Abstract
Background:
Optic neuritis (ON) is often the presenting symptom in inflammatory central nervous system demyelinating disorders.
Objective:
To compare the frequency and pattern of optic chiasm involvement in patients with aquaporin-4-immunoglobulin G (AQP4-IgG) associated ON to patients with myelin oligodendrocyte glycoprotein- immunoglobulin G (MOG-IgG) associated ON.
Methods:
Retrospective review of all patients evaluated at Mayo Clinic, Stanford University and Ramathibodi Hospital who were found to have: (1) ON; (2) either MOG-IgG or AQP4-IgG by cell based assay and (3) magnetic resonance imaging (MRI) at the time of ON. MRI was reviewed for contrast enhancement of the optic chiasm and the pattern of involvement.
Results:
One-hundred and fifty-four patients (74 AQP4-IgG and 80 MOG-IgG) were included. Among patients with AQP4-IgG-ON, 20% had chiasmal involvement, compared with 16% of patients with MOG-IgG-ON (p=0.66). In patients with chiasmal involvement, longitudinally extensive optic nerve enhancement (from orbit extending to chiasm) was identified in 54% of MOG-IgG-ON patients, compared with 7% of AQP4-IgG-ON patients (p=0.01).
Conclusion:
Chiasmal involvement of MOG-IgG-ON and AQP4-IgG-ON occur at more similar frequencies than previously reported. Furthermore, MOG-IgG-ON chiasmal involvement is more likely to be part of a longitudinally extensive optic nerve lesion.
Keywords: Demyelination, Neuromyelitis Optica, Relapsing/remitting, MRI
Introduction:
Distinct features and imaging advances have shaped our understanding of inflammatory central nervous system demyelinating disorders and have led to the separation of neuromyelitis optica spectrum disorder (NMOSD) from multiple sclerosis. Furthermore, the discovery of immunoglobulin G (IgG) against aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) has greatly improved our understanding of these inflammatory disorders, who that share optic neuritis (ON) as a common presenting symptom.1, 2
However, it has become increasingly evident that MOG-IgG associated disorder (MOGAD) and AQP4-IgG positive NMOSD have other differences in presentation, histopathology, therapeutic response, and outcomes, including features of ON. 3-5 Prior studies have evaluated the magnetic resonance imaging (MRI) characteristics of ON with respect to the optic chiasm. It has been reported in small studies that the optic chiasm is involved in 44-64% of AQP4-IgG-ON cases on MRI, whereas MOG-IgG-ON affects the chiasm at 0-15%, suggesting that optic chiasm involvement may be a diagnostic feature of AQP4-IgG-ON. 6-8 Our aim was to characterize chiasm involvement in a larger cohort than previously studied.
Methods:
This was a retrospective, observational case series of patients with clinical diagnosis of ON seen at Mayo Clinic from 2001 to 2020, Stanford University from 2017-2020, and Ramathibodi Hospital (Bangkok, Thailand) from 2018 to 2019. Each clinical site received approval or exemption from review by their respective Institutional Review Boards.
Criteria for inclusion were (1) clinically documented history of ON; (2) either a positive serum test for MOG-IgG or AQP4-IgG by live cell based assay; (3) MRI obtained within 4 weeks of ON attack.
Medical records were reviewed for demographics and serologic results. Characterization of the optic chiasm at the time of ON, was extracted from radiologic reports. The chiasm was deemed involved if there was active contrast enhancemenT or T2 hyperintensity affecting any part of the chiasm. A longitudinally extensive optic nerve lesion was deemed present when the optic chiasm was involved as a result of enhancement of the orbital portion of the optic nerve extending the length of the optic nerve into the chiasm. Posterior chiasmal involvement was deemed present when there was chiasm enhancement with less than 50% involvement of the adjacent intracranial portion of the optic nerve. (Figure 1).
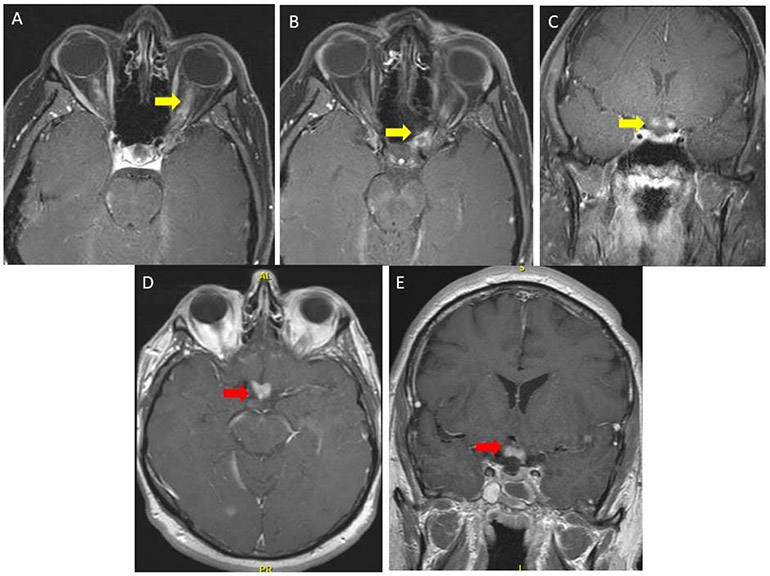
Figure 1. MRI Depicting Optic Chiasm Involvement in Longitudinally Extensive and Isolated Lesions.
Magnetic resonance imaging (MRI) contrasting myelin oligodendrocyte glycoprotein immunoglobulin G optic neuritis (MOG-IgG-ON) and aquaporin 4 (AQP4)-IgG neuromyelitis optica spectrum disorder (NMOSD) ON. Panels A, B (axial images), and C (coronal image) show contrasted, T1-weighted, fat suppression images of the orbits demonstrating bilateral optic nerve enhancement that involves the entire optic nerve and the optic nerve sheath that extends into the optic chiasm (yellow arrows) in a patient with MOG-IgG-ON. Panel D (axial) and E (coronal) shows isolated chiasmal enhancement (red arrows) in a patient with AQP4-IgG-ON.
Categorical and continuous variables were analyzed and compared by chi square test and t-test respectively. For non-normal distributions, Mann-Whitney rank sum test was used for analysis.
Results:
One hundred and fifty-four patients met the inclusion criteria. Seventy-four were AQP4-IgG-ON and 80 were MOG-IgG-ON; none were seropositive for both. The median time, in days, from symptom onset to imaging was 10 for AQP4-IgG-ON and 10.5 for MOG-IgG-ON (p=0.49).
Of the 74 patients with AQP4-IgG-ON, the median age was 44 years (range 7-78), 85% were female, 69% were white and 22% were black. Among these, 15 of 74 (20 %) had optic chiasm involvement at time of ON, with 14 of 15 present in the first ON attack. One of 15 (6.7%) had chiasmal involvement from a longitudinally extensive optic nerve lesion (Table 1).
Table 1.
Comparison of Demographic and Radiographic Characteristics of Patients with Aquaporin-4 IgG Optic Neuritis (AQP4-IgG-ON) and Myelin Oligodendrocyte Glycoprotein IgG (MOG-IgG-ON)
| Demographics and Radiological Findings |
AQP4-IgG-ON No. /Total No. (%) |
MOG-IgG-ON No. /Total No. (%) |
MOG-IgG-ON vs. APQ4-IgG-ON P valuea |
|---|---|---|---|
| Median age at ON onset (range), y | 44 (7-78) | 32 (2-79) | .007 |
| Female | 63/74 (85) | 50/80 (63) | .005 |
| Ethnicity, % | |||
| White | 69 | 58 | |
| Black | 22 | 6 | |
| Asian | 3 | 18 | |
| Hispanic | 1 | 11 | |
| Other/unknown | 5 | 8 | |
| Optic chiasm involvement | 15/74 (20) | 13/80 (16) | .66 |
| Female | 12/15 (80) | 9/13 (70) | |
| White | 11/15 (73) | 9/13 (70) | |
| Black | 3/15 (20) | 0 | |
| Asian | 0 | 1/13 (8) | |
| Hispanic | 1/15 (7) | 2/13 (15) | |
| Other | 0 | 1/13 (8) | |
| Prior ON without chiasm involvement | 1/15 (7) | 1/13 (8) | |
| Chiasmal involvement with longitudinally extensive optic nerve lesion | 2/15 (13) | 7/13 (54) | .04 |
| Posterior optic chiasm lesion | 12/15 (80) | 3/13 (23) | 0.008 |
| Extra-optic nerveb involvement and ON | 14/74 (19) | 17/80 (21) | 0.72 |
| Without chiasmal involvement | 12/59 (20) | 16/67 (24) | 0.63 |
| With chiasmal involvement | 2/15 (13) | 1/13 (8) | 0.63 |
Statistical comparison between MOG-IgG-ON and AQP4-IgG-ON.
Extra-optic nerve refers to cases with other symptomatic MRI lesions, such as transverse myelitis or area postrema involvement at the time of ON.
Of the 80 patients with MOG-IgG-ON, the median age was 32 years (range 2-79), 63% were female, 58% were white and 6% were black. Among these, 13 of 80 (16%) had optic chiasm involvement on imaging at time of ON, with 12 of 13 present in the first ON attack. Seven of 13 (54%) had chiasmal involvement from a longitudinally extensive optic nerve lesion (Table 1).
The AQP4-IgG-ON cohort was older (p=0.007) and more female predominant (p=0.005) compared with the MOG-IgG-ON cohort. There was no significant difference in the frequency of optic chiasm involvement in AQP4-IgG-ON and MOG-IgG-ON (p=0.66). Chiasm involvement as a longitudinally extensive optic nerve lesion was more likely in MOG-IgG-ON (p=0.01), whereas posterior chiasmal involvement was more common in AQP4-IgG-ON (p=0.008) (Figure 1; Table 1).
Discussion:
The involvement of the optic chiasm in AQP4-IgG-ON and MOG-IgG-ON was previously suggested as a differentiating feature between these two entities and significantly higher in AQP4-ON.6-8 Our data, from a large cohort of AQP4-IgG NMOSD and MOGAD patients showed that there was no significant difference in the frequency of chiasmal involvement in ON. The chiasm was involved in 16% of MOG-IgG-ON, while the optic chiasm was affected in 20% of AQP4-IgG-ON, which is lower than previous studies that reported chiasm involvement as high as 64%.6-8 A recent study focusing on the outcomes of MOG-IgG-ON reported similar relative percentages of chiasm involvement to our study of 22% for AQP4-IgG-ON and 15% for MOG-IgG ON9, which further supports that optic chiasm involvement is more similar between the two entities than previously recognized. We believe the higher frequency of chiasm involvement in AQP4-IgG-ON reported in the past was likely due to smaller sample sizes and potential selection bias, while larger studies, such as ours, allows a more representative spectrum of the disease to be appreciated.
We also found a difference in the characteristics of optic chiasm involvement between AQP4-IgG-ON and MOG-IgG-ON. Chiasmal involvement of MOG-IgG-ON was more likely as a longitudinally extensive optic nerve lesion, while AQP4-IgG-ON was more likely to have posterior chiasmal involvement. Similar to prior studies, our data support an older age and female predominance in AQP4-IgG-ON.3-5, 9, 10
Limitations of our study include the retrospective design and referral bias of the contributing institutions as tertiary centers, which could lead to increased cases of more severe ON. The geographic locations of the involved centers could also bias ethnicity proportions. Future studies of these differences, preferably of a prospective design that includes a blinded review of imaging, and increased demographic diversity would increase the generalizability of the results
In summary, chiasmal involvement of ON in AQP4-IgG-positive NMOSD and MOGAD occur at more similar frequencies than previously reported with this occurring in 20% and 16% respectively. In addition, MOG-IgG-ON chiasmal involvement is more likely as a longitudinally extensive optic nerve lesion; whereasAQP4-IgG-ON is more likely a posterior chiasmal lesion. Our findings emphasize the need for appropriate diagnostic testing when the optic chiasm is affected in ON, which can be stratified further based on patient demographics and pattern of involvement.
Acknowledgments
Stanford Funding: NEI P30 026877: An unrestricted grant from Research to Prevent Blindness to the Stanford Department of Ophthalmology
Footnotes
Disclosures:
D. Tajfirouz reports no disclosures.
T. Padungkiatsagul reports no disclosures.
S. Beres reports no disclosures.
H.E. Moss reports an unrestricted grant for research to prevent blindness - P30 026877.
S. Pittock reports grants, personal fees and non-financial support from Alexion Pharmaceuticals, Inc.; grants from Grifols, Autoimmune Encephalitis Alliance; grants, personal fees, non-financial support and other from MedImmune, Inc.; Dr. Pittock has a patent # 9,891,219 (Application#12-573942) “Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive”.
E. Flanagan is a site principal investigator in a randomized placebo-controlled clinical trial of Inebilizumab (A CD19 inhibitor) in neuromyelitis optica spectrum disorders funded by MedImmune/Viela Bio.
A. Kunchok has previously received research support from Biogen.
S. Shah reports no disclosures.
M. T. Bhatti reports no disclosures.
J.J. Chen reports no disclosures.
REFERENCES
- 1.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018; 15: 134. 2018/05/05. DOI: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobo-Calvo A, Ruiz A, Maillart E, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology 2018; 90: e1858–e1869. 2018/04/27. DOI: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 3.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–3138. 2017/11/15. DOI: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol 2018; 195: 8–15. 2018/07/29. DOI: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajfirouz DA, Bhatti MT and Chen JJ. Clinical Characteristics and Treatment of MOG-IgG-Associated Optic Neuritis. Curr Neurol Neurosci Rep 2019; 19: 100. 2019/11/28. DOI: 10.1007/s11910-019-1014-z. [DOI] [PubMed] [Google Scholar]
- 6.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry 2016; 87: 446–448. 2015/03/10. DOI: 10.1136/jnnp-2014-310206. [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler 2016; 22: 470–482. 2015/07/15. DOI: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 8.Dutra BG, Jose da Rocha A, Nunes RH, et al. Neuromyelitis Optica Spectrum Disorders: Spectrum of MR Imaging Findings and Their Differential Diagnosis-Erratum. Radiographics 2018; 38: 662. 2018/03/13. DOI: 10.1148/rg.2018184002. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Tan S, Chan TCY, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol 2018; 102: 1372–1377. 2018/01/25. DOI: 10.1136/bjophthalmol-2017-311177. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa H, Kezuka T, Shikishima K, et al. Epidemiologic and Clinical Characteristics of Optic Neuritis in Japan. Ophthalmology 2019. 2019/06/15. DOI: 10.1016/j.ophtha.2019.04.042. [DOI] [PubMed] [Google Scholar]