Abstract
Background:
A case series suggested efficacy for lithium to treat agitation in dementia, but no placebo-controlled trials have been conducted.
Objectives:
To evaluate low-dose lithium treatment of agitation in Alzheimer’s disease (AD).
Method:
In a four-site trial, patients with AD and agitation/aggression score ≥ 4 on the Neuropsychiatric Inventory (NPI) were randomized, double-blind, to lithium carbonate 150–600 mg daily or placebo for 12 weeks. Primary efficacy outcome was change in NPI agitation/aggression; secondary efficacy outcome was treatment response (30% reduction in NPI score for agitation/aggression plus psychosis and a Clinical Global Impression (CGI) score of much or very much improved). Safety profile of lithium was assessed.
Results:
Fifty-eight of 77 patients (75.3%) completed the trial. In linear mixed effects model analyses, lithium was not significantly superior to placebo for agitation/aggression. Proportion of responders was 31.6% on lithium and 17.9% on placebo (χ2=1.26, p=0.26). Moderate or marked improvement (CGI) was greater on lithium (10/38=36.8%) than placebo (0/39=0%, Fisher’s exact test p<0.001). In exploratory analyses, improvement on lithium was greater than placebo on NPI delusions and irritability/lability (p’s<0.05). Lithium showed greater reduction than placebo in patients with high Young Mania Rating Scale scores (β=5.06; 95%CI,1.18 to 8.94,p=0.01). Oral dose and serum levels demonstrated similar associations with efficacy outcomes. Lithium did not differ significantly from placebo on safety outcomes.
Conclusions:
Low-dose lithium was not efficacious in treating agitation but was associated with global clinical improvement and excellent safety. A larger trial may be warranted of likely lithium-responsive behavioral symptoms that overlap with mania.
OBJECTIVE
The most common type of dementia, Alzheimer’s disease (AD), is characterized by progressive cognitive and functional decline. Nearly all patients with Alzheimer’s disease (AD) develop neuropsychiatric symptoms, which include psychosis, agitation, depression, anxiety, and insomnia.1 Agitation, which can include aggressive behavior,2 occurs in one-third of community-dwelling patients with AD and is more common in nursing homes.1,3 Delusions and hallucinations occur in 15–30% and 5–15% of patients with AD, respectively.1, 3, 4 Agitation, aggression, and psychosis are distressing to patients and caregivers, difficult to treat, and associated with increased caregiver burden, accelerated cognitive deterioration, institutionalization, and increased mortality.5–7 Behavioral approaches have been recommended to treat agitation in AD.8 Trials of caregiver-focused education with behavior modification showed on average a small effect size, but published studies were not double-blind and lacked a comparator group with similar staff intervention time.9 Aggression prevention training was ineffective in a large, controlled trial of patients with dementia.10 In patients with AD who develop moderate to severe agitation and psychosis, pharmacological treatment typically is needed.11, 12
There is no FDA-approved treatment for agitation or psychosis in AD. Some antipsychotics demonstrated mild to moderate efficacy for agitation and psychosis in placebo-controlled trials,13, 14 but were associated with safety concerns, including increased mortality in dementia, that now carry a boxed warning.15 Citalopram, an SSRI antidepressant, reduced some measures of agitation in a placebo-controlled trial, but the dose of 30 mg/day was associated with QT prolongation on the electrocardiogram and worse global cognition.16 Anticonvulsants have not shown efficacy17; other medications have not demonstrated consistent efficacy compared to placebo in randomized, double-blind trials and new treatments remain in development.18
Lithium is an established treatment for bipolar disorder.19 Lithium’s effects on multiple neurotransmitters have led to uncertainty about its specific mechanism of action in bipolar and other disorders.20 Lithium is a mood-stabilizer and is effective in reducing disruptive behaviors in bipolar mania; this characteristic led to initial efforts to use lithium to treat behavioral changes in dementia. In early case reports of individual patients with dementia and behavioral symptoms, lithium treatment with serum levels of 0.6–1.6 mmol/l was associated with significant adverse effects.21, 22 In our published case series of six patients with dementia, agitation and psychosis unresponsive to antipsychotics improved appreciably on low-dose lithium, particularly in three patients with AD (oral dose 300–600 mg daily, serum lithium 0.30– 0.32 mmol/L), with minimal to no side effects.23 In two placebo-controlled trials of lithium treatment of cognitive decline in AD and amnestic mild cognitive impairment, respectively, low doses of lithium with blood levels of 0.2–0.8 mmol/L were well-tolerated.24, 25 Lithium treatment of agitation or psychosis has not been evaluated in a placebo-controlled clinical trial in any type of dementia. Based on our initial case series and the reported lack of side effects with low-dose lithium in placebo-controlled cognitive enhancer trials, we conducted a preliminary, randomized, double-blind, low-dose lithium treatment trial for agitation with or without psychosis in AD.26 In this trial (Lit-AD), we compared the efficacy and side effects of lithium to placebo.
METHODS
Study Design
The Lit-AD study was an investigator-initiated, multicenter, randomized, placebo-controlled, double-blind, parallel group trial. The study rationale and design, including eligibility criteria, protocol schedule, and statistical analysis plan were published.26 The four academic sites were New York State Psychiatric Institute/Columbia University Irving Medical Center, New York, NY (NYSPI/CUIMC, lead coordinating site); University of Miami Miller School of Medicine, Miami, FL (UM); McLean Hospital, Harvard University, Belmont, Massachusetts (MH); and University of Texas Southwestern Medical Center, Dallas TX (UTSW). The study was conducted from May 2014 to January 2020 and recruitment stopped at the end of the project funding period.
The institutional review board (IRB) at each site approved the study protocol, consent forms, and amendments. The study was registered with ClinicalTrials.gov (trial registration number: NCT02129348). Written informed consent was obtained from all patients and their informants, including surrogate consent when required. An independent data and safety monitoring board reviewed adverse events and study progress, and its recommendations were followed.
Participants and Procedures
Participants were outpatients and recruited primarily from memory clinics and physician referrals. As described elsewhere in detail,26 salient inclusion criteria were a diagnosis of possible or probable Alzheimer’s disease by National Institute on Aging (NIA) criteria,27 score ≥4 on the Neuropsychiatric Inventory (NPI) domain score for agitation/aggression,28 Folstein Mini Mental State Exam (MMSE) range 5–26,29 and availability of an informant. Patients with current major depression or suicidality, alcohol/substance dependence in the prior 6 months, bipolar or other psychotic disorder, and specific neurological disorders were excluded.26 Medical exclusion criteria were tremor causing functional impairment in order to minimize the potential toxicity of lithium, falls in the prior month, untreated thyroid disease (low T4 or high TSH), and serum creatinine >1.5 mg/100ml or eGFR < 44ml/min/1.73m2. The relatively low GFR threshold was based on published recommendations, which account for the fact that approximately half of individuals in the general population over 70 years of age have an abnormally low GFR because of a required age adjustment in the standard GFR calculation.30, 31 Additional exclusion criteria were heart rate <50/min, QTc >460 msec and use of hydrochlorothiazide >25 mg/day or furosemide >10 mg/day or other diuretics at comparable doses. Stable doses for minimum one month of cholinesterase inhibitors, memantine, antidepressants and antipsychotics were permitted. Lorazepam ≤1 mg daily was permitted as rescue medication.
Generic lithium carbonate 150 mg tablets were purchased and over-encapsulated by the NYSPI research pharmacy to match placebo capsules with inert filler. At baseline (week 0), patients were prescribed lithium carbonate 150 mg or placebo daily. At week 2 and at subsequent 2-week intervals until 12 weeks, blood was drawn 12–16 hours after the nighttime dose. An unblinded physician who did not participate in study ratings or clinical management received the serum lithium level results. This physician communicated to the study physician the real lithium level for patients on lithium and a comparable “sham” level for patients on placebo without revealing which was real and which was sham. The study physician adjusted the oral dose in increments of 150 mg/day up to a maximum of 600 mg/day based on both clinical status and serum real/sham lithium levels targeted to reach 0.2–0.6 mmol/l.
Randomization and Masking
Patients were randomized to lithium or placebo, 1:1, stratified by site, for 12 weeks. Randomization, developed by the statistician and executed by the NYSPI pharmacy, was stratified within each site by the presence of psychosis (NPI score ≥4 on delusions or hallucinations) with randomization sequences balanced in blocks of four. All study personnel and patients were masked to treatment assignment. After the final study visit, a psychiatrist independent of the study was unmasked and clinically treated the patient while study personnel remained blind.
Outcome Measures
The primary pre-specified efficacy endpoint was change from baseline in the NPI agitation/aggression domain score. The secondary pre-specified efficacy endpoint was clinical response defined by a 30% decrease in NPI core score (sum of domain scores for agitation/aggression, delusions, and hallucinations) together with a Clinical Global Impression (CGI) behavior change score of 1 or 2 (much improved or very much improved). Exploratory efficacy endpoints were change in other NPI domain scores related to disruptive behaviors and mood (irritability/lability, anxiety, depression, euphoria, disinhibition, aberrant motor activity), and the Young Mania Rating Scale (YMRS) total score.32 Additional pre-specified exploratory endpoints were changes in the Zarit Caregiver Burden Interview total score and the Basic Activities of Daily Living (BADL) Scale.33
Secondary endpoints for side effects included the Treatment Emergent Symptoms Scale (TESS) and adverse and serious adverse events as reported by the patient/informant. Serum creatinine and estimated glomerular filtration rate (eGFR) were assessed at baseline, 6 and 12 weeks. Other assessments were completed at baseline and 12 weeks: complete blood count with chemistry panel; thyroid function tests; Simpson Angus Scale for tremor, extrapyramidal, and other neurological signs; timed Get Up and Go test for mobility; Mini Mental Status Examination (MMSE) and Severe Impairment Battery (SIB) for cognition. The Cumulative Illness Rating Scale for Geriatrics (CIRS-G) documenting medical burden, was completed at baseline. Apolipoprotein E genotype was determined at LGC Genomics (Beverly, MA, USA) using the KASP genotyping assay.
Statistical Analysis
A detailed description of the statistical analysis plan was published.26 Analyses were based on the Intent-To-Treat (ITT) principle, including every patient who was randomized. All tests were performed at two-tailed significance α=0.05.
To test the primary hypothesis, i.e., lithium will significantly reduce agitation/aggression compared to placebo, a linear mixed effects model (MEM) was used with visit (baseline versus 12 weeks), group (placebo versus lithium) and their interactions as fixed effects and a random intercept per patient to account for within-subject correlation due to repeated measurements. Differences in the least squares means (treatment effects) in each group with 95% confidence intervals were derived. For mixed effects regression, the degrees of freedom were estimated by the Kenward-Roger method.34 For the secondary hypothesis and related categorical outcomes, χ2 test or Fisher’s exact test was used. For other secondary and exploratory efficacy outcomes, MEM was used for continuous outcomes as appropriate, including change in TESS scores and other adverse effect outcomes. Standardized effect sizes using Cohen’s d for continuous outcomes35 and odds ratios for categorical outcomes are reported.
We used the Repeated Measures and Sample Size (RMASS) program (http://www.rmass.org) for power analysis for longitudinal studies. For the originally projected sample size of 80 patients, under moderate or weak within-subject correlation we estimated 80% power to detect a medium to large effect size of d=0.5 if dropout was 15% and correlation was 0.36. The actual sample size was 77 patients with 24.7% dropout, thereby lowering statistical power.
RESULTS
Participant Characteristics
Patient characteristics at baseline did not differ between treatment groups (Table 1). Patient flow is described in Figure 1. Fifty-eight of 77 participants (75.3%) completed the trial. Reasons for early terminations were similar in the lithium and placebo groups (Figure 1).
Table 1.
Patient Characteristics at baseline by treatment assignment.
| Characteristics | Lithium (n=38) |
Placebo (n=39) |
|---|---|---|
| Age, mean (SD), years | 75.6 (8.3) | 74.3 (6.9) |
| Women, No. (%) | 23 (60.5) | 23 (59) |
| Race/Ethnicity, No. (%) | ||
| White, non-Hispanic | 30 (78.9) | 32 (82.1) |
| African American, non-Hispanic | 4 (10.5) | 3 (7.7) |
| Hispanic/Latino | 3 (7.9) | 2 (5.1) |
| Asian | 1 (2.6) | 2 (5.1) |
| Married, No. (%) | 24 (63.2) | 28 (71.8) |
| Spouse informant, No. (%) | 24 (63.2) | 28 (71.8) |
| Education, mean (SD), years | 13.3 (4.8) | 12.8 (3.8) |
| Agitation without psychosis, No. (%) | 16 (42.1) | 18 (46.2) |
| Agitation with psychosis, No. (%) | 22 (57.9) | 21 (53.8) |
| CGI severity for behavior, mean (SD) | 4.5 (1.0) | 4.7 (1.0) |
| CGI severity global, mean (SD) | 4.5 (0.8) | 4.6 (0.8) |
| NPI total score, mean (SD) | 44.9 (21.1) | 41.8 (18.6) |
| NPI agitation/aggression, mean (SD) | 7.7 (3.1) | 7.8 (2.9) |
| NPI delusions, mean (SD) | 5.5 (4.5) | 4.8 (4.6) |
| NPI hallucinations, mean (SD) | 1.9 (3.5) | 2.6 (4.1) |
| Young Mania Rating Scale (YMRS), mean (SD) | 10.6 (8.1) | 9.1 (5.5) |
| Mini Mental State Exam (MMSE), mean (SD) | 14.3 (5.9) | 14.5 (5.8) |
| Severe Impairment Battery (SIB), mean (SD) | 79.9 (16.3) | 79.1 (18.8) |
| Basic Activities of Daily Living (BADL), mean (SD) | 4.6 (1.7) | 4.4 (1.7) |
| Zarit Caregiver burden, mean (SD) | 38.2 (16.4) | 40.2 (13.7) |
| Concomitant medications, No. (%) | ||
| Cholinesterase inhibitors | 23 (60.5) | 29 (74.4) |
| Memantine | 17 (44.7) | 21 (53.8) |
| Antipsychotics | 14 (36.8) | 21 (53.8) |
| Antidepressants | 24 (63.2) | 25 (64.1) |
| Lorazepam, No. (%) | 1 (2.6) | 6 (15.4) |
| Cumulative Illness Rating Scale-Geriatric (mean (SD)) | 4.3 (3.3) | 4.0 (2.5) |
| Treatment Emergent Symptom Scale (mean (SD)) | 5.7 (5.9) | 5.5 (5.4) |
| Simpson-Angus scale, mean (SD) | 2.4 (2.5) | 2.7 (3.4) |
| Get up & go time seconds, mean (SD) | 18.6 (18.8) | 17.3 (15.0) |
| Creatinine, mg/dl, mean (SD) | 0.9 (0.2) | 0.9 (0.2) |
| eGFR, ml/min/1.73m2, mean (SD), >60 counts as 60 | 58.9 (2.9) | 58.2 (3.6) |
| Thyroid disease, No. (%) | 10 (26.3) | 5 (12.8) |
| Apolipoprotein E ε4 positive, n (%)* | 22 (61.1) | 24 (61.5) |
CGI: Clinical Global Impression: range 1–7 (higher scores indicate greater severity). NPI: Neuropsychiatric Inventory: range 0–144 (higher score indicates greater symptoms). NPI domain (agitation/aggression, delusions, hallucinations): range 0–12 (higher scores indicate greater symptoms). Young Mania Rating Scale: range 0–60 (higher scores indicate greater symptoms). Mini Mental State Exam: range 0–30 (higher scores indicate better cognition). Severe Impairment Battery: range 0–100 (higher scores indicate better cognition). Basic Activities of Daily Living: range 0–6 (higher scores indicate better functioning). Zarit Caregiver Burden Interview: range 0–88 (higher scores indicate greater burden). CIRS-G: Cumulative Illness Rating Scale-Geriatric: range 0–48 (higher scores indicate more medical illnesses). Treatment Emergent Symptom Scale: range 0–26 (higher scores indicate more somatic symptoms). Simpson Angus Scale for Extrapyramidal Signs: range 0–40 (higher scores indicate increased severity of signs). eGFR: estimated Glomerular Filtration Rate: range 0–60 (higher scores indicate better kidney function).
2 participants in the lithium group had missing values.
Figure 1.
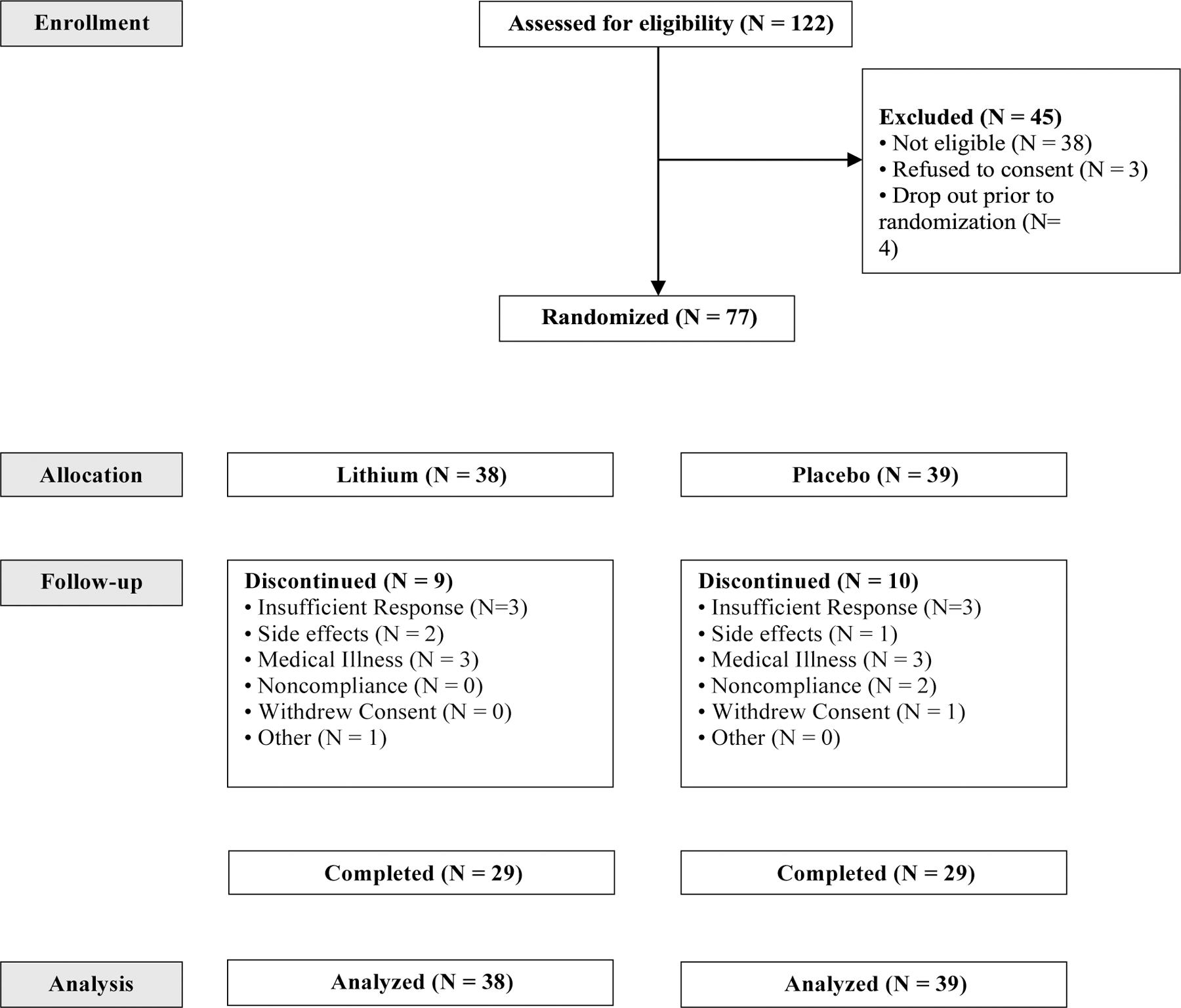
Patients with Alzheimer’s disease and agitation randomized to lithium or placebo for 12 weeks.
Key Outcomes
In MEM analyses (Table 2), NPI agitation/aggression scores were reduced on both lithium (mean reduction 3.17; 95%CI, 1.72 to 4.62; t(66)=4.36, p<.0001) and placebo (mean reduction 2.52; 95%CI, 1.07 to 3.97; t(66)=3.48, p=.001) with no treatment group by time effect (B=0.65; 95%CI, −1.35 to 2.66; t(66)=0.63, p=0.53).
Table 2.
Primary, Secondary and Exploratory Efficacy Outcomes Analyses.
| Change (Baseline - Week 12) (95% CI) | Treatment Effect Difference (95% CI) | Effect Size (d)1 | P value | ||
|---|---|---|---|---|---|
| Lithium | Placebo | ||||
| Primary Outcome | |||||
| NPI agitation/aggression | 3.2 (1.7 to 4.6) | 2.5 (1.1 to 4.0) | 0.7 (−1.4 to 2.7) | 0.23 | t(66)=0.63, 0.53 |
| Secondary Outcome | |||||
| Proportion of Responders n (%)2 | 12/38 (31.6) | 7/39 (17.9) | 2.11 (0.73 to 6.13)2 | - | χ2(1)=1.26, 0.26 |
| Exploratory Efficacy Outcomes | |||||
| CGI Behavior Change n (%)2 | 12/38 (31.6) | 8/39 (20.5) | 1.79 (0.64–5.04)2 | - | χ2(1)=1.33, 0.25 |
| CGI Global Change n (%) | 10/38 (36.8) | 0/39 (15.4) | NA4 | - | <0.0013 |
| NPI total | 14.1 (6.1 to 22.1) | 8.8 (0.8 to 16.7) | 5.3 (−5.6 to 16.4) | 0.34 | t(65)=0.95, 0.35 |
| NPI psychosis | 3.4 (1.3 to 5.5) | 1.5 (−0.6 to 3.6) | 1.9 (−1.0 to 4.8) | 0.48 | t(62)=1.31, 0.20 |
| NPI core score | 6.6 (3.5 to 9.7) | 4.1 (1.0 to 7.2) | 2.5 (−1.8 to 6.8) | 0.41 | t(64)=1.14, 0.26 |
| NPI Hallucinations | 1.0 (0.1 to 1.9) | 1.3 (0.5 to 2.2) | −0.4 (−1.6 to 0.8) | −0.21 | t(60)=−0.59,0.56 |
| NPI Delusions | 2.4 (0.9 to 4.0) | 0.2 (−1.4 to 1.7) | 2.3 (0.2 to 4.4) | 0.76 | t(63)=2.11,0.04 |
| NPI Depression | 0.6 (−0.4 to 1.5) | 0.1 (−0.8 to 1.1) | 0.5 (−0.8 to 1.8) | 0.26 | t(63)=0.72,0.48 |
| NPI Anxiety | 1.7 (0.3 to 3.1) | 2.0 (0.6 to 3.5) | −0.3 (−2.3 to 1.6) | −0.12 | t(65)=−0.33,0.75 |
| NPI Elation | 0.2 (−0.1 to 0.5) | 0.1 (−0.2 to 0.4) | 0.1 (−0.3 to 0.5) | 0.18 | t(58)=0.49,0.63 |
| NPI Disinhibition | −0.3 (−1.2 to 0.5) | 0.3(−0.6 to 1.2) | −0.7 (−1.9 to 0.6) | −0.38 | t(60)=−1.04,0.30 |
| NPI Irritability/lability | 3.0 (1.4 to 4.7) | 0.7 (−0.9 to 2.3) | 2.3 (0.1 to 4.6) | 0.72 | t(67)=2.03,0.05 |
| NPI aberrant motor behavior | 0.8 (−0.4 to 2.0) | 0.6 (−0.6 to 1.8) | 0.2 (−1.5 to 1.8) | 0.09 | t(60)=0.24,0.81 |
| NPI apathy | 0.1 (−1.3 to 1.5) | 0.7 (−0.8 to 2.07) | −0.6 (−2.5 to 1.4) | −0.21 | t(64)=−0.59,0.56 |
| NPI nighttime behaviors (sleep) | 0.7 (−0.6 to 2.1) | −0.4 (−1.8 to 0.9) | 1.1 (−0.7 to 3.0) | 0.43 | t(63)=1.19,0.24 |
| NPI appetite | 0.6 (−0.5 to 1.6) | 0.6 (−0.5 to 1.6) | 0.0 (−1.4 to 1.5) | 0.00 | t(63)=0.00, 1.00 |
| BADL | 0.3 (−0.1 to 0.7) | 0.1 (−0.3 to 0.6) | 0.2 (−0.4 to 0.8) | 0.18 | t(59)=0.49,0.63 |
| YMRS | 3.1 (0.9 to 5.3) | 1.1 (−1.1 to 3.3) | 2.0 (−1.1 to 5.1) | 0.46 | t(62)=1.28,0.21 |
| Zarit Caregiver Burden | 2.8 (−1.1 to 6.6) | −0.4 (−4.2 to 3.3) | 3.2 (−2.1 to 8.4) | 0.44 | t(58)=1.19,0.24 |
| Safety Outcomes | |||||
| TESS | 0.6 (−1.3 to 2.4) | 0.7 (−1.1 to 2.5) | −0.1 (−2.6 to 2.4) | −0.04 | t(62)=−0.11,0.91 |
| Simpson-Angus Scale | −0.0 (−1.1 to 1.0) | 0.0 (−1.0 to 1.0) | 0.1 (−1.5 to 1.4) | −0.02 | t(62)=−0.07,0.94 |
| Get Up and Go Time | 3.5 (−2.6 to 9.5) | 0.6 (−5.5 to 6.7) | 2.9 (−5.6 to 11.3) | 0.24 | t(65)=0.67,0.50 |
| MMSE | 0.9 (−0.3 to 2.2) | 0.9 (−0.4 to 2.1) | 0.0 (−1.7 to 1.8) | 0.01 | t(57)=0.05,0.96 |
| SIB | 2.1 (−1.1 to 5.4) | −0.0 (−3.3 to 3.2) | 2.2 (−2.3 to 6.6) | 0.35 | t(56)=0.94,0.35 |
| Change in Creatinine week 0: | |||||
| to week 6, abnormal (%) | 2/35 (5.7) | 1/33 (3) | 1.94 0.17–22.46)2 | - | 13 |
| to week 12, abnormal (%) | 0/29 (0) | 0/28 (0) | NA4 | - | ㅡ |
| Change in eGFR from week 0 | |||||
| to week 6, abnormal (%) | 3/35 (8.6) | 2/33 (6.1) | 1.45 (0.15 to 18.40)2 | - | 13 |
| to week 12, abnormal (%) | 1/29 (3.4) | 1/28 (3.6) | 0.96 0.06 to 16.21)2 | - | 13 |
Linear mixed effect models were used to estimate means of change scores by treatment group, lithium versus placebo, for continuous outcomes.
CGI: Clinical Global Impression: range 1–7 (higher scores indicate greater severity). NPI: Neuropsychiatric Inventory: range 0–144 (higher score indicates greater symptoms). NPI domain (agitation/aggression, hallucinations, delusions, depression, anxiety, elation, disinhibition, irritability, lability, aberrant motor behavior, apathy, nighttime behavior (sleep), appetite): range 0–12 (higher scores indicate greater symptoms). NPI psychosis (sum of scores for NPI domains: delusions and hallucinations): range 0–24 (higher score indicates greater symptoms). NPI core score (sum of scores for NPI domains: agitation/aggression, delusions, and hallucinations): range 0–36 (higher score indicates greater symptoms). Basic Activities of Daily Living: range 0–6 (higher scores indicate better functioning). Young Mania Rating Scale: range 0–60 (higher scores indicate greater symptoms). Zarit Caregiver Burden Interview: range 0–88 (higher scores indicate greater burden). Treatment Emergent Symptom Scale: range 0–26 (higher scores indicate more somatic symptoms). Simpson Angus Scale for Extrapyramidal Signs: range 0–40 (higher scores indicate increased severity of signs). Mini Mental State Exam: range 0–30 (higher scores indicate better cognition). Severe Impairment Battery: range 0–100 (higher scores indicate better cognition). eGFR: estimated Glomerular Filtration Rate: range 0–60 (higher scores indicate better kidney function).
Cohen’s d for continuous variables.
Odds ratio (OR) for categorical variables. CGI change measures for behavior and global classified as 1 or 2 (much/very much improved) versus 3–7 (mildly improved to very much worse). Chi-square test was used.
Fisher’s exact test used because of cells with low frequency.
OR was not computed due to a zero cell.
The proportion of responders was 31.6% (12/38) on lithium and 17.9% (7/39) on placebo, a non-significant difference (χ2(1)=1.26, p=0.26, Table 2) when using the last available observation for dropouts. This pattern was similar when dropouts were considered as non-responders (28.9% responders on lithium versus 12.8% on placebo; χ2(1)=2.14, p=0.14) or after dropouts were excluded (37.9% responders on lithium versus 17.2% on placebo; χ2(1)=2.12, p=0.14). Change in both NPI core score (≥30% versus <30%) and CGI behavior change (1 or 2 versus ≥3) were required for response; these two measures were then analyzed separately. NPI core score reduction ≥30% did not differ between lithium (24/38=63.2%) and placebo (20/39=51.3%), χ2(1)=0.68, p=0.41. CGI behavior change on lithium (12/38=31.6%) was not greater than on placebo (8/39=20.5%, χ2(1)=0.72, p=0.40). CGI global change, which incorporated behavior, cognition, and function, showed greater improvement on lithium (10/38=36.8%) compared to placebo (0/39=0%, Fisher’s exact test p<0.001) when using the last available observation for dropouts. Similar results were observed in 58 completers (CGI behavior change, lithium 37.9% versus placebo 20.7%, χ2(1)=1.33, p=0.25; CGI global change, lithium 31% versus placebo 0%, Fisher’s exact test p=0.002).
Other measures
In secondary analyses, NPI total score reduction on lithium (mean 14.11; 95%CI, 6.14 to 22.08; t(64)=3.54, p=0.001) was not significantly greater than placebo (mean 8.77; 95%CI, 0.83 to 16.72; t(65)=2.21, p=0.03), B=5.34, 95%CI, −5.62 to 16.40; t(65)=0.95, p=0.35. For psychosis, delusions improved more on lithium (mean 2.44; 95%CI, 0.90 to 3.98; t(63)=3.16, p=0.002) than placebo (mean 0.15; 95%CI −1.39 to 1.68; t(63)=0.19, p=0.85), B=2.29, 95%CI, 0.16 to 4.42; t(63)=2.11, p=0.04, but change in hallucinations did not differ between the two groups (B=−0.36; 95%CI, −1.57 to 0.84; t(60)=−0.59, p=0.56).
Other NPI domains were evaluated in exploratory analyses. Irritability/lability improved more on lithium (mean 3.04; 95%CI 1.43 to 4.66; t(66)=3.77, p<.0001) than placebo (mean 0.72; 95%CI −0.88 to 2.33; t(67)=0.90, p=0.37), B=2.32; 95%CI 0.10 to 4.55; t(67)=2.03, p=0.046. Lithium showed a non-significant advantage over placebo on depression and elation while placebo showed a non-significant advantage over lithium on anxiety (Table 2, p’s>0.3). For the NPI domains of delusions and irritability/lability that were significant, baseline NPI domain severity (median split) did not moderate the lithium effect (p’s>0.18). For reduction in YMRS scores (symptom improvement), lithium’s advantage over placebo was not significant. Lithium was superior to placebo in patients with high YMRS scores defined by a median split (B=5.06; 95%CI 1.18 to 8.94; t(62)=2.61, p=0.01) but not in patients with low YMRS scores (B=−0.91; 95%CI −4.78 to 2.96; t(63)=−0.47, p=0.641). The time by treatment group by YMRS group interaction was significant (B=5.97, 95%CI 0.72 to 11.33; t(63)=2.18, p=0.03). In the entire sample, greater reduction in YMRS scores correlated significantly with CGI behavior change (Spearman’s r: −0.34, p=0.002) and CGI global change (Spearman’s r: −0.31, p=0.006). For CGI behavior change, this correlation was significant for both lithium (r: −0.34, p<0.04) and placebo (r:−0.34, p<0.04), and for CGI global change the correlation was significant for lithium (r:−0.32, p<0.05) but not for placebo (r:−0.23, p=0.16).
The results of the primary, secondary, and exploratory efficacy analyses did not change when age, sex, baseline MMSE, baseline CIRS-G, apolipoprotein E e4 genotype, and antidepressant or antipsychotic use (yes/no dichotomous variables) were included separately or together as covariates.
Adherence and serum levels
Caregivers ensured administration of capsules and pill counts indicated >75% capsules prescribed were taken by all patients. Serum levels were consistent with the assigned treatment condition in all cases. In the lithium group, final oral daily dose did not differ between responders (mean 262.5 mg SD 67.84) and non-responders (mean 264.0 mg SD 213.37; p=0.98), and final serum lithium levels in mmol/l did not differ between responders (mean 0.35 SD 0.14) and non-responders (mean 0.28 SD 0.23; p=0.29).
Safety and Adverse Events
There was no significant treatment group effect for change in somatic side effects (TESS), heart rate, systolic or diastolic blood pressure, creatinine levels and eGFR. SAS score and Get Up and Go Time did not differ between the treatment groups, indicating that low dose lithium did not lead to the neurological side effects of extrapyramidal signs and tremor. The cognitive measures of MMSE and SIB scores, and BADL, did not differ between treatment groups, indicating a lack of adverse effects on cognition and function (Table 2). One patient on lithium and none on placebo had an abnormal creatinine level by week 12, and 3 patients in each group had abnormal eGFR by week 12. Increase in TSH occurred in 4 patients on lithium of whom 3 had prior thyroid disease with thyroid replacement therapy, and 1 patient on placebo showed decreased T4 levels. Lorazepam use at baseline (lithium n=1, placebo n=6; Table 1) and change in lorazepam use during the trial did not differ significantly between lithium and placebo. The rate of Serious Adverse Events (SAEs) during the trial did not differ between lithium (26.3%) and placebo (23.0%), and specific adverse events did not differ significantly between the two treatment groups with increased agitation (greater on placebo) and falls (greater on lithium) being the most common (Table 3).
Table 3.
Patients Experiencing Adverse Events.
| Adverse Event | Lithium (n=38) | Placebo (n=39) | P value* |
|---|---|---|---|
| Increased agitation | 6 (15.8%) | 11 (28.2%) | 0.27 |
| Fall | 7 (18.4%) | 3 (7.7%) | 0.19 |
| Diarrhea | 3 (7.9%) | 4 (10.3%) | 1.0 |
| Reduced Kidney Function | 3 (7.9%) | 2 (5.1%) | 0.68 |
| Vomiting | 4 (10.5%) | 1 (2.6%) | 0.2 |
| Gait instability | 4 (10.5%) | 0 (0%) | 0.055 |
| UTI | 2 (5.3%) | 2 (5.1%) | 1.0 |
| Insomnia | 2 (5.3%) | 1 (2.6%) | 0.62 |
| Pneumonia | 2 (5.3%) | 1 (2.6%) | 0.62 |
| Tremor | 3 (7.9%) | 0 (0%) | 0.12 |
| Abdominal pain | 2 (5.3%) | 0 (0%) | 0.24 |
| Contact dermatitis | 1 (2.6%) | 1 (2.6%) | 1.0 |
| Headache | 1 (2.6%) | 1 (2.6%) | 1.0 |
| Increased anxiety | 2 (5.3%) | 0 (0%) | 0.24 |
| Increased confusion | 1 (2.6%) | 1 (2.6%) | 1.0 |
| Muscle pain | 0 (0%) | 2 (5.1%) | 0.49 |
| Myoclonus | 1 (2.6%) | 1 (2.6%) | 1.0 |
| URI | 0 (0%) | 2 (5.1%) | 0.49 |
Adverse events that occurred in at least two patients in the entire sample are listed.
Fisher’s exact test was used.
CONCLUSIONS
Lithium did not differ from placebo on the primary outcome measure of change in agitation/aggression. The substantial reduction in agitation on placebo decreased the likelihood of identifying a lithium-placebo difference. The proportion of responders on lithium (31.6%) was not significantly greater than on placebo (17.9%). The observed difference in proportion of responders of 13.7% is similar to the 14% difference between citalopram and placebo on a global impression change scale, mADCS-CGIC, in the CitAD trial for agitation in AD.16 In four published placebo-controlled trials of risperidone for agitation and psychosis in AD, the risperidone-placebo difference in CGI change score did not exceed 11% in any trial.14 In Lit-AD, the lack of significance was most likely due to the small sample size with slightly less recruitment and more attrition than originally estimated, thereby limiting statistical power.
In exploratory analyses, six NPI domains showed greater improvement on lithium than placebo with delusions and irritability/lability reaching statistical significance (Table 2). During long-term follow-up in AD, delusions, irritability/lability, agitation/aggression, and aberrant motor behavior are the domains that contribute to persistently high NPI scores and represent symptom profiles that often lead to therapeutic intervention.36 Irritability and lability are common in AD and are features of mania in bipolar disorder. In Lit-AD, lithium was superior to placebo in reducing high YMRS scores. The advantage for lithium over placebo in the clinical global improvement measure must be viewed with circumspection because cognitive and ADL measures did not improve. There were indications that clinical global improvement was associated with reduction in manic symptoms assessed by the YMRS. The pattern of results indirectly suggests that lithium, which is a mood stabilizer, may have efficacy for behavioral symptoms in AD that overlap with those in mania. To our knowledge, the YMRS has not been evaluated in other studies of patients with dementia.
Pharmacokinetic changes with aging reduce the volume of distribution and renal clearance, leading to higher lithium serum levels and increased adverse effects.37 In Lit-AD, low doses of lithium resulted in low serum lithium levels that did not lead to increased somatic side effects nor impairment in kidney function that can occur with prolonged, high-dose lithium use.19 Long-term lithium treatment can impair thyroid function.19 In this short-duration trial, worsening thyroid function in four patients is difficult to interpret because three of them were either on thyroid replacement therapy or had a history of thyroidectomy. Lithium did not differ from placebo in neurological signs on the Simpson-Angus scale. These signs, particularly tremor and ataxia, can occur in older adults receiving high doses of lithium for bipolar disorder.37 Falls and gait instability were non-significantly greater on lithium, increased agitation was greater on placebo. These findings, which have potential implications for lithium use in clinical practice, need replication in a larger study.
The absence of cognitive decline on low dose lithium stands in contrast to cognitive worsening with antipsychotics in CATIE-AD38 and the SSRI citalopram in the CitAD trial.16 Electronic health record studies suggest that long-term lithium use in bipolar disorder may improve cognition,39, 40 and a preliminary trial suggested possible efficacy for cognition in amnestic MCI.25 In Lit-AD, the lack of differences on MMSE and SIB suggest that lithium’s advantage over placebo on CGI global change was not primarily due to cognitive improvement.
Therapeutic dose window
In Lit-AD, low doses of lithium were associated with global improvement and an excellent safety profile. Theoretically, higher oral doses of lithium may have improved efficacy, but high doses in older adults may compromise safety.19, 41 In a large treatment trial of mania in older adults with bipolar disorder, lithium doses averaging 780 mg daily with moderately high blood levels (0.8–0.99 mmol/L) were associated with a high dropout rate of 51% by 9 weeks of treatment.32 In AD, a preliminary report showed that micro-doses of lithium may slow cognitive deterioration,42 but the limited efficacy observed with low doses in Lit-AD makes it unlikely that even lower doses would have improved efficacy further for behavioral symptoms.
Strengths and limitations of the study
Strengths of the study include: 1) first randomized trial comparing lithium to placebo to treat agitation and other behavioral symptoms in AD; 2) study sample broadly representative of patients with AD; 3) double-blind randomized treatment assignment with placebo comparator; 4) rigorous adherence to protocol with blinded ratings; 5) sham versus real lithium blood levels used to maintain the blind; 6) low oral doses of lithium led to low blood levels; 7) global improvement on lithium compared to placebo; 8) excellent safety profile; 9) lack of significant differences in side effects strengthened maintenance of the blind.
Limitations of the study include: 1) patients comprised a sample of convenience in U.S. academic medical centers that may not generalize to other settings; 2) relatively short duration of treatment; 3) lack of biomarker confirmation of AD diagnosis increasing sample heterogeneity may have contributed to the negative outcome, though the high proportion of apoE e4 positive patients was consistent with reported apoE e4 positivity in most AD samples; 4) difference for lithium versus placebo in the primary outcome measure of agitation/aggression was not significant; 5) small sample size limited statistical power whereby the observed small to medium effect sizes for several secondary and exploratory efficacy outcomes were not significant at the p<.05 level; 6) baseline antipsychotic and benzodiazepine use was greater in the placebo group but were not significant covariates in efficacy analyses; 7) no correction for multiple comparisons for exploratory analyses in this preliminary study.
Conclusions
Low dose lithium did not show evidence of efficacy in treating agitation, which may have been due to limited statistical power. Lithium treatment was associated with global clinical improvement and an excellent safety profile. The findings in this preliminary study suggest the need for a larger clinical trial with precision pharmacological targeting of likely lithium-responsive behavioral symptoms that may overlap with symptoms of mania. Lithium can be difficult to use in older adults because of the need to monitor blood levels and the risk of adverse effects, but the generally favorable side effect profile observed with low doses and low blood levels suggests potential clinical applicability if efficacy is established in a larger clinical trial.
Highlights.
-
What is the primary question addressed by this study?
The main goal was to evaluate the efficacy and side effects of low-dose lithium to treat agitation in Alzheimer’s disease (AD).
-
What is the main finding of this study?
In 77 patients with AD and agitation randomized to lithium or placebo in a four-site study, lithium was not significantly superior to placebo in treating agitation/aggression but demonstrated excellent safety. Compared to patients who received placebo, patients who received lithium showed greater improvement in Clinical Global Impression scores and greater improvement in patients with high Young Mania Rating Scale scores.
-
What is the meaning of the finding?
Low-dose lithium was not efficacious in treating agitation in AD but was associated with global clinical improvement, reduction in behavioral symptoms that overlap with mania, and excellent safety.
Acknowledgements
The study was supported by grant R01AG047146 from the National Institute on Aging, NIH, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: ClinicalTrials.gov Identifier NCT02129348
Disclosure of Interest
Dr. Devanand reports receipt of grants to his institution from the National Institute on Aging and the Alzheimer’s Association; paid consultancy for Acadia, Biogen, BXCel, Eisai, Genentech, Sunovion, and Green Valley.
Dr. Crocco reports receipt of grants to her institution from the National Institute on Aging, Novartis, Avanir, and Neurim.
Dr. Forester reports receipt of grants to his institution from the National Institute on Aging, Rogers Family Foundation, Eisai, and Eli Lilly; paid consultancy for Biogen.
Dr. Husain reports receipt of grants to his institution from the National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Neurological Disorders and Stroke, National Institute on Aging, Stanley Medical Research Institute.
Dr. Lee reports receipt of grants to her institution from the National Institute on Aging.
Dr. Huey reports receipt of grants to his institution from the National Institute on Aging.
Dr. Vahia reports receipt of grants to his institution from the National Institute on Aging, Once upon a time Foundation, NASA, and paid honorarium from the American Journal of Geriatric Psychiatry.
Drs. Andrews, Deliyannides, Pelton, Luca, and Ms. Simon-Pearson and Ms. Imran report receipt of grants to the institution from the National Institute on Aging.
Contributor Information
D. P. Devanand, Division of Geriatric Psychiatry, New York State Psychiatric Institute, New York, USA; Department of Psychiatry, Columbia University Medical Center, New York, USA; Department of Neurology and Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University Medical Center, New York, USA.
Elizabeth Crocco, Center for Cognitive Neuroscience and Aging, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine Miami, Florida, USA.
Brent P. Forester, Division of Geriatric Psychiatry, McLean Hospital, Harvard Medical School, Belmont, MA, USA.
Mustafa M. Husain, Departments of Psychiatry and Neurology, University of Texas Southwestern Medical Center, Dallas, TX USA.
Seonjoo Lee, Department of Psychiatry, Columbia University Medical Center, New York, USA; Mental Health Data Science, Department of Psychiatry, Columbia University Medical Center and New York State Psychiatric Institute, New York, USA.
Ipsit V. Vahia, Division of Geriatric Psychiatry, McLean Hospital, Harvard Medical School, Belmont, MA, USA.
Howard Andrews, Department of Psychiatry, Columbia University Medical Center, New York, USA; Mental Health Data Science, Department of Psychiatry, Columbia University Medical Center and New York State Psychiatric Institute, New York, USA.
Laura Simon-Pearson, Division of Geriatric Psychiatry, New York State Psychiatric Institute, New York, USA.
Nadia Imran, Departments of Psychiatry and Neurology, University of Texas Southwestern Medical Center, Dallas, TX USA.
Luminita Luca, Center for Cognitive Neuroscience and Aging, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine Miami, Florida, USA.
Edward D. Huey, Division of Geriatric Psychiatry, New York State Psychiatric Institute, New York, USA; Department of Psychiatry, Columbia University Medical Center, New York, USA; Department of Neurology and Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University Medical Center, New York, USA.
Deborah A. Deliyannides, Division of Geriatric Psychiatry, New York State Psychiatric Institute, New York, USA; Department of Psychiatry, Columbia University Medical Center, New York, USA.
Gregory H. Pelton, Division of Geriatric Psychiatry, New York State Psychiatric Institute, New York, USA; Department of Psychiatry, Columbia University Medical Center, New York, USA.
Data Availability
The data that support the findings of this study are available from the corresponding author, DPD, upon reasonable request.
REFERENCES
- 1.van der Linde RM, Dening T, Stephan BCM, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209(5):366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Intl Psychogeriatr. 2015;27:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Jacobs DM, Tang MX, Del Castillo-Castaneda C, Sano M, Marder K, et al. The course of psychopathologic symptoms in mild to moderate Alzheimer’s disease. Arch Gen Psychiatry. 1997;54:257–263. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. [DOI] [PubMed] [Google Scholar]
- 5.Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64:1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller EA, Schneider LS, Rosenheck RA. Predictors of nursing home admission among Alzheimer’s disease patients with psychosis and/or agitation. Int Psychogeriatr. 2011;23(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 9.Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946–53. Erratum in: Am J Psychiatry 2013;170(2):227. [DOI] [PubMed] [Google Scholar]
- 10.Kunik ME, Stanley MA, Shrestha S, Ramsey D, Richey S, Snow L, et al. Aggression Prevention Training for Individuals with Dementia and Their Caregivers: A Randomized Controlled Trial Am J Geriatr Psychiatry 2020; 28:662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2012;5:245–255. [DOI] [PubMed] [Google Scholar]
- 12.Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367:1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–1538. [DOI] [PubMed] [Google Scholar]
- 14.Katz I, de Deyn PP, Mintzer J, Greenspan A, Zhu Y, Brodaty H, et al. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer’s disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry. 2007;22:475–484. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LS, Dagerman KS, Insel P. Risk of Death with Atypical Antipsychotic Drug Treatment for Dementia: Meta-analysis of Randomized Placebo-Controlled Trials. JAMA. 2005;294(15):1934–1943. [DOI] [PubMed] [Google Scholar]
- 16.Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of Citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tariot PN, Schneider LS, Cummings J, Thomas RG, Raman R, Jakimovich LJ, et al. ; Alzheimer’s disease cooperative study group: Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68(8):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl SM. New hope for Alzheimer’s dementia as prospects for disease modification fade: symptomatic treatments for agitation and psychosis. CNS Spectr. 2018. October;23(5):291–297. [DOI] [PubMed] [Google Scholar]
- 19.McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721–728. [DOI] [PubMed] [Google Scholar]
- 20.Won E, Kim YK. An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms. Int J Mol Sci. 2017. December 11;18(12):2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havens WW 2nd, Cole J. Successful treatment of dementia with lithium. J Clin Psychopharmacol. 1982;2:71–72. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S, et al. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23:704–711. [DOI] [PubMed] [Google Scholar]
- 23.Devanand DP, Pelton GH, D’Antonio K, Strickler JG, Kreisl WC, Noble J, et al. Low-dose Lithium treatment for agitation and psychosis in Alzheimer’s disease and Frontotemporal dementia: a case series. Alzheimer Disease & Associated Disorders. 2017;31:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel H, Ewers M, Bürger K, Annas P, Mörtberg A, Bogstedt A, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 2009;70: 922–931. [PubMed] [Google Scholar]
- 25.Forlenza OV, Radanovic M, Talib LL, et al. Disease modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomized controlled trial. Br J Psychiatry. 2019; 215:668–674. [DOI] [PubMed] [Google Scholar]
- 26.Devanand DP, Strickler JG, Huey ED, Crocco E, Forester BP, Husain MM, et al. Lithium Treatment for Agitation in Alzheimer’s disease (Lit-AD): Clinical rationale and study design. Contemp Clin Trials. 2018;71:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 30.Janowsky DS, Buneviciute J, Hu Q, Davis JM. Lithium-induced renal insufficiency: a longitudinal study of creatinine increases in intellectually disabled adults. J Clin Psychopharmacol. 2011;31:769–773. [DOI] [PubMed] [Google Scholar]
- 31.Stevens LA, Viswanathan G, Weiner DE. CKD and ESRD in the elderly: Current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young RC, Mulsant BH, Sajatovic M, Gildengers AG, Gyulai L, Al Jurdi RK, et al. GERI-BD: A Randomized Double-Blind Controlled Trial of Lithium and Divalproex in the Treatment of Mania in Older Patients with Bipolar Disorder. Am J Psychiatry. 2017;174:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. [DOI] [PubMed] [Google Scholar]
- 34.Kenward MG, & Roger JH Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997; 53:983–997. [PubMed] [Google Scholar]
- 35.Rouder JN, Morey RD, Speckman PL, Province JM. Default Bayes factors for ANOVA designs. J Math Psychol. 2012;56:356–374. [Google Scholar]
- 36.Vik-Mo AO, Giil LM, Borda MG, Ballard C, Aarsland D. The Individual Course of Neuropsychiatric Symptoms in People with Alzheimer’s and Lewy Body Dementia: 12-year Longitudinal Cohort Study. Br J Psychiatry. 2020; 216:43–48. [DOI] [PubMed] [Google Scholar]
- 37.Sproule BA. Hardy BG, Shulman KI. Differential pharmacokinetics of lithium in elderly patients. Drugs Aging. 2000;16:165–177. [DOI] [PubMed] [Google Scholar]
- 38.Vigen CL, Mack WJ, Keefe RS, Sano M, Sultzer DL, Stroup TS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168(8):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessing LV, Søndergård L, Forman JL, Andersen PK. Lithium Treatment and Risk of Dementia. Arch Gen Psychiatry. 2008;65(11):1331–1335. [DOI] [PubMed] [Google Scholar]
- 40.Gerhard T, Devanand DP, Huang C, Crystal S, Olfson M. Lithium treatment and risk for dementia in adults with bipolar disorder: population-based cohort study. Br J Psychiatry. 2015;207:46–55. [DOI] [PubMed] [Google Scholar]
- 41.Sajatovic M, Madhusoodhan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging. 2005; 22;39–54. [DOI] [PubMed] [Google Scholar]
- 42.Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013;10:104–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, DPD, upon reasonable request.


