Abstract
Objectives:
The objective of this study was to identify novel serum biomarkers specific to postoperative delirium following major cardiac surgery to provide insight into the pathological processes involved in delirium and its sequelae.
Design:
Nested, case-control study.
Setting:
Cardiac surgical intensive care unit in a single-site hospital setting.
Participants:
Twenty-four older adults (>60 years old) undergoing major cardiac surgery with cardiopulmonary bypass.
Interventions:
None.
Measurements and Main Results:
The primary outcome was a positive screen for delirium from postoperative day 1 through 3 based on criteria included in the long form of the Confusion Assessment Method. We applied a multiplexed proteomic approach using proximity extension assays to identify and quantify proteins found in serum collected on the day of surgery and postoperative day 1 in delirious and non-delirious patient cohorts. We identified an increase in serum fibroblast growth factor (FGF)-21 levels in the delirious cohort from a pre-surgery baseline of (mean ±stdev) 5.0 (±1.1) log2 abundance (95% confidence interval (CI) [4.3 to 5.7]) to 6.7 (±1.6) log2 abundance (95% CI [5.7 to 7.7], p=0.01) post-surgery. We identified a similar increase in FGF-23 from a pre-surgery baseline of 1.7 (±1.3) log2 abundance (95% CI [0.8 to 2.5]) to 3.4 (±2.2) log2 abundance (95% CI [2.0 to 4.8]), p=0.06) post-surgery. We also identified an increase in interleukin-6 serum levels in the delirious cohort from a pre-surgery baseline of 3.8 (±1.1) log2 abundance (95% CI [3.1 to 4.5]) to 8.7 (±1.9) log2 abundance (95% CI [7.5 to 9.9], p<0.0001) post-surgery. However, the increase in interleukin-6 serum levels of the non-delirious cohort also met our threshold for statistical significance (p<0.0001). Finally, we identified an increase in monocyte chemotactic protein-3 serum levels in the delirious cohort from a pre-surgery baseline of 4.1 (±0.9) log2 abundance (95% CI [3.6 to 4.7]) to 6.1 (±2.0) log2 abundance (95% CI [4.8 to 7.4], p=0.009) post-surgery.
Conclusion:
FGF-21, FGF-23, interleukin-6, and monocyte chemotactic protein-3 serum levels were increased postoperatively in patients that developed delirium following major cardiac surgery. This study identifies two members of the FGF family as potential putative systemic biomarkers for postoperative delirium after cardiac surgery, suggesting a possible role for metabolic recovery in the pathophysiological mechanisms underlying neurocognitive dysfunction.
Keywords: Delirium, FGF, neurodegeneration, cognition, metabolism, cardiac surgery, cardiopulmonary bypass, inflammation
Introduction
Delirium is an acute brain dysfunction characterized by disturbances in attention, awareness, and cognition not explained by a preexisting neurocognitive disorder.1 Delirium disproportionally affects older individuals (>65 years) and is associated with increased mortality, prolonged hospitalization, and long-term cognitive deficits. In the United States alone, an estimated $39.2 billion dollars (95% confidence interval (CI) [$25.7 billion-$42.2 billion]) are spent on healthcare costs associated with postoperative delirium.2 Older patients account for nearly double the number of hospitalization stays compared to younger individuals (45–64 years)3 and are at increased risk for developing delirium while in the hospital.4
Metabolic disturbance, organ failure, sleep disturbance, hyperthermia, and central nervous system insults are a few of the etiological factors that may contribute to developing delirium.5, 6 Delirium commonly occurs after critical illness, surgery, and invasive diagnostic procedures and is associated with increased mortality, prolonged hospitalization, long-term cognitive deficits, and higher healthcare costs.7, 8 In terms of mechanism, systemic activation of the inflammatory cascade involving interleukin-6 (IL-6) and C-reactive protein have been associated with delirium in humans.9, 10 While blood-brain barrier breakdown and microglial activation appear to be involved in neurocognitive dysfunction,11, 12 the pathophysiological mechanisms underlying delirium in the context of the aging brain and the response to the physiological and biological changes associated with surgery and invasive diagnostic procedures remain unclear. Thus, our ability to modify the public health burden of postoperative delirium is presently limited by the lack of objective biomarkers that can be related to delirium onset or its sequelae. The discovery of systemic biomarkers of delirium may provide a defined biochemical signature associated with delirium progression, resolution, and/or response to treatment (e.g., prognostic biomarkers) or as factors correlated with delirium onset (e.g., ‘disease’ biomarkers). We hypothesize that development of delirium may be associated with fluctuations in systemic factors that can be detected in serum and relate to the underlying pathophysiology involved in neurocognitive dysfunction. The identification of prognostic or predictive biomarkers of delirium may aid in identifying patients that are at high-risk for developing postoperative delirium, independent of age, as a means to personalize postoperative care and develop therapeutic interventions to improve patient-centered outcomes.
Here, we performed a nested case-control study of 24 patients to identify proteins found in serum that might provide insights into the underlying pathological processes involved in delirium and potentially serve as prognostic or diagnostic biomarkers.
Methods
Study Design
This nested, case-control study involves an unbiased proteomic screen of serum from age-and sex-matched non-delirious control and delirious patients, as an approved substudy of the Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS) trial.13 All recruited patients provided written and informed consent prior to enrollment in the MINDDS study in accordance with institutional and federal guidelines (Institutional Review Board Protocol #: 20168000742). A sample size of n=24 patients (n=12 non-delirious control patients and n=12 delirious patients) was selected to determine biochemical differences in serum protein levels. Study details for the MINDDS trial have been previously described.13 As the MINDDS trial is ongoing, the researchers remained blinded to treatment with placebo or the adrenergic sedative, dexmedetomidine. Seven of the 12 control patients and 7 of the 12 delirious patients did not receive either placebo or dexmedetomidine. Briefly, inclusion criteria included patients aged ≥60 years old who were undergoing cardiac surgery with an expected same-day admission to the CSICU for >24 hours for sample collection. Exclusion criteria was applied to patients with greater than two days in the ICU during the month prior to surgery, renal or liver failure, and severe neurocognitive damage. All patients underwent cognitive and physical function assessments before and after surgery. The 3D Cognitive Assessment Method (3D CAM) and Telephone-Montreal Cognitive Assessment (T-MoCA) were performed preoperatively to establish baseline cognitive scores. The Patient-Reported Outcomes Measurement Information System (PROMIS) clinical outcome assessments were performed preoperatively to define baseline physical and mental functional scores (PROMIS v.1.1 Global Health Physical, PROMIS v.1.1 Global Health Mental, PROMIS v1.2 Physical Function 8b, PROMIS v1.0 Applied Cognition-Abilities-Short Form 8a, PROMIS Sleep Disturbance-Short Form 4a, and PROMIS v1.0 Pain Interference-Short Form 8a).14–18 Cognitive assessments were performed twice daily for three days following surgery using the Confusion Assessment Method (CAM) short- and long-forms. The T-MoCA and PROMIS questionnaires were performed preoperatively and at postoperative days 30, 90, and 180. Details regarding patient-centered clinical outcomes, including delirium onset, delirium severity, mortality, and hospital length of stay were recorded.
Serum Isolation and Processing
Blood samples were collected before surgery and the morning of postoperative day one in a glass serum separator tube (BD Vacutainer tube, BD Life Sciences, Franklin Lakes, NJ) and allowed to clot for 30 minutes, followed by centrifugation at 3000 rpm at 22°C for 30 minutes. The supernatant was then collected, aliquoted, and stored at −20°C for at least 24 hours, followed by transfer to −80°C until further processing.
Protein Analysis
Serum samples were analyzed using proximity extension assays (Olink Inflammation Panel, v.3004 and Olink Neuro Exploratory Panel (v.3901)) that utilize targeted antibodies coupled to oligonucleotide sequences for amplification and quantification by real-time polymerase chain reaction (Olink Proteomics Inc., Watertown, MA).19, 20 Serum samples (∼40 μL/patient sample) were added to individual wells of a 96-well plate, sealed, and sent on dry ice to the Olink Proteomics Analysis laboratory (Watertown, MA). Internal controls were included within the biochemical analysis to verify specificity and quality control of the incubation, extension, and detection steps. The average intra-assay and inter-assay % coefficient of variation for the proximity extension assay method has been reported as 8% and 12%, respectively.19 Biological replicates were compared for significant differences (>2-linear fold increase and p<0.05) between control and delirium cohorts. Graphs of the proteins detected in serum samples from each cohort and individual patient responses from preoperative to postoperative states are provided within the Supplemental Materials (Appendix A and B).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism (version 8.4.3) (GraphPad Software, San Diego, CA). Our primary hypothesis to determine significant biomarker regulation in the delirium cohort compared to the control group was assessed with an ordinary two-way ANOVA and a Šídák’s multiple comparisons test for each protein detected. Each biomarker was first analyzed using a univariate analysis. A multivariable analysis of each protein considering the timepoint and cohort (control versus delirium) was performed. A Kolmogorov-Smirnov normality test was used to determine if the data were normally distributed. Secondary comparisons of clinical features were assessed with a Mann-Whitney U test. Pearson correlation coefficients (r) were calculated using multiple variables, e.g., age, BMI, CPB time, FGF-21pre-op, FGF-21post-op, IL-6pre-op, and IL-6post-op with significance determined using a two-tailed p-value. Multiple logistic regression models were applied to identify biomarker selectivity, specificity, and predictive power using bivariate analyses. The odds ratio was calculated for delirium based on FGF-21post-op with a Wald confidence interval reported. A p<0.05 was considered statistically significant.
Results
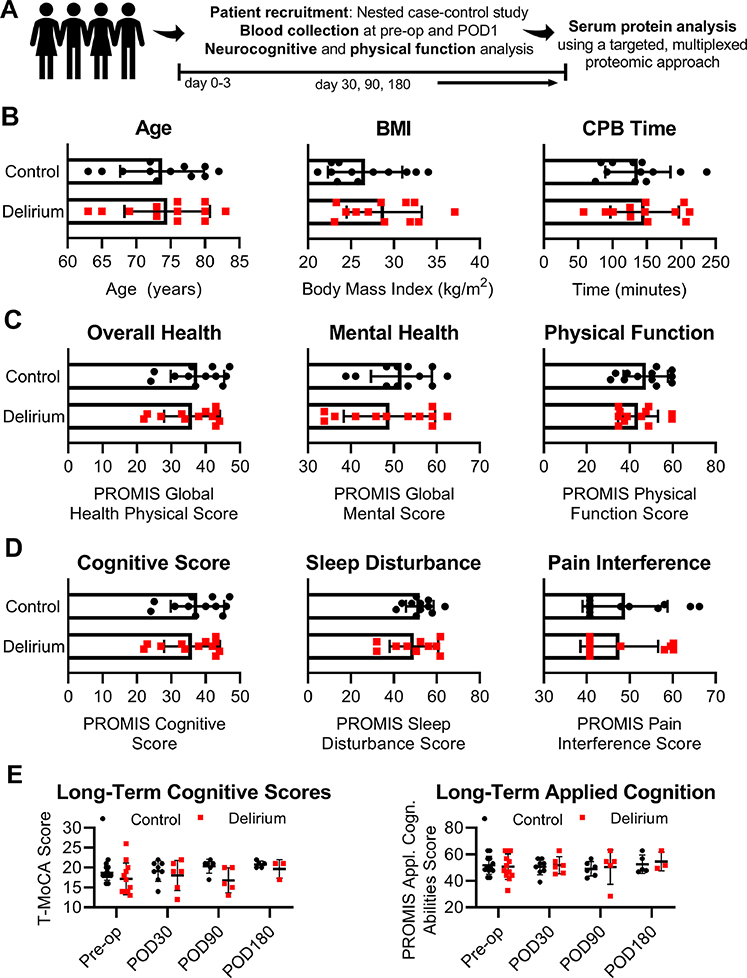
Control and delirium cohorts were defined by matching patients by age- and sex- with no significant differences in body mass index, cardiopulmonary bypass time, or overall cognitive or physical health detected between groups at the preoperative evaluation, thereby excluding underlying dementia or other late-stage neurodegenerative diseases (Fig. 1).
Figure 1.
Clinical characteristics and physical and cognitive assessments of patients enrolled in a substudy to evaluate serum biomarkers detected at pre- and postoperative states in control and delirium cohorts. (A) Study design and data collection; (B) Age, body mass index (BMI), and total cardiopulmonary bypass (CPB) time; (C) Patient-Reported Outcomes Measurement Information System (PROMIS) scores of the patients’ overall physical health, and mental and physical function reported at pre-op; (D) PROMIS cognitive score, sleep disturbance, and pain interference scores reported at pre-op; and (E) temporal evaluation of overall cognitive abilities and applied cognition from pre- to postoperative day (POD) 30, 90, and 180. Bars show mean ± standard deviation. Statistical significance was evaluated using a Mann-Whitney U test.
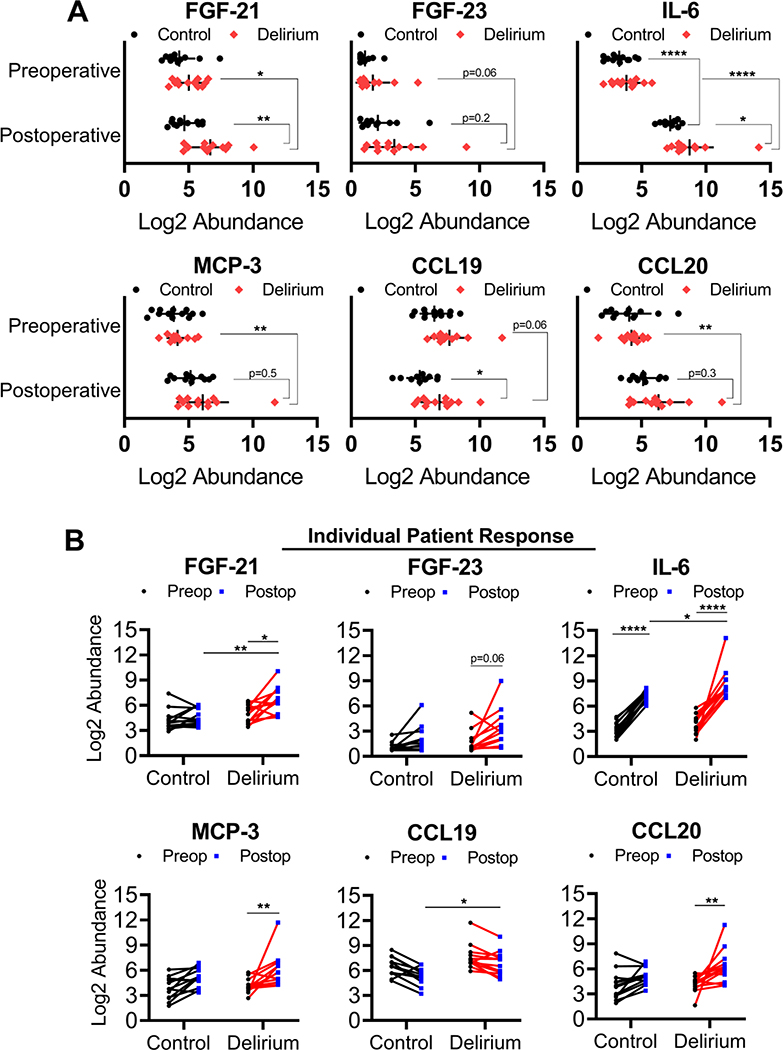
We applied a sensitive, multiplexed proteomics-based approach using proximity extension assays to identify the relative abundance of select proteins found in serum samples. We detected a specific and significant increase in a pleiotropic hormone and metabolic regulator, known as fibroblast growth factor (FGF)-21, in serum from patients that developed delirium following surgery compared to patients that did not develop delirium (mean postoperative values = 6.7 (±1.6) log2 abundance, 95% CI [5.7 to 7.7] and 4.6 (±1.0) log 2 abundance, 95% CI [4.0 to 5.3] for delirious and control cohorts, respectively, p=0.002) (Fig. 2A). FGF-23 was elevated postoperatively in the delirious cohort (3.4 (±2.2), 95% CI [2.0 to 4.8]), though not statistically significant, compared to the control group (2.1 (±1.5), 95% CI [1.1 to 3.0], p=0.2). In agreement with previous reports, we also detected a significant increase in the pro-inflammatory factor, IL-6, in the delirium cohort following surgery (8.7 (±1.9) log2 abundance, 95% CI [7.5 to 9.9], p=0.02]) compared to non-delirious control patients (7.2 (±0.6) log2 abundance, 95% CI [6.8 to 7.6]). Monocyte chemotactic protein-3 (MCP-3) and chemokine (C-C motif) ligand (CCL) 20 levels at the postoperative state were not significantly different between delirious patients (6.1 (±2.0) log2 abundance, 95% CI [4.8 to 7.4] and 6.3 (±2.0) log2 abundance, 95% CI [5.0 to 7.6], respectively) and non-delirious patients (5.1 (±1.2) log2 abundance, 95% CI [4.4 to 5.9], p=0.5; and 5.1 (±1.0) log2 abundance, 95% CI [4.5 to 5.8], p=0.3, respectively). CCL19 decreased with surgery in both cohorts with a significant difference detected between delirious and control patients at the postoperative state (mean postoperative values = 6.9 (±1.5) log2 abundance, 95% CI [5.9 to 7.8] and 5.3 (±1.0) log2 abundance, 95% CI [4.7 to 6.0] for delirious and control cohorts, respectively, p=0.04).
Figure 2.
Select proteins detected in serum from patients with delirium compared to age- and sex-matched control patients. (A) FGF-21, FGF-23, IL-6, MCP-3, CCL19, and CCL20 expression in control and delirium cohorts at preoperative day 0 and postoperative day (POD) 1; (B) The individual patient response of FGF-21, FGF-23, IL-6, MCP-3, CCL19, and CCL20 from preoperative day 0 to POD1. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 based on an ordinary two-way ANOVA with Šídák’s multiple comparisons test.
In terms of individual patient responses from preoperative to postoperative states, FGF-21 was increased at least 2-linear fold in 8 out of 12 patients (67%) that screened positive for delirium and 3 out of 12 patients (25%) that screened negative for delirium (mean preoperative values = 5.0 (±1.1) log2 abundance, 95% CI [4.3 to 5.7] and 4.3 (±1.3) log2 abundance, 95% CI [3.5 to 5] for delirious and control cohorts, respectively) (Fig. 2B). Similarly, FGF-23 was increased at least 2-linear fold following surgery in 8 out 12 delirious patients (67%) and 4 out of 12 patients (33%) that screened negative for delirium (mean preoperative values = 1.7 (±1.3) log2 abundance, 95% CI [0.8 to 2.5] and 1.1 (±0.6) log2 abundance, 95% CI [0.7 to 1.4] for delirious and control cohorts, respectively) (Fig. 2B). IL-6 was increased from preoperative levels in every patient following surgery regardless of the development of delirium (mean preoperative values = 3.8 (±1.1) log2 abundance, 95% CI [3.1 to 4.5] and 3.3 (±0.9) log2 abundance, 95% CI [2.7 to 3.8] for delirious and control cohorts, respectively) (Fig. 2B). MCP-3 was increased at least 2-linear fold following surgery in 7 out of 12 delirious patients (58%) and 8 out of 12 non-delirious patients (67%) (mean preoperative values = 4.1 (±0.9) log2 abundance, 95% CI [3.6 to 4.7] and 3.8 (±1.3), 95% CI [3.0 to 4.7] for delirious and control cohorts, respectively). Though overall postoperative CCL19 levels were higher in the delirious cohort, most patients (11 of the 12 delirious patients (92%) and 11 of the 12 non-delirious patients (92%)) showed a reduction in CCL19 serum levels following surgery ranging from 1.03–4.1 and 1.3–4.8 linear fold-change in the delirious and control cohorts, respectively (mean preoperative values = 7.6 (±1.5) log2 abundance, 95% CI [6.7 to 8.6] and 6.5 (±1.2) log2 abundance, 95% CI [5.7 to 7.2] for delirious and control cohorts, respectively). Showing a similar pattern to MCP-3, CCL20 increased at least 2-linear fold postoperatively in most patients (8 out of 12 delirious patients (67%) and 7 out of 12 non-delirious patients (58%) from preoperative serum levels (mean preoperative value = 4.2 (±1.0) log2 abundance, 95% CI [3.6 to 4.9] and 4.0 (±1.7) log2 abundance, 95% CI [2.9 to 5.1] for delirious and control cohorts, respectively).
The positive and negative predictive power of FGF-21 for delirium was 73% and 69%, respectively, with a sensitivity and specificity for delirium of 80% and 75%, respectively, based on multiple logistic regression analyses (odds ratio 3.7, 95% CI [1.3 to 10.2]). Delirium severity scores in the delirium cohort were higher on postoperative day one, highly variable through postoperative day three, and correlated with elevated average FGF-21 levels on postoperative day one compared to non-delirious control patients (Supplemental Fig. 2). Significant Pearson’s correlation (r) were identified for postoperative FGF-21 and IL-6 serum levels (r=0.53, p=0.007) with comparable positive correlations for the presence of delirium (r=0.6, p=0.004 and r=0.49, p=0.014, respectively) (Supplemental Fig. 3).
Discussion
In this study, we utilized a multiplexed proteomic approach to screen for >150 proteins in serum isolated from a sub-population of patients (n=24) enrolled in the MINDDS trial.13 We found that after cardiac surgery with cardiopulmonary bypass, IL-6 serum levels increased in all patients and were 2.8-linear fold higher on average in patients with delirium. Interestingly, postoperative serum FGF-21 levels were higher by an average of 4.3-linear fold in patients that developed delirium compared to non-delirious control patients. To-date, studies have focused heavily on the role of pro-inflammatory pathways and sensitization of the blood-brain barrier in the pathobiology of delirium,9, 21 however, the role of systemic metabolism in the development of postoperative delirium remains unclear.
FGF-21 is a potent endocrine factor secreted by the liver known to influence circadian behavior, insulin sensitivity, and physical activity in animal models.22, 23 Initially identified for its importance in metabolic regulation and stimulation of glucose and fatty acid uptake, FGF-21 and related mimetics have been studied in animal and human clinical trials as a potential treatment for Type II diabetes mellitus, obesity and metabolic disorders.24–27 Beneficial neuroprotective properties of FGF-21 in insulin-resistant mouse models have also been described.28, 29 FGF-21 has also been reported as a strong biomarker of mitochondrial dysfunction in human populations,30 which is predictive of increased mortality during sepsis31 and a hallmark of aging.32
In terms of downstream signaling, both FGF-21 and FGF-23 share high-affinity binding interactions with the klotho family of receptors (βKlotho and αKlotho for FGF-21 and FGF-23, respectively), as obligate co-receptors to the FGF receptor (FGFR1-R4).33, 34 FGF-23 is a bone-derived hormone involved in phosphate reabsorption and Vitamin D metabolism.35 Elevated circulating FGF-23 levels have been associated with increased incident dementia in a community-based cohort36 and cognitive impairment in a cohort of hemodialysis patients,37 though associations between FGF-23 levels and baseline cognitive function or development of cognitive impairment over time in well-functioning older adults remain disputed.38 Of note, the increase in FGF-21 (and FGF-23) in patients that developed postoperative delirium in our study occurred at postoperative day 1, with no significant difference detected between cohorts preoperatively suggesting an acute response to surgery, rather than constitutive differences in kidney or liver function at baseline. However, the presence of numerous confounding factors in the development of postoperative delirium and the dynamic changes in circulating protein levels must be considered. Further studies evaluating changes in protein biomarkers associated with postoperative delirium in a larger cohort will require stratification based on the presence of a metabolic disorder, kidney and liver function, and Vitamin D levels, among others.
Given the importance of metabolic stores and energy switching in the brain, we posit that systemic FGF-21 may be an early signal of cerebral metabolic insufficiency and inflammation, two putative processes involved in developing delirium.5 The increase in serum FGF-21 in postoperative delirious patients may also be presenting as a compensatory mechanism to counteract a systemic or localized metabolic failure, in a similar manner to that found in Type II diabetic patients with elevated basal levels of circulating FGF-21.39, 40 As delirium is a clinical diagnosis with no current biochemical approach for standardized detection, the detection of elevated FGF-21 in delirious patients following major cardiac surgery is a promising finding in the search for objective delirium biomarkers.
Limitations of this study include the small sample size (n=24) and the dependency of statistical significance on individual samples in evaluating associations between increased IL-6, MCP-3, CCL19 and CCL20 with delirium. We also detected a modest sensitivity and specificity of FGF-21 for delirium (80% and 75%, respectively). While we performed cognitive evaluations up to 180 days following surgery, the progressive reduction in clinical data collected with time (e.g., as patients were lost to follow-up, n=3 in the delirious cohort by postoperative day 180) leads to difficulty in determining any differences in control and delirium cohorts in regard to long-term effects of an episode of postoperative delirium on permanent cognitive dysfunction.
Our findings show an increase in FGF-21 in a small cohort of patients with delirium, which may represent an initial signal of mitochondrial dysfunction and oxidative stress, both prominent features also associated with chronic neurodegenerative diseases.41, 42 Thus, principled insights into the regulation of FGF-21, and potentially FGF-23, as biomarkers of acute neurocognitive dysfunction may be highly relevant to developing therapeutics to prevent long-term neurocognitive decline that may follow an episode of delirium. Further studies evaluating if FGF-21 may be prognostic for delirium severity and whether its upregulation precedes or follows development of postoperative delirium are warranted. The long-term goals of this work will investigate whether mitochondrial dysfunction and metabolic stores (two factors that regulate FGF-21 expression) influence the predisposition of elderly patients to delirium and whether therapies that induce mitochondrial preconditioning prior to surgical stress can improve postoperative neurocognitive function and recovery.
Supplementary Material
Acknowledgements
National Institutes of Health and National Institute on Aging (R01AG053582) (OA) and research funds from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (OA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.American Psychiatric Association. DSM-5 Task Force: Diagnostic and statistical manual of mental disorders: DSM-5. (ed 5th). Washington, D.C., American Psychiatric Association, 2013. [Google Scholar]
- 2.Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surg 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACL: 2018 Profile of Older Americans, US Department of Health and Human Services, Washington, DC, 2017, pp. 13. [Google Scholar]
- 4.Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–1165. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33:1428–1457. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi A, Ahern TL, Henderson SO. Excited delirium. West J Emerg Med 2011;12:77–83. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997;45:174–178. [DOI] [PubMed] [Google Scholar]
- 8.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA 1990;263:1097–1101. [PubMed] [Google Scholar]
- 9.Vasunilashorn SM, Ngo LH, Chan NY, et al. Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J Gerontol A Biol Sci Med Sci 2019;74:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeilJ B, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One 2019;14:e0226412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nation DA, Sweeney MD, Montagne A, et al. Blood—brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019;25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019;10:5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelton KT, Qu J, Bilotta F, et al. Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018;8:e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose M, Bjorner JB, Becker J, et al. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol 2008;61:17–33. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33:781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg M, Eriksson A, Tran B, et al. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res 2011;39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasunilashorn SM, Dillon ST, Inouye SK, et al. High C-Reactive Protein Predicts Delirium Incidence, Duration, and Feature Severity After Major Noncardiac Surgery. J Am Geriatr Soc 2017;65:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill CM, Laeger T, Dehner M, et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep 2019;27:2934–2947.e2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bookout AL, de Groot MH, Owen BM, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 2013;19:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng L, LamK SL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol 2020;16:654–667. [DOI] [PubMed] [Google Scholar]
- 26.Schlein C, Talukdar S, Heine M, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab 2016;23:441–453. [DOI] [PubMed] [Google Scholar]
- 27.Gaich G, Chien JY, Fu H, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18:333–340. [DOI] [PubMed] [Google Scholar]
- 28.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed Pharmacother 2018;97:1663–1672. [DOI] [PubMed] [Google Scholar]
- 29.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav 2016;85:86–95. [DOI] [PubMed] [Google Scholar]
- 30.Suomalainen A, Elo JM, Pietiläinen KH, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol 2011;10:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360:219–223. [DOI] [PubMed] [Google Scholar]
- 32.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kharitonenkov A, Dunbar JD, Bina HA, et al. FGF-21/FGF-21 receptor interaction and activation is determined by βKlotho. Journal of Cellular Physiology 2008;215:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006;281:6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Journal of bone and mineral research 2004;19:429–435. [DOI] [PubMed] [Google Scholar]
- 36.McGrath ER, Himali JJ, Levy D, et al. Circulating fibroblast growth factor 23 levels and incident dementia: The Framingham heart study. PLoS One 2019;14:e0213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drew DA, Tighiouart H, Scott TM, et al. FGF-23 and cognitive performance in hemodialysis patients. Hemodialysis International 2014;18:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew DA, Katz R, Kritchevsky S, et al. Fibroblast growth factor 23 and cognitive impairment: The health, aging, and body composition study. Plos one 2020;15:e0243872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng X, Zhu B, Jiang F, et al. Serum FGF-21 Levels in Type 2 Diabetic Patients. Endocr Res 2011;36:142–148. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Cheung BMY, Tso AWK, et al. High Plasma Level of Fibroblast Growth Factor 21 Is an Independent Predictor of Type 2 Diabetes. Diabetes Care 2011;34:2113–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 2001;21:3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol 1994;36:747–751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.