLoS ONE 2010;5:e12191. 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caesar R, Nygren H, Oresic M, Bäckhed F. Interaction between dietary lipids and gut microbiota regulates hepatic cholesterol metabolism. J Lipid Res 2016;57:474–81. 10.1194/jlr.M065847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Martinez-Guryn K, Hubert N, Frazier K, Urlass S, Musch MW, Ojeda P, et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host and Microbe 2018;23:458–469.e5. 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson EL, Heaver SL, Waters JL, Kim BI, Bretin A, Goodman AL, et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun 2020;11:2471. 10.1038/s41467-020-16274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kübeck R, Bonet-Ripoll C, Hoffmann C, Walker A, Müller VM, Schüppel VL, et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol Metab 2016;5:1162–74. 10.1016/j.molmet.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr 2010;104:919–29. 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- [13].Miyamoto J, Igarashi M, Watanabe K, Karaki S, Mukouyama H, Kishino S, et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun 2019;10:4007. 10.1038/s41467-019-11978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011;93:950–62. 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metabolism 2015;22:658–68. 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DiNicolantonio JJ, O’Keefe JH. Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Missouri Medicine 2017;114:303–7. [PMC free article] [PubMed] [Google Scholar]
- [17].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- [18].Su HM, Brenna JT. Simultaneous measurement of desaturase activities using stable isotope tracers or a nontracer method. Anal Biochem 1998;261:43–50. 10.1006/abio.1998.2706. [DOI] [PubMed] [Google Scholar]
- [19].Gómez-Cortés P, Tyburczy C, Brenna JT, Juárez M, de la Fuente MA. Characterization of cis-9 trans-11 trans-15 C18:3 in milk fat by GC and covalent adduct chemical ionization tandem MS. J Lipid Res 2009;50:2412–20. 10.1194/jlr.M800662-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oh PL, Martínez I, Sun Y, Walter J, Peterson DA, Mercer DF. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am J Transplant 2012;12:753–62. 10.1111/j.1600-6143.2011.03860.x. [DOI] [PubMed] [Google Scholar]
- [21].Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. 2019. [Google Scholar]
- [22].Bray JR, Curtis JT. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecological Monographs 1957;27:325–49. 10.2307/1942268. [DOI] [Google Scholar]
- [23].McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013;8:e61217. 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- [25].Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 2015;67:1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- [26].Lenth RV. Least-Squares Means: The R Package lsmeans. Journal of Statistical Software 2016;69:1–33. 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- [27].Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- [28].Di Rienzi SC, Jacobson J, Kennedy EA, Bell ME, Shi Q, Waters JL, et al. Resilience of small intestinal beneficial bacteria to the toxicity of soybean oil fatty acids. ELife 2018;7:1968. 10.7554/eLife.32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mitsutake S, Zama K, Yokota H, Yoshida T, Tanaka M, Mitsui M, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem 2011;286:28544–55. 10.1074/jbc.M111.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metabolism 2014;20:687–95. 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- [31].Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 2019;11:eaan5662. 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host & Microbe 2019;25:789–802.e5. 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- [33].Lee M- T, Le HH, Johnson EL. Dietary sphinganine is selectively assimilated by members of the mammalian gut microbiome. J Lipid Res 2020:jlr.RA120000950. 10.1194/jlr.RA120000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moriya T, Satomi Y, Murata S, Sawada H, Kobayashi H. Effect of gut microbiota on host whole metabolome. Metabolomics 2017;13:101. 10.1007/s11306-017-1240-9. [DOI] [Google Scholar]
- [35].Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 2010;51:1101–12. 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr 1995;62:564–71. 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- [37].Nikkari T, Luukkainen P, Pietinen P, Puska P. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med 1995;27:491–8. 10.3109/07853899709002458. [DOI] [PubMed] [Google Scholar]
- [38].Vessby B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol 2003;14:15–9. 10.1097/01.mol.0000052859.26236.5f. [DOI] [PubMed] [Google Scholar]
- [39].Zock PL, Mensink RP, Harryvan J, de Vries JH, Katan MB. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am J Epidemiol 1997;145:1114–22. 10.1093/oxfordjournals.aje.a009074. [DOI] [PubMed] [Google Scholar]
- [40].Fievez V, Colman E, Castro-Montoya JM, Stefanov I, Vlaeminck B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Animal Feed Science and Technology 2012;172:51–65. 10.1016/j.anifeedsci.2011.12.008. [DOI] [Google Scholar]
- [41].Knez M, Stangoulis J, Glibetic M, Tako E. The Linoleic Acid: Dihomo-γ-Linolenic Acid Ratio (LA:DGLA)—An Emerging Biomarker of Zn Status. Nutrients 2017;9:825. 10.3390/nu9080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zurier RB, Quagliata F. Effect of Prostaglandin E 1 on Adjuvant Arthritis. Nature 1971;234:304–5. 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]
- [43].Wang X, Lin H, Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis 2012;11:25. 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Perez MA, Magtanong L, Dixon SJ, Watts JL. Dietary Lipids Induce Ferroptosis in Caenorhabditis elegans and Human Cancer Cells. Developmental Cell 2020:S1534580720304986. 10.1016/j.devcel.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jenkins BJ, Seyssel K, Chiu S, Pan P-H, Lin S-Y, Stanley E, et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Sci Rep 2017;7. 10.1038/srep44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wahlström A, Sayin SI, Marschall H- U, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metabolism 2016;24:41–50. 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- [47].Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front Physiol 2019;10:185. 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Harfoot CG. Lipid metabolism in the rumen. Prog Lipid Res 1978;17:21–54. 10.1016/0079-6832(78)90004-6. [DOI] [PubMed] [Google Scholar]
- [49].Salsinha AS, Pimentel LL, Fontes AL, Gomes AM, Rodríguez-Alcalá LM. Microbial Production of Conjugated Linoleic Acid and Conjugated Linolenic Acid Relies on a Multienzymatic System. Microbiol Mol Biol Rev 2018;82:e00019–18, /mmbr/82/4/e00019–18.atom. 10.1128/MMBR.00019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Choi S, Snider AJ. Obesity-Related Diseases. Mediators of Inflammation 2015;2015:1–12. 10.1155/2015/520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chaurasia B, Tippetts TS, Monibas RM, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis 2019:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, et al. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metabolism 2015;22:266–78. 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, Oemrawsingh R, et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. Journal of Lipid Research 2018;59:1729–37. 10.1194/jlr.P081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Moore ES, Daugherity EK, Karambizi DI, Cummings BP, Behling-Kelly E, Schaefer DMW, et al. Sex-specific hepatic lipid and bile acid metabolism alterations in Fancd2-deficient mice following dietary challenge. Journal of Biological Chemistry 2019;294:15623–37. 10.1074/jbc.RA118.005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Apostolopoulou M, Gordillo R, Koliaki C, Gancheva S, Jelenik T, De Filippo E, et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Dia Care 2018;41:1235–43. 10.2337/dc17-1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Mouse weight, fat pad mass, and food consumption on soybean oil diets. A. Body weight of animals over time relative to body weight at weaning when placed on SBO diets. B. Body weight of animals at euthanasia relative to weight at weaning. C. Epididymal fat mass relative to body weight at euthanasia. D. Average calorie intake for each animal. Feed intake was measured per cage. For A and D, error bars indicate standard deviations across all animals in a study. For B and C, dark lines indicate the 50% quartile, and the two thinner lines show the 25% and 75% quartiles. See Supplementary Table 1 for mean values. LF: low fat; HF: high fat; CNV1: conventional 1; CNV2: conventional 2; CNV3: conventional 3; GF: germfree 1; LG: low-germ.
Supplementary Figure S2. Plasma FAs C14:0 and C16:0 iso differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S3. Plasma FAs C15:0, C16:0, and C16:1n-7 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S4. Plasma FAs C18:0 iso, C18:1n-7/9/?, and C18:2n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S5. Plasma FAs C18:3n-6, C18:3n-3, and C20:3n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S6. Plasma FAs ClnA.7 and C22:5n-6 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.
Supplementary Figure S7. Plasma FAs C20:5n-3, C24:1n-9, ClnAs4, and ClnA.2 differ by diet, gavage, and/or microbial status. Data are plotted as in Figure 5. N=3 to 148 per group.