Abstract
Aim and objective
The purpose of this research was to analyze the role of chitosan in the remineralization of enamel and dentin.
Materials and methods
An electronic search was done for articles published from January 2009 to January 2020. A manual search was done from bibliographies of selected articles for relevant articles that were unexplored. Only in vitro studies conducted on the application of chitosan for remineralization of enamel and dentin were included in the study.
Results
Of the 162 articles that were searched, only 15 in vitro studies were selected for the study. These studies met the inclusion criteria and were published from January 2009 to January 2020.
Conclusion
The review provides insight into the mechanism of remineralization of enamel and dentin. The properties of chitosan make it an ideal biomaterial that can be employed in the formulation of a novel remineralizing gel. However, more in vivo studies, clinical trials, and research are essential to transform chitosan-based remineralizing gels from research to clinical use.
Clinical significance
This review article opens a new window of opportunities for remineralizing enamel and dentin which have been long considered a challenging job.
How to cite this article
Nimbeni SB, Nimbeni BS, Divakar DD. Role of Chitosan in Remineralization of Enamel and Dentin: A Systematic Review. Int J Clin Pediatr Dent 2021;14(4):562–568.
Keywords: Casein phosphopeptide-amorphous calcium phosphate, Chitosan, Demineralization, Dental caries, Fluorides, Remineralization
Introduction
Chitosan is a biopolymer derived from 70% deacetylation of chitin in a basic solution. Chitin is a naturally occurring complex carbohydrate present in the exoskeleton of shrimps, crustaceans, and insects. Chitosan was first discovered by C. Rougeut in 1859 and named chitosan by Hoppe-Seyler in 1894. The structure of chitosan was discovered by Darmon and Rudall in 1950, after which various studies were conducted on chitosan-derived biomaterials in the biomedical field.1
Chitosan has been used as a direct and indirect pulp capping agent, an antimicrobial agent against E. faecalis. It is one of the ingredients in the triple antibiotic intracanal medicament. It is used for the removal of the smear layer during biomechanical preparation of root canals, guided tissue regeneration, guided bone regeneration, and to promote healing after periodontal surgeries. Various restorative materials like glass ionomer cements, composites, and dental adhesives have been modified using chitosan to enhance their antimicrobial property and adhesion to the tooth structure. In oral surgery, it has been used for hemostasis, oral reconstruction, bone substitute, and repair of the temporomandibular joint disc.2 The most widely studied property of chitosan is its remineralization property and its role in enamel and dentin regeneration.3
Remineralization of enamel is different from the remineralization of dentin. In remineralization of enamel, the demineralized tissue is remineralized again and is made sturdier to acid attacks compared to the natural enamel which existed before.4 Remineralization of dentin involves the regeneration of a new mineralized collagen matrix and the formation of hydroxyapatite crystals that block the dentinal tubules and protect the pulp–dentin complex.5 Remineralization of dentin is more difficult compared to the remineralization of enamel either in a clinical setup or in the laboratory.6 The remineralization systems have been classified into fluoride-based and non-fluoride-based remineralization systems.
Though fluoride is considered the cornerstone of remineralization in dentistry,7 it has also been classified as a neurotoxin, which elevates safety alarms among the common public. There is a very fine demarcation between the optimum fluoride concentration and the lethal fluoride dose.8 There is a need to adopt a biological approach to dental caries management, which includes the preservation of teeth and restoration only when required.9
The modern non-fluoride-based remineralizing systems have two mechanisms of action. Some remineralizing agents regenerate the tissue lost due to the lesion by the tissue engineering method. Such systems are called biomimetic systems.10 They attempt to remineralize the dental tissues and are more regenerative than reparative.11 The other systems of non-fluoride-based remineralization are calcium phosphate systems, polyphosphate systems, and natural products. The mechanism of action of these products is by supply and precipitation of calcium and phosphate ions over the subsurface and superficial lesions, which convert into HA and fluorapatite crystals during phase transformation.12 The motto of this research was to evaluate the role of chitosan in the remineralization of enamel and dentin.
Materials and Methods
Literature searches were performed from January 2009 to January 2020. The ensuing keywords and Boolean operatives were utilized in the search: Chitosan, remineralization, demineralization, dental caries, casein phosphopeptide-amorphous calcium phosphate (ACP), fluorides.
The literature search was conducted in January 2020, using the following database:
PubMed: The national library of medicines online search interface for Medline and preMEDLINE (https://www.ncbi.nlm.nih.gov/entrez/query.fcgi).
Scopus: Scopus is Elsevier's abstract and citation database launched in 2004 (https://www.scopus.com/home.uri).
Google India http://www.google.co.in.
The primary focus or the search involved systematic reviews (evidence level Ia), experimental studies (evidence levels IIb), and case reports were considered. The level of evidence of articles was categorized according to the guidelines of the Oxford Center for Evidence-Based Medicine (http://www.cebm.net/levels_of_evidence.asp).3 The search results have been explained in Table 1.
Table 1.
Search results
| Database | Search string | Hits | Selected papers |
|---|---|---|---|
| Scopus | TITLE-ABS-KEY (Chitosan AND Remineralization AND Enamel) | 22 | 3 |
| PubMed | ((Chitosan) AND (Remineralization)) AND (Enamel) | 14 | 7 |
| Google Scholar | TX chitosan AND TX enamel AND TX remineralization | 91 | 2 |
| Additional records identified through other sources | (Chitosan) AND (Remineralization) AND (dentin) | 35 | 3 |
Review Results
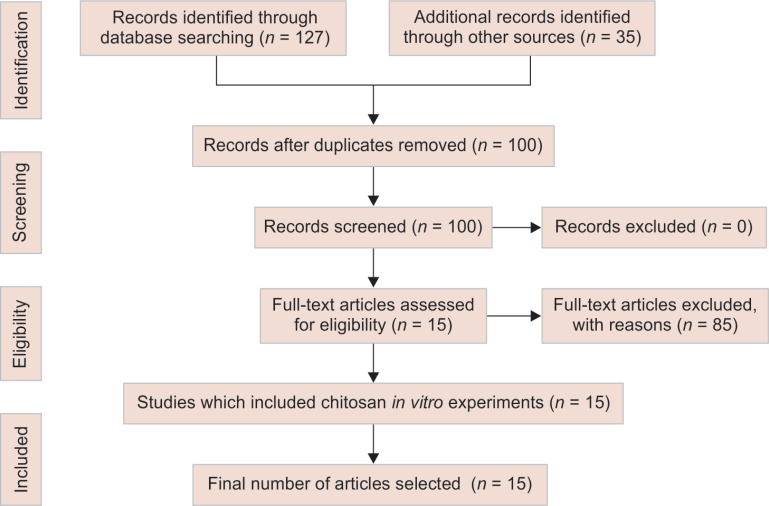
Of the 162 articles that we got from the search, only 15 articles were selected that were published on the in vitro studies conducted on the application of chitosan for remineralization of enamel and dentin were included in the study. The selection of articles is explained in the Flowchart 1—PRISMA flowchart. Table 2 represents the analysis of the selected articles.
Flowchart 1.
PRISMA flowchart
Table 2.
Analysis of the selected articles
| No | Title | Author | Year | Study type | Form in which chitosan is used | Type of teeth | Tissue remineralized | Result |
|---|---|---|---|---|---|---|---|---|
| 1 | Chitosan-bioglass complexes promote subsurface remineralization of incipient human carious enamel lesions | Zhang et al. | 2019 | In vitro | Chitosan bio-glass complex | Human third molars | Enamel | Chitosan bio-glass complex promoted subsurface remineralization |
| 2 | Remineralization of enamel white spot lesions pretreated with chitosan in the presence of salivary pellicle | Zhang et al. | 2018 | In vitro | Chitosan solution | Human third molars | Enamel | Chitosan enhances white spot lesion remineralization |
| 3 | Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate | Zhang et al. | 2014 | In vitro | Phosphorylated chitosan-amorphous calcium phosphate nano complexes | Human third molars | Enamel | Phosphorylated chitosan-amorphous calcium phosphate nano complexes caused significant subsurface remineralization |
| 4 | In vitro subsurface remineralization of artificial enamel white spot lesions pretreated with chitosan | Zhang et al. | 2018 | In vitro | Chitosan solution | Human third molars | Enamel | Chitosan improved the remineralization efficiency of the remineralizing solution |
| 5 | Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides | Xiao et al. | 2017 | In vitro | Carboxymethyl chitosan- amorphous calcium phosphate nanocomplex solution | Human third molars | Enamel | Carboxymethyl chitosan-amorphous calcium phosphate nanocomplex solution brought about biomineralization of enamel |
| 6 | Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries | Chen et al. | 2015 | In vitro | Carboxymethyl chitosan-amorphous calcium phosphate nanocomplex solution | Human third molars | Dentin | Completely demineralized dentin was partially remineralized by biomimetic strategy |
| 7 | Chitosan effect on dental enamel de-remineralization: an in vitro evaluation | Arnaud et al. | 2010 | In vitro | Chitosan solution | Human teeth | Enamel | Chitosan inhibits demineralization |
| 8 | Amelogenin-chitosan matrix for human enamel regrowth: effects of viscosity and supersaturation degree | Ruan et al. | 2014 | In vitro | Amelogenin chitosan hydrogel | Human third molars | Enamel | Under optimal conditions, the CS-AMEL system helped in the formation of enamel-like organized apatite crystals |
| 9 | Anti-biofilm and remineralization effects of chitosan hydrogel containing amelogenin-derived peptide on initial caries lesions | Ren et al. | 2018 | In vitro | chitosan hydrogel containing amelogenin-derived peptide | Bovine incisors | Enamel | The chitosan hydrogel containing the amelogenin-derived peptide |
| Inhibited S. mutans biofilm and promote the remineralization of the initial enamel caries | ||||||||
| 10 | Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel—A model study | Simeonov et al. | 2019 | In vitro | hybrid chitosan/calcium phosphates microgels | Human permanent molars and premolars | Enamel | Chitosan had superior bio adhesiveness by which it continuously supplied calcium and phosphate ions for remineralization. Chitosan has an anti-microbial property to enhance remineralization |
| 11 | Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems | Ruan et al. | 2016 | In vitro | Amelogenin chitosan hydrogel | Human third molars | Enamel | CS-AMEL hydrogel effectively formed a new organized layer of enamel-like crystals on the surface of erosive lesions |
| 12 | Carboxymethyl chitosan/amorphous calcium phosphate and dentin remineralization | Santoso et al. | 2019 | In vitro | Carboxymethyl chitosan- amorphous calcium phosphate gel | Human teeth | Dentin | Carboxymethyl chitosan-amorphous calcium phosphate gel induced dentin remineralization |
| 13 | Induced synthesis of hydroxyapatite by chitosan for enamel remineralization | Tian et al. | 2012 | In vitro | Chitosan hydrogel | Human molars | Enamel | Chitosan-enhanced biomimetic remineralization |
| 14 | The effect of carboxymethyl chitosan/amorphous calcium phosphate to guide tissue remineralization of dentin collagen | Annisa et al. | 2019 | In vitro | Carboxymethyl chitosan-amorphous calcium phosphate solution | Human premolar | Dentin | Chitosan improved intrafibrillar and extrafibrillar remineralization |
| 15 | Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentine modified with phosphorylated chitosan | Zhang et al. | 2011 | In vitro | Phosphorylated chitosan | Human third molar | Dentin | Chitosan facilitated biomimetic surface remineralization |
Discussion
The objective behind remineralizing enamel is the deposition of minerals back into the tissue and blocking the demineralized pores. Any remineralizing agent needs a vehicle to carry the agent into the tooth and supply the minerals for remineralization. The demineralized enamel surface is negatively charged due to the leaching out of calcium ions. The positive charge of chitosan helps in better adherence to the negatively charged demineralized lesion surface. In a study conducted by Arnaud et al., 12 sets of human teeth samples were collected and subjected to de-remineralization in DE4.0 and DE4.8 solutions with measured pH for 3 hours. Later the samples were removed, cleaned, dried, and immersed in the remineralizing solution for 21 hours. This remineralization–demineralization cycle was conducted for 5 days with freshly prepared solutions each day. The remineralization–demineralization cycle created artificial caries-like lesions with a depth of 50 to 60 μm. One set of teeth was kept as a control group and the remaining samples were brushed with chitosan of varying strengths between 2.5 and 5.0 mg/mL for 60 to 90 seconds. Optical coherence tomography (OCT) was done to assess the amount of diffusion of chitosan into the enamel. It was observed, OCT results indicated that for chitosan concentrations of 2.5 and 5.0 g/mL the dispersion was deep into the DEJ. From this study, it was proved that chitosan has a better ability to penetrate deeper into the enamel, and thus it can carry the required ions more in-depth into the lesion.13
Chitosan and its derivatives have developed as novel biomaterials due to their low toxicity, biodegradability, biocompatibility, and biological activity. Among all the derivatives of chitosan, phosphorylated chitosan exhibited bactericidal properties along with other properties like biocompatibility and osteoinductive property.14 This was proved in a study conducted by Zhang et al. where phosphorylated chitosan was used on partially demineralized dentin for biomimetic surface remineralization. It was observed that phosphorylated chitosan induced significant deposition of calcium and phosphate ions on the surface of partly demineralized dentin promoting crystal nucleation.15
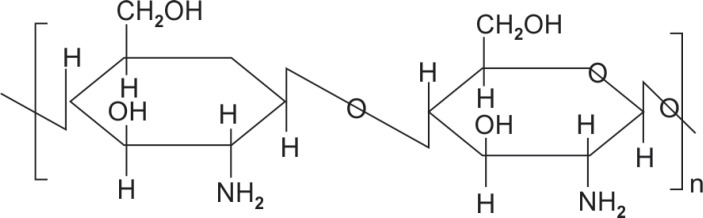
The biogenetic formation of calcified dental tissues such as enamel is a complicated multistep procedure that includes calcium and potassium starting from soft tissues made up of type I collages to a mineralized tissue made up of hydroxyapatite crystals and extracellular matrix. Since enamel is non-living tissue and cannot remineralize on its own numerous in vitro methods have been developed to artificially remineralize enamel.16 Chitosan is one such mucopolysaccharide containing 2-acetamido-2-deoxy-b-D-glucose through a β (1-4) linkage as shown in Figure 1.17
Fig. 1.
Chemical structure of chitosan
Chitosan is considered a bioactive material as its functional group can induce apatite nucleation.18 In a study conducted by Tian et al., a phosphorylation model was planned to induce crystallization of hydroxyapatite in vitro in a controlled manner. It was witnessed that hydroxyapatite nanorods could be controlled, followed by the in situ crosslinking process, and activated by pH and ionic strength. The dentinal tubules were clogged by neonatal hydroxyapatite layer and this was combined with a continuous structure of columns crystal with a dimension of 10 to 40 nm. Simultaneously, X-ray diffraction (XRD) reports exhibited that the precipitation was calcium fluoride phosphate, and Ca:P was 1.6. Moreover, there were column crystals arranged equivalently inside, similar to the crystal array on top of the enamel rod. The outcomes proposed that chitosan monolayer could be valuable in the intonation of mineral behavior throughout in situ dental tissue engineering.19
With the development of biomimetic remineralization, various concepts like the non-classical concept of nucleation have come up which suggests that amorphous antecedents of hydroxyapatite (HAP) crystals are convoluted in the natural mineralization of dental hard tissues by direct transformation to HAP crystals.20 Numerous in vitro studies have suggested that analogs of proteins like casein phosphopeptide, derived from milk can aid in biomineralization. In combination with ACP, CPP–ACP serves as a reservoir of mineral ions that promote remineralization.21 Another such material is phosphorylated chitosan which retards spontaneous precipitation of calcium ions and incorporates them into the precipitate.22 In a study conducted by Zhang et al., nanocomplexes of phosphorylated chitosan combined with ACP also called Pchi–ACP was used to remineralize demineralized enamel. A remineralizing solution was prepared consisting of Pchi–ACP nanocomplexes. It was synthesized by a combination of CaCl2 and K2HPO4 into a Pchi–ACP solution. This solution was standardized by transmission electron microscopy (TEM), XRD, Fourier-transform infrared (FTIR), and selected area electron diffraction (SAED). The solution was tested on enamel lesions and compared for remineralization potential with fluoridated solutions using micro-CT and field emission electron microscope. It was observed that nanocomplexes of Pchi–ACP brought about significant remineralization of the enamel subsurface lesions on par with remineralization brought about by fluoridated solutions. From the study, it could be concluded that the use of naturally occurring protein analogs convoluted in biomineralization could be an effective and alternative strategy to remineralize enamel.23
Biomimetic production of enamel-mirroring material is being regarded as the future of preventive dentistry as correction of enamel defects with restorative materials is being considered unsuitable due to weak mechanical strength and poor retention. Numerous biomimetic systems have been tried and tested in vitro but their clinical application for remineralization has been under question.24 According to a study conducted by Ruan et al. on the amelogenin-chitosan (CS-AMEL) hydrogel biomimetic system, the consequences of increasing viscosity and supersaturated on the dimensions and alignment of synthetic crystals were evaluated by using scanning electron microscopy and XRD. It was observed that increasing the viscosity of chitosan from 1 to 2% significantly enhanced the orientation of the crystals and with more supersaturation, there was the synthesis of larger and irregular enamel-like crystals. Thus, it was concluded that ideal conditions for the production of enamel-like crystals in an organized manner using amelogenin-chitosan (CS-AMEL) hydrogel were: 2% (w/v) chitosan, 2.5 mM calcium, and 1.5 mM phosphate (degree of supersaturation = 8.23), and 200 μg/mL of amelogenin.25
Remineralization of dentin can be classified into extrafibrillar remineralization, and intrafibrillar remineralization, also called as guided-tissue remineralization (GTR). The remineralization that occurs in GTR strengthens the mechanical properties of dentin. This remineralization requires a non-collagen protein called dentine matrix protein 1 (DMP1), i.e., dentin matrix protein 1. This protein acts as a binder between Ca2+ ions and hydroxyapatite crystals of the teeth. This protein can be replaced by several analogous proteins that perform the same function. One such example is carboxymethyl chitosan (CMC). In a research conducted by Santoso et al., CMC/ACP was used for remineralization of dentin, and it was observed that CMC/ACP significantly improved the process of remineralization and strengthened dentin.26
In a similar study by Annisa et al., CMC/ACP was used to substitute the role of dentin matrix protein 1. The ability of CMC/ACP to attain intrafibrillar and extrafibrillar remineralization on demineralized dentin was studied, and it was seen that from day 7 to day 14, there was a marked rise in the levels of calcium and phosphate ions in the CMC/APC crystals. Transmission electron microscopy analysis showed that the CMC/ACP group displayed more intrafibrillar remineralization and extrafibrillar remineralization compared to other remineralization agents.27
Remineralization of demineralized dentin in deep carious lesions is a challenging task in clinical settings. In indirect pulp capping, though efforts have been made to remineralize the affected dentin collagen with calcium hydroxide, its effects have not been explicated.28 The study conducted by Chen et al. was intended to remineralize the affected dentine in a tooth model with a deep cavity utilizing nanocomplexes of CMC/ACP based on mirroring the stabilizing effect of DMP1 on ACP in the remineralization of dentine. The outcomes of this in vitro study showed that CMC could stabilize ACP and form nanocomplexes of CMC/ACP, which converted into scaffoldings. In both the models, i.e., single layer collagen model and tooth model of deep caries, ACP nanoparticles were discharged from scaffolds of CMC/ACP nanocomplexes which accomplished mineralization of intrafibrillar collagen employing bottom-up approach, therefore simplifying remineralization of demineralized dentine.
As nanocomplexes of CMC/ACP showed encouraging effects of remineralization on affected dentine through biomimetic approach, CMC/ACP could be considered a prospective indirect pulp capping agent for the treatment of deep caries.29
Chitosan is a polycationic mucoadhesive polymer that allows a sustained release of bioactive substances over a while. It is biodegradable, and the by-products are non-toxic. Moreover, the presence of a primary amine group at the C-2 position allows modification of chitosan on repeat units.30 Many modifications of chitosan have been tried as remineralizing agents for enamel and dentin. According to a study conducted by Zhang et al., chitosan was used for pretreatment of white spot lesions followed by the application of bioglass and bioglass with polyacrylic acid slurry. It was observed that chitosan improved the remineralization ability, and the subsurface was seen to have higher mineral content.31
Similarly, in a study conducted by Ruan et al., amelogenin-chitosan hydrogel was used as a remineralizing agent. This hydrogel was prepared by mixing 75% chitosan with purified amelogenin rP172 obtained from recombinant DNA technology. This gel was used for remineralizing surface erosive lesions and artificial incipient lesions. The results demonstrated that CS-AMEL hydrogel effectively formed a new organized enamel-like crystal layer on the surface of erosive lesions. It could also repair incipient lesions by regrowing organized crystals and also reduced the depth of the lesions.32
In another study by Ren et al., chitosan was combined with amelogenin-derived peptide QP5 which formed a hydrogel with antibiotic and tooth remineralizing properties. The antibiotic property was contributed by chitosan which acted as an antibiotic carrier and remineralizing property was contributed by QP5. It was observed that CS-QP5 hydrogel inhibited S. mutans biofilm formation and also promoted remineralization of initial enamel carious lesions.33
Correspondingly, in a similar study conducted by Zhang et al., subsurface remineralization efficacy of chitosan–bioglass complex on artificial white spot lesion was assessed. It was concluded that the chitosan–bioglass complex promoted subsurface mineral deposition in the presence of a salivary pellicle.34
Enamel formation is biological mineralization that requires the collaborative effects of inorganic and organic constituents.35 The organic components disintegrate and the inorganic components grow to nucleate, form crystals that align themselves in an orderly manner to convert into nanoparticles. These nanoparticles are made up of ACP and proteins that are concomitant with biomineralization and ACP can convert themselves into hydroxylapatite crystals. While the crystals align themselves, the particles vanish and form crystals parallel to the c-axis of enamel.36 Among the organic proteins used in biomineralization, the most commonly used are amelogenin and casein phosphoproteins. But the associated disadvantages with these materials are the low capability to stabilize high-ion supersaturation and allergic reaction in patients with lactogen intolerance, respectively.37 According to a study conducted by Xiao et al., CMC/ACP nanocomplex solution was first prepared by sequential adding of CMC, calcium chloride, and dipotassium phosphate in distilled water. Amorphous calcium phosphate nanoparticles were degraded using 1% NaClO from CMC/ACP nanocomplexes. Transmission electron microscopy was used to study the structure of the complexes at different phases. The organizing of ACP nanoparticles on the surface of demineralized enamel was guided by chimeric peptides. The remineralization effect was tested by XRD/scanning electron microscope (SEM)/confocal laser scanning microscopy (CLSM). It was observed and concluded that the ACP nanoparticles could arrange themselves in oriented groups before transformation into crystals, and the enamel-like crystals were firmly attached to the demineralized surface and possessed robust mechanical properties.38
White spot lesions are the initial clinical manifestations of dental caries. They are made up of an intact surface with porous subsurface. Right measures to remineralize the white spot lesion at the correct time can prevent cavitation and further progress of dental caries.39 Saliva and salivary pellicles can be used as an aid to combat demineralization by supersaturating the saliva with ions needed in remineralization. The pellicle is found to be a tenacious layer, insoluble in oral fluids, and has an intricate biological part in harmonizing the demineralization and remineralization cycle.40 According to a study conducted by Zhang et al., remineralization of white spot lesions was attempted wherein the lesions were pretreated with chitosan bioactive glass slurry in the presence of a 3-minute-old salivary pellicle. It was observed that white spot lesions pretreated with chitosan bioglass slurry have superior biomechanical properties and a compact subsurface in comparison to other remineralizing actions of other agents. However, the author suggested that a long-term study be required with a mature pellicle.41 In a similar study conducted by Simeonov et al., a new hybrid chitosan/calcium phosphates microgel was formulated to remineralize the demineralized enamel. Chitosan macromolecules were used as guides for hybrid microgel formation they acted as prototypes for the in situ creation of amorphous to crystalline nonstoichiometric hydroxyapatite crystals. The chitosan macromolecules had superior biological properties like antimicrobial property, bio-adhesiveness, and reservoir of Ca2+ and PO42− which aided in remineralization. It was concluded that this method or remineralization was cost-effective, efficient in treating early enamel demineralization.42
Conclusion
A thorough understanding of the demineralization–remineralization cycle in the oral cavity is essential for the formulation of new remineralizing agents. At present, most of the available non-fluoride remineralizing agents either enhance fluoride uptake by the tooth or reduce the associated fluoride risks. Natural remineralizing agents like chitosan, which have a proven biocompatible nature, antibiotic properties, and biodegradability, have found a wide range of applications in regenerative dentistry for remineralization of teeth and regeneration of dental tissues like enamel and dentin. Due to the active regions available in the structure of chitosan, it can be easily combined with various bioactive materials which act as a reservoir for ions required in remineralization. Moreover, its non-toxic and no allergic nature after degradation has increased its application in tissue engineering. However, to transform chitosan-based remineralizing gels from research to clinical use, more in vivo studies, clinical trials, and research are essential.
Clinical Significance
This article provides new scope for non-fluoride-based remineralization of enamel and dentin which mimics the natural process of mineralization of teeth by using naturally available non-toxic, biodegradable substances like chitosan.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Muzzarelli RA, Muzzarelli C. Chitosan chemistry: relevance to the biomedical sciences. Adv Polym Sci. 2005;186:151–209. [Google Scholar]
- 2.Kmiec M, Pighinelli L, Tedesco MF, et al. Chitosan-properties, and applications in dentistry. Adv Tissue Eng Regen Med. 2017;2(4):00035. [Google Scholar]
- 3.Phillips B, Ball C, Sackett D, et al. Oxford Centre for Evidence-based Medicine-Levels of Evidence. Since November 1998. Updated by Jeremy Howick March 2009.
- 4.Cao Y, Mei ML, Li QL, et al. Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prism-like tissue. ACS Appl Mater Interfaces. 2014;6(1):410–420. doi: 10.1021/am4044823. [DOI] [PubMed] [Google Scholar]
- 5.Ning T-Y, Xu X-H, Zhu L-F, et al. Biomimetic mineralization of dentin induced by agarose gel loaded with calcium phosphate. J Biomed Mater Res Part B. 2012;100B(1):138–144. doi: 10.1002/jbm.b.31931. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Ma G, Nie J, et al. Restorative dental resin functionalized with methacryloxy propyl trimethoxy silane to induce reversible in situ generation of enamel-like hydroxyapatite. J Mat Sci. 2018;53(24):16183–16197. doi: 10.1007/s10853-018-2533-8. [DOI] [Google Scholar]
- 7.Chow LC, Vogel GL. Enhancing remineralization. Oper Dent. 2001;6:27–38. [Google Scholar]
- 8.Grandjean P. Developmental fluoride neurotoxicity: an updated review. Environ Health. 2019;18(1):110. doi: 10.1186/s12940-019-0551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail AI, Tellez M, Pitts NB, et al. Caries management pathways preserve dental tissues and promote oral health. Community Dent Oral Epidem. 2013;41(1):e12–e40. doi: 10.1111/cdoe.12024. [DOI] [PubMed] [Google Scholar]
- 10.Duggal MS, Toumba KJ, Amaechi BT, et al. Enamel demineralization in situ with various frequencies of carbohydrate consumption with and without fluoride toothpaste. J Dent Res. 2001;80(8):1721–1724. doi: 10.1177/00220345010800080801. [DOI] [PubMed] [Google Scholar]
- 11.Amaech BT. Remineralization therapies for initial caries lesions. Curr Oral Health Rep. 2015;2(2):95–101. doi: 10.1007/s40496-015-0048-9. [DOI] [Google Scholar]
- 12.Walsh T, Worthington HV, Glenny AM, et al. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2010;1(1):CD007868. doi: 10.1002/14651858.CD007868.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Arnaud TM, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: an in vitro evaluation. J Dent. 2010;38(11):848–852. doi: 10.1016/j.jdent.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 15.Xu Z, Neoh KG, Lin CC, et al. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentine modified with phosphorylated chitosan. J Biomed Mater Res Part B: Appl Biomater. 2011;98(1):150–159. doi: 10.1002/jbm.b.31844. [DOI] [PubMed] [Google Scholar]
- 16.Robinson C, Brookes SJ, Kirkham J, et al. In vitro studies of the penetration of adhesive resins into artificial caries-like lesions. Caries Res. 2001;35(2):136–141. doi: 10.1159/000047445. [DOI] [PubMed] [Google Scholar]
- 17.Hudson SM, Jenkins DW. Chitin and chitosan, encyclopedia of polymer science and technology. NJ: Wiley Interscience; 2001. [Google Scholar]
- 18.Peng X, Zhang L, Kennedy JF. Release behavior of microspheres from cross-linked N-methylated chitosan encapsulated ofloxacin. Carbohyd Polym. 2006;65(3):288–295. doi: 10.1016/j.carbpol.2006.01.014. [DOI] [Google Scholar]
- 19.Tian K, Peng M, Fei W, et al. Adv Mat Res. Vol. 530. Trans Tech Publications Ltd.; 2012. Induced synthesis of hydroxyapatite by chitosan for enamel remineralization. pp. 40–45. [Google Scholar]
- 20.Gebauer D, Colfen H. Prenucleation clusters and non-classical nucleation. Nano Today. 2011;6(6):564–584. doi: 10.1016/j.nantod.2011.10.005. [DOI] [Google Scholar]
- 21.Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health—chemistry and clinical applications. Curr Pharm Des. 2007;13(8):793–800. doi: 10.2174/138161207780363086. [DOI] [PubMed] [Google Scholar]
- 22.Kim SK, Park PJ, Jung WK, et al. Inhibitory activity of phosphorylated chitooligosaccharides on the formation of calcium phosphate. Carbohydr Polym. 2005;60(4):483–487. doi: 10.1016/j.carbpol.2005.03.014. [DOI] [Google Scholar]
- 23.Zhang X, Li Y, Sun X, et al. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J Mater Sci Mater Med. 2014;25(12):2619–2628. doi: 10.1007/s10856-014-5285-2. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Yang JJ, Li JY, et al. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials. 2013;34(21):5036–5047. doi: 10.1016/j.biomaterials.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 25.Ruan Q, Siddiqah N, Li X, et al. Amelogenin-chitosan matrix for human enamel regrowth: effects of viscosity and supersaturation degree. Connect Tissue Res. 2014;55((Suppl 1(0 1)):):150–154. doi: 10.3109/03008207.2014.923856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoso T, Djauharie NK, Kamizar,, et al. Carboxymethyl chitosan/amorphous calcium phosphate and dentin remineralization. J Int Dent Med Res. 2019;129(1):84–87. [Google Scholar]
- 27.Annisa RN, Djauharie N, Suprastiwi E, et al. The effect of carboxymethyl chitosan/amorphous calcium phosphate to guide tissue remineralization of dentin collagen. Int J App Pharma. 2019;11:181–183. doi: 10.22159/ijap.2019.v11s1.16300. [DOI] [Google Scholar]
- 28.Gupta A, Sinha N, Logani A, et al. An ex vivo study to evaluate the remineralizing and antimicrobial efficacy of silver diamine fluoride and glass ionomer cement type VII for their proposed use as indirect pulp capping materials—Part I. J Conserv Dent. 2011;14(2):113–116. doi: 10.4103/0972-0707.82603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Cao S, Wang H, et al. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS ONE. 2015;10(1):e0116553. doi: 10.1371/journal.pone.0116553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venter J, Kotze A, Auzelyvelty R, et al. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int J Pharm. 2006;313(1-2):36–42. doi: 10.1016/j.ijpharm.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Boyes V, Festy F, et al. In-vitro subsurface remineralization of artificial enamel white spot lesions pre-treated with chitosan. Dent Mater. 2018;34(8):1154–1167. doi: 10.1016/j.dental.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Ruan Q, Liberman D, Bapat R, et al. Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems. J Biomed Eng Inform. 2016;2(1):119–128. doi: 10.5430/jbei.v2n1p119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Q, Li Z, Ding L, et al. Anti-biofilm, and remineralization effects of chitosan hydrogel containing amelogenin-derived peptide on initial caries lesions. Regen Biomater. 2018;5(2):69–76. doi: 10.1093/rb/rby005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Lynch RJM, Watson TF, et al. Chitosan-bioglass complexes promote subsurface remineralization of incipient human carious enamel lesions. J Dent. 2019;84:67–75. doi: 10.1016/j.jdent.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Beniash E, Metzler RA, Lam RS, et al. Transientamorphous calcium phosphate in forming enamel. J StructBiol. 2009;166(2):133. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Wang L, Qin Y, et al. How amelogenin orchestrates the organization ofhierarchical elongated microstructures of apatite. J PhysChem B. 2010;114(6):2293–2300. doi: 10.1021/jp910219s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed NA, El-Ghany NAA. Synthesis and antimicrobialactivity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose. 2012;19(6):1879–1891. doi: 10.1007/s10570-012-9789-y. [DOI] [Google Scholar]
- 38.Xiao Z, Que K, Wang H, et al. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimeric peptides. Dent Mater. 2017;33(11):1217–1228. doi: 10.1016/j.dental.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Kidd EAM. Essential of dental caries: the disease and its management. 3rd ed., Oxford: Oxford University Press; 2005. [Google Scholar]
- 40.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent. 2003;28(1):47–52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Lynch RJM, Watson TF, et al. Remineralisation of enamel white spot lesions pre-treated with chitosan in the presence of salivary pellicle. J Dent. 2018;72:21–28. doi: 10.1016/j.jdent.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Simeonov M, Gussiyska A, Mironova J, et al. Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel – a model study. Eur Poly J. 2019;119:14–21. doi: 10.1016/j.eurpolymj.2019.07.005. [DOI] [Google Scholar]