Abstract
Background
We report the clinical, radiological, laboratory, and neuropathological findings in support of the first diagnosis of lethal, small-vessel cerebral vasculitis triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric patient.
Patient Description
A previously healthy, eight-year-old Hispanic girl presented with subacute left-sided weakness two weeks after a mild febrile illness. SARS-CoV-2 nasopharyngeal swab was positive. Magnetic resonance imaging revealed an enhancing right frontal lobe lesion with significant vasogenic edema. Two brain biopsies of the lesion showed perivascular and intraluminal lymphohistiocytic inflammatory infiltrate consistent with vasculitis. Despite extensive treatment with immunomodulatory therapies targeting primary angiitis of the central nervous system, she experienced neurological decline and died 93 days after presentation. SARS-CoV-2 testing revealed positive serum IgG and positive cerebrospinal fluid IgM. Comprehensive infectious, rheumatologic, hematologic/oncologic, and genetic evaluation did not identify an alternative etiology. Postmortem brain autopsy remained consistent with vasculitis.
Conclusion
This is the first pediatric presentation to suggest that SARS-CoV-2 can lead to a fatal, postinfectious, inflammatory small-vessel cerebral vasculitis. Our patient uniquely included supportive cerebrospinal fluid and postmortem tissue analysis. While most children recover from the neurological complications of SARS-CoV-2, we emphasize the potential mortality in a child with no risk factors for severe disease.
Keywords: SARS-CoV-2, COVID-19, Pediatrics, Stroke, Cerebral vasculitis
Introduction
We describe the comprehensive evaluation and ultimately fatal course of an eight-year-old girl who presented with left-sided weakness and was found to have a brain lesion. After extensive evaluation the brain lesion was felt to be vasculitis triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The lesion was progressive and refractory to numerous immunotherapies targeting cerebral vasculitis, and the patient unfortunately passed away. This is the first reported pediatric case of lethal small-vessel cerebral vasculitis triggered by SARS-CoV-2, as evidenced by positive cerebrospinal fluid (CSF) SARS-CoV-2 IgM antibodies. Clinicians should be cognizant of the potential mortality of this postinfectious neurological complication of SARS-CoV-2 in children without medical comorbidities.
Patient presentation
This eight-year-old previously healthy fully immunized (excluding vaccination for SARS-CoV-2, which had not yet been developed) Hispanic girl presented with left hemiparesis. Two weeks prior, she experienced several hours of fever, cough, and headache. Her family noticed left foot weakness after a fall, followed by left arm weakness. On presentation she exhibited left arm weakness (4/5 strength), leg weakness (proximal 4/5, distal 0/5 strength), hyperreflexia, ankle hypertonia, and a left Babinski sign. Her birth and family history were unremarkable.
Laboratory and imaging studies
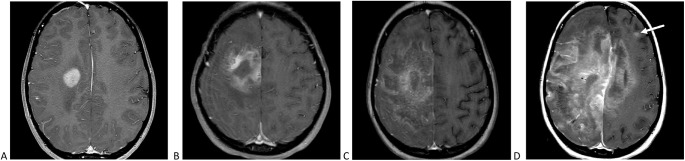
SARS-CoV-2 nasopharyngeal polymerase chain reaction (PCR) test on hospital day one prior to anesthesia for magnetic resonance imaging (MRI) was positive. MRI of the brain and cervical spine (Fig 1 A) demonstrated a right frontal lobe enhancing lesion with vasogenic edema. Incidental encephalomalacia in the left basal ganglia indicative of remote infarct was noted. Differential diagnosis was tumefactive demyelination versus tumor.
FIGURE 1.
Imaging findings. (A) Axial T1-weighted magnetic resonance image with contrast through the frontal lobes shows an enhancing lesion with diffusion restriction centered in the right corona radiata, including extension into the precentral gyrus, with extensive surrounding edema. The cortical component had hemorrhagic staining. Examination performed two weeks later (B) shows increased size of the enhancing component, with central nonenhancement. (C) Contrast-enhanced image six weeks after presentation demonstrates further progression of the lesion, with enhancement extending throughout much of the entire cerebral hemisphere. (D) Ten weeks after presentation the lesion had spread throughout the right hemisphere, with substantial extension across the corpus callosum into the left hemisphere, with noncontiguous peripheral lesions apparent (arrow).
Due to diagnostic uncertainty, a brain biopsy was performed on hospital day four. Pathology demonstrated infarctlike necrosis with perivascular lymphohistiocytic inflammatory infiltrates, the majority being T lymphocytes (Fig 2 A-D). Bacterial and fungal cultures and viral immunohistochemistry were negative. Tissue analysis favored vasculitis and excluded neoplasm and demyelination. Extensive evaluation for infectious, autoimmune, hematologic, oncologic, and genetic etiologies was done (Supplemental Table 1A and 1B) and was normal except for positive mycoplasma serology, which was considered incidental.
FIGURE 2.
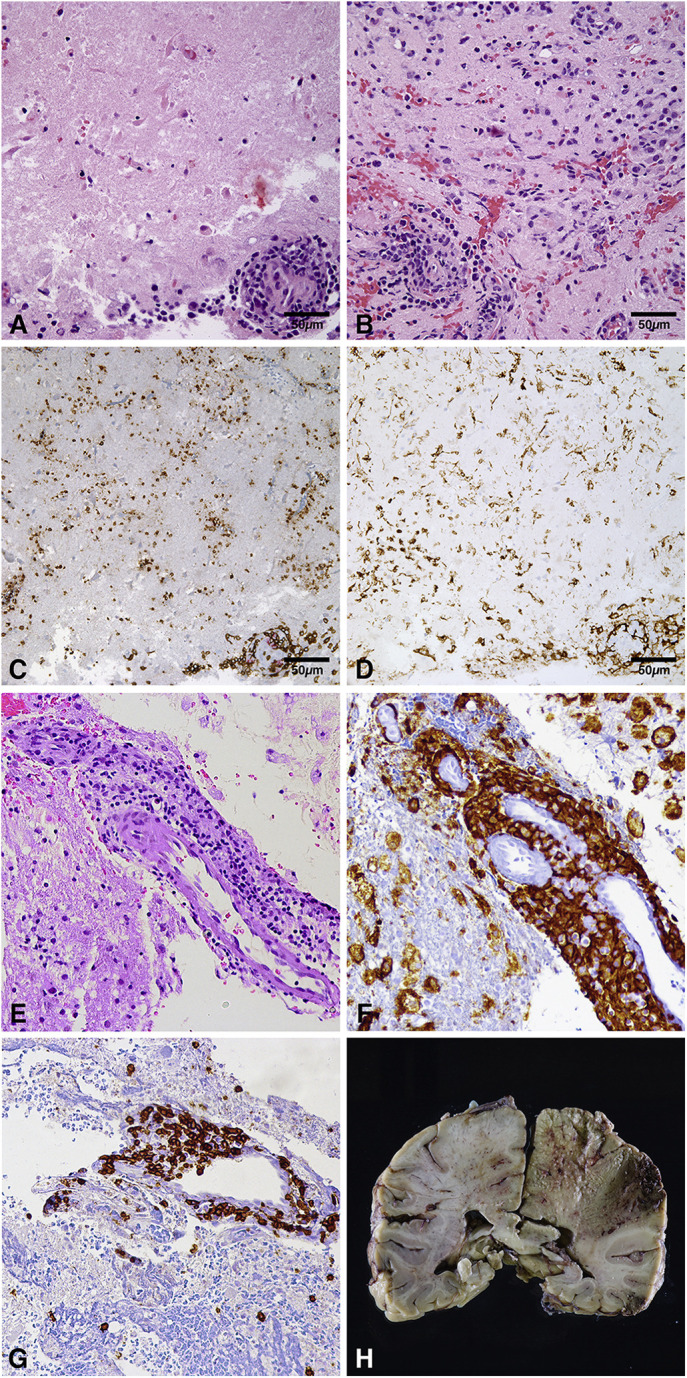
Brain biopsies and autopsy findings. (A-D) Initial biopsy. (A) Relatively bland ischemic necrosis with cellular loss of nuclear basophilia along with perivascular inflammation. (B) Patchy areas of intraparenchymal inflammation and gliosis. (C) CD3 (brown) and CD20 (red) dual chromogen immunohistochemistry revealing predominance of CD3-positive T lymphocytes in the vascular walls and perivascular infiltrates. (D) CD163 highlights vascular and intraparenchymal histiocytes. (E-G) Second biopsy. (E) Similar findings to previous biopsy including transmural and perivascular inflammation, but also with areas of cystic change and foamy histiocytes (right upper corner). (F) CD163 highlighting histiocytes. (G) CD3 highlighting T lymphocytes. (H) Coronal section of brain at autopsy revealing confluent areas of necrosis from cortex to corpus callosum and basal ganglia, hemorrhage near previous biopsy. Microscopic images are 400× magnification. The color version of this figure is available in the online edition.
On hospital day nine, serum SARS-CoV-2 IgG qualitative testing was positive. Multiple subsequent nasopharyngeal SARS-CoV-2 PCR tests were negative (Supplemental Table 1D). SARS-CoV-2 testing of fresh frozen brain biopsy tissue was negative. At the time of evaluation there was no validated CSF SARS-CoV-2 test.
Treatment and further evaluation
Initial suspicion for tumefactive demyelination led to treatment with intravenous (IV) methylprednisolone followed by plasmapheresis. During plasmapheresis, arm weakness worsened and left lower face weakness developed. MRI showed enlarged lesion with extension into the corpus callosum (Fig 1B). Given biopsy concern for vasculitis, cyclophosphamide was administered. Weekly MRI studies demonstrated progression of the lesion despite all interventions (Fig 1C).
A second brain biopsy was performed to rule out inadequate sampling of neoplasm by the first biopsy. Pathology showed parenchymal destruction with features of small-vessel lymphohistiocytic vasculitis and no neoplastic cells (Fig 2E-G). A second dose of cyclophosphamide was administered, followed by rituximab and IV immunoglobulin. Her neurological examination continued to decline.
Sixty-two days after presentation she developed headache and status epilepticus followed by acute deterioration of her neurological function. Imaging revealed expansion of the lesion with midline shift. Levetiracetam, neuroprotective measures for elevated intracranial pressure, and broad-spectrum antimicrobials (including azithromycin, antifungal, and parasitic coverage) were initiated. IV methylprednisolone was restarted, and infliximab was given for presumed treatment-resistant vasculitis.
Laboratory testing at that time revealed anemia, leukopenia, thrombocytopenia, and elevated inflammatory markers (Supplemental Table 1C). Persistent fevers developed. Bone marrow biopsy demonstrated hypocellularity, but no hemophagocytosis nor signs of malignancy. Due to fevers, cytopenias, hyperferritinemia, and elevation in liver enzymes, a diagnosis of secondary macrophage activation syndrome was postulated for which she received a course of anakinra for additional immunosuppression and central nervous system (CNS) penetration.
Unrelenting clinical decline and progression of MRI findings (Fig 1D) led to transition to comfort care, and she died 93 days after presentation.
Postmortem evaluation
Brain autopsy (Fig 2H) showed a large necrotic region and smaller satellite lesions. Histology revealed diffuse acute hypoxic/ischemic changes along with a massive parenchymal infarct, multifocal hemorrhages, and perivascular inflammatory infiltrates confined to areas adjacent to the large areas of infarction. There were scattered small parenchymal infarcts of various ages which did not appear to show surrounding inflammatory infiltrate. The atypical lymphohistiocytic vessel-associated inflammatory infiltrate had a similar morphology to the prior biopsies, including T-lymphocyte predominance. Nonlesional background brain showed no significant inflammation. Electron microscopy on fragments of brain did not show evidence of viral particles.
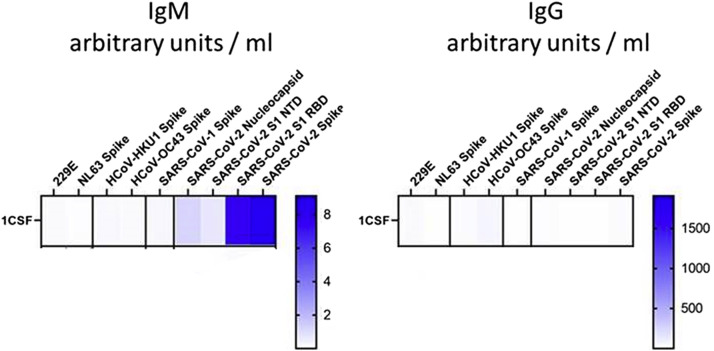
Postmortem, the Centers for Disease Control and Prevention tested for SARS-CoV-2 on CSF from hospitalization day two, which returned strongly positive for SARS-CoV-2 IgM (Fig 3 ). The extensive negative evaluation, lack of evidence on autopsy for an alternative diagnosis, and presence of SARS-CoV-2 IgM in the initial CSF led to the hypothesis that the underlying disease was a reactive inflammatory vasculitis secondary to SARS-CoV-2.
FIGURE 3.
Cerebrospinal fluid (CSF) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing. CSF testing showing strong SARS-CoV-2 IgM positivity on left and negative SARS-CoV-2 IgG on right. The color version of this figure is available in the online edition.
Discussion
Solitary CNS lesions present a diagnostic challenge. Initial differential diagnoses were high-grade glioma versus tumefactive demyelination, followed by CNS hemophagocytic lymphohistiocytosis, infection, and vasculitis. Acute disseminated encephalomyelitis (ADEM) was not considered due to lack of encephalopathy. Tumefactive demyelinating lesions are rare in children, and biopsy is often required for definitive diagnosis,.1
Two brain biopsies excluded neoplasm. The first biopsy revealed extensive necrosis with perivascular inflammation and rare microthrombi, raising the question of vasculitis, which was bolstered by the second biopsy demonstrating dense transmural inflammatory infiltrate. Small-vessel primary angiitis of the CNS in childhood (SV-cPACNS) typically presents with encephalopathy, seizure, ataxia, headache, or neuropsychiatric disturbances.2 The typical histopathological pattern in SV-cPACNS is predominately intramural/perivascular activated T-cell lymphocytic infiltrate.3 PACNS can rarely present as a tumorlike lesion in 7% of adults and isolated case reports of children.4 , 5 As viral infections are known to trigger CNS vasculitis, we questioned if SARS-CoV-2 could incite a necrotizing vasculopathy. In this patient, the presence of chronic encephalomalacia in the left basal ganglia suggested a possible risk for arteriopathy.
SARS-CoV-2 arose in 2019 and has spread globally. In the United States, as of July 2021, children under 18 years represent 12.6% of cases.6 Neurological manifestations of SARS-CoV-2 include stroke, cerebral venous sinus thrombosis, seizure, meningitis, encephalitis, ADEM, acute fulminant cerebral edema, posterior reversible encephalopathy syndrome, myelitis, Guillain-Barré syndrome, cranial neuropathies, headache, myositis, and weakness.7, 8, 9
Children with SARS-CoV-2 and neuroimaging findings are reported to recover well, with exceptions being deaths from fatal coinfections (four patients), acute fulminant cerebral edema in the setting of status epilepticus (three patients), stroke (four patients, one of whom is the patient described in this article), and severe encephalopathy (four patients).7 , 9 CNS vasculitis has been reported in adults with SARS-CoV-2.10 In pediatrics, isolated vascular events from focal cerebral arteriopathies have been reported.8
It has been postulated that proinflammatory cytokines associated with SARS-CoV-2 lead to enhanced permeability of the blood-brain barrier, allowing antibodies and other proinflammatory mediators into the brain parenchyma, perpetuating a postinfectious CNS inflammatory response.11
SARS-CoV-2-related neuropathology findings in the literature are described as hypoxic-ischemic changes secondary to respiratory/cardiovascular compromise, acute thrombotic ischemic and/or hemorrhagic infarcts within the brain parenchyma, or lesions similar to those seen in ADEM. Rare cases in the adult population have shown focal or diffuse perivascular T lymphocytic infiltrate,10 similar to what was encountered in our patient.12
Mycoplasma has been associated with CNS disease including vasculitis, ADEM, Guillain-Barré syndrome, and encephalitis mimicking brain mass.13 , 14 A mechanism of vasculitic necrosis associated with Mycoplasma has been proposed; however, this has only been reported in the thalamus, basal ganglia, pons, and splenium of the corpus callosum, which is not consistent with our patient's presentation, nor is the enlargement of our patient's lesion after treatment with azithromycin.14
Although multisystem inflammatory syndrome in children (MIS-C) has arisen during the SARS-CoV-2 pandemic, our patient never met diagnostic criteria due to lack of daily fevers, other additional clinical features consistent with MIS-C, or significant inflammatory response at time of presentation.15 Furthermore, symptoms of MIS-C present approximately four weeks from acute SARS-CoV-2 infection; thus MISC-C would not have been considered when fevers and inflammatory markers rose two months after her initial presentation. Instead, these findings were attributed to possible reactive macrophage activation syndrome.
Conclusion
We suspect that our patient's progressive cerebral infarction was caused by SV-cPACNS, precipitated by an exaggerated immune response to SARS-CoV-2. The time course of preceding illness, positive SARS-CoV-2 testing (nasopharyngeal PCR, serum and plasma IgG, and CSF IgM), and lack of systemic inflammation at presentation are suggestive of a postinfectious mechanism of disease. We believe this is the first well-documented child with SARS-CoV-2-related fatal, postinfectious, inflammatory CNS vasculitis.
Acknowledgments
We thank the family for allowing us to report this case and the kindness and graciousness they showed us throughout their child's illness. Informed consent (written and oral) was obtained from family for this publication. We thank the inpatient RNs for the wonderful care they gave to the patient, our medical colleagues in many subspecialities who let us discuss the case with them (particularly Dr. Maureen O'Brien and Dr. Alexei Grom), and our colleagues at outside institutions who provided second opinions on imaging and pathology results. Finally, we are very grateful to Natalie J. Thornburg, PhD, and her team at the Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, the Centers for Disease Control and Prevention, for testing our patient's laboratory samples and sharing the results with us, including Fig 3.
Footnotes
Authors' Contributions: All authors contributed to the conception and design of the study, data acquisition and analysis, and drafting the article. Final approval was obtained from all authors before submission.
Declarations of Interests: None.
Funding/support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Previous presentation of the information in this manuscript: Our patient was included in very limited detail in the study “Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome,” published in JAMA Neurology in March 2021. In that study's text our patient is the fourth stroke patient who died, and her course is summarized in one sentence within the body of the manuscript. In supplemental e-Table 4 she is presented as Case 3. The authors of that study had limited access to data and no access to imaging on our patient, and their manuscript was published before our patient's postmortem autopsy was performed and the CSF SARS-CoV-2 IgM result was known.
Disclosures: Authors have no potential, perceived, or real conflicts of interest to report relative to this study. This research was not funded by any sponsor. Study design, collection, analysis and interpretation of data, and all subsequent writing as well as decisions on manuscript submission were made at authors' discretion without undue influence. Drafting and revision of manuscript was performed by all study authors without honorarium, grant, or other form of payment.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pediatrneurol.2021.11.003.
Supplementary data
References
- 1.Hanumanthe S.B., Francisco C., Hart J., Graves J., Waubant E. Biopsy-supported tumefactive demyelination of the central nervous system in children. J Child Neurol. 2016;31:1528–1533. doi: 10.1177/0883073816664667. [DOI] [PubMed] [Google Scholar]
- 2.Smitka M., Bruck N., Engellandt K., Hahn G., Knoefler R., von der Hagen M. Clinical perspective on primary angiitis of the central nervous system in childhood (cPACNS) Front Pediatr. 2020;8:281. doi: 10.3389/fped.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbers J., Halliday W., Hawkins C., Hutchinson C., Benseler S.M. Brain biopsy in children with primary small-vessel central nervous system vasculitis. Ann Neurol. 2010;68:602–610. doi: 10.1002/ana.22075. [DOI] [PubMed] [Google Scholar]
- 4.Salvarani C., Brown R.D., Christianson T.J.H., et al. Primary central nervous system vasculitis mimicking brain tumor: comprehensive analysis of 13 cases from a single institutional cohort of 191 cases. J Autoimmun. 2019;97:22–28. doi: 10.1016/j.jaut.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Denny A.M., Das S.K. A case of central nervous system vasculitis presenting as a mass-like lesion. Childs Nerv Syst. 2019;35:1223–1226. doi: 10.1007/s00381-018-04034-7. [DOI] [PubMed] [Google Scholar]
- 6.CDC CDC COVID Data Tracker. Center of Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#demographics Available at:
- 7.Lindan C.E., Mankad K., Ram D., et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297:E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmons G.M., Rempe T., Bevins E.A., et al. CNS lymphocytic vasculitis in a young woman with COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1048. doi: 10.1212/NXI.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexopoulos H., Magira E., Bitzogli K., et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7:e893. doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younger D.S. Postmortem neuropathology in COVID-19. Brain Pathol. 2021;31:385–386. doi: 10.1111/bpa.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpkins A., Strickland S.M., Oliver J., et al. Complete resolution of advanced Mycoplasma pneumoniae encephalitis mimicking brain mass lesions: report of two pediatric cases and review of literature. Neuropathology. 2012;32:91–99. doi: 10.1111/j.1440-1789.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 14.Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. 2016;7:23. doi: 10.3389/fmicb.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). Center of Disease Control and Prevention. https://www.cdc.gov/mis/index.html Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.