Abstract
The uveal tract consists of the iris, the ciliary body and the choroid; these three distinct tissues form a continuous layer within the eye. Uveitis refers to inflammation of any region of the uveal tract. Despite being grouped together anatomically, the iris, ciliary body and choroid are distinct functionally, and inflammatory diseases may affect only one part and not the others. Cellular structure of tissues direct their function, and understanding the cellular basis of the immune environment of a tissue in health, the “steady state” on which the perturbations of disease are superimposed, is vital to understanding the pathogenesis of those diseases. A contemporary understanding of the immune system accepts that haematopoietic and yolk sac derived leukocytes, though vital, are not the only players of importance. An array of stromal cells, connective tissue cells such as fibroblasts and endothelial cells, may also have a role in the inflammatory reaction seen in several immune-mediated diseases. In this review we summarise what is known about the cellular composition of the uveal tract and the roles these disparate cell types have to play in immune homeostasis. We also discuss some unanswered questions surrounding the constituents of the resident leukocyte population of the different uveal tissues, and we look ahead to the new understanding that modern investigative techniques such as single cell transcriptomics, multi-omic data integration and highly-multiplexed imaging techniques may bring to the study of the uvea and uveitis, as they already have to other immune mediated inflammatory diseases.
Keywords: uvea, uveitis, iris, ciliary body, choroid
Introduction
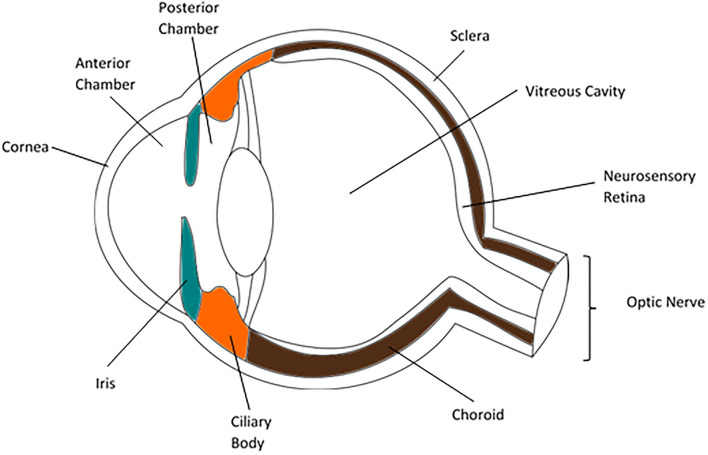
The eye has several functions that make it unique amongst organs. It is required to be optically clear along the visual axis to allow light to pass through with minimal interruption, to focus light reflected or produced from sources at variable distances, and to transduce information carried by light into neural impulses to be transmitted to the brain for integration and spatial interpretation. The anatomy of the globe reflects these functions. The cornea and crystalline lens refract light through the transparent aqueous and vitreous and onto the neurosensory retina, where it is detected by photopigments and neural impulses are generated. The uveal tract is the middle vascular and pigmented layer of the eye and is comprised of the iris, ciliary body and choroid. These structures have a range of functions; forming the aperture through which light is focused, producing the internal fluids of the eye, absorbing unwanted light from outside or reflected within the eye and providing arterial supply and venous drainage to other layers of the globe (Figure 1). Anatomical lymphatic vessels have not been convincingly described in the healthy uvea in most animals studied, though they are found in the conjunctiva (1).
Figure 1.
Diagram of a cross section of an eye, highlighting the iris (blue), ciliary body (orange) and choroid (brown).
The uvea is the focus for a group of diseases falling under the umbrella term of uveitis. Inflammation within the eye may profoundly interrupt its key functions, causing impairment of vision alongside other well-documented symptoms of inflammation. Clinical classification of uveitis is anatomical, describing the area of the eye affected. Anterior uveitis is confined to the anterior chamber, intermediate uveitis mainly affects the vitreous and posterior uveitis the choroid or retina. Panuveitis describes a case affecting each of these areas (2). Clinically distinct conditions cause uveitis at different sites, and inflammation may extend into adjacent tissues.
The immunological environment of the eye is endowed with highly tuned regulatory immunological properties and thus recognised as an immune privileged site. By this, it is meant that circumstances that would usually lead to marked inflammatory reactions at other body sites do not produce such damaging effects, which allows the core visual function of the eye to continue (3–5). Despite ocular immune privilege however, uveitis is not uncommon and is a major cause of blindness worldwide (6). Historically inflammation has been thought of as a process driven mainly by leukocytes, but it is now acknowledged that the stroma of an affected organ also has a vital role to play in inflammatory conditions. Stroma is an umbrella term for tissue types traditionally seen as fulfilling a structural role for organs, distinct from the parenchyma that provides a specific function unique to that organ. Far from being inactive bystanders however, stromal cells are often active players in inflammatory conditions, and may take on distinct phenotypes in inflamed tissues (7, 8).
The uveo-centric nature of many ocular inflammatory diseases makes an understanding of the basic cellular constituents of the uveal immune system fundamental to a wider understanding of ocular inflammation and infection. This review discusses what is known about the immune environment of the three anatomical structures that make up the uveal tract, both in terms of leukocytes, classical immune cells, and the wider stromal cellular population. We will focus predominantly on the healthy eye, the resting state over which inflammatory states are superimposed in disease.
Iris
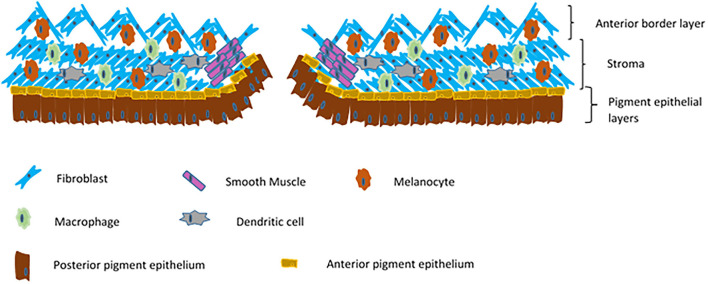
The iris is a contractile diaphragm of tissue with a central aperture, the pupil. It separates the anterior from posterior chambers of the eye and is continuous peripherally with the ciliary body and trabecular meshwork. From anterior to posterior its constituents are the anterior border layer, the stroma, the anterior iris myoepithelium which makes up the dilator pupillae muscle, and the posterior pigment epithelium. The anterior border layer is discontinuous and made up of fibroblasts and melanocytes along with collagen fibrils. Defects in this layer allow contact between the aqueous and the iris stroma, which also consists mainly of collagens, fibroblasts and melanocytes. Within the iris stroma sits the sphincter pupillae muscle, the pupil constrictor. This smooth muscle structure lies close to the pupillary margin. The cells of the anterior pigment myoepithelium are arranged with their apices pointing posteriorly, the anterior basal portions of the cells have contractile elements producing the dilator pupillae muscle. The apices of these cells are pigmented and abut the apices of the darkly pigmented posterior pigment epithelium (Figure 2).
Figure 2.
Schematic of a cross section of an iris.
The leukocyte population of the healthy iris is, to our current knowledge, largely composed of cells of the myeloid lineage. However, much of the work that has been undertaken to identify the immune cell population of healthy eye tissue has been performed in rodents; the eyes of Lewis rats have been widely studied due to their common use for the Experimental Autoimmune Uveitis (EAU) model of uveitis (9). Dendritic cells are the prototypical antigen presenting cell, and are frequently seen in sections and whole mounts of Lewis rat iris (10, 11), as well as those of other rat strains (12), as defined by the presence of MHC class II molecules on their surface, dendritiform morphology and lack of staining for macrophage markers. Dendritic cells are similarly seen within the iris of mice and are thought to be functionally immature, lacking the co-stimulatory molecules CD80 and CD86 required to activate a T-cell response (13). It is known that freshly isolated antigen presenting cells from the mouse iris lack the ability to trigger lymphocyte proliferation in vitro (14), though they may gain this ability when they receive maturation signals, as rat iris dendritic cells do when cultured with GM-CSF (15). A study using bone marrow chimeric rats showed that the dendritic cell population of the iris has a residence half-life of 3 days, and replenishment of dendritic cells is a bone marrow dependant process, consistent with other tissues (16).
Some authors have advocated pig eyes as being more similar to human eyes than those of rodents, and the porcine iris also contain numerous dendritic cells, in addition to other myeloid lineage cells (17). Dendritic cells are also widespread in the human iris (18). A subset of dendritic cells in human iris tissue have been shown to express the Toll-Like Receptor (TLR) 4 complex, including MD2 and CD14 which is involved in response to lipopolysaccharide. These have a largely perivascular niche, as well as being more common in the iris root than elsewhere in the iris (19). Dendritic cells are now commonly sub-classified as conventional (or myeloid), or plasmacytoid dendritic cells, and each of these subtypes is specialised in defence against specific pathogen types or danger signals (20, 21). Dendritic cells within the uveal tract as a whole have been found to be myeloid dendritic cells, no plasmacytoid dendritic cells have so far been identified (22).
In Lewis rat iris tissue, resident macrophages are widespread (10, 11) and commonly have a perivascular niche, predominantly along the arterial circulation, as well as a perineural distribution (23). This is in contrast to the dendritic cells also identified in the same sections which were more evenly spaced without apparent preference for perivascular or perineural areas. The morphology of the rat iris macrophages documented by McMenamin et al. was variable. Macrophages within the rat iris have a slower turnover rate than dendritic cells in the same tissue, and resupply is also bone marrow dependant (16) indicating that at least a portion of these cells derive from the monocyte-macrophage lineage rather than being primitive yolk-sac derived tissue resident macrophages (24). Mouse irises have a density of tissue macrophages similar to that of rats (13), though it is acknowledged that there are strain based differences in immune cell numbers within the same species. Recent work with the C57BL6/J mouse strain identified three subtypes of tissue macrophage within the iris, defined by surface markers on flow cytometry as MCH II− (69% of iris macrophages), MHC II + CD11c− (23%), and MHC II + CD11c+ (8%). Lineage tracing using a tamoxifen inducible Cre recombinase to force Green Fluorescent Protein (GFP) production in Cx3cr1+ cells suggested the MCH II− population to be largely long lived self-replenishing tissue macrophages, while the two MCH II+ populations were majority circulating monocyte derived macrophages. These lineage tracing experiments, however, examined single cell suspensions of whole eyes, rather than the iris alone (25). Work from other groups using similar methods has also shown that the murine iris contains both long lived tissue macrophages and short lived, presumed monocyte derived, macrophages (26).
Pig irises, in common with those of rodents, also contain macrophages which are positive for CD163. The healthy human iris stroma has been found to contain a network of tissue macrophages similar to that of rodent irises (23), though heavier pigmentation makes study of human tissues by microscopy more challenging. Further work corroborated these findings, locating macrophages as defined by staining with the PM-2K monoclonal antibody (27), a marker that is believed to label tissue macrophages specifically (28). Healthy porcine irises examined as wholemounts contain no granulocytes (17), a finding which is thought to hold across species (27), though work looking at granulocytes in healthy eye tissue is rare.
Mast cells are also of the myeloid lineage, and are present in tissues throughout the body. Mast cells are rare in the rodent iris (27, 29). Cook and colleagues have reviewed the role of mast cells in ocular disease, including a discussion on the relative distribution of mast cells throughout ocular tissues in different animals, which does vary widely (30). This is a helpful reminder that immunological differences are important to consider when extrapolating data generated from animal experiments to humans. In the human iris, mast cells are present, though they are fewer in number than in other regions of the uvea such as the choroid (31). Human mast cells are classified based on the protease content of the granules present in the cytoplasm which are released on activation; they may contain both tryptase and chymotryptase (TC mast cells) or tryptase alone (T mast cells), and these cell subtypes have distinct relative tissue distributions (32). A third minor population containing chymase only is also described (33). Within the normal human iris stroma TC mast cells are by far the most prevalent and have a scattered distribution, while mast cells of the sphincter pupillae muscle are largely of the tryptase containing subtype. Interestingly T mast cells are also the dominant subtype in the ciliary muscle (31). The relative dearth of mast cells in the iris compared to the choroid has been hypothesised to relate to the lack of capillary fenestrations in the iris vessels (31).
It is widely thought that the uvea as a whole is entirely devoid of lymphocytes, outside of circulating cells in blood vessels (3). However, there are occasional references to lymphocytes in the healthy iris. In Lewis rats T-cells may be seen very rarely in the normal iris (10, 11), within the rat iris the rare T-cells are of the αβ T-cell receptor type, with no γδ T-cells apparent (16). Occasional T-cells have been identified in pig iris wholemounts, though B-cells are noted to be absent (17). Normal human iris biopsies have also been reported to contain occasional T-lymphocytes, but not B-lymphocytes or plasma cells (34). Indeed B-cells are not found in normal uveal tissue in any species (27). Over the last decade interest in, and understanding of, tissue resident lymphocytes has grown markedly (35) and Resident Memory T-cells (TRM) have been located in several tissues (36, 37). Other lymphocyte classes such as Innate Lymphoid Cells (ILCs), and non-classical TCR expressing T-cells, for example γδ T-cells, are commonly tissue resident (36). It is possible then that the rare description of T-lymphocytes within the uvea represent one of these populations, with the caveat that circulating blood contamination must be carefully considered as a possible source.
The vast majority of the iris tissue mass is made up of structural cell types, such as epithelial cells of the anterior and posterior epithelial layers, fibroblasts of the stroma and melanocytes, which are present in considerable numbers in the human iris and throughout the rest of the uveal tract. Traditionally considered to be involved only in protection from the effects of the ultraviolet frequencies of the electromagnetic spectrum, melanocytes are increasingly acknowledged to have more diverse roles (38). Melanocytes in other tissues have been noted to constitutively express numerous TLRs, which have been shown to be functional upon presentation of the appropriate Pathogen Associated Molecular Patterns (PAMP) (39, 40). There is evidence that skin melanocytes respond via TLRs to some pathogens (41) and melanocytes in the iris also respond to Lipopolysaccharide (LPS) stimulation by producing IL-8 and MCP-1 (42). Furthermore, treating cultured uveal melanocytes with IL-1β causes an increase in IL-6 production, partially mediated by the NF-κB transcription factor (43). Additionally, uveal melanocytes including those from the iris, constitutively produce low levels of Matrix Metalloproteinase 8 (MMP8) and increase production under control of TNFα (44). MMP8 has a role in tissue remodelling and inflammation, and is best known as an extracellular protease produced by neutrophils. It is also implicated in several inflammatory disease processes (45). Supportive evidence for a role for melanocytes and other ocular stromal cells in ocular immunity is that non-bone marrow derived cells appear to be capable of driving inflammation in the murine endotoxin induced uveitis model (46). Elsewhere in the body there is data supporting an immune role for melanocytes including the production of inflammatory mediators and cytokines and potentially in phagocytosis and non-professional antigen presentation (47). Some evidence exists for uveal melanocytes functioning as antigen presenting cells in disease states, but not in healthy tissue (48).
There is substantial evidence for iris melanocyte involvement in the ocular immune environment. A second pigmented cell type in the iris is the posterior pigmented epithelium, the most posterior layer of the iris which is continuous with the non-pigmented ciliary epithelium, in turn continuous with the neurosensory retina. The developmental origin of the iris posterior pigment epithelia, the ciliary body non-pigmented epithelium, and the neurosensory retina has long been thought to be from the inner layer of the embryonic optic cup (49), which itself arises from the developing forebrain and is therefore ultimately of neuroectodermal origin. More recent evidence from histological studies of human foetal tissue at different developmental ages suggests that both the anterior and posterior iris epithelium may arise from the outer layer of the optic cup, which curves inward around the rim of the cup during iris development (50). This finding requires corroboration, but it would link both iris epithelial layers with the retinal pigment epithelium (RPE), which also arises from the outer layer of the optic cup. The cells of the posterior pigment epithelium layer of the iris have also been found to have properties suggesting an immune modulatory role. Human iris pigment epithelial cells removed from post-mortem eyes within 16 h produce mRNA for the TLRs 2, 3, 4, and 6, with TLR4 mRNA showing the strongest expression, and translation of these receptors is confirmed by western blot (4). Functionality of these TLRs in aiding the eye's immune response was demonstrated by production of IL-8 and MCP-1 in response to LPS (ligand for TLR4), Pam3CSK4.3HCl (TLR1/2), Poly(I:C) (TLR3) and MALP-2 (TLR2/6). These responses were in turn blocked by TLR inhibition (51). Other components of the LPS receptor complex made up of TLR4, CD14, and MD-2 are also expressed by human iris pigment epithelial cells, which have been shown to produce IL-6 on stimulation with LPS (52). As seen with melanocytes and cells of the RPE, iris pigment epithelial cells have phagocytic capacity (53, 54), though there is no evidence of a functional role for iris epithelium phagocytosis in immunity or inflammation.
The iris pigment epithelial contribution to the ocular immune system extends to a postulated role in ocular immune privilege. Mouse iris pigment epithelial cells can directly inhibit T-cell activation in a cell contact dependant process, measured by T-cell mitogenesis assay (55). This process appears to be at least partially dependant on CD86-CTLA-4 interactions between iris pigment epithelium and lymphocytes (56) and is not dependant on inducing apoptosis (57). In addition to direct actions on lymphocytes, iris pigment epithelium has an inhibitory effect of the ocular immune response in an indirect manner by the induction of T-regulatory lymphocytes (Tregs). CD8+, but not CD4+ T-cells are converted to a Treg phenotype by a contact dependant mechanism by mouse iris pigment epithelium (58). Notably, the TGFβ signalling pathway appears to be involved in Treg induction and Treg mediated suppression of T-cell activation (59–61). Thrombospondin 1 has also been implicated in CD8+ Treg generation by contact with iris pigment epithelium (62). Treg controlled reduction of lymphocyte activation in the mouse eye supresses several arms of the immune response, including T-helper 1 (Th1), Th2 and Th17, but different mechanisms apply to suppression of each arm. It has been shown that PD-L1 binding to PD-1 is involved with Th1 response suppression, but not Th17 response suppression (63). Pigment epithelia from other parts of the eye such as the retinal pigment epithelium possess similar capacity to induce Treg formation (64). Work with human iris pigment epithelial cells in culture has suggested that similar mechanisms are in play, with contact dependant and TGFβ dependant suppression of T-cell proliferation and IFN-γ production (65). Cultured human iris pigment epithelial cells also express PD-L1 and PD-L2, and the PD-1 pathway may be involved in control of immune responses in the human eye, as it is in the mouse (66). Clinical evidence for this comes in the form of drug related uveitis which can be triggered by checkpoint inhibitor therapy used in the treatment of a range of solid and haematological cancers (67, 68). The PD-1 pathway is commonly targeted by checkpoint inhibitors such as pembrolizumab and nivolumab which block PD-1 directly, and atezolizumab, avelumab, and durvalumab which act on PD-L1. These are prominent amongst those checkpoint inhibitors associated with several forms of uveitis, most often anterior uveitis (67, 68).
Lymphatic vessels are vital structures found throughout most of the body, they drain fluid from the interstitium into the systemic venous circulation and bring antigen presenting cells such as dendritic cells to lymph nodes to allow interaction with cells of the adaptive immune system (7). Lymphatic drainage of the conjunctiva of the ocular surface is well-characterised (69), but the internal ocular environment appears to lack lymphatic vessels (70, 71) and this property is thought to contribute to ocular immune privilege. Some iris macrophages in mice do display lymphatic markers such as LYVE-1 (69), a protein that, when expressed with podoplanin, is considered to mark lymphatic endothelial cells. A further population of LYVE-1+, Sca-1+, CD34+ cells were also identified in the murine iris root by the same authors and hypothesised to represent lymphatic progenitors (69). In the human iris numerous LYVE-1, CD68 double positive cells have been described, again thought to be macrophages (71). A fascinating property of bone marrow derived macrophages in the cornea has been described, which may provide the eye with functional lymphatics in times when inflammatory processes have been triggered. CD11b+ bone marrow derived macrophages have been observed to form tube like structures within inflamed mouse corneas, these cells also express LYVE-1, podoplanin and PROX-1 which are well-known markers of lymphatic endothelium (72). Therefore, macrophages seem capable of forming lymphatics in previously alymphatic structures when required. This has not yet been documented in the uvea, but it does represent a mechanism for providing a highly adaptive lymphatic drainage to an organ in times of need.
Notwithstanding the controversy and uncertainty around the lymphatic drainage of the internal compartments of the eye, it is clear that soluble antigens from within the eye do make their way to lymph nodes in the head and neck (73). The drainage of aqueous humour from the eye is also very well-characterised, mainly leaving the eye via the trabecular meshwork and Schlemm's canal, with a minor drainage pathway being described via the uveoscleral outflow tract. The uveoscleral, or unconventional, pathway involves aqueous humour moving directly through the tissue of the ciliary body and the perivascular spaces of the sclera (74), it is an ill-defined route as compared to the trabecular meshwork. Schlemm's canal itself is a unique structure, its endothelial cells express some lymphatic markers as well as those found on blood vascular endothelial cells (75, 76).
Ciliary Body
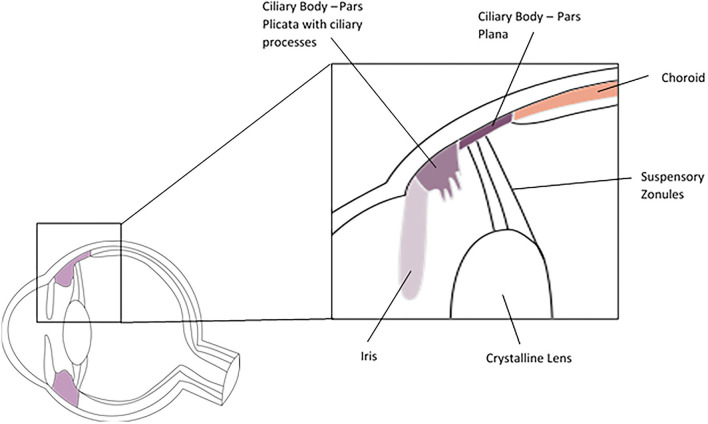
The ciliary body is an annular structure with a triangular cross section. It is continuous with the iris anteriorly and the choroid posteriorly and is divided into the pars plicata anteriorly and pars plana posteriorly (Figure 3). The pars plicata is formed into the ciliary processes, which produce the aqueous humour. The pars plana is posterior to the par plicata and extends to the ora serrata, where the neurosensory retina begins. Histologically the ciliary body epithelium consists of the pigmented and non-pigmented epithelia, which form a bilayer on the inner surface of the ciliary body facing the aqueous and vitreous, the stroma and the ciliary muscle. The inner non-pigmented ciliary epithelium is in direct contact with the aqueous and vitreous, non-pigmented epithelial cells of the pars plicata produces aqueous by active secretion and the cells are held together by tight junctions. The non-pigmented epithelial cells of the pars plana are also secretory and synthesise the vitreal collagens and the suspensory zonules (of Zinn) of the crystalline lens, which are primarily comprised of fibrillin and pass from the pars plana to insert into the lens capsule. The outer pigmented ciliary epithelium is continuous with the retinal pigment epithelium at the ora serrata. The stroma of the ciliary body comprises fibroblasts, melanocytes and occasional leukocytes. Within the stroma sits the ciliary muscle which is a smooth muscle structure allowing accommodation when the eye focuses on near objects. Due to its close anatomical association with the iris, the ciliary body is often considered in conjunction with the iris in studies of its cellular composition (77).
Figure 3.
Cross section of the ciliary body.
The presence of dendritic cells and macrophages is well-documented within the ciliary body, as with the iris. Dendritic cells within the ciliary body of the rat have been noted to sit with the cell bodies between the pigmented and non-pigmented ciliary epithelium (12). Tissue macrophages are less common than dendritic cells in the epithelial layers, but are seen frequently in the underlying lamina propria on which the epithelial layers sit (12) and are also found in large numbers in the ciliary body stroma from where they are seen to follow the course of blood vessels to the ciliary processes (23). Within the ciliary body of the mouse, the stroma contains large numbers of tissue macrophages, and as with the rat dendritic cells these are found in the plane of the epithelial layers. Dendritic cells within healthy mouse uveal tissue appear to lack the necessary co-stimulatory molecules to activate T-lymphocytes, suggesting that further maturation is required prior to antigen presentation (13). Similar to the iris, the murine ciliary body contains both long lived tissue resident macrophages and shorter lived, monocyte replenished macrophages (26). Comparatively little work has been done on the immune cell composition of the human ciliary body, even in comparison to other areas of the uveal tract, but from what data is available it appears that the distribution of macrophages and dendritic cells is similar between rodent and human ciliary bodies (23). A small study found numerous CD163+ macrophages in the stroma of the healthy human ciliary body, while CD68+ were absent (78). This has been used to support the theory that, under homeostatic conditions, the ciliary body microenvironment is “M2” polarised in terms of the M1 vs. M2 macrophage paradigm, although making this determination on the basis of CD68 and CD163 alone has been challenged (79).
Mast cells are similarly rare in the rodent ciliary body and iris (80) but where present are mostly found in the stroma of the ciliary body proper and the ciliary processes (27). In the human ciliary body, the microanatomical distribution of mast cells is quite diverse. The stroma and ciliary muscle has a higher density of mast cells than the iris, and these are split between a TC mast cell dominant population in the stroma and a T mast cell dominant ciliary muscle (31). The ciliary processes have a very low density of mast cells, but those that are present are almost exclusively TC mast cells (31). The healthy ciliary body contains very few, if any, T-cells and no B-cells when examined by immunohistological techniques (14), though these may fail to detect rare cell populations.
The stroma of the ciliary body is similar in cellular composition to that of the iris, and as with studies on the immune cells of the ciliary body much of the work on the ciliary body stroma is done in conjunction with iris tissue. Ciliary body melanocytes have similar properties to those from the iris in terms of constitutive and induced expression of inflammatory cytokines and other mediators (42–44). The stromal immune environment of the ciliary body does, however, have some differences to that of the iris. The ciliary body pigment epithelium, unlike that of the iris, does not seem to have the capacity to induce Treg formation (58). In this regard it also differs from the retinal pigment epithelium, which has also shown Treg induction capacity (81). Ciliary body pigment epithelium does have an immune regulatory role, which while involving cell-cell contact (57) is less dependent on contact than that of the iris pigment epithelium (55) and involves secretion of immune mediators (62) including TGFβ (82). These findings support the hypothesis of an immune skewing “educational gate” element to the blood aqueous barrier (83), which is formed in part by the ciliary epithelium. Another element to this barrier, analogous in many ways to the blood brain barrier, are the tight junctions between the endothelial cells of the iris vasculature.
The non-pigmented ciliary body epithelium is the innermost part of the ciliary body and is in direct contact with the internal eye microenvironment, unlike the iris non-pigmented epithelium. This layer of cells in human eyes has been found to express both TLR4 and CD14 and these are functional in response to LPS (84). Interestingly this same study makes no mention of the staining pattern of iris pigment epithelium when antibodies to TLR4 and CD14 are used, despite other studies demonstrating expression in this tissue, only mentioning staining in the iris stroma. The ciliary body, in common with the rest of the internal tissues of the eye, does not have classical lymphatic vessels, though like the iris the human ciliary body does contain macrophages positive for LYVE-1, a marker also found on lymphatic endothelium (71). This leaves open the possibility for “ducible lymphatics” as have been described in the cornea (72).
Choroid
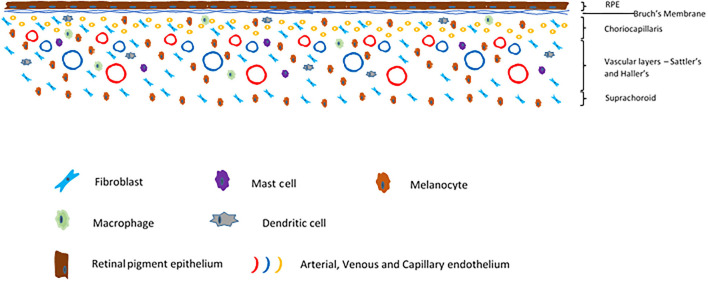
The choroid is a highly vascular, pigmented layer of tissue deep to the sclera and superficial to the neurosensory retina and retinal pigment epithelium. It is continuous anteriorly with the ciliary body. The choroid is made up of five histological layers; the inner Bruch's membrane, the choriocapillaris, the two vascular layers Sattler's and Haller's, and the outer suprachoroid which merges into the inner lamina fusca of the sclera outside it (Figure 4). Developmentally, these arise from the mesenchyme which surrounds the embryonic optic cup. Bruch's membrane is a thin connective tissue layer which itself is subdivided into five layers; the retinal pigment epithelium basement membrane, the inner collagenous layer, the middle elastic zone, the outer collagenous layer and the basement membrane of the choriocapillaris (85). The choriocapillaris is a very dense and highly anastomotic network of capillaries which supply the outer neurosensory retina. Of particular note is that the photoreceptors receive their oxygenation and nutrition from these capillaries. The choroid has among the highest blood flow by weight of any tissue (86, 87). This high blood flow keeps high oxygen saturation even in the venous blood, as even the metabolically demanding photoreceptors do not extract all available oxygen from the arterial supply (22). The capillaries of the choriocapillaris are arranged into lobules, each of which is supplied by an arteriole from Sattler's layer, which lies superficial to the choriocapillaris. Sattler's layer contains numerous arterioles and venules which are supplied from and drain to larger arteries and veins in Haller's layer, which is immediately superficial. The outermost choroidal layer is the suprachoroid which is a stromal layer primarily containing fibroblasts, melanocytes and scattered resident immune cells of the myeloid lineage, as does the stroma of the choroid as a whole. The endothelium of the choriocapillaris is fenestrated (88), in stark contact to the non-fenestrated retinal circulation which exhibit tight junctions between endothelial cells Tight junctions also exist between cells of the RPE. Thus the choroid links the eye with the systemic circulation, while the neurosensory retina has barriers which contribute to its immune privilege, akin to those in the central nervous system.
Figure 4.
Schematic of a cross section of the choroid.
As with the iris and ciliary body, the majority of experimental data on the resident immune cell contingent of the normal choroid comes from animal models, rodent models in particular. Macrophages and dendritic cells are both found in abundance (27). Butler and colleagues utilised whole mounts of Lewis Rat choroid dissected from the retina, deep to it, and sclera, superficially, to examine the resident immune cell population by immunohistochemistry (10). They identified tissue resident macrophages based on immunostaining for CD163, known as a relatively specific marker for tissue resident macrophages (89), and found them to be widespread within the choroid and concentrated around blood vessels. Many of these resident macrophages also stained positive for sialoadhesin (90), a marker known to be present on macrophages within lymphoid organs and identified on resident macrophage populations at other sites such as the liver and lung in the rat (90). In humans, sialoadhesin (CD169) is not expressed on circulating monocytes but is on a wide range of tissue resident macrophages (91) and some activated macrophages in inflamed tissues (91, 92). In contrast to macrophages of the iris and ciliary body, which have been shown to be a mix of long-lived locally self-replenishing and monocyte derived cells, macrophages in the healthy choroid may be almost exclusively monocyte derived according to mouse experimental data (26).
Macrophages in the healthy human choroid are often found in contact with the capillaries in the choriocapillaris (93). Dendritic cells by contrast, identified based on MHC II expression, lack of staining for CD163 and their dendritiform morphology, were present at a greater density but primarily located close to the retinal pigment epithelium (10). The microanatomical localisation of dendritic cells within the choroid gave greater clarity to earlier work on rat tissue which showed their presence in perivascular tissue (94), and other studies have indicated that there are two groups of dendritic cells present which are discernible by differential levels of MHC II expression, perhaps indicating a maturation process within the tissue (95). Wholemounts of pig choroid also revealed numerous tissue resident macrophages, again with a marked perivascular preponderance, as well as dendritic cells (17). The normal human choroid outside Bruch's membrane is also populated by numerous CD68+ macrophages (96), which in the healthy state do not express inducible nitric oxide synthase, a marker of activation (97). Recent single-cell RNA sequencing data has suggested that human choroidal macrophages may be clustered into at least four subtypes based on transcriptome, with a fifth cluster primarily found in patients with neovascular age related macular degeneration (25). Two of the identified clusters with a large contribution from healthy choroidal tissue upregulated genes involved in angiogenesis compared to the other macrophage clusters, suggesting specific functional differences between macrophage subtypes within the choroid.
Mast cells have been identified and studied in the choroid of numerous species (29, 30). That they have been found within the choroid of almost all vertebrates (98) suggests a strong evolutionary drive towards their conservation. Mast cells are more numerous in the choroid than in other parts of the uveal tract in many mammals (99), including humans (31). In the rat, choroidal mast cells are more numerous towards the posterior pole of the eye than at the equator (99). Within the human choroid, the great majority of mast cells possess granules containing both tryptase and chymase and are located in the inner of the two large vascular layers. Bruch's membrane, the choriocapillaris and the suprachoroid are mostly lacking in mast cells (31). There is evidence from studies in rats that mast cells may be involved in the pathogenesis of Experimental Autoimmune Uveitis (EAU) (100), and it is interesting that different strains of rats commonly used for rodent studies show different susceptibilities to EAU which correlate to the density of mast cells found within the choroid of those strains (101).
Within the choroid, similarly to other regions of the uveal tract, it is widely held that there are no resident lymphocytes. On this point, however, experimental evidence is mixed. Some studies have found no evidence of T or B-cells within the choroid of the rat (95) or the pig (17). However, others indicate small numbers of T-cells are found within the normal choroid of Lewis rats, at a reported density of 16 ± 7 cells/mm2, an order of magnitude lower than the density of resident macrophages (10). Other studies corroborate these findings and, unusually, many of the T-cells identified in the choroid of both mice and humans have been found to express the γδ T-cell receptor (102), which is not the case in other parts of the uvea (16). Analysis of single cell RNA sequencing data from combined mouse retinal pigment epithelium and choroid revealed 5 immune cell clusters (103), identified by the authors as macrophages, mast cells, T-cells and two distinct dendritic cell clusters. Single cell RNA sequencing has also been performed on combined RPE-choroid of eyes from human deceased donors, though some had ocular pathology that could impact the leukocyte population present. Interestingly, in this series leukocytes clustered into B-cells, T/NK-cells, macrophages and mast cells. No mention is made of dendritic cells, which other investigators have found to make up a large part of the choroidal leukocyte population (104). It is therefore apparent that the generally held view that there are no lymphocytes within the choroid, as with other areas of the uvea, is not necessarily wholly accurate. However, if present, lymphocytes are undoubtedly less frequent within the uvea than cells of innate immune lineages.
A common theme across all three uveal sites is that stromal cells have a vital role in immune homeostasis and immune activity. This is equally true in the choroid. The choroid is an exceptionally vascular tissue (86, 87). Vascular endothelial cells have been considered in some quarters to be simply structural components of blood vessels with few other physiological roles, but it is becoming apparent that they have key roles to play in tissue and organ homeostasis and regeneration after injury (105). Within the choroid of mice, endothelial cells have a different transcriptomic profile on RNA sequencing to endothelial cells from other organs (103). The Indian Hedgehog (IHH) gene, encoding a member of the hedgehog family, is more highly expressed in choroidal endothelial cells than in other locations, and downstream from this signalling molecule is a pathway that is necessary for choroidal mast cell survival. Additionally, IHH knock out animals have an exaggerated choroidal inflammatory response to NaIO3-induced RPE death (103). IHH expression in human choroid was also found to be higher than in comparator tissues, leaving open the possibility of a similar regulatory circuit in humans (103). Human choroidal endothelial cells also express functional TLRs 3, 4, and 9 (106), while elsewhere in the uveal tract the endothelial cells of the non-fenestrated iris capillaries are known to express components of the LPS receptor complex, which induce inflammatory cytokine production when stimulated (84). Work applying single cell transcriptomics to the human choriocapillaris also suggests age dependent changes in the transcriptome of endothelial cells. For instance, endothelial cells from older donors exhibit enriched gene expression in pathways associated with trans-endothelial leukocyte migration compared to tissue from younger individuals, with the caveat that some of the tissue from the older donor cohort came from patients with changes consistent with age related macular degeneration (107).
The human choroid is highly pigmented, as is the choroid of other primates (85). Indeed melanocytes are the most numerous cells in the choroid (108), and as with melanocytes at other uveal locations choroidal melanocytes express low levels of some cytokines constitutively, with production increasing in response to LPS (42). Choroidal melanocytes also respond to TNFα stimulation by increasing production of MMP8 (44). Cultured human choroidal melanocytes express mRNA for TLRs 1-10, and of these functional response to ligands has been demonstrated for TLRS 1-6 based on IL-8 and MCP-1 production (108). Human choroidal melanocytes also have capacity to produce a range of chemokines downstream from IFN-γ and TNF-α stimulation (109). Curiously, Jehs et al. describe a donor dependant effect of melanocytes on monocyte migration, with melanocytes from some eyes increasing migration on a transwell migration assay when stimulated with IFN-γ and TNFα, while those from other eyes decrease migration; this is not explained by an identifiable difference in secreted cytokines. Finally, choroidal melanocytes display an ability to inhibit activated T-cell proliferation, suggesting a role in immune modulation (109). It is likely that some choroidal leukocytes are impacted by interactions with the retinal pigment epithelium, given its direct contact with the innermost aspect of the choroid, Bruch's membrane, and given that choroidal dendritic cells are observed to have some processes in contact with RPE cells (27). The RPE, like the pigment epithelia of the iris and ciliary body, appears to have a role in immune regulation of leukocytes within the eye (4, 81) and matured dendritic cells cultured with RPE cells markedly reduce production of pro-inflammatory cytokines as well as inhibiting mixed lymphocyte reactions (110). These immunoregulatory functions, along with tight junctions between RPE cells, have led to the identification of the RPE as the outer blood retinal barrier, while the tight junctions between endothelial cells of the retinal vessels act as the inner blood retinal barrier (83, 111). It is, therefore, important to remember that the uvea has close anatomical associations with other structures with their own immunological functions.
Despite making up a large proportion of the cell population of the choroid, fibroblasts remain under-studied in this tissue. One stromal cell that has attracted growing attention on several fronts is the mesenchymal stem cell (MSC). MSCs are non-haematopoietic stem cells which have the capacity to differentiate into tissues derived from embryonic mesoderm. In an attempt to standardise nomenclature, a 2006 position statement from the International Society for Cellular Therapy established criteria for identifying MSCs, and this has been widely adopted (112). Early work on mesenchymal stem cells isolated them from bone marrow (113), but it has been possible to isolate them from numerous adult tissues, and a perivascular niche for them has been described (114). MSCs have been isolated from human choroidal tissue, and shown in vitro to have anti-angiogenic properties, limiting the growth of cultured choroidal vascular endothelial cells (115). Work on a mouse model has demonstrated that intravitreal administration of bone marrow derived MSCs reduced expression of numerous inflammatory mediators, including IL-1α, IL-6, iNOS and TNF, and also reduced infiltration of immune cells into the retina under inflammatory conditions (116). This is in keeping with a broader paradigm of immunoregulatory functions of MSCs, which has been reviewed elsewhere (117), and suggests a possible anti-inflammatory role for MSCs in the uvea.
Controversy surrounds the presence or absence of lymphatics within the mammalian choroid. Some evidence exists from microscopy to suggest structures with the features of lymphatic vessels (118), but more recent efforts incorporating immunostaining for lymphatic markers suggest that classical lymphatics are absent from the choroid, in the human at any rate (119). Some researchers have suggested that avian choroid does contain lymphatic vessels (120). Unusually, these are described as directly draining into choroidal veins rather than connecting to systemic lymphatics and draining into the great veins (121). Similar to the iris, murine choroid has been shown to contain a population of LYVE-1 positive macrophages (69) and a population of similar cells double positive for LYVE-1 and CD 68 has been demonstrated in human choroidal samples (119). It is clear that the question of the lymphatic drainage of the internal ocular environment has not yet been settled.
Discussion of any tissue in isolation runs the risk of creating artificial conceptual silos, which are not reflected in biology. This may be particularly true in an organ such as the eye, which has several layers distinct in structure and function and yet separated by no more than potential spaces. A discussion of the immunological environment of the choroid should therefore acknowledge its role in supporting the key function of the eye – the transduction of light to neural signal by the retina. The retina itself is a distinct immune environment, a full discussion of which is beyond the purview of this review, but its chief immune cellular player is the microglial cell (122). These long lived cells of the embryonic yolk sac derived macrophage lineage are not a homogenous population, with differences observed between populations found in the inner and outer plexiform layers (26). Tight control of microglial activation is required to avoid the damaging effects of inflammation on retinal function. This is in part achieved by microglial CD200R binding by CD200 on retinal neurons and vascular endothelium (123).
Discussion
Histopathological study of the normal and diseased uvea in humans and experimental animals has provided a longstanding knowledgebase on the anatomical organisation of its parenchyma and stroma, and the broad outline of the cellular component of the uveal immune system is well-known. This is summarised in Tables 1–3. Myeloid lineage cells make up the large majority of the resident leukocytes, as is the case in many tissues. However, there is persisting uncertainty as to the complete leukocyte complement of the uveal niche; though some sources report T-cells within the healthy uveal tract of several animals, this is controversial. Little work has been published identifying whether these may be cells which are circulating within the blood or whether they are truly present within uveal tissues and are perhaps resident lymphocytes such as have been described in several other tissues (37). Work to elucidate the functional properties of several of the resident leukocytes of the different sectors of the uveal tract also continues. For example, the tissue macrophage populations of the uveal sub-compartments have not been fully characterised, and our understanding of these cells in the uvea is limited when compared to other tissues. Macrophages in some tissues are largely derived from the embryonic yolk sac, such as the microglia of the central nervous system (124) while those at other sites such as the lung and kidney have a contribution from haematopoietic stem cells via circulating monocytes (125). It is becoming clear that uveal macrophages are a mix of long-lived self-replicating and monocyte derived cells (25, 26), though whether the long-lived subset is ultimately embryonic yolk sac derived is not known. Functional biology of macrophages within the uveal tissues is also poorly understood when compared to many other tissues.
Table 1.
Summary table of the immune functions of iris cell types.
| Cell | Immune function | References |
|---|---|---|
| Cells of haematopoietic origin | ||
| Dendritic cell (cDC subtype) | Antigen presentation, functionally immature when in situ | (10–16, 19, 22) |
| Tissue macrophage | Cytokine production and antigen presentation | (10, 11, 16, 24–27) |
| Mast cell | Sentinel function, immediate response to pathogens Cytokine production | (27, 29–33) |
| Parenchymal and stromal cells | ||
| Melanocyte | Response to PAMPs, limited cytokine production | (39–44) |
| Iris pigment epithelium | Response to PAMPs, limited cytokine production | (51, 52) |
| Inhibition of T-cell activation, induction of T-reg cells | (55, 56, 58–60, 62, 66) | |
Table 3.
Summary table of the immune functions of choroidal cell types.
| Cell | Immune function | References |
|---|---|---|
| Cells of haematopoietic origin | ||
| Dendritic cell (cDC subtype) | Antigen presentation | (27, 94, 95) |
| Tissue macrophage | Cytokine production and antigen presentation | (10, 25, 93, 96, 97) |
| Mast cell | Sentinel function, immediate response to pathogens Cytokine production | (29–31, 98, 99, 101) |
| Parenchymal and stromal cells | ||
| Melanocyte | Response to PAMPs | (42, 108) |
| Response to TNFα and IFNγ | (44, 109) | |
| Inhibition of T-cell proliferation | (109) | |
| Vascular endothelium | Response to PAMPs | (84, 106) |
Table 2.
Summary table of the immune functions of ciliary body cell types.
| Cell | Immune function | References |
|---|---|---|
| Cells of haematopoietic origin | ||
| Dendritic cell | Antigen presentation | (12) |
| Tissue macrophage | Cytokine production and antigen presentation | (23, 26) |
| Mast cell | Sentinel function, immediate response to pathogens Cytokine production | (27, 31) |
| Parenchymal and stromal cells | ||
| Melanocyte | Response to PAMPs, limited cytokine production | (42–44) |
| Pigmented ciliary epithelium | Immunoregulatory role | (57, 62, 82, 83) |
| Non-pigmented ciliary epithelium | Response to LPS | (84) |
Our knowledge and understanding of much of the uveal stroma is sparse. Fibroblasts make up a large proportion of the stromal cells in each part of the uveal tract, but little has been done to identify the specific properties of this cell type in the eye and within the uvea the fibroblast population remains largely uncharacterised. Within other tissues fibroblast heterogeneity is well-established, and different fibroblast populations have been shown in different microanatomical niches of the same organ. Key examples of this are the gut, where fibroblast populations vary along the crypt-villus axis (126); and the skin, where papillary and reticular dermal fibroblasts have distinct transcriptome profiles (127). The function of fibroblasts is also being revisited. It has long been accepted that these cells are involved in the production of the extracellular matrix, but the crucial role of the fibroblast to immune regulation and tissue defence is becoming increasingly apparent, and is reviewed elsewhere (128, 129). The focus of discussion here has been on cellular components of the uvea, but a significant part of the mass of the iris, ciliary body and choroid comes from the extracellular matrix, made up of a wide array of matrix proteins, the matrisome. These proteins, including collagens, proteoglycans and glycoproteins, have well-documented structural roles as well as functions in cell attachment and the binding of growth factors (130). Extracellular matrix is ubiquitous, though the precise composition differs between locales, so it is unsurprising that it has a role in host defence. Both direct antimicrobial and antiviral effects of extracellular matrix proteins, and indirect effects based on chemotactic functions towards host immune cells have been documented (131).
Recent technological advances have taken our understanding of the cellular subtypes and organisation within organs and tissues to a considerably more advanced state than was previously possible. In particular, the advent of bulk and then single cell RNA sequencing has allowed the transcriptomic phenotyping of tissues and individual cells, permitting the construction of a database of cell types defined by the genes they express. The construction of just such a database is the aim of the Human Cell Atlas project (132), which has teams working in several fields in an attempt to fully define the extent of the different cell populations that make up the human body. This project can provide the link between the known human DNA code and the individual cells that are responsible for function. Within the eye, single cell RNA sequencing has already been used to great effect in the study of the human retina (133), trabecular meshwork and uveoscleral aqueous outflow route (134, 135) and corneal limbus (136), but of the uveal tract only the choroid (in conjunction with the RPE) has so far been examined in this way (104, 107). Voigt et al. show a number of cell clusters within the combined RPE/choroid analysis, including the interesting finding of T-cells which need to be further defined. A limitation of their studies is, however, the profiling of both healthy tissue and those with dry and wet age related macular degeneration in the same analysis, at a time when then baseline transcriptome of the healthy uvea is not yet understood. Defining the cell types present in an organ by their transcriptome is only part of the task of producing an atlas. It is also vital to understand the niches within an organ which particular cell types inhabit. For this reason, the new generation of highly multiplexed immunofluorescence imaging techniques (137) which allow the localisation of tens of protein markers on a single tissue sample, along with the rapidly advancing world of spatial transcriptomics (138) will be vital in localising cellular subtypes to their microanatomical position.
Developing a more complete understanding of the immunological aspects of uveal homeostasis is not of merely academic interest. Uveitis as a spectrum of disease is a major cause of blindness (139). Worldwide, the prevalence of uveitis varies markedly across countries, as does the underlying cause (140). Defining cell populations found in different anatomical locations can shed light on localised disease processes, and many forms of uveitis affect the uvea alone (141). Of greater interest to the wider medical community are uveitides associated with systemic disease. The most common form of uveitis, acute anterior uveitis, is often, but not exclusively, associated with carriage of the HLA-B27 MHC class I allele (6), which links it to a group of conditions known as the seronegative spondyloarthropathies. Recent discoveries in this field include that of a resident T-cell in the healthy entheses of mice which may have a role in the enthesitis characteristic of many spondyloarthritides (142), a finding which was corroborated in human spinal entheses where both γδ T-cells (143) and conventional T-cells expressing a TRM phenotype (144) have been found. The many extra-articular associations of the seronegative spondyloarthropathies include anterior uveitis, inflammatory bowel disease and psoriasis (145), and it is hoped that improved understanding of the biology of each location in this Joint-Eye-Skin-Gut axis will shed light on similarities that may explain these associations.
Single cell RNA sequencing has already transformed our understanding of the cellular constituents of some disease states. For example, single cell sequencing has shown that in joints affected by rheumatoid arthritis there are distinct subtypes of fibroblast making up the lining layer of the synovium and the sublining layer, and these have different contributions to disease states (146). These fibroblast groups have the same physical appearance on microscopy, and express some of the same surface markers. It is only by applying transcriptomic techniques that the differences between populations can be robustly identified. Similarly, distinct subtypes of monocytes and T-cells can be defined in rheumatoid and osteoarthritic tissue (147). The stage is therefore set for similar technologies and techniques to be employed to further our understanding of inflammatory eye disease, elucidating the leukocyte and stromal cell types and axes that drive the initiation, maintenance, and resolution of the inflammatory state seen in the uveitides. In doing this we will be able to better understand the available targets for treatment of the many forms of uveitis which cause so much visual morbidity globally.
The immunology of the uvea is a fascinating field, where leukocytes and stromal cells keep delicate balance to permit inflammatory activation where required, but maintain the immune tolerance for which the ocular environment is well-known amongst immunologists and physicians. With recently developed and emerging technologies it is clear that there is great scope to expand our understanding of both stromal and leukocyte biology in the uvea for the future benefit of the many patients who suffer from the debilitating symptoms that accompany uveitis.
Author Contributions
IR conceived the article, performed the literature search, and drafted the article. CB conceived the article and critically revised the work. SS, AF, JS, MC, ADD, and AKD critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
MC and CB were funded through Medical Research Grant: MR/S025308/1.
Conflict of Interest
JS is now an employee of Janssen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Chen L. Ocular lymphatics: state-of-the-art-review. Lymphology. (2009) 42:66–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G . Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. (2005) 140:509–16. 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front Immunol. (2012) 3:338. 10.3389/fimmu.2012.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mochizuki M, Sugita S, Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res. (2013) 33:10–27. 10.1016/j.preteyeres.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 5.Hori J, Vega JL, Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocul Immunol Inflamm. (2010) 18:325–33. 10.3109/09273948.2010.512696 [DOI] [PubMed] [Google Scholar]
- 6.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. (2018) 26:2–16. 10.1080/09273948.2016.1196713 [DOI] [PubMed] [Google Scholar]
- 7.Buckley CD, Barone F, Nayar S, Benezech C, Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. (2015) 33:715–45. 10.1146/annurev-immunol-032713-120252 [DOI] [PubMed] [Google Scholar]
- 8.Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. (2008) 153 (Suppl. 1):S241–6. 10.1038/sj.bjp.0707487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal RK, Silver PB, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Biol. (2012) 900:443–69. 10.1007/978-1-60761-720-4_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler TL, McMenamin PG. Resident and infiltrating immune cells in the uveal tract in the early and late stages of experimental autoimmune uveoretinitis. Investig Ophthalmol Visual Sci. (1996) 37:2195–210. [PubMed] [Google Scholar]
- 11.McMenamin PG, Crewe J, Kijlstra A. Resident and infiltrating cells in the rat iris during the early stages of experimental melanin protein-induced uveitis (EMIU). Ocul Immunol Inflamm. (1997) 5:223–33. 10.3109/09273949709085063 [DOI] [PubMed] [Google Scholar]
- 12.McMenamin PG, Holthouse I, Holt PG. Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye: distribution, phenotype and relation to retinal microglia. Immunology. (1992) 77:385–93. [PMC free article] [PubMed] [Google Scholar]
- 13.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. (1999) 83:598–604. 10.1136/bjo.83.5.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson JSP, Bradley D, Streilein JW. Immunoregulatory properties of bone marrow-derived cells in the iris and ciliary body. Immunology. (1989) 67:96–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Steptoe RJ, Holt PG, McMenamin PG. Functional studies of major histocompatibility class II-positive dendritic cells and resident tissue macrophages isolated from the rat iris. Immunology. (1995) 85:630–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Steptoe RJ, Holt PG, McMenamin PG. Origin and steady-state turnover of major histocompatibility complex Class II-positive dendritic cells and resident-tissue macrophages in the iris of the rat eye. Journal of Neuroimmunology. (1996) 68:67–76. 10.1016/0165-5728(96)00070-7 [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zwart R, Yang P, Kijlstra A. Macrophages and MHC class II positive dendritiform cells in the iris and choroid of the pig. Curr Eye Res. (2003) 26:291–6. 10.1076/ceyr.26.4.291.15432 [DOI] [PubMed] [Google Scholar]
- 18.Lin W, Liu T, Wang B, Bi H. The role of ocular dendritic cells in uveitis. Immunol Lett. (2019) 209:4–10. 10.1016/j.imlet.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 19.Chang JH, McCluskey P, Wakefield D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest Ophthalmol Vis Sci. (2004) 45:1871–8. 10.1167/iovs.03-1113 [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Denniston AK, Hirani S, Hannes S, Nussenblatt RB. Role of dendritic cell subsets in immunity and their contribution to noninfectious uveitis. Surv Ophthalmol. (2015) 60:242–9. 10.1016/j.survophthal.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. (2018) 154:3–20. 10.1111/imm.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester JVXH, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. (2010) 243:282–304. 10.1111/j.0105-2896.2009.00873.x [DOI] [PubMed] [Google Scholar]
- 23.McMenamin PG, Crewe J, Morrison S, Holt PG. Immunomorphologic studies of macrophages and MHC class II-positive dendritic cells in the iris and ciliary body of the rat, mouse, and human eye. Investig Ophthalmol Visual Sci. (1994) 35:3234–50. [PubMed] [Google Scholar]
- 24.Shepard JL, Zon LI. Developmental derivation of embryonic and adult macrophages. Curr Opin Hematol. (2000) 7:3–8. 10.1097/00062752-200001000-00002 [DOI] [PubMed] [Google Scholar]
- 25.Droho S, Thomson BR, Makinde HM, Cuda CM, Perlman H, Lavine JA. Ocular macrophage origin and heterogeneity during steady state and experimental choroidal neovascularization. J Neuroinflammation. (2020) 17:341. 10.1186/s12974-020-02010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, Mathew R, et al. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity. (2019) 50:723–37.e7. 10.1016/j.immuni.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMenamin PG. The distribution of immune cells in the uveal tract of the normal eye. Eye. (1997) 11:183–93. 10.1038/eye.1997.49 [DOI] [PubMed] [Google Scholar]
- 28.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. (2009) 4:e7475. 10.1371/journal.pone.0007475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smelser GK, Silver S. The distribution of mast cells in the normal eye: a method of study. Exp Eye Res. (1963) 2:134–40. 10.1016/S0014-4835(63)80005-6 [DOI] [PubMed] [Google Scholar]
- 30.Cook EB, Stahl JL, Barney NP, Graziano FM. Ocular Mast cells characterization in normal and disease states. Clin Rev Allergy Immunol. (2001) 20:243–68. 10.1385/CRIAI:20:2:243 [DOI] [PubMed] [Google Scholar]
- 31.May CA. Mast cell heterogeneity in the human uvea. Histochem Cell Biol. (1999) 112:381–6. 10.1007/s004180050420 [DOI] [PubMed] [Google Scholar]
- 32.Irani AA, Schechter NM, Craig SS, Deblois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Nati Acad Sci USA. (1986) 83:4464–8. 10.1073/pnas.83.12.4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidner N, Austen KF. Heterogeneity of mast cells at multiple body sites: fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. (1993) 189:156–62. 10.1016/S0344-0338(11)80086-5 [DOI] [PubMed] [Google Scholar]
- 34.Wakefield DM P., Palladinetti P. Distribution of lymphocytes and cell adhesion molecules in iris biopsy specimens from patients with uveitis. Arch Ophthalmol. (1992) 110:121–5. 10.1001/archopht.1992.01080130123040 [DOI] [PubMed] [Google Scholar]
- 35.Mami-Chouaib F, Tartour E. Editorial: tissue resident memory T cells. Front Immunol. (2019) 10:1018. 10.3389/fimmu.2019.01018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol. (2019) 37:521–46. 10.1146/annurev-immunol-042617-053214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo PA, Miron M, Farber DL. Location, location, location: tissue resident memory T cells in mice and humans. Sci Immunol. (2019) 4:eaas9673. 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, et al. What are melanocytes really doing all day long.? Exp Dermatol. (2009) 18:799–819. 10.1111/j.1600-0625.2009.00912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn JH, Park TJ, Jin SH, Kang HY. Human melanocytes express functional toll-like receptor 4. Exp Dermatol. (2008) 17:412–7. 10.1111/j.1600-0625.2008.00701.x [DOI] [PubMed] [Google Scholar]
- 40.Jin SH, Kang HY. Activation of toll-like receptors 1, 2, 4, 5, and 7 on human melanocytes modulate pigmentation. Ann Dermatol. (2010) 22:486–9. 10.5021/ad.2010.22.4.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapia CV, Falconer M, Tempio F, Falcon F, Lopez M, Fuentes M, et al. Melanocytes and melanin represent a first line of innate immunity against Candida albicans. Med Mycol. (2014) 52:445–54. 10.1093/mmy/myu026 [DOI] [PubMed] [Google Scholar]
- 42.Hu DN, Bi M, Zhang DY, Ye F, McCormick SA, Chan CC. Constitutive and LPS-induced expression of MCP-1 and IL-8 by human uveal melanocytes in vitro and relevant signal pathways. Invest Ophthalmol Vis Sci. (2014) 55:5760–9. 10.1167/iovs.14-14685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu DN, Chen M, Zhang DY, Ye F, McCormick SA, Chan CC. Interleukin-1beta increases baseline expression and secretion of interleukin-6 by human uveal melanocytes in vitro via the p38 MAPK/NF-kappaB pathway. Invest Ophthalmol Vis Sci. (2011) 52:3767–74. 10.1167/iovs.10-6908 [DOI] [PubMed] [Google Scholar]
- 44.Hu DN, Rosen RB, Chan CC, Yang WE, Yang SF. Uveal melanocytes express high constitutive levels of MMP-8 which can be upregulated by TNF-alpha via the MAPK pathway. Exp Eye Res. (2018) 175:181–91. 10.1016/j.exer.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 45.Dejonckheere E, Vandenbroucke RE, Libert C. Matrix metalloproteinase8 has a central role in inflammatory disorders and cancer progression. Cytokine Growth Factor Rev. (2011) 22:73–81. 10.1016/j.cytogfr.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 46.Kezic J, Taylor S, Gupta S, Planck SR, Rosenzweig HL, Rosenbaum JT. Endotoxin-induced uveitis is primarily dependent on radiation-resistant cells and on MyD88 but not TRIF. J Leukoc Biol. (2011) 90:305–11. 10.1189/jlb.0111036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong Y, Song B, Chen HD, Gao XH. Melanocytes and skin immunity. J Investig Dermatol Symp Proc. (2015) 17:37–9. 10.1038/jidsymp.2015.14 [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto T, Murata T, Inomata H. Class II major histocompatibility complex on melanocytes of Vogt-Koyanagi-Harada disease. Arch Ophthalmol. (1991) 109:1270–4. 10.1001/archopht.1991.01080090096030 [DOI] [PubMed] [Google Scholar]
- 49.Davis-Silberman N, Ashery-Padan R. Iris development in vertebrates; genetic and molecular considerations. Brain Res. (2008) 1192:17–28. 10.1016/j.brainres.2007.03.043 [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Xiong K, Lu L, Gu D, Wang S, Chen J, et al. Developmental origin of the posterior pigmented epithelium of iris. Cell Biochem Biophys. (2015) 71:1067–76. 10.1007/s12013-014-0310-0 [DOI] [PubMed] [Google Scholar]
- 51.Mai K, Chui JJ, Di Girolamo N, McCluskey PJ, Wakefield D. Role of toll-like receptors in human iris pigment epithelial cells and their response to pathogen-associated molecular patterns. J Inflam. (2014) 11:1–12. 10.1186/1476-9255-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chui JJ, Li MW, Di Girolamo N, Chang JH, McCluskey PJ, Wakefield D. Iris pigment epithelial cells express a functional lipopolysaccharide receptor complex. Invest Ophthalmol Vis Sci. (2010) 51:2558–67. 10.1167/iovs.09-3923 [DOI] [PubMed] [Google Scholar]
- 53.Sakuragi M, Tomita H, Abe T, Tamai M. Changes of phagocytic capacity in basic fibroblast growth factor-transfected iris pigment epithelial cells in rats. Curr Eye Res. (2001) 23:185–91. 10.1076/ceyr.23.3.185.5465 [DOI] [PubMed] [Google Scholar]
- 54.Rezai K, Lappas A, Farrokh-Siar L, Kohen L, Wiedemann P, Heimann K. Iris pigment epithelial cells of long evans rats demonstrate phagocytic activity. Exp Eye Res. (1997) 65:23–9. 10.1006/exer.1997.0307 [DOI] [PubMed] [Google Scholar]
- 55.Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. (2003) 11:91–105. 10.1076/ocii.11.2.91.15914 [DOI] [PubMed] [Google Scholar]
- 56.Sugita S, Streilein JW. Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4. J Exp Med. (2003) 198:161–71. 10.1084/jem.20030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida M, Takeuchi M, Streilein W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Investigative Ophthalmology & Visual Science. (2000) 41:811-21. [PubMed] [Google Scholar]
- 58.Sugita S, Keino H, Futagami Y, Takase H, Mochizuki M, Stein-Streilein J, et al. B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells. Invest Ophthalmol Vis Sci. (2006) 47:5376–84. 10.1167/iovs.05-1354 [DOI] [PubMed] [Google Scholar]
- 59.Sugita S, Ng TF, Lucas PJ, Gress RE, Streilein JW. B7+ iris pigment epithelium induce CD8+ T regulatory cells; both suppress CTLA-4+ T cells. J Immunol. (2006) 176:118–27. 10.4049/jimmunol.176.4.2670-c [DOI] [PubMed] [Google Scholar]
- 60.Sugita S, Futagami Y, Horie S, Mochizuki M. Transforming growth factor beta-producing Foxp3(+)CD8(+)CD25(+) T cells induced by iris pigment epithelial cells display regulatory phenotype and acquire regulatory functions. Exp Eye Res. (2007) 85:626–36. 10.1016/j.exer.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 61.Yoshida MK T., Streilein J.W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 2. Generation of TGF-b–producing regulatory T cells. Investig Ophthalmol Visual Sci. (2000) 41:3862–70. [PubMed] [Google Scholar]
- 62.Futagami Y, Sugita S, Vega J, Ishida K, Takase H, Maruyama K, et al. Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J Immunol. (2007) 178:6994–7005. 10.4049/jimmunol.178.11.6994 [DOI] [PubMed] [Google Scholar]
- 63.Sugita S, Horie S, Yamada Y, Keino H, Usui Y, Takeuchi M, et al. Suppression of bystander T helper 1 cells by iris pigment epithelium-inducing regulatory T cells via negative costimulatory signals. Invest Ophthalmol Vis Sci. (2010) 51:2529–36. 10.1167/iovs.09-4460 [DOI] [PubMed] [Google Scholar]
- 64.Sugita S, Horie S, Nakamura O, Maruyama K, Takase H, Usui Y, et al. Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2alpha during inflammatory conditions. J Immunol. (2009) 183:5013–22. 10.4049/jimmunol.0901623 [DOI] [PubMed] [Google Scholar]
- 65.Horie S, Sugita S, Futagami Y, Kawaguchi T, Kamoi K, Shirato S, et al. Human iris pigment epithelium suppresses activation of bystander T cells via TGFbeta-TGFbeta receptor interaction. Exp Eye Res. (2009) 88:1033–42. 10.1016/j.exer.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 66.Hattori T, Kezuka T, Usui Y, Okunuki Y, Takeuchi M, Maruyama K, et al. Human iris pigment epithelial cells suppress T-cell activation via direct cell contact. Exp Eye Res. (2009) 89:358–64. 10.1016/j.exer.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 67.Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM, et al. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm. (2020) 28:854–9. 10.1080/09273948.2019.1583347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parikh RA, Chaon BC, Berkenstock MK. Ocular complications of checkpoint inhibitors and immunotherapeutic agents: a case series. Ocul Immunol Inflamm. (2020) 1–6. 10.1080/09273948.2020.1766082 [DOI] [PubMed] [Google Scholar]
- 69.Xu H, Chen M, Reid DM, Forrester JV. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci. (2007) 48:2162–71. 10.1167/iovs.06-0783 [DOI] [PubMed] [Google Scholar]
- 70.Birke K, Lutjen-Drecoll E, Kerjaschki D, Birke MT. Expression of podoplanin and other lymphatic markers in the human anterior eye segment. Invest Ophthalmol Vis Sci. (2010) 51:344–54. 10.1167/iovs.08-3307 [DOI] [PubMed] [Google Scholar]
- 71.Kaser-Eichberger A, Schrodl F, Trost A, Strohmaier C, Bogner B, Runge C, et al. Topography of lymphatic markers in human iris and ciliary body. Invest Ophthalmol Vis Sci. (2015) 56:4943–53. 10.1167/iovs.15-16573 [DOI] [PubMed] [Google Scholar]
- 72.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. (2005) 115:2363–72. 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boonman ZF, van Mierlo GJ, Fransen MF, Franken KL, Offringa R, Melief CJ, et al. Intraocular tumor antigen drains specifically to submandibular lymph nodes, resulting in an abortive cytotoxic T cell reaction. J Immunol. (2004) 172:1567–74. 10.4049/jimmunol.172.3.1567 [DOI] [PubMed] [Google Scholar]
- 74.Alm A, Nilsson SF. Uveoscleral outflow–a review. Exp Eye Res. (2009) 88:760–8. 10.1016/j.exer.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 75.Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. (2014) 12:e1001912. 10.1371/journal.pbio.1001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park DY, Lee J, Park I, Choi D, Lee S, Song S, et al. Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity. J Clin Invest. (2014) 124:3960–74. 10.1172/JCI75392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chinnery HR, McMenamin PG, Dando SJ. Macrophage physiology in the eye. Pflugers Arch. (2017) 469:501–15. 10.1007/s00424-017-1947-5 [DOI] [PubMed] [Google Scholar]
- 78.Cherepanoff S, Hasic E, McMenamin P, Gillies M. CD163+ and CD68+ cells in the adult human eye. In: ARVO Annual Meeting Abstract (2013). [Google Scholar]
- 79.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE. (2013) 8:e80908. 10.1371/journal.pone.0080908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q, Whitcup SM, Fujino Y, Nussenblatt RB, Chan CC. The role of mast cells in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. (1993) 34:256–9. [PubMed] [Google Scholar]
- 81.Mochizuki M. Regional immunity of the eye. Acta Ophthalmol. (2010) 88:292–9. 10.1111/j.1755-3768.2009.01757.x [DOI] [PubMed] [Google Scholar]
- 82.Sugita S, Futagami Y, Smith SB, Naggar H, Mochizuki M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp Eye Res. (2006) 83:1459–71. 10.1016/j.exer.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 83.Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. (2013) 13:206–18. 10.1038/nri3391 [DOI] [PubMed] [Google Scholar]
- 84.Brito BE, Zamora DO, Bonnah RA, Pan Y, Planck SR, Rosenbaum JT. Toll-like receptor 4 and CD14 expression in human ciliary body and TLR-4 in human iris endothelial cells. Exp Eye Res. (2004) 79:203–8. 10.1016/j.exer.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 85.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. (2010) 29:144–68. 10.1016/j.preteyeres.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca ims) : a Study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. (1973) 15:15–29. 10.1016/0014-4835(73)90185-1 [DOI] [PubMed] [Google Scholar]
- 87.Urs R, Ketterling JA, Yu ACH, Lloyd HO, Yiu BYS, Silverman RH. Ultrasound imaging and measurement of choroidal blood flow. Transl Vis Sci Technol. (2018) 7:5. 10.1167/tvst.7.5.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SA, Kim SJ, Choi YA, Yoon HJ, Kim A, Lee J. Retinal VEGFA maintains the ultrastructure and function of choriocapillaris by preserving the endothelial PLVAP. Biochem Biophys Res Commun. (2020) 522:240–6. 10.1016/j.bbrc.2019.11.085 [DOI] [PubMed] [Google Scholar]
- 89.Van den Heuvel MM, Tensen CP, Van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, et al. Regulation of CD163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukocyte Biol. (1999) 66:858–66. 10.1002/jlb.66.5.858 [DOI] [PubMed] [Google Scholar]
- 90.Frei K, Steger C, Samorapoompichit P, Lucas T, Förster O. Expression and function of sialoadhesin in rat alveolar macrophages. Immunol Lett. (2000) 71:167–70. 10.1016/S0165-2478(99)00180-7 [DOI] [PubMed] [Google Scholar]
- 91.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. (2001) 97:288–96. 10.1182/blood.V97.1.288 [DOI] [PubMed] [Google Scholar]
- 92.Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, et al. The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol Dial Transplant. (2005) 20:2704–13. 10.1093/ndt/gfi105 [DOI] [PubMed] [Google Scholar]
- 93.McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM, Lutty GA. Distribution and quantification of choroidal macrophages in human eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. (2016) 57:5843–55. 10.1167/iovs.16-20049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choudhury A, Al-Pakalnis V, Bowers W. Characterization and functional activity of dendritic cells from rat choroid. Exp Eye Res. (1994) 59:297–304. 10.1006/exer.1994.1111 [DOI] [PubMed] [Google Scholar]
- 95.Forrester JV, Lumsden L, Duncan L, Dick AD. Choroidal dendritic cells require activation to present antigen and resident choroidal macrophages potentiate this response. Br J Ophthalmol. (2005) 89:369–77. 10.1136/bjo.2004.054197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. (2010) 94:918–25. 10.1136/bjo.2009.165563 [DOI] [PubMed] [Google Scholar]
- 97.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 98.McMenamin PG, Polla E. Mast cells are present in the choroid of the normal eye in most vertebrate classes. Vet Ophthalmol. (2013) 16 (Suppl. 1):73–8. 10.1111/vop.12035 [DOI] [PubMed] [Google Scholar]
- 99.Godfrey WA. Characterisation of the choroidal mast cell. Tr Am Ophth Soc. (1987) 85:557. [PMC free article] [PubMed] [Google Scholar]
- 100.de Kozak Y, Sainte-Laudy J, Benveniste J, Faure J-P. Evidence for immediate hypersensitivity phenomena in experimental autoimmune uveoretinitis. Eur J Immunol. (1981) 11:612–7. 10.1002/eji.1830110805 [DOI] [PubMed] [Google Scholar]
- 101.Mochizuki M, Kuwabara T, Chan CC, Nussenblatt RB, Metcalfe DD, Gery I. An association between susceptibility to experimental autoimmune uveitis and choroidal mast cell numbers. J Immunol. (1984) 133:1699–701. [PubMed] [Google Scholar]
- 102.Zhao Z, Liang Y, Liu Y, Xu P, Flamme-Wiese MJ, Sun D, et al. Choroidal gammadelta T cells in protection against retinal pigment epithelium and retinal injury. FASEB J. (2017) 31:4903–16. 10.1096/fj.201700533R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lehmann GL, Hanke-Gogokhia C, Hu Y, Bareja R, Salfati Z, Ginsberg M, et al. Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid. J Exp Med. (2020) 217:e20190730. 10.1084/jem.20190730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, et al. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci USA. (2019) 116:24100–7. 10.1073/pnas.1914143116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. (2016) 529:316–25. 10.1038/nature17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stewart EA, Wei R, Branch MJ, Sidney LE, Amoaku WM. Expression of Toll-like receptors in human retinal and choroidal vascular endothelial cells. Exp Eye Res. (2015) 138:114–23. 10.1016/j.exer.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 107.Voigt AP, Whitmore SS, Mulfaul K, Chirco KR, Giacalone JC, Flamme-Wiese MJ, et al. Bulk and single-cell gene expression analyses reveal aging human choriocapillaris has pro-inflammatory phenotype. Microvasc Res. (2020) 131:104031. 10.1016/j.mvr.2020.104031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cioanca AV, McCluskey PJ, Eamegdool SS, Madigan MC. Human choroidal melanocytes express functional Toll-like receptors (TLRs). Exp Eye Res. (2018) 173:73–84. 10.1016/j.exer.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 109.Jehs T, Faber C, Udsen MS, Jager MJ, Clark SJ, Nissen MH. Induction of chemokine secretion and monocyte migration by human choroidal melanocytes in response to proinflammatory cytokines. Invest Ophthalmol Vis Sci. (2016) 57:6568–79. 10.1167/iovs.15-18524 [DOI] [PubMed] [Google Scholar]
- 110.Sugita S, Kawazoe Y, Imai A, Usui Y, Iwakura Y, Isoda K, et al. Mature dendritic cell suppression by IL-1 receptor antagonist on retinal pigment epithelium cells. Invest Ophthalmol Vis Sci. (2013) 54:3240–9. 10.1167/iovs.12-11483 [DOI] [PubMed] [Google Scholar]
- 111.Coca-Prados M. The blood-aqueous barrier in health and disease. J Glaucoma. (2014) 23 (8 Suppl. 1):S36–8. 10.1097/IJG.0000000000000107 [DOI] [PubMed] [Google Scholar]
- 112.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. (2006) 8:315–7. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 113.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. (1999) 284:143–7. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 114.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. (2008) 3:301–13. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 115.Alexander N, Walshe J, Richardson NA, Futrega K, Doran MR, Harkin DG, et al. Stromal cells cultivated from the choroid of human eyes display a mesenchymal stromal cell (MSC) phenotype and inhibit the proliferation of choroidal vascular endothelial cells in vitro. Exp Eye Res. (2020) 200:108201. 10.1016/j.exer.2020.108201 [DOI] [PubMed] [Google Scholar]
- 116.Hermankova B, Kossl J, Bohacova P, Javorkova E, Hajkova M, Krulova M, et al. The immunomodulatory potential of mesenchymal stem cells in a retinal inflammatory environment. Stem Cell Rev Rep. (2019) 15:880–91. 10.1007/s12015-019-09908-0 [DOI] [PubMed] [Google Scholar]
- 117.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. 10.1038/s41581-018-0023-5 [DOI] [PubMed] [Google Scholar]
- 118.Krebs W, Krebs IP. Ultrastructural evidence for lymphatic capillaries in the primate choroid. Arch Ophthalmol. (1988) 106:1615–6. 10.1001/archopht.1988.01060140783055 [DOI] [PubMed] [Google Scholar]
- 119.Schroedl F, Brehmer A, Neuhuber WL, Kruse FE, May CA, Cursiefen C. The normal human choroid is endowed with a significant number of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1)-positive macrophages. Invest Ophthalmol Vis Sci. (2008) 49:5222–9. 10.1167/iovs.08-1721 [DOI] [PubMed] [Google Scholar]
- 120.Junghans BM, Crewther SG, Crewther DP, Pirie B. Lymphatic sinusoids exist in chick but not rabit choroid Australian and New Zealand. J Ophthalmol. (1997) 25:103–5. 10.1111/j.1442-9071.1997.tb01772.x [DOI] [PubMed] [Google Scholar]
- 121.De Stefano M E, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Investig Ophthalmol Vis Sci. (1997) 38:1241–60. [PubMed] [Google Scholar]
- 122.Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol. (2019) 173:18–40. 10.1016/j.pneurobio.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 123.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. (2002) 161:1669–77. 10.1016/S0002-9440(10)64444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. (2010) 330:841–5. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. (2012) 336:86–90. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- 126.Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DB, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. (2019) 178:714–30.e22. 10.1016/j.cell.2019.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janson DG, Saintigny G, van Adrichem A, Mahe C, El Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol. (2012) 132:2565–72. 10.1038/jid.2012.192 [DOI] [PubMed] [Google Scholar]
- 128.Bautista-Hernandez LA, Gomez-Olivares JL, Buentello-Volante B, Bautista-de Lucio VM. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur J Microbiol Immunol. (2017) 7:151–7. 10.1556/1886.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. (2021). 10.1038/s41577-021-00540-z [DOI] [PubMed] [Google Scholar]
- 130.Hynes RO, Naba A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. (2012) 4:a004903. 10.1101/cshperspect.a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomlin H, Piccinini AM. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology. (2018) 155:186–201. 10.1111/imm.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, et al. The human cell atlas. Elife. (2017) 6:e27041. 10.7554/eLife.27041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liang Q, Dharmat R, Owen L, Shakoor A, Li Y, Kim S, et al. Single-nuclei RNA-seq on human retinal tissue provides improved transcriptome profiling. Nat Commun. (2019) 10:5743. 10.1038/s41467-019-12917-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Zyl T, Yan W, McAdams A, Peng YR, Shekhar K, Regev A, et al. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc Natl Acad Sci USA. (2020) 117:10339–49. 10.1073/pnas.2001250117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patel G, Fury W, Yang H, Gomez-Caraballo M, Bai Y, Yang T, et al. Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc Natl Acad Sci USA. (2020) 117:12856–67. 10.1073/pnas.2001896117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li DQ, Kim S, Li JM, Gao Q, Choi J, Bian F, et al. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul Surf. (2021) 20:20–32. 10.1016/j.jtos.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. (2020) 40:135–53. 10.1002/cac2.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Asp M, Bergenstrahle J, Lundeberg J. Spatially resolved transcriptomes-next generation tools for tissue exploration. Bioessays. (2020) 42:e1900221. 10.1002/bies.201900221 [DOI] [PubMed] [Google Scholar]
- 139.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. (2004) 88:1159–62. 10.1136/bjo.2003.037226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. (2013) 23:705–17. 10.5301/ejo.5000278 [DOI] [PubMed] [Google Scholar]
- 141.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol. (2013) 156:228–36. 10.1016/j.ajo.2013.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. (2012) 18:1069–76. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 143.Cuthbert RJ, Watad A, Fragkakis EM, Dunsmuir R, Loughenbury P, Khan A, et al. Evidence that tissue resident human enthesis gammadeltaT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis. (2019) 78:1559–65. 10.1136/annrheumdis-2019-215210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watad A, Rowe H, Russell T, Zhou Q, Anderson LK, Khan A, et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis. (2020) 79:1044–54. 10.1136/annrheumdis-2020-217309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.El Maghraoui A. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. (2011) 22:554–60. 10.1016/j.ejim.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 146.Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. (2018) 9:789. 10.1038/s41467-018-02892-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. (2019) 20:928–42. 10.1038/s41590-019-0378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]