Abstract
Objective
This study investigated the association between soluble scavenger receptor differentiation antigen 163 (sCD163) and the severity and prognosis of renal injury in lupus nephritis (LN).
Methods
Serum sCD163 levels in 121 Eastern Chinese patients with LN who underwent renal biopsy were determined by enzyme-linked immunosorbent assays. Clinical data were collected, and the glomerular filtration rate and disease activity score of lupus were calculated. Pathological classification was performed, and renal pathological scores were assessed by the activity index (AI) and chronic index (CI). Kaplan–Meier survival curves were drawn to evaluate prognosis.
Results
The pathological classification, AI and CI scores in the high sCD163 group were increased. The sCD163 levels were positively correlated with serum creatinine, blood urea nitrogen, AI scores and CI scores and negatively correlated with the estimated glomerular filtration rate. Kaplan–Meier survival analysis showed that the incidence of renal endpoint events was increased in the high sCD163 group compared with the normal sCD163 group.
Conclusion
The serum sCD163 level correlates with the severity of LN and is an important indicator of poor renal prognosis in patients with LN.
Keywords: Soluble CD163, lupus nephritis, renal injury, macrophage, autoimmune, serum marker
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease, often involving the skin, joints, blood, blood vessels and kidneys. Lupus nephritis (LN) is the most relevant manifestation in 50% to 70% of patients with SLE, and 25% of patients with LN eventually develop end-stage renal disease (ESRD). 1 Immune cells and their secreted soluble effector molecules participate in the development of LN, and adaptive immunity mediated by B lymphocytes and T lymphocytes plays an important role. 2 As a critical component of innate and adaptive immunity, macrophages have several immune functions, such as antigen presentation, phagocytosis and cytokine production. Dysregulated expression of macrophage surface markers, cytokine production, phagocytosis and clearance of cellular debris promote the abnormal activation and proliferation of lymphocytes, leading to the development of autoantigenic and inflammatory responses. Thus, the important role of macrophages in LN has attracted increasing attention. 3
Scavenger receptor differentiation antigen 163 (CD163) is a single-chain transmembrane glycoprotein belonging to the first subgroup of the scavenger receptor superfamily B, with a molecular weight of approximately 130 kDa. sCD163 is present in serum and other tissue fluids and is a specific marker of macrophage activation. The expression of sCD163 and heme oxidase is upregulated in systemic inflammatory responses, which may be related to the initiation of the waterfall secretion of anti-inflammatory factors. 4 Serum sCD163 concentrations in peripheral blood reflect disease severity in autoimmune diseases, such as rheumatoid arthritis, systemic sclerosis and polymyositis/dermatomyositis.5–7 In addition, the number and frequency of CD163-positive cells (M2 macrophages) tend to increase in acute post-streptococcal glomerulonephritis, suggesting that the severity of endothelial injury might be related to the difference in the phenotypes of infiltrating inflammatory cells. 8 Analyses of the correlation between sCD163 and SLE disease activity have produced inconsistent results,9–11 and there are few related studies. This study analyzed the clinical characteristics, laboratory parameters and renal pathology of patients with LN and explored the relevance between sCD163 and renal injury to identify new serum markers for the diagnosis and evaluation of LN, thereby providing a theoretical basis for novel therapeutic targets in LN.
Materials and methods
Study population
Patients with LN who underwent renal biopsy from 1 January 2014 to 1 June 2018 at tertiary hospitals (Affiliated Hospital of Nantong University, Affiliated Hospital of Jiangnan University and Nanjing Medical University Affiliated Wuxi Second Hospital) in Eastern China were selected as the study subjects, and healthy individuals were selected as the control group. All patients with LN were scored on the Disease Activity Index using the 2000 revision of the SLE Disease Activity Index (SLEDAI). Participants were followed-up until the occurrence of a renal endpoint event or the study termination date (1 January 2019). Renal endpoints in this study were defined as the occurrence of ESRD, estimated glomerular filtration rate (eGFR) reduction >50% or ≥2-fold increase in serum creatinine (SCr). The inclusion criteria were as follows: (1) patients met both SLE and LN diagnostic criteria 12 and (2) definite LN diagnosis by renal biopsy. The following exclusion criteria were used: (1) patients with other autoimmune diseases, such as scleroderma or polymyositis/dermatomyositis, (2) patients with chronic infections and severe infections within the last 3 months, (3) patients with severe diseases of the respiratory, circulatory, hematological and endocrine systems and (4) patients with malignant tumors. The study was approved by the appropriate ethics committees of the three hospitals (Affiliated Hospital of Nantong University, Affiliated Hospital of Jiangnan University and Nanjing Medical University Affiliated Wuxi Second Hospital; 2013-K030), and all patients or their families were fully informed and signed informed consent forms prior to study implementation.
Biochemical analyses
All subjects were required to fast for at least 8 hours, and 3 to 4 mL of blood were drawn and sent to the hospital laboratory. SCr, blood urea nitrogen (BUN), complement C3, complement C4 and immunoglobulins (Ig A, Ig M, Ig G) were tested using an automatic biochemical analyzer (Hitachi 7600, Hitachi, Tokyo, Japan). In addition, 24-hour urinary protein was quantified by immunoturbidimetry. Autoantibodies [anti-Smith (anti-Sm), anti-double stranded DNA (anti-dsDNA), anti-Sjögren’s-syndrome-related antigen A (anti-SSA), anti-Sjögren’s-syndrome-related antigen B (anti-SSB), anti-ribonucleoprotein (anti-RNP)] were measured using an American Delrin BN-II automatic immunoassay analyzer.
The Modification of Diet in Renal Disease 13 formula was used to calculate the eGFR as follows: eGFR = 186 ×SCr−1.154 ×Age−0.203 × (0.742 in women). The results were expressed as mL · min−1 (1.73 m2)−1.
Ethylenediaminetetraacetic acid anticoagulated blood samples were taken, and the serum was separated by centrifugation and stored at −20°C. To detect sCD163, an enzyme-linked immunosorbent assay (ELISA) was performed using a human soluble CD163 ELISA Kit (My Biosource, San Diego, CA, USA) in accordance with the manufacturer’s instruction. The detection range of this kit is 1.56 to 100 ng/mL.
Histological analysis
All enrolled patients underwent ultrasound-guided renal biopsy for pathological examination. The pathological classification of LN was performed using the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) criteria, and the activity index (AI) and chronicity index (CI) were scored. 14 AI includes capillary endothelial hyperplasia, fibrillar-like necrosis, nuclear fragmentation, cellular crescents, hyaline thrombosis/platinum ear changes, leukocyte infiltration and/or interstitial inflammatory cell infiltration; CI includes glomerulosclerosis, fibrillar crescents, interstitial fibrosis and renal tubular atrophy. Each lesion was scored 0 to 3. The AI was 0 to 24 points, and the CI was 0 to 12 points.
Statistical methods
Normally distributed measures are expressed as the mean ± standard deviation (SD), t-tests were used for group comparisons, and Pearson’s analysis was used for correlations. Non-normally distributed data are expressed as median and quartiles [P50 (P25, P75)], with the Mann–Whitney U test for group comparisons and Spearman’s analysis for correlations. Count data were expressed as frequencies and rates, and comparisons of rates were made using the cube test. The Kaplan–Meier method was used to plot survival curves for renal survival analysis, and the log-rank test was used for comparison of survival rates. IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA) was used for data processing, and P < 0.05 was considered to indicate a statistically significant difference.
Results
Comparison of serum sCD163 between the two groups
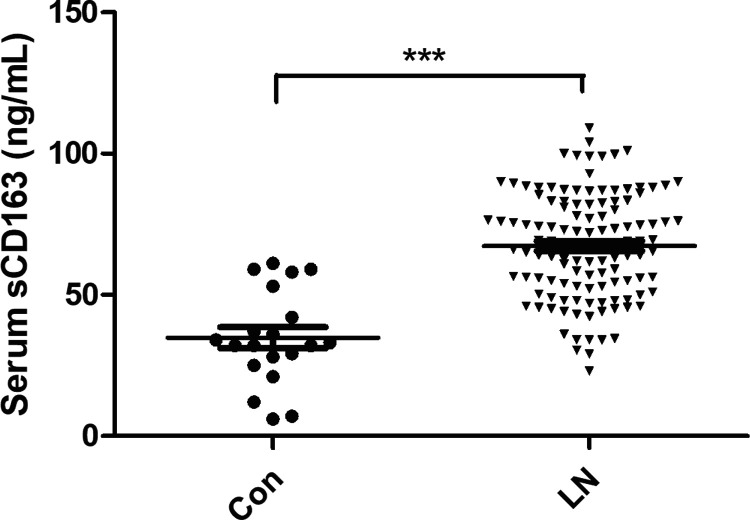
In the present study, we defined a measured value greater than the mean + 2SD of healthy controls (69.7 ng/mL) as a high serum sCD163 level and divided 121 patients with LN into a normal sCD163 group (72 patients) and a high sCD163 group (49 patients). Serum sCD163 levels were measured by ELISA in all patients with LN and 20 healthy controls. sCD163 levels in the LN group were higher than those in the control group (67.04 ± 18.70 and 34.98 ± 17.49 ng/mL, respectively), and the difference was statistically significant (P < 0.001) (Figure 1).
Figure 1.
Serum sCD163 in LN and Con groups. Patients with LN who underwent renal biopsy were included as the study subjects (n = 121), and healthy individuals (n=20) were selected as the Con group. Serum sCD163 levels were measured by enzyme-linked immunosorbent assays. ***P < 0.001.
sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis; Con: control.
Patient characteristics
The clinical data of patients with LN in the normal sCD163 group and high sCD163 group are shown in Table 1. All participants were between 21 and 54 years old. The age and gender differences between the two groups were not statistically significant. Compared with patients with LN in the normal sCD163 group, patients in the high sCD163 group had significantly elevated 24-hour urine protein levels, SCr and BUN (all P < 0.05) and decreased eGFR (P < 0.001). The SLEDAI scores and positivity rates of C3, C4, IgA, IgM, IgG, anti-dsDNA, anti-Sm, anti-SSA, anti-SSB and anti-RNP were not statistically different between the two groups.
Table 1.
Comparison of clinical data between the high sCD163 group and normal sCD163 group in patients with LN.
| High sCD163 group | Normal sCD163 group | t/Z/χ2 | P | |
|---|---|---|---|---|
| N (men) | 49 (6) | 72 (7) | 0.193 | 0.660 |
| Age (years) | 36.88 ± 14.03 | 33 ± 12.67 | −1.034 | 0.243 |
| SLEDAI scores | 14.55 ± 5.02 | 13.23 ± 4.70 | −1.31 | 0.221 |
| 24-h urinary protein (g/24 h) | 3.42 ± 0.83 | 2.46 ± 0.66 | −3.35 | 0.033 |
| SCr (μmol/L) | 80.26 ± 23.75 | 56.75 ± 20.08 | −4.008 | <0.001 |
| BUN (mmol/L) | 10.01 ± 5.87 | 6.18 ± 3.90 | −5.233 | <0.001 |
| eGFR [mL · min−1·(1.73 m2) −1] | 70.32 ± 28.36 | 101.24 ± 30.02 | 6.231 | <0.001 |
| C3 (mg/L) | 0.53 ± 0.19 | 0.56 ± 0.17 | 0.401 | 0.685 |
| C4 (mg/L) | 0.17 ± 0.09 | 0.16 ± 0.08 | 0.872 | 2.021 |
| IgA (g/L) | 2.62 ± 1.08 | 2.40 ± 0.87 | −0.985 | 0.624 |
| IgM (g/L) | 1.24 ± 0.55 | 1.20 ± 0.64 | −0.651 | 0.722 |
| IgG (g/L) | 11.04 ± 3.32 | 12.27 ± 4.05 | 0.505 | 0.665 |
| Anti-dsDNA antibody (%) | 30 (61.22) | 40 (55.56) | 0.384 | 0.535 |
| Anti-Sm antibody (%) | 17 (34.69) | 28 (40.28) | 0.220 | 0.639 |
| Anti-SSA antibody (%) | 32 (65.31) | 48 (66.67) | 0.024 | 0.877 |
| Anti-SSB antibody (%) | 7 (14.29) | 8 (11.11) | 0.271 | 0.603 |
| Anti-RNP antibody (%) | 23 (46.94) | 35 (48.61) | 0.033 | 0.857 |
Data are the mean ± standard deviation. sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis; SLEDAI scores: SLE Disease Activity Index; SCr: serum creatinine; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate; C3: complement 3; C4: complement 4; IgA: immunoglobulin A; IgM: immunoglobulin M; IgG: immunoglobulin G; anti-Sm: anti-Smith; anti-dsDNA: anti-double stranded DNA; anti-SSA: anti-Sjögren’s-syndrome-related antigen A; anti-SSB: anti-Sjögren’s-syndrome-related antigen B; anti-RNP: anti-ribonucleoprotein.
In the high sCD163 group, the baseline glucocorticoid treatment rate was 100%, and the baseline immunosuppressive treatment rate was 95.91%, including 69.39% who received cyclophosphamide and 53.06% who received hydroxychloroquine. In addition, 48.98% of patients were using one immunosuppressant at baseline, 46.94% were using two immunosuppressants, and 1.63% were using three or more immunosuppressants. In the normal sCD163 group, the baseline glucocorticoid treatment rate was 100%, and the baseline immunosuppressive treatment rate was 93.88%, including 65.31% who received cyclophosphamide and 55.10% who received hydroxychloroquine. Moreover, 53.06% of patients were treated with one immunosuppressive agent, 42.86% with two immunosuppressive agents, and 2.65% with three or more immunosuppressive agents. There was no significant difference in baseline rates between the two groups.
Renal pathological grades
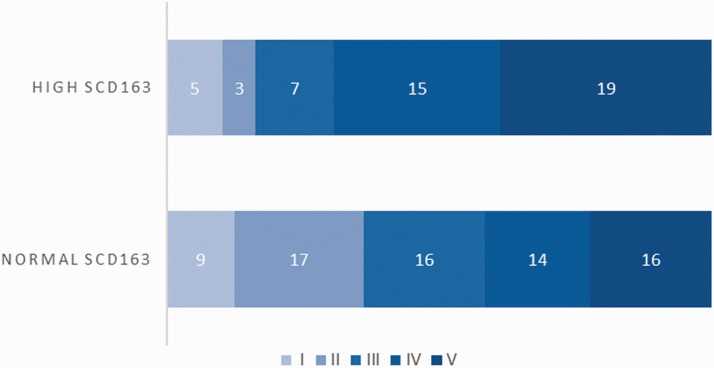
The renal pathological classification of all patients with LN was obtained by renal biopsy, and according to the pathological classification of LN established by the ISN/RPS in 2018, 14 patients with LN were divided into six grades: mild lesion grade (I), mesenteric hyperplasia grade (II), focal segmental grade (III), diffuse hyperplasia grade (IV), membranous grade (V) and sclerosis grade (VI). Because the number of LN cases with V + III (three cases) and V + IV (two cases) in this study was small, both grades were classified as grade V. The distribution of LN grades I, II, III, IV and V in the high sCD163 group and normal sCD163 group are shown in Figure 2. Patients with LN in the high sCD163 group had a higher pathological classification grade compared with those in the normal sCD163 group, and the difference was statistically significant (Mann–Whitney U value = 1264, P = 0.007).
Figure 2.
Grades of renal pathology in patients with high sCD163 and normal sCD163. One hundred twenty-one patients with LN were divided into a normal sCD163 group (72 patients) and a high sCD163 group (49 patients). The renal pathological classification of all patients with LN was obtained by renal biopsy, and patients were divided into grades I (mild lesion grade), II (mesenteric hyperplasia grade), III (focal segmental grade), IV (diffuse hyperplasia grade) and V (membranous grade).
sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis.
Renal pathology scores
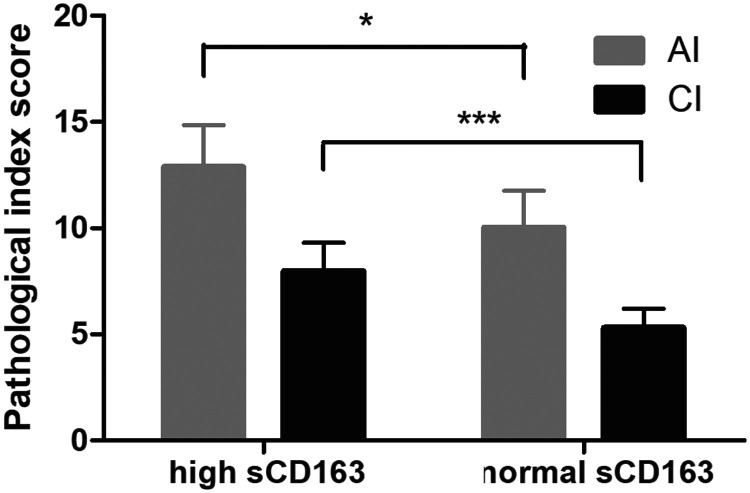
The AI and CI scores of renal lesions in patients with LN were analyzed by a semi-quantitative method. The AI was scored 0 to 24 points, and the CI was scored 0 to 12 points. The AI and CI scores of patients with high sCD163 levels were increased compared with those of patients with normal sCD163 levels. The difference was statistically significant (all P < 0.05), as shown in Figure 3.
Figure 3.
Comparison of renal pathological scores between the high sCD163 group and normal sCD163 group. One hundred twenty-one patients with LN were divided into a normal sCD163 group (72 patients) and a high sCD163 group (49 patients). The AI and CI scores of renal lesions in patients with LN were analyzed by a semi-quantitative method. The AI was scored 0 to 24 points, and the CI was scored 0 to 12 points. *P < 0.05 and ***P < 0.001.
sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis; AI: activity index; CI: chronicity index.
Correlation between serum sCD163 and renal injury index parameters in patients with LN
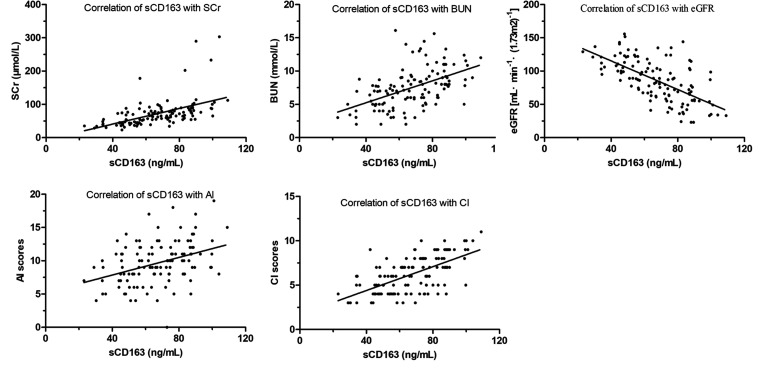
Renal injury was assessed by SCr, BUN, eGFR and pathological indicators, including the pathological grade and renal pathology score. The correlation analysis showed that sCD163 levels in patients with LN were positively correlated with SCr (r = 0.521, P < 0.01), BUN (r = 0.511, P < 0.01), AI (r = 0.387, P < 0.01) and CI (r = 0.611, P < 0.01) and negatively correlated with eGFR (r = −0.632, P < 0.01), as shown in Figure 4.
Figure 4.
Correlation analysis of serum sCD163 and renal injury index parameters in patients with LN. All subjects fasted for at least 8 hours, and blood samples were obtained for biochemical analyses. Spearman’s analysis was performed to determine the correlations between renal injury parameters and sCD163 levels.
sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis; SCr: serum creatinine; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate; AI: activity index; CI: chronicity index.
sCD163 as a predictive marker of renal endpoint events
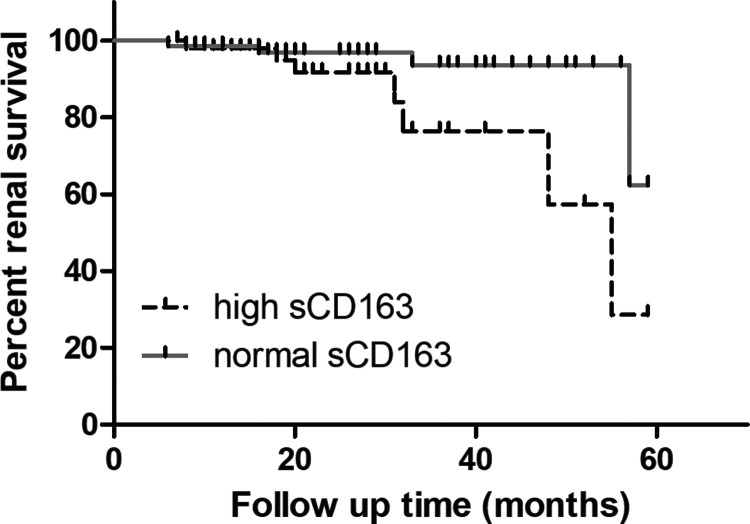
Renal endpoints in this study were defined as the occurrence of ESRD, eGFR reduction >50% or ≥2-fold increase in SCr. After a mean follow-up of 25 (10, 34) months, eight patients showed a 2-fold increase in SCr, and three patients developed ESRD. The renal survival curves of patients in the high sCD163 group and normal sCD163 group are shown in Figure 5. The renal survival rate of patients in the high sCD163 group was lower than that in the normal sCD163 group (85.71% vs 94.44%, respectively, P = 0.017) (Figure 5).
Figure 5.
Kaplan–Meier survival curves of renal outcome events in both groups. One hundred twenty-one patients with LN were divided into a normal sCD163 group (72 patients) and a high sCD163 group (49 patients). The renal survival rate was determined by Kaplan–Meier analysis.
sCD163: soluble scavenger receptor differentiation antigen 163; LN: lupus nephritis.
Discussion
Transmembrane glycoprotein CD163, a scavenger receptor expressed in monocytes and macrophages, is an M2 macrophage serum marker. In vivo, CD163 exists in two forms: a transmembrane macromolecule on mononuclear/macrophage membranes [membrane chimeric CD163 (mCD163)] and sCD163 in blood or other tissue fluids. sCD163 is a shedding product of mCD163. The expression of CD163 is influenced by several factors. For example, interleukin 6 (IL6), IL10 and glucocorticoids stimulate the expression of CD163, whereas tumor necrosis factor-α, interferon-γ (IFN-γ) and platelet factor 4 inhibit the expression of CD163. 15 During acute and chronic inflammatory responses, CD163 is shed from the surface of activated macrophages and released into the peripheral blood in a soluble form. Thus, serum sCD163 is a marker of macrophage activation.
In the present study, serum sCD163 was measured in peripheral blood samples by ELISA and found to be significantly higher in patients with LN than in healthy controls, which is consistent with the studies by Nakayama et al. 9 and Zizzo et al. 10 Recent studies16,17 reported not only significantly elevated serum sCD163 levels in patients with SLE but also significantly increased skin and kidney CD163+ macrophage infiltration, and researchers have proposed that CD163 might be involved in the pathogenesis of SLE. We found that serum sCD163 was significantly elevated in patients with LN, indicating that macrophage activation is involved in the development of LN and that sCD163 may act as an inflammatory mediator in the immunopathogenic process of LN. Macrophages may participate in the pathogenesis of LN through the following mechanisms. First, macrophages express specific markers on their surface, recognize the surrounding environment, and respond to endogenous and exogenous stimuli to influence the immune response and participate in the pathogenesis of LN. 18 Second, dysregulated cytokine secretion aberrantly activates and enhances the adaptive immune response. Macrophages promote T- and B-cell activation and are the main source of these cytokines, such as IL-6 and IL-10, which stimulate IgG and IFN-1 production, thereby inducing B cell differentiation into antibody-secreting plasma cells. 19 In addition, B-cell activating factor is mainly derived from macrophages and promotes B-cell survival and proliferation. 20 Third, functional defects in macrophage phagocytosis and pathogen clearance and apoptotic and necrotic cells may provide autoantigens for the development of LN. The markedly impaired clearance by macrophages in patients with LN may be related to the decreased expression of CD44 on the surface of macrophages, 21 reduced differentiation of CD34+ hematopoietic stem cells into mature macrophages 22 and decreased complement levels. 23 Thus, macrophage dysfunction plays an increasingly important role in the pathogenesis of LN, and the specific mechanisms require further investigation.
Our study found that patients with LN in the high sCD163 group had elevated SCr and BUN and decreased eGFRs compared with those in the normal sCD163 group. Furthermore, we found that eight patients showed a 2-fold increase in SCr, and three patients developed ESRD. These results reveal that serum sCD163 levels reflect the severity of kidney damage in LN, suggesting that serum sCD163 may be important for the evaluation of kidney damage severity in LN. Urinary protein not only reflects the presence of glomerular lesions in patients with LN but also has a direct toxic effect on renal mesangial cells and tubules, leading to the accumulation of extracellular matrix and aggravating renal damage. 24 We found that the 24-hour urinary protein level in the high sCD163 group was significantly increased compared with that in the normal sCD163 group. The SLEDAI score is commonly used in clinics to evaluate SLE disease activity, and with a gradual increase in the SLEDAI score, the activity level of lupus becomes increasingly intense. 14 We found no statistically significant difference in SLEDAI scores between the high sCD163 group and normal sCD163 group. This may be because there are several factors included in the SLEDAI scoring criteria, and renal factors account for a small proportion. The results of our study were similar to those of Nakayama et al. 9 In contrast, Zizzo et al. 10 found that serum sCD163 was positively correlated with SLEDAI scores in 40 patients with SLE. Activated macrophages expressing CD163 (M2) are the most abundant subtype of macrophages in renal biopsies from patients with LN. Studies 25 have shown that urinary sCD163 levels are elevated in patients with active LN and are associated with renal SLEDAI scores. A decrease in urinary sCD163 levels after treatment may help monitor the response to treatment in LN. As a specific antibody for SLE, increased titers of anti-dsDNA antibodies are associated with LN activity, and anti-dsDNA antibodies can be used as an independent risk factor for LN. Anti-RNP antibodies are a hallmark of mixed connective tissue disease and are closely associated with various clinical symptoms of autoimmune diseases, such as SLE, Sjogren’s syndrome and systemic sclerosis. 14 We found that although the high sCD163 group had an increased positivity rate of anti-dsDNA antibodies and reduced positivity rate of anti-RNP antibodies compared with the normal sCD163 group, the difference between the two was not statistically significant. These findings suggest that a high sCD163 level has a minimal effect on the positive rate of autoantibodies and that the two may play independent roles in promoting the progression of LN.
LN pathological classification and renal pathology scores are important for diagnosis, treatment and prognosis. The AI in the renal pathology score is closely related to clinical manifestations and usually reflects the degree of acute inflammatory damage to the kidney tissues. The CI is proportional to prognosis and represents the degree of chronic kidney damage. A cohort study of 1814 Chinese patients with LN identified pathological typing at renal biopsy as an independent risk factor for ESRD. 26 In this study, we analyzed the renal pathological data of 121 patients with LN and concluded that the pathological classification, AI scores and CI scores of patients in the high sCD163 group were significantly different from those in the normal sCD163 group, and further analysis indicated that there was a correlation between serum sCD163 and AI and CI scores. This result further illustrates that elevated serum sCD163 levels may be associated with the severity of renal injury and poor prognosis in patients with LN.
In conclusion, sCD163 may participate in the immunopathological process of LN as an inflammatory mediator. Serum sCD163 levels correlate with the severity of LN and are an important indicator of poor renal prognosis in patients with LN, suggesting that macrophage activation is involved in the development of LN. Serum sCD163 shows potential as an effective biological marker for LN diagnosis, differential diagnosis and prognosis evaluation and provides a new therapeutic target to reduce morbidity and improve prognosis.
Acknowledgments
We thank Dr Yang Huang, Department of Rheumatology, Affiliated Hospital of Jiangnan University, for excellent technical assistance and Dr Yingji Cao, Department of Nephrology, Affiliated Hospital of Nantong University, for help with renal biopsy assessments.
Footnotes
Author contributions: Jianhua Wu designed the study and drafted the manuscript. Guangxia Yang and Naifeng Guo participated in data collection. Jun Yin performed statistical analyses. All authors critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Nantong technology projects (MS12017002-2).
ORCID iD: Jianhua Wu https://orcid.org/0000-0001-8868-3122
References
- 1.Bomback AS. An Update on Therapies for Proliferative Lupus Nephritis: How Certain Can We Be About the Evidence? Am J Kidney Dis 2018; 72: 758–760. doi: 10.1053/j.ajkd.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillai S. T and B lymphocytes in fibrosis and systemic sclerosis. Curr Opin Rheumatol 2019; 31: 576–581. doi: 10.1097/bor.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 3.Terai S, Ueda-Hayakawa I, Nguyen CTH, et al. Palisaded neutrophilic and granulomatous dermatitis associated with systemic lupus erythematosus: possible involvement of CD163(+) M2 macrophages in two cases, and a review of published works. Lupus 2018; 27: 2220–2227. doi: 10.1177/0961203318809892 [DOI] [PubMed] [Google Scholar]
- 4.Moller HJ. Soluble CD163. Scand J Clin Lab Invest 2012; 72: 1–13. doi: 10.3109/00365513.2011.626868 [DOI] [PubMed] [Google Scholar]
- 5.Greisen SR, Moller HJ, Stengaard-Pedersen K, et al. Soluble macrophage-derived CD163 is a marker of disease activity and progression in early rheumatoid arthritis. Clin Exp Rheumatol 2011; 29: 689–692. [PubMed] [Google Scholar]
- 6.Peng QL, Zhang YL, Shu XM, et al. Elevated Serum Levels of Soluble CD163 in Polymyositis and Dermatomyositis: Associated with Macrophage Infiltration in Muscle Tissue. J Rheumatol 2015; 42: 979–987. doi: 10.3899/jrheum.141307 [DOI] [PubMed] [Google Scholar]
- 7.Fabriek BO, Moller HJ, Vloet RP, et al. Proteolytic shedding of the macrophage scavenger receptor CD163 in multiple sclerosis. J Neuroimmunol 2007; 187: 179–186. doi: 10.1016/j.jneuroim.2007.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Arai M, Mii A, Kashiwagi T, et al. The severity of glomerular endothelial cell injury is associated with infiltrating macrophage heterogeneity in endocapillary proliferative glomerulonephritis. Sci Rep 2021; 11: 13339. doi: 10.1038/s41598-021-92655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama W, Jinnin M, Makino K, et al. CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. Eur J Dermatol 2012; 22: 512–517. doi: 10.1684/ejd.2012.1756 [DOI] [PubMed] [Google Scholar]
- 10.Zizzo G, Guerrieri J, Dittman LM, et al. Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Res Ther 2013; 15: R212. doi: 10.1186/ar4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino A, Katsumata Y, Kawasumi H, et al. Usefulness of soluble CD163 as a biomarker for macrophage activation syndrome associated with systemic lupus erythematosus. Lupus 2019; 28: 986–994. doi: 10.1177/0961203319860201 [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. doi: 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, et al. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med 1999; 139: 461–470. [DOI] [PubMed] [Google Scholar]
- 14.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018; 93: 789–796. [DOI] [PubMed] [Google Scholar]
- 15.Etzerodt A, Berg RM, Plovsing RR, et al. Soluble ectodomain CD163 and extracellular vesicle-associated CD163 are two differently regulated forms of ‘soluble CD163' in plasma. Sci Rep 2017; 7: 40286. doi: 10.1038/srep40286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olmes G, Buttner-Herold M, Ferrazzi F, et al. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther 2016; 18: 90. doi: 10.1186/s13075-016-0989-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo N, Tsuboi N, Furuhashi K, et al. Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 2016; 31: 2023–2033. doi: 10.1093/ndt/gfw214 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Ptacek TS, Brown EE, et al. Fcgamma receptors: structure, function and role as genetic risk factors in SLE. Genes Immun 2009; 10: 380–389. doi: 10.1038/gene.2009.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jego G, Palucka AK, Blanck JP, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003; 19: 225–234. [DOI] [PubMed] [Google Scholar]
- 20.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 2001; 97: 198–204. doi: 10.1182/blood.v97.1.198 [DOI] [PubMed] [Google Scholar]
- 21.Cairns AP, Crockard AD, McConnell JR, et al. Reduced expression of CD44 on monocytes and neutrophils in systemic lupus erythematosus: relations with apoptotic neutrophils and disease activity. Ann Rheum Dis 2001; 60: 950–955. doi: 10.1136/ard.60.10.950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Tang J, Mok MY, et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 2003; 48: 2888–2897. doi: 10.1002/art.11237 [DOI] [PubMed] [Google Scholar]
- 23.Bijl M, Reefman E, Horst G, et al. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann Rheum Dis 2006; 65: 57–63. doi: 10.1136/ard.2005.035733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2019. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Yadav A, Aggarwal A. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clin Rheumatol 2021; 40: 941–948. doi: 10.1007/s10067-020-05343-6 [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Liang D, Zhang H, et al. Long-term renal outcomes in a cohort of 1814 Chinese patients with biopsy-proven lupus nephritis. Lupus 2015; 24: 1468–1478. doi: 10.1177/0961203315593166 [DOI] [PubMed] [Google Scholar]