Abstract
Background
Patients with some types of immunodeficiency can experience chronic or relapsing infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This leads to morbidity and mortality, infection control challenges, and the risk of evolution of novel viral variants. The optimal treatment for chronic coronavirus disease 2019 (COVID-19) is unknown.
Objective
Our aim was to characterize a cohort of patients with chronic or relapsing COVID-19 disease and record treatment response.
Methods
We conducted a UK physician survey to collect data on underlying diagnosis and demographics, clinical features, and treatment response of immunodeficient patients with chronic (lasting ≥21 days) or relapsing (≥2 episodes) of COVID-19.
Results
We identified 31 patients (median age 49 years). Their underlying immunodeficiency was most commonly characterized by antibody deficiency with absent or profoundly reduced peripheral B-cell levels; prior anti-CD20 therapy, and X-linked agammaglobulinemia. Their clinical features of COVID-19 were similar to those of the general population, but their median duration of symptomatic disease was 64 days (maximum 300 days) and individual patients experienced up to 5 episodes of illness. Remdesivir monotherapy (including when given for prolonged courses of ≤20 days) was associated with sustained viral clearance in 7 of 23 clinical episodes (30.4%), whereas the combination of remdesivir with convalescent plasma or anti-SARS-CoV-2 mAbs resulted in viral clearance in 13 of 14 episodes (92.8%). Patients receiving no therapy did not clear SARS-CoV-2.
Conclusions
COVID-19 can present as a chronic or relapsing disease in patients with antibody deficiency. Remdesivir monotherapy is frequently associated with treatment failure, but the combination of remdesivir with antibody-based therapeutics holds promise.
Key words: COVID-19, SARS-CoV-2, immunodeficiency, therapeutic monoclonal, remdesivir
Abbreviations used: COVID-19, Coronavirus disease 2019; CRP, C-Reactive protein; CVID, Common variable immunodeficiency; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; XLA, X-linked agammaglobulinemia
Introduction
Antibody-deficient patients experience chronic infection with certain viruses,1 , 2 and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection can also become persistent or relapsing.3, 4, 5, 6, 7, 8 This creates a risk of chronic ill health, permanent lung disease, intrahost evolution of viral variants,9 and social isolation. The optimal treatment is not yet established.
We conducted a UK physician survey collecting anonymized data on immunosuppressed adults with prolonged or relapsing coronavirus disease 2019 (COVID-19) (with ≥21 days’ duration and/or ≥2 episodes of clinical illness). The survey was sent to all immunologists in the United Kingdom via a professional network, to infectious diseases and other specialists via the COVID-19 Therapeutics Advice and Support Group, and to individual clinicians who were known to have managed patients in the target group. Data were derived solely from information collected as part of routine clinical care and were provided in fully anonymized form.
Results and discussion
A total of 31 responses were received (Table I ). The median duration of the patients' symptomatic disease was 62 days (maximum 300 days); the median time between first recorded and most recent positive SARS-CoV-2 PCR was 48 days (>200 days in 4 patients).
Table I.
Demographic and background data on 31 patients included in the survey
| Variable | Value |
|---|---|
| Age (y), median (range) | 49 (20-80) |
| Male sex, no. (%) | 20 (64.5%) |
| Diagnosis, no. | |
| CVID | 5 |
| XLA | 8 |
| Other primary hypogammaglobulinemia | 3 |
| Secondary hypogammaglobulinemia, previous anti-CD20 treatment∗ | 12 |
| Secondary hypogammaglobulinemia, no previous anti-CD20† treatment | 2 |
| Unspecified | 1 |
| IgG concentration (g/L), median (IQR) | |
| Trough level for patients undergoing immunoglobulin replacement before COVID-19 diagnosis (n = 19) | 8.8 (6.7-12.3) |
| At presentation with COVID-19 for patients not previously on immunoglobulin replacement (n = 12) | 4.3 (1.6-5.0) |
| IgA concentration (n = 27) (g/L), median (IQR) | 0 (0-0.57) |
| IgM concentration (n = 27) (g/L), median (IQR) | 0 (0-0.18) |
| B-cell count (n = 27) (× 109/L), median (IQR) | 0 (0-0.004) |
| CD4+ T-cell count (n = 26) (× 109/L), median (IQR) | 0.46 (0.23-0.85) |
| CD8+ T-cell count (n = 24) (× 109/L), median (IQR) | 0.35 (0.27-0.80) |
| White ethnicity, no. (%) | 26 (83.9%) |
| Other comorbidity present, no. (%)‡ | 13 (41.9%) |
| Episodes of clinical illness with COVID-19, no. | |
| Total | 62 |
| Range per patient | 1-5 |
| Mean per patient | 2 |
| Total median duration of illness per patient at the time of survey§ | 64 d |
| Patient was viremic at any time | |
| Yes | 7 |
| No | 5 |
| Not known | 19 |
| SARS-CoV-2 antibodies in serum during infection | |
| Positive | 3‖ |
| Negative | 18 |
| Not tested | 9 |
| Patient was receiving immunoglobulin replacement therapy at time of survey | |
| Yes | 21 |
| No, the patient died | 3 |
| No, the patient does not meet NHS England criteria | 6 |
| No, the patient experienced significant side effects | 1 |
IQR, interquartile range; NHS, National Health Service.
Follicular lymphoma (n = 3), mantle cell lymphoma (n = 2), other lymphoma (n = 2), Waldenstrom macroglobulinemia (n = 2), chronic lymphocytic leukemia (n = 1), acute myeloid leukemia with stem cell transplant (n = 1), and rheumatoid arthritis (n = 1).
B-cell acute lymphocytic leukemia with chimeric antigen receptor T-cell therapy (n = 1) and renal transplant (n = 1).
Diabetes mellitus, hypertension, ischemic heart disease, other heart diseases (eg, arrhythmia, valvular heart disease), asthma, chronic obstructive pulmonary disease, and other chronic respiratory disease.
In some instances, details were not provided for all episodes of illness (eg, for those managed in the community), and this figure is therefore likely to be an underestimate.
All patients had received antibody-based therapies.
The median patient age was 49 years. Of the 31 patients, 17 (54.8%) had a primary immunodeficiency causing hypogammaglobulinemia with a high frequency of X-linked agammaglobulinemia (XLA), and 14 of 31 (45.2%) had secondary immunodeficiency, mostly due to previous anti-CD20 treatment. Accordingly, patients demonstrated a striking absence of peripheral blood B cells, with a median count of zero; the maximum B-cell count recorded was 0.056 × 109/L, with a maximum of 3% of blood lymphocytes. In contrast, T-cell counts were relatively well preserved (Table I). IgG trough levels were within an acceptable range for those receiving replacement therapy before their COVID-19 diagnosis but low in those not previously receiving treatment. IgA and IgM concentrations were generally very low or absent.
Patients with XLA had a confirmed genetic diagnosis or absent Bruton tyrosine kinase (Btk) expression, and 1 patient had confirmed Wiskott-Aldrich syndrome. Other patients with primary immunodeficiencies, including those with common variable immunodeficiency (CVID), did not have a genetic diagnosis. Causes of secondary antibody deficiency are listed in Table I.
Only 13 of the 31 patients (41.9%) had other comorbidities. Of the 31 patients, 30 (97%) had been hospitalized at least once because of clinical COVID-19 illness. The patients presented with typical symptoms of COVID-19: 30 of 31 (96.7%) had a cough and 27 of 31 (87.1%) had a fever. The results of chest imaging (chest radiograph and/or computed tomography scan) were compatible with COVID-19 in 30 of 31 patients and not reported in 1 patient. Where measured, viremia was relatively common (7 of 12 patients [58.3%]). No patients had received a COVID-19 vaccine before presenting with infection. Three patients (9.7%) had died at the time of the survey (1 of them died of an unrelated infection after clearing SARS-CoV-2). Among those patients who had cleared the virus and were alive, 6 still had persistent symptoms but 14 had fully recovered; all of those who had not cleared the virus but were alive (n = 8) had persistent symptoms.
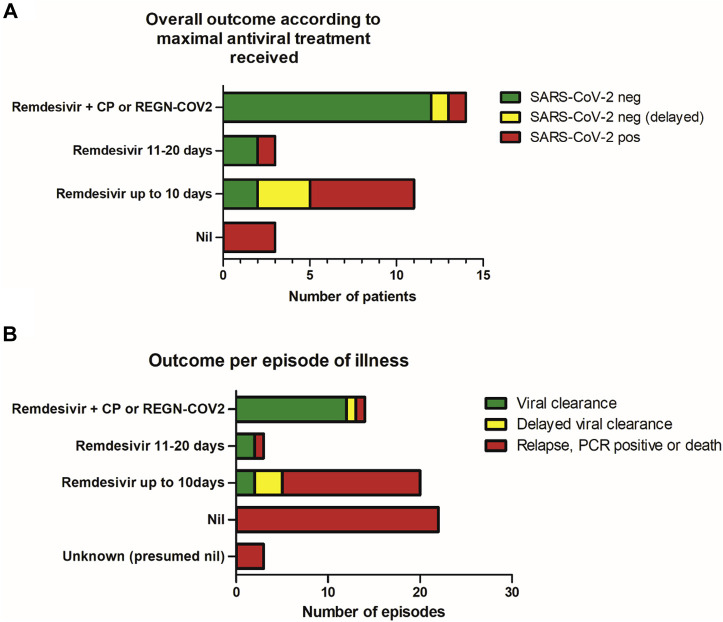
We recorded virologic outcome per patient according to the maximal antiviral treatment received (Fig 1 , A), defined as remdesivir or antibody-based therapy (convalescent plasma and SARS-CoV-2–specific mAbs). Among the 20 patients who cleared infection (median symptom duration 51.5 days), 13 received a combination of remdesivir plus antibody-based therapy (8 received REGN-COV2 and 5 received convalescent plasma), 5 received remdesivir monotherapy (≤10 days per course), and 2 received remdesivir monotherapy (for >10 days in a single course). Viral clearance was delayed in 3 patients treated with remdesivir monotherapy and 1 patient treated with combination therapy. Among the 11 patients who did not clear the infection (median symptom duration 70 days), 1 received combination therapy (remdesivir plus convalescent plasma), 6 received remdesivir monotherapy (for ≤10 days), 1 received remdesivir monotherapy (for >10 days), and 3 did not receive any antiviral treatment (P = .006 according to the chi-square test for treatment difference between the groups). According to logistic regression controlling for age, sex and underlying diagnosis, the odds ratio of clearing infection with combination therapy versus with remdesivir monotherapy was 23.1 (95% CI = 1.3-424.9 [P = .035]).
Fig 1.
A, Eventual virologic outcome according to maximal treatment received in 31 antibody-deficient patients with chronic or relapsing COVID-19. B, Outcome per episode of clinical illness (n = 62 episodes) among the patient group. CP, Convalescent plasma; neg, negative; pos, positive.
The patient who received combination therapy but failed to clear SARS-CoV-2 died after a monophasic illness lasting 23 days. The patient with delayed clearance received sequential therapy (remdesivir followed 3 weeks later by REGN-COV2), whereas the other patients received the therapies contemporaneously.
We also analyzed each of the 62 episodes of clinical COVID-19 illness (Fig 1, B). We classified outcomes as unfavorable (persistent PCR positivity, clinical relapse, death) or viral clearance. The episodes with a successful outcome are described earlier in this report. Among the 42 episodes with an unfavorable outcome, 3 did not have any treatment details available, 22 occurred in patients who did not receive any treatment, 15 episodes were treated with remdesivir monotherapy for no more than 10 days, 1 was treated with remdesivir monotherapy for more than 10 days, and 1 was treated with combination therapy (as stated earlier) (P < .0001 according to the chi-square test for difference in outcome according to treatment received). Overall, 16 of the 23 episodes (69.6%) treated with remdesivir monotherapy had an unfavorable outcome, with viral clearance occurring in 7 of the 23 episodes (30.4%) (it was delayed in 3 instances).
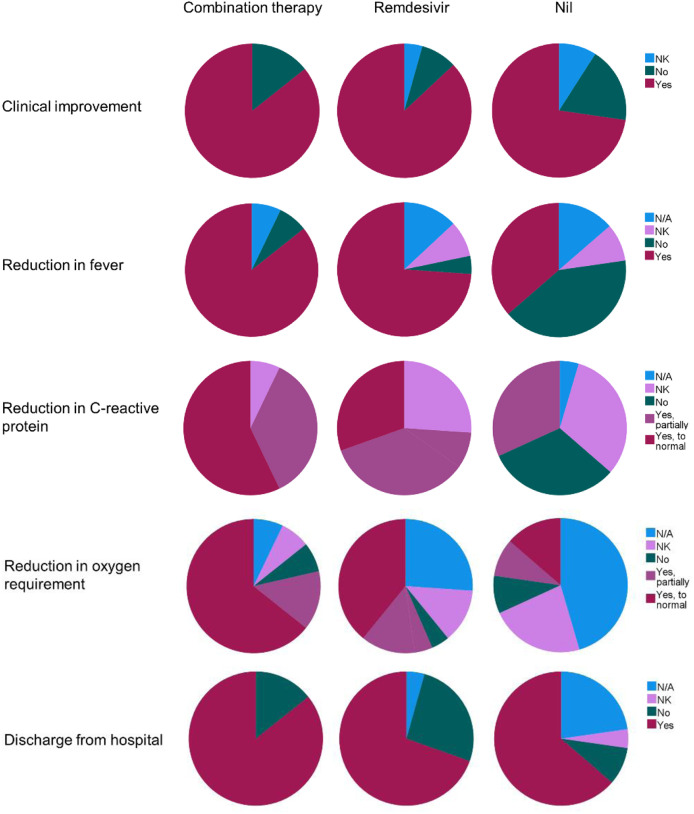
We also analyzed clinical outcomes (overall clinical improvement, reduction in fever, reduction in serum C-reactive protein (CRP) level, reduction in oxygen requirement, and discharge from the hospital) per episode of illness (see Fig E1 in the Online Repository at www.jacionline.org). Many untreated episodes demonstrated some spontaneous improvement with discharge from the hospital. However, fever and CRP level often did not improve significantly. In contrast, for the vast majority of episodes treated with remdesivir or combination therapy (and where outcome data were available), there was improvement in fever, CRP level, and oxygen requirement regardless of sustained viral clearance.
Fig E1.
Proportion of clinical episodes in which patients experienced clinical improvement, reduction in fever, reduction in serum C-reactive protein concentration, reduction in oxygen requirement, and discharge from the hospital according to treatment received: combination therapy (remdesivir plus convalescent plasma or REGN-COV2 monoclonals [n = 14]), remdesivir monotherapy (n = 23), or no treatment (n = 22). N/A, not applicable; NK, not known.
Immunosuppressive and immunomodulatory therapeutic approaches were tried in some patients. The most commonly prescribed treatment was dexamethasone or other corticosteroids (in 25 of 62 episodes [40.3%]). Other treatments given were tocilizumab (in 3 episodes), anakinra (in 4 episodes), and intravenous immunoglobulin (in 2 episodes). One patient additionally received inhaled tissue plasminogen activator. Of the 62 clinical episodes, 6 (9.7%) were treated with immunosuppressive or immunomodulatory treatments without antiviral treatments; none of these resulted in viral clearance.
Overall, our survey of 31 antibody-deficient patients with COVID-19 has demonstrated a high burden of morbidity among this population and has highlighted the fact that combination therapy with antivirals and antibody-based therapeutics is associated with the highest rate of viral clearance.
Compared with the general population of individuals hospitalized with COVID-19, our cohort tended to be younger and have less comorbidity.10 The underlying immunodeficiency was almost invariably characterized by antibody deficiency, including a high frequency of patients with XLA or prior anti-CD20 treatment: there was a striking absence or profound reduction in peripheral blood B-cell counts across this cohort. Some patients in the cohort may have broader immune compromise, including impaired T-cell function, whereas monocyte function may be impaired in XLA; however, the composition of this cohort nevertheless implicates B cells and antibodies as key immunologic components required to clear SARS-CoV-2 infection.
There are several case reports of COVID-19 outcomes among antibody-deficient patients who have not received any specific proven antiviral therapy. Some of these case patients have died,7 , 11 but full recovery from mild illness11 , 12 and even severe disease with intubation13 , 14 has been reported in others.
In our surveyed patients, all untreated episodes had an unfavorable outcome of persistent PCR positivity and/or clinical relapse or death, although patients were included in the analysis only if they had at least 21 days' duration of symptoms and/or at least 2 episodes of clinical illness, which introduced a deliberate bias. Thus, although the published literature confirms that mild disease and spontaneous clearance of infection is a possible outcome in these groups of patients, chronic or relapsing disease15 may well require specific therapy.
We found that a significantly higher proportion of patients had sustained clearance of SARS-CoV-2 after treatment with combination antiviral therapy (remdesivir plus antibody therapy, with 13 of 14 [92.8%] achieving viral clearance) as compared with those who were treated with remdesivir alone (with 7 of 14 [50%] achieving viral clearance, often delayed) or who received no specific treatment (with 0 of 3 achieving viral clearance).
Remdesivir monotherapy was given in 23 of 62 clinical episodes in our surveyed patients and led to mixed outcomes; 16 of 23 episodes (69.6%) had an unfavorable outcome and 7 of 23 (30.4%) had viral clearance (delayed in 3 cases). However, remdesivir treatment generally led to an improvement in physiologic parameters (fever, CRP level, and oxygen requirement) even if there was no viral clearance and/or a subsequent clinical relapse. Remdesivir may therefore have utility in unwell patients when there is no access to antibody-based therapies (although there may be a risk of selection of resistant viral subpopulations).
Failure to clear SARS-CoV-2 from antibody-deficient patients after remdesivir monotherapy has been reported in similar patients.3 , 5 , 8 , 16, 17, 18 However, a good response to remdesivir in antibody deficiency has been documented in other cases.11 , 19 Notably, in the patients in our cohort, remdesivir was often used outside the UK commissioning guidelines at the time (ie, beyond 10 days from symptom onset), and some patients were treated with prolonged courses with up to 20 days’ duration. As the numbers were small, it is unclear whether this approach was more successful than the “standard” (as per clinical trials) courses with up to 10 days’ duration.
None of the patients included in our survey had received anti–SARS-CoV-2 antibody therapy alone. However, successful treatment with convalescent plasma alone has been described,4, 5, 6, 7 , 19 including in 16 of 17 B-cell–depleted patients with chronic COVID-19,16 8 of 14 treated patients with secondary immunodeficiency,20 and 2 patients with CVID and severe COVID-19.21 , 22 In contrast, monotherapy with convalescent plasma19 or REGN-COV-28 was not successful in other cases.
Combination therapy with remdesivir and convalescent plasma or REGN-COV2 was generally a successful strategy in our surveyed patients. Successful outcomes with combination therapy have also been demonstrated in other similar patients,17, 18, 19 , 23 , 24 but this strategy failed in a patient with CVID who died at day 30 despite maximal therapy,25 similar to a patient in our cohort.
In summary, among predominantly B-cell– and antibody-deficient patients with chronic or relapsing COVID-19, a significantly higher proportion experienced sustained clearance of SARS-CoV-2 after treatment with remdesivir plus antibody therapy than after treatment with remdesivir alone or no specific treatment. Currently, providing access to antibody-based treatments is challenging in many parts of the world, and access to any antiviral therapeutic is often restricted to those with early disease. Although appropriate for immunocompetent individuals, this approach does not address the needs of chronically infected patients. Indeed, many of the patients reported here were treated only as part of large, open-label trials, in which they could equally have been randomized to the standard of care, or as a result of favorable decisions from pharmaceutical companies’ compassionate use committees.
From both clinical and public health perspectives, we encourage improved access to treatment for these patients via protocols or studies with careful monitoring for outcome. Well-conducted observational studies can provide vital data on rare patients such as these and may even be preferable to large, randomized controlled studies in which recruitment would be challenging and use of placebos could present ethical issues.
Clinical implications.
COVID-19 can become chronic in patients with immunodeficiency, and the optimal treatment for this situation remains unknown. Here, we have demonstrated that the combination of antivirals and antibody-based therapeutics is highly effective.
Footnotes
Disclosure of potential conflict of interest: T. Simpson has received speakers’ fees from Gilead. S. Jolles reports advisory board, speaker, conference, drug safety monitoring board, and project support from CSL Behring, Shire, Takeda, BioCryst Pharmaceuticals, Swedish Orphan Biovitrum, Biotest, Binding Site, LFB, Octapharma, Grifols, UCB Pharma, Sanofi, Pharming, Weatherden, and Zarodex Therapeutics, Limited, and he is a member of the IPOPI SAFE Taskforce and COVIC19 Trial Group. M. Brown is the UK chief investigator for a Gilead remdesivir trial (GS-US-540-9012), local principal investigator for a Gilead remdesivir trial (GS-US-540-5773/5774), and local coinvestigator for the AstraZeneca PROVENT trial. D. Lowe has received travel and subsistence costs for consultancy work for CSL Behring and fees for roundtable discussion from Merck; in addition, he is chief investigator for the COVID-19 antiviral FLARE trial (NCT04499677), and he holds research grants from LifeArc, the UK Medical Research Council, Blood Cancer UK, Bristol-Myers Squibb and the British Society for Antimicrobial Chemotherapy. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
References
- 1.Brown L.-A.K., Ruis C., Clark I., Roy S., Brown J.R., Albuquerque A.S., et al. A comprehensive characterization of chronic norovirus infection in immunodeficient hosts. J Allergy Clin Immunol. 2019;144:1450–1453. doi: 10.1016/j.jaci.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kainulainen L., Vuorinen T., Rantakokko-Jalava K., Osterback R., Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 2010;126:120–126. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckland M.S., Galloway J.B., Fhogartaigh C.N., Meredith L., Provine N.M., Bloor S., et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11:6385. doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mira E., Yarce O.A., Ortega C., Fernandez S., Pascual N.M., Gomez C., et al. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8:2793–2795. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin H., Reed J.C., Liu S.T.H., Ho H.-E., Lopes J.P., Ramsey N.B., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8:3594–3596.e3. doi: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London J., Boutboul D., Lacombe K., Pirenne F., Heym B., Zeller V., et al. Severe COVID-19 in patients with B cell alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2021;41:356–361. doi: 10.1007/s10875-020-00904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambaut A., Loman N., Pybus O., Barclay W., Barrett J., Carabelli A., et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. 2021. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 Available at:
- 10.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Normal L., et al. Features of 20133UK patients in hospital with covid -19using the ISARIC WHO Clinical Characterisation Protocol :prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I., et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Foca E., et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aljaberi R., Wishah K. Positive outcome in a patient with coronavirus disease 2019 and common variable immunodeficiency after intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2020;125:349–350. doi: 10.1016/j.anai.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fill L., Hadney L., Graven K., Persaud R., Hostoffer R. The clinical observation of a patient with common variable immunodeficiency diagnosed as having coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125:112–114. doi: 10.1016/j.anai.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarhini H., Recoing A., Bridier-Nahmias A., Rahi M., Lambert C., Martres P., et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223:1522–1527. doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hueso T., Pouderoux C., Péré H., Beaumont A.-L., Raillon L.-A., Ader F., et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136:2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malsy J, Veletzky L, Heide J, Hennigs A, Gil-Ibanez I, Stein A, et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19 [e-pub ahead of print]. Clin Infect Dis 10.1093/cid/ciaa1637. Accessed July 20, 2021. [DOI] [PMC free article] [PubMed]
- 18.Helleberg M., Niemann C.U., Moestrup K.S., Kirk O., Lebech A.-M., Lane C., et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2020;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodionov R.N., Biener A., Spieth P., Achleitner M., Holig K., Aringer M., et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. Lancet Microbe. 2021;2:e138. doi: 10.1016/S2666-5247(21)00030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Damme K.F.A., Tavernier S., Van Roy N., De Leeuw E., Declercq J., Bosteels C., et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and severe COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro L.C., Benites B.D., Ulaf R.G., Nunes T.A., Costa-Lima C., Addas-Carvalho M., et al. Rapid clinical recovery of a SARS-CoV-2 infected common variable immunodeficiency patient following the infusion of COVID-19 convalescent plasma. Allergy Asthma Clin Immunol. 2021;17:14. doi: 10.1186/s13223-021-00518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iaboni A., Wong N., Betschel S.D. A patient with X-linked agammaglobulinemia and COVID-19 infection treated with remdesivir and convalescent plasma. J Clin Immunol. 2021;41:923–925. doi: 10.1007/s10875-021-00983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinot M., Jary A., Fafi-Kremer S., Leducq V., Delagreverie H., Garnier M., et al. Emerging RNA-dependent RNA polymerase mutation in a remdesivir-treated B-cell immunodeficient patient with protracted coronavirus disease 2019. Clin Infect Dis. 2021;73:e1762–e1765. doi: 10.1093/cid/ciaa1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullur J., Wang A., Feldweg A. A fatal case of coronavirus disease 2019 in a patient with common variable immunodeficiency. Ann Allergy Asthma Immunol. 2021;126:90–92. doi: 10.1016/j.anai.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]