Abstract
Background
The AOT is an atypical tumor of odontogenic origin that comprises about 0.1% of jaw tumors and cysts as well as up to 3% of odontogenic tumors (OTs).
Aim and objective
This review describes the clinical, radiographical, histopathological, and immunohistochemical properties of adenomatoid odontogenic tumor (AOT) and reports an occurrence of an AOT in a boy, 13 years of age.
Case description
A male, 13 years of age, presented with a swelling with respect to the left maxilla, painless, and with obvious facial asymmetry. The orthopantomogram and computed tomography scan revealed a large unilocular radiolucency in the left maxilla with permanent lateral incisor embedded within the lesion and permanent canine pushed away from its normal position. After complete enucleation of the cyst under local anesthesia and extraction of associated impacted permanent teeth and retained deciduous teeth related to the lesion, the defect was filled with a bone graft and closed. Postoperative follow-up was uneventful.
Conclusion
An accurate diagnosis should be established through clinical, radiographical, and pathological correlations in order to be able to differentiate AOT from other conditions for early diagnosis.
Clinical significance
This report highlights the salient features of the AOT to be able to correctly diagnose and manage the lesion.
How to cite this article
Kamble A, Shimpi MR, Dash JK, et al. Adenomatoid Odontogenic Tumor of the Maxilla in a 13-year-old Patient: A Rare Case Report with a Review of Literature. Int J Clin Pediatr Dent 2021;14(4):596–600.
Keywords: Adenomatoid odontogenic tumor, Jaw, Maxilla, Neoplasm, Odontogenic, Tumor
Background
Gnathic neoplasms affecting children and adolescents are most frequently benign.1 Those occurring in the jaws include bone tumors and odontogenic tumors (OTs), which only present themselves in the jaws. OT are anomalies, derived from odontogenic tissues or their residuum, entrapped within the bones of the jaw or the adjoining soft tissues.2 They encompass a diversified category of lesions that arise from the epithelium and/or odontogenic ectomesenchyme and remnants. While a part of these lesions constitutes hamartomas with a wide range of differentiation, the remaining are benign or malignant, with varying aggressiveness and may metastasize.
The potential origin of OTs is varied as follows:2
The pre-functional dental lamina distal to the mandibular third molars
The post-functional dental lamina, that is, cell rests of Serre, cell rests of Malassez, and the reduced enamel organ epithelium
The basal cell layer of the epithelium of the gingiva
The dental papilla
The dental follicle, and
The periodontal ligament.
The AOT is a true benign, noninvasive, non-aggressive neoplasm, that is, a distinctive lesion of the maxillofacial region, or the gingival overlying dentulous areas or alveolar mucosa of non-teeth-bearing areas.
In this paper, we report an unusual and rare instance of a 13-year-old male presenting with an extraoral swelling in the maxillary left region extending from the maxillary central incisor till the permanent maxillary left first molar. The lesion was initially diagnosed as an inflammatory cyst, and after histopathological investigations, a final diagnosis of AOT was made. The paper presents a review of literature discussing the unique clinical, radiographical, and histopathological features seen in AOT along with several immunohistochemical markers of the tumor.
Case Description
A male, 13 years of age, reported to the Department of Paediatric Dentistry, Bharati Vidyapeeth Dental College and Hospital, Pune, with the chief complaint of a swelling, asymptomatic in nature, present from approximately 6 months, with respect to the left side of the maxilla, which expanded with time. The medical, family, and dental histories were non-contributory. Extraoral examination disclosed a large swelling with respect to the left side of the maxilla with obvious facial asymmetry (Fig. 1). In the intraoral examination, a large painless swelling with respect to the left maxillary labial vestibule was revealed, extending from the distal surface of the central incisor till the mesial aspect of the first molar. The deciduous maxillary left lateral incisor was retained and the canine was erupting buccally (Fig. 2). An orthopantomogram revealed a large unilocular radiolucency with internal opacities in the left maxilla with permanent lateral incisor embedded within the lesion and permanent canine pushed away from its normal position. The premolars appeared to be deviated from their normal eruptive pathway (Fig. 3A). Posterioranterior (PA) and lateral views of the skull showed the displacement of the canine superiorly. Preoperative noncontrast axial section of jaws and paranasal sinus revealed unilocular, expansile cystic lesion measuring 3 cm × 2.7 cm × 6.4 cm in the left antero-inferior aspect of the maxilla. The lesion showed punctuated “snow flake” calcification with unerupted permanent teeth lying within it. The cystic lesion seemed to bulge into the nasal cavity causing superior displacement of the inferior turbinate (Fig. 3B). The operative noncontrast enhanced computed tomography taken in coronal section demonstrated cystic nature of lesion and local expansive changes (Fig. 3C). Fine-needle aspiration cytology was performed: 5 mL of fluid was withdrawn from the lesion (Fig. 4). Histopathological examination showed occasional squamous cells and histiocytes against a hemorrhagic background. After correlating clinically and radiographically, a provisional diagnosis of inflammatory cyst was made.
Fig. 1.

Extraoral clinical examination revealing asymmetry of the face
Fig. 2.
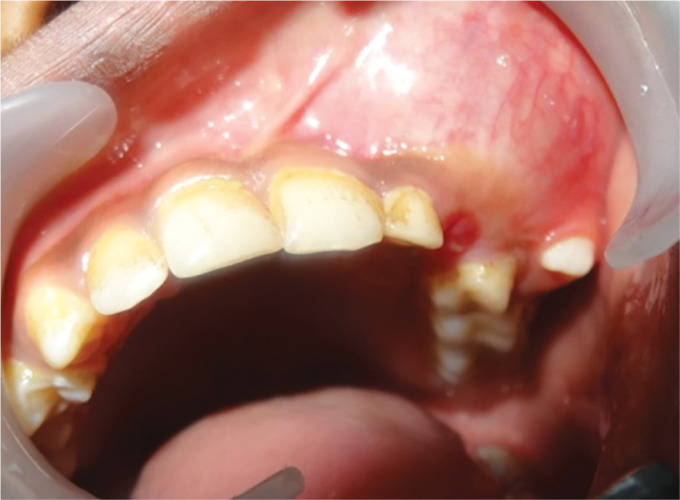
Intraoral clinical examination illustrating a swelling in the left maxillary vestibule
Figs 3A to C.
Radiographical investigations: (A) Orthopantomogram; (B) PA and lateral views of the skull; (C) Axial view of cone-beam computed tomography showing a well-demarcated radiolucency extending from the maxillary left central incisor till the permanent first molar
Fig. 4.

Fine-needle aspiration to withdraw fluid content from within the lesion
After carrying out hematological investigations and obtaining informed consent, local anesthesia was administered to the patient. A trapezoidal flap was raised extending from the mesial surface of the central incisor till the distal surface of the permanent first molar (Fig. 5A). The cyst lining was dissected off the surrounding bone and the lesion was entirely enucleated and of the deciduous lateral incisor, canine, deciduous first and second molars, the permanent canine, premolars, and permanent first molar were extracted (#62, 63, 64, 65, 21, 22, 23, 24 in FDI system) under local anesthesia (Fig. 5B). Following the placement of a bone graft (B-Ostin) (Fig. 5C), the surgical site was closed using black silk sutures (4–0) (Fig. 5D). The lesion measured 3 cm × 2.7 cm × 6.4 cm (Fig. 5E). After inspecting the defect for the impacted permanent lateral incisor in vain, it was located within the lesion after making an incision through it (Fig. 5F).
Figs 5A to F.
Treatment performed: (A) Surgical window created on anterior surface of the cyst; (B) Enucleation of cyst; (C) Placement of bone graft material; (D) Closure of surgical site using black silk 4–0 sutures; (E) Enucleated mass with the extracted teeth; (F) Impacted permanent lateral incisor located within the lesion
There was no complication seen postoperatively. After the surgery, the entire specimen was histopathologically examined, revealing the characteristics of AOT. The lesion was lined by odontogenic epithelium with occasional cellular projection in the cystic lumen. The capsule of the tumor was distinctly identifiable (Fig. 6A). Polyhedral and cuboidal epithelial cells in this fibro-collagenous stroma displayed characteristic duct-like structures with the classical “rosette” pattern that is associated with AOT lined with columnar epithelial cells with polarized nuclei (Fig. 6B) and filled with eosinophilic material with small foci of calcified material (Fig. 6C). The treatment plan further includes the provision of a removable partial denture for the patient until the completion of skeletal growth, after which a fixed prosthesis can be planned.
Figs 6A to C.
Histopathological examination: (A) Capsular lining of the lesion; (B) Duct-like epithelial structures along with the rosette pattern arrangement of tumor cells; (C) Eosinophilic deposits along with small foci of calcified material dispersed within the lesion
Discussion
The AOT is an odontogenic epithelial tumor which is a distinct entity of the maxillofacial skeleton affecting young patients. Dreibladt published one of the first reports on the AOT in 1907, terming it as “pseudoadenoameloblastoma” while Ghosh et al. termed it as “adamantinoma.” It was first recognized as a unique lesion by Stafne in 1948. Unal described it under various names—adenoameloblastoma, cystic complex composite odontoma, ameloblastic odontogenic tumor, odontogenic adenomatoid tumor, etc.3
Marx proposed “adenomatoid odontogenic cyst,” stating that the lesion presents as a cyst, arising from Hertwig's epithelial root sheath (HERS), with proliferation intraluminally, filling the cystic space, giving a solid impression.4
WHO in 1971, adopted the expression “AOT” proposed by Philipsen and Birn, defining the lesion as “a tumor of the odontogenic epithelium with duct-like structures with varying degrees of inductive changes in the connective tissue. The tumor may be partially cystic, and in some cases, a solid lesion may be present as masses in the wall of large cyst.”5
Usually, non-neoplastic causes of swellings associated with the jaw in young adults are the presence of an apical cyst, calcifying odontogenic cyst, dentigerous cyst, odontogenic keratocyst, or central giant cell granuloma, while the common neoplastic causes include AOT, unicystic ameloblastoma, calcifying epithelial OT, ameloblastic fibroma and ameloblastic fibro-odontoma.
Reports vary greatly in relation to the predominant OT in the age group of 3 to 18. From the early 1990s to the early 2000s, 65 cases of AOT have been published.6 The average age was 13.2 years (with a range of 3–28 years). The male:female ratio was 1:2.3. The AOT is predominantly found in the maxilla (maxilla:mandible 2.6:1) and the anterior region of the jaw is more likely to be involved than the posterior region.6 The tumor is most often diagnosed in the second decade of life. The extent of the lesion is approximately 2 to 7 cm, gradually increasing in size over time, resulting in a painless enlargement of the jaws.
The origin of AOT is controversial. It is believed that its origin may either be from the odontogenic epithelium that lines a dentigerous cyst or derived from the residual dental lamina system. The lesion grows into or adjacent to a dental follicle as per the “envelopmental” theory.7
Chen et al. described an AOT derived from a dentigerous cyst where the tumor surrounded a canine, proposing an envelopmental focalization or “hybrid variant.”8
Clinical features of AOT generally pivot on complaints associated with a missing tooth and asymptomatic gradually growing swelling. Radiologically, a unilocular cystic mass enclosing the unerupted tooth is seen.9 This case was presented with an extra-oral swelling in the left maxilla. A unilocular radiolucency extending from the distal surface of the central incisor till the mesial surface of the first molar was seen. The histopathological characteristics of AOTs are very specific, and all variants of AOT reveal similar histopathological characteristics.10 In this case, the lesion was lined by odontogenic epithelium enclosed within a capsule. Polyhedral and cuboidal epithelial cells within a fibro-collagenous stroma arranged in the classical “rosette” pattern were identified along with the presence of eosinophilic material with small foci of calcified material. Table 1 gives an account of the clinical, radiographical, and histopathological features of AOT.
Table 1.
Clinical, radiographical, and histopathological features of three variants of AOT
| Histological variants (Philipsen and Reichart7 1969) | Prevalence (Handschel et al.,6 2005) | Clinical features (Acharya et al.,9 2014) | Radiographical features (Handschel et al.,6 2005) | Histopathological features (similar in the case of all three variants) (Bonardi et al.,10 2015) |
|---|---|---|---|---|
| Follicular | 71% | Associated with the crown and often part of the root of an impacted (unerupted) tooth | A well-circumscribed unilocular radiolucency associated with the crown and often part of the root of an unerupted tooth | A well-encapsulated structure with a circumscribed intraluminal, thick, and fibrous proliferation of the epithelium showing false ducts, spiral, or rosette forms. The ducts may be coated with cylindrical or cubical cells with nuclei polarized away from the lumen. The lumen that may be empty or may contain an eosinophilic, uncalcified amorphous material of undetermined origin called “tumor droplets” is seen |
| Extra-follicular | 15% | Usually not associated with an impacted tooth | Located between, above, or superimposed upon the roots of erupted permanent teeth | |
| Peripheral | 4% | Occurs in the gingival tissue of tooth-bearing areas | Erosion of the adjacent cortical bone is seen |
Immunohistochemical investigations have also been carried out for the diagnosis of AOT. It involves the identification of monoclonal and polyclonal antibodies which are used to detect specific antigens in the sections of the tissues. Several immunohistochemical markers of the tumor include keratin, vimentin,11 amelogenin,12 enamelin,13 and matrix metalloproteinase (MMP-7 and MMP-26).14
The treatment of choice for AOT is the surgical resection of the tumor where complete enucleation of the lesion along with the removal of teeth associated with the lesion is carried out, according to Kumar15 et al., Bonardi10 et al., and Yadav16 et al. For intra-bony defects secondary to AOT, guided tissue regeneration coupled with the placement of a membrane is suggested after the removal of the tumor for rapid filling of large defects. In the present case, the enucleation of the entire cyst was carried out along with the removal of teeth associated with the lesion. The defect was augmented with bone graft material to aid in the formation of bone.
Recurrence of AOT is exceptionally rare. Toida et al. reported two cases, with recurrences with intracranial extension in one of them.17 Philipsen and Reichart reported recurrence in only three cases out of 750 studied.7
Even though the prognosis of the tumor is excellent, long-term follow-up is necessary to assess the fate of the involved teeth and to check for recurrences, if any.
Conclusion
The AOT is a rarely occurring benign epithelial OT that can be treated by the excision under local anesthesia. An accurate diagnosis should be established through clinical, radiographical, and pathological correlations in order to be able to differentiate AOT from other conditions that may present in the similar clinical and radiographical manners, thus preventing extensive or mutilating surgery in the process. Immunohistochemical investigations validate cytoskeletal characteristics and can be used to identify the tumor. The routinely undertaken treatment modality is enucleation and curettage for AOT. However, further investigations are required to identify the origin of the tumor and to accurately differentiate it from other lesions with the same clinical presentation.
Clinical Significance
This case report highlights the various clinical, radiographical, and histopathological features of AOT and presents a case of an extensive tumor that required surgical correction. Often being incorrectly diagnosed as a dentigerous cyst, due to its radiographical and histopathological characteristics, an accurate diagnosis of AOT should be established and treatment should be carried out accordingly so as to prevent the mutilating surgery.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Keszler A, Guglielmotti MB, Dominguez FV. Oral pathology in children. Frequency, distribution and clinical significance. Acta Odont Latinoamer. 1990;5(1):39–48. [PubMed] [Google Scholar]
- 2.Steenland HS. Epithelioma adamantinum. J Exper Med. 1905;6(4-6):377–389. doi: 10.1084/jem.6.4-6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unal T, Cetingul E, Gunbay T. Peripheral adenomatoid odontogenic tumour: birth of a term. J Clin Pediatr Dent. 1995;19(2):139–142. [PubMed] [Google Scholar]
- 4.Marx RE, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Hanover Park, Ill, USA: Quientessence Publishing; 2003. [Google Scholar]
- 5.Philipsen HP, Birn H. The adenomatoid odontogenic tumour, ameloblastic adenomatoid tumour or adenoameloblastoma. Acta Pathologica et Microbiologica Scandinavica. 1969;75(3):375–398. [PubMed] [Google Scholar]
- 6.Handschel J, Depprich RA, Zimmermann AC, et al. Adenomatoid odontogenic tumour of the mandible: review of the literature and report of a rare case. Head Face Med. 2005;1(1):3. doi: 10.1186/1746-160X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipsen HP, Samman N, Ormiston IW, et al. Variants of the adenomatoid odontogenic tumour with a note on tumour origin. J Oral Pathol Med. 1992;21(8):348–352. doi: 10.1111/j.1600-0714.1992.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen YK, Hwang IY, Chen JY, et al. Adenomatoid odontogenic tumour arising from a dentigerous cyst: a case report. Internat J Pediat Otorhinolaryng Extra. 2007;2(4):257–263. doi: 10.1016/j.pedex.2007.08.003. [DOI] [Google Scholar]
- 9.Acharya S, Goyal A, Rattan V, et al. Dentigerous cyst or adenomatoid odontogenic tumour: clinical radiological and histopathological dilemma. Case Rep Med. 2014;2014:514720. doi: 10.1155/2014/514720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonardi JP, da Costa FH, Matheus RA, et al. Rare presentation of adenomatoid odontogenic tumour in a patient: a case report. Oral Maxillofac Surg. 2016;20(2):215–217. doi: 10.1007/s10006-015-0537-y. [DOI] [PubMed] [Google Scholar]
- 11.Tatemoto Y, Tanaka T, Okada Y, et al. Adenomatoid odontogenic tumour: Co-expression of keratin and vimentin. Virchows Arch A Pathol Anat Histopathol. 1988;413(4):341–347. doi: 10.1007/BF00783027. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Yamada K, Kasai T, et al. Immunohistochemical expression of amelogenins in odontogenic epithelial tumours and cysts. Virchows Arch A Pathol Anat Histopathol. 1991;418(4):319–325. doi: 10.1007/BF01600161. [DOI] [PubMed] [Google Scholar]
- 13.Saku T, Okabe H, Shimokawa H. Immunohistochemical demonstration of enamel proteins in odontogenic tumours. J Oral Pathol Med. 1992;21(3):113–119. doi: 10.1111/j.1600-0714.1992.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 14.Souza Freitas V, Ferreira de Araújo CR, Alves PM, et al. Immunohistochemical expression of matrilysins (MMP-7 and MMP-26) in ameloblastomas and adenomatoid odontogenic tumours. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):417. doi: 10.1016/j.tripleo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Kharti A, Kalra N, et al. Adenomatoid odontogenic tumour of maxilla in a 14-year-old child. J Pediat Dent. 2014;2(2):61–64. doi: 10.4103/2321-6646.137692. [DOI] [Google Scholar]
- 16.Yadav S, Tyagi S, Kumar P, et al. Surgical management of maxillary adenomatoid odontogenic tumour in paediatric patient: a clinical report. J Sci Soc. 2013;40(1):51–56. doi: 10.1053/j.seminoncol.2013.09.010. [DOI] [Google Scholar]
- 17.Toida M, Hyodo I, Okuda T, et al. Adenomatoid odontogenic tumours. report of two cases and survey of 126 cases in Japan. J Oral Maxillofac Surg. 1990;48(4):407–408. doi: 10.1016/0278-2391(90)90441-4. [DOI] [PubMed] [Google Scholar]





