Abstract
Objective
To explore whether dihydroartemisinin (DHA) can block interleukin (IL)-6-induced epithelial–mesenchymal transition (EMT) in laryngeal squamous cell carcinoma (LSCC).
Methods
The expression of SLUG, signal transducer and activator of transcription 3 (STAT3), and microRNA (miR)-130b-3p was measured. In addition, a dual-luciferase reporter assay was performed to examine the interaction of miR-130b-3p with STAT3.
Results
We found that IL-6 can promote EMT and invasion in LSCC cells, whereas DHA can inhibit these two processes. However, DHA alone does not influence EMT and cancer invasion. Furthermore, DHA upregulated miR-130b-3p, which can downregulate STAT3 and β-catenin protein expression and decrease the activity of the IL-6/STAT3 signaling pathway. Moreover, we found that miR-130b-3p can target STAT3 directly.
Conclusions
DHA can block IL-6-triggered EMT and invasion in LSCC, and during these processes, DHA increases miR-130b-3p expression to decrease the activation of the IL-6/STAT3 and β-catenin signaling pathways. These findings may provide new insights into strategies for suppressing and even preventing LSCC metastasis.
Keywords: Dihydroartemisinin, microRNA-130b-3p, signal transducer and activator of transcription 3, β-catenin, laryngeal squamous cell carcinoma, epithelial–mesenchymal transition, interleukin-6
Introduction
Laryngeal cancer affects millions of people worldwide, and the most common type is laryngeal squamous cell carcinoma (LSCC). The risk factors for laryngeal cancer include alcohol consumption, smoking, and human papillomavirus infection. 1 Current management options, depending on the stage, include surgery, chemotherapy, and radiotherapy.2,3 It has been reported that 3.21% of patients have distant metastasis, 4 especially in the lungs and brain. In addition, it was stated that patients with laryngeal cancer and distant metastasis have poorer prognosis, and the 3-year survival rate is estimated to be 30%. 4 To date, many studies have revealed the mechanisms of laryngeal cancer metastasis, including epithelial–mesenchymal transition (EMT). During this process, cell–cell adhesion and cellular polarity are lost, and cancer cells obtain the ability to migrate and invade. 5 There are multiple factors that can induce EMT in cancer cells, such as chemotherapy, hypoxia, and cytokines. 6 Of these factors, interleukin (IL)-6 has been discovered to promote EMT in various cancers, including breast cancer, 7 but its role in laryngeal cancer has not been extensively explored. However, one study found that the serum concentration of IL-6 in patients with laryngeal cancer was much higher than that in healthy individuals and that the serum IL-6 concentration was positively correlated with the progression of laryngeal cancer. 8 Therefore, we hypothesized that IL-6 may be involved in the progression and metastasis of laryngeal cancer.
Dihydroartemisinin (DHA) is a derivative of artemisinin that is currently used to treat malaria. Clinical and experimental studies have found that the drug can alleviate arthritis by suppressing inflammation. 9 In addition, DHA downregulated IL-6 to reduce inflammation in a rat arthritis model. 10 Apart from its anti-inflammatory effects, DHA exerts anti-cancer effects. It can induce apoptosis in leukemic cells via Noxa-mediated pathways. 11 Furthermore, it can suppress the invasion, migration, and growth of gastric cancer cells by decreasing the activation of phosphoinositide 3-kinase/protein kinase B and Snail. 12 For these reasons, we aimed to determine whether DHA can counteract IL-6-induced EMT in laryngeal cancer.
DHA has been demonstrated to exert its anti-inflammatory and anti-cancer effects through microRNAs (miRNAs). For example, DHA decreases inflammation in vascular smooth muscle cells by regulating the miR-376b-3p/KLF15 pathway. 13 DHA also inhibits prostate cancer cells through the Jumonji and AT-rich interactive domain 2/miR-7/miR-34a-dependent downregulation of AXL tyrosine receptor kinase. 14 Notably, miRNAs participate in EMT in laryngeal cancer. For instance, miR-217 can block EMT in laryngeal cancer, 15 whereas miR-10b can stimulate EMT in laryngeal cancer. 16 miR-130b-3p is considered a tumor suppressor because it can decrease growth, angiogenesis, migration, and invasion in cancer.17,18 However, its role in EMT has rarely been explored.
This study examined whether DHA can counteract IL-6-induced EMT in laryngeal cancer.
Materials and methods
Cells and cell culture
This study was approved by the Ethics Committee of Bethune International Peace Hospital (approval number: 2017-KY-02). AMC-HN-8 and Tu212 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco, Thermo Fischer Scientific, Waltham, MA, USA). The cells were cultured in an incubator with 5% CO2 at 37°C and used for experiments when their confluence reached approximately 70%.
Cell viability assay
The viability of AMC-HN-8 and Tu212 cells was measured using the Cell Counting Kit-8 (CCK-8) assay (MedChemExpress, Monmouth Junction, NJ, USA). Briefly, the cells were seeded into 96-well plates at a density of approximately 3 × 104 cells/well. Next, CCK-8 solution (10 μL) was added to each well, and the plates were placed in an incubator (37°C) for 1 hour. Finally, the absorbance of each sample was determined at 450 nm.
Western blotting
The cells were lysed using radioimmunoprecipitation assay buffer on ice to extract the total protein. The samples were placed onto 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (Weiao Biotechnology, Shanghai, China) and subjected to electrophoresis. Next, the separated proteins in the gels were transferred onto polyvinylidene fluoride membranes (Thermo Fisher Scientific) and blocked with 4% bovine serum albumin. Thereafter, these membranes were incubated with primary antibodies at 4°C overnight. After incubation, the blots were blocked with secondary antibodies at 24°C for 1 hour. The protein bands were visualized with High-sig Electrochemiluminescence Western Blotting Substrate (Tenon, Shanghai, China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control for the blots.
Reagents and antibodies
DHA was purchased from MedChemExpress and dissolved in dimethyl sulfoxide. Recombinant IL-6 protein was purchased from Abcam (ab208325; Cambridge, UK). Primary antibodies against E-cadherin (1:1000; ab40772; Abcam), SLUG (1:1000; ab51772; Abcam), Snail (1:1000; ab216347; Abcam), signal transducer and activator of transcription 3 (STAT3, 1:1000; ab68153; Abcam), phosphorylated (p)-STAT3 (Ser727; 1:1000; ab32143; Abcam), β-catenin (1:1000; ab32572; Abcam), and GAPDH (1:1000; ab8245; Abcam) were employed in this research.
Reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was obtained from LSCC cells using Trizol reagent (TaKaRa Bio, Shiga, Japan). miRNA was extracted using a Molpure Cell/Tissue miRNA Kit (Yeasen, Shanghai, China). In addition, mRNA was transcribed using SuperScript IV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). miRNAs were transcribed using a TaqMan MicroRNA Reverse Transcription Kit (Invitrogen). The mRNA expression of genes in this research was determined using a SYBR Premix Ex Taq II kit (Tli RNase H Plus; TaKaRa Bio). U6 small nucleolar RNA and GAPDH were used as endogenous controls for miR-130b-3p and the other genes, respectively. The primers used in this research are presented in Table 1.
Table 1.
Sequences of primers used in this study.
| Gene | Primer 5′→3′ |
|---|---|
| SLUG | F: TGTGACAAGGAATATGTGAGCC |
| R: TGAGCCCTCAGATTTGACCTG | |
| STAT3 | F: ATCACGCCTTCTACAGACTGC |
| R: CATCCTGGAGATTCTCTACCACT | |
| miR-130b-3p | F: CCCGACACTCTTTCCCTGT |
| R: GACCGATGCCCTTTCATCATTG | |
| GAPDH | F: GGAGCGAGATCCCTCCAAAAT |
| R: GGCTGTTGTCATACTTCTCATGG | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
F, forward primer; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; R, reverse primer; STAT3, signal transducer and activator of transcription 3.
Dual-luciferase reporter assay
When the cell confluence reached approximately 60%, cells cultured in 96-well plates were transfected or co-transfected with pGL3-STAT3-wild-type (WT) and pGL3-STAT3-mutant (MUT) as well as negative control (NC) mimics, miR-130b-3p mimics, NC inhibitors, or miR-130b-3p inhibitors. After 48 hours of transfection, luciferase activity was determined according to the manufacturer’s instructions (Promega, Madison, WI, USA).
Transwell assay
The Transwell assay was used in this research to evaluate invasion. Initially, Matrigel (Corning, Corning, NY, USA) was placed on top of the Transwell insert filter membrane (Millipore, Burlington, MA, USA) and incubated at 37°C for 30 minutes. Next, 100 μL of the cell suspension (1 × 106 cells/mL) were placed on the Transwell insert filter membrane and allowed to settle for 15 minutes. In the meantime, IL-6 (20 ng/mL) was added to the bottom of the lower chamber. After 48 hours, the culture medium, Transwell insert, and Matrigel on the filter membrane were carefully removed. Thereafter, 70% ethanol was added to each well, and the Transwell insert was immersed into ethanol for fixation. Crystal violet (0.2%) was added to each well to stain the cells in the filter membrane. Finally, after the crystal violet solution was carefully removed, the stained cells were visualized and numbered under an inverted microscope.
Assessment of binding between miR-130b-3p and STAT3
starBase (http://starbase.sysu.edu.cn) was used to predict the possible binding sequence between miR-130b-3p and STAT3. WT and MUT luciferase constructs (pGL3-STAT3-WT and pGL3-STAT3-MUT) were created. Next, AMC-HN-8 and Tu212 cells were seeded in 24-well plates at a density of 2 × 106 cells per well. The cells were transfected with pGL3-STAT3-WT or pGL3-STAT3-MUT and then treated with an NC mimic, an miR-130b-3p mimic, an NC inhibitor, or an miR-130b-3p inhibitor. Next, luciferase activity was examined using the Dual-Luciferase® Reporter Assay System according to the manufacturer’s instructions (Promega, Madison, WI, USA).
Statistical analysis
Each experiment in this study was independently performed five times, and all data were analyzed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA). Data are expressed as the mean ± standard deviation. The statistical significance between comparisons was assessed using Student’s t-test and one-way analysis of variance followed by the Bonferroni post hoc test. Two-tailed P < 0.05 was considered statistically significant.
Results
IL-6 may upregulate SLUG mRNA expression in LSCC cells
To determine the appropriate concentration of IL-6 for inducing the EMT, we treated cells with different IL-6 concentrations for 24 hours and assessed SLUG mRNA expression using RT-qPCR because this gene is involved in EMT and is regulated by IL-6 via the STAT3 signaling pathway.19,20 As presented in Figure 1a, IL-6 upregulated the mRNA expression of SLUG in LSCC cells in a concentration-dependent manner (P < 0.05), and at an IL-6 concentration of 20 ng/mL, SLUG mRNA expression was increased significantly. For this reason, 20 ng/mL IL-6 was used in our experiments. Next, the cells were treated with DHA for 24 hours, and cell viability was evaluated using the CCK-8 assay. DHA decreased cell viability in a concentration-dependent manner (Figure 1b). After the concentration of IL-6 was determined, the cells were treated with various DHA concentrations for 24 hours to evaluate the effects on the mRNA expression of SLUG. However, DHA did not alter SLUG mRNA expression (Figure 1c). Considering the cell viability results and mRNA expression of SLUG, 30 μM DHA was used in our subsequent experiments. These findings indicate that IL-6 can induce EMT in LSCC, but DHA alone may not inhibit this process.
Figure 1.
DHA downregulates the mRNA expression of SLUG in IL-6-treated LSCC cells. (a) SLUG mRNA expression in cells following IL-6 stimulation. (b) Relative cell viability was examined using the CCK-8 assay after AMC-HN-8 and Tu212 cells were treated with DHA. (c) SLUG mRNA expression in two LSCC cell lines upon treatment with different DHA concentrations. NS, non-significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control group (0 ng/mL IL-6). NS, non-significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control group (0 ng/mL DHA).
CCK-8, Cell Counting Kit-8; DHA, dihydroartemisinin; IL-6, interleukin 6; LSCC, laryngeal squamous cell carcinoma.
DHA inhibits IL-6-induced EMT and invasion in LSCC cells
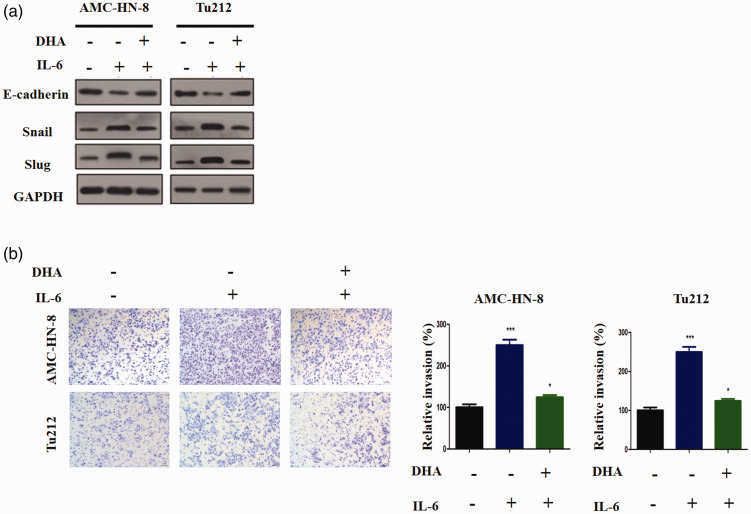
LSCC cells were co-treated with IL-6 and DHA for 24 hours. Following IL-6 treatment, the cells displayed lower E-cadherin expression but higher Snail and SLUG protein expression (Figure 2a). However, compared with the findings for treatment with IL-6 alone, E-cadherin protein expression was much higher in the IL-6/DHA co-treatment group, and both Snail and SLUG protein expression was downregulated. Therefore, DHA appears to block IL-6-induced EMT in LSCC. In addition, the Transwell assay was performed in our research to evaluate cellular invasion. The results illustrated that IL-6 increased the invasiveness of these cells (P < 0.001), whereas DHA decreased IL-6-triggered cellular invasion (P < 0.05, Figure 2b). Thus, we conclude that IL-6-induced EMT and invasion in LSCC may be blocked via DHA.
Figure 2.
DHA inhibits EMT and invasion triggered by IL-6. (a) Western blots for E-cadherin, Snail, and SLUG. (b) The Transwell assay was used examine the invasive properties of AMC-HN-8 and Tu212 cells following different treatments. NS, non-significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control group.
DHA, dihydroartemisinin; EMT, epithelial–mesenchymal transition; IL-6, interleukin 6.
DHA upregulates miR-130b-3p in LSCC cells
It has been reported that miR-130b-3p can suppress cancer growth; 17 thus, we opted to focus on this miRNA. First, the cells were treated with DHA alone, and the RT-qPCR results revealed that DHA increased miR-130b-3p expression (P < 0.001, Figure 3a). Next, the two cell lines were exposed to IL-6, and we found that IL-6 moderately downregulated miR-130b-3p expression (P < 0.05, Figure 3b). Next, the cells were subjected to IL-6 and DHA co-treatment, and our findings suggested that the expression of miR-130b-3p was upregulated in the IL-6/DHA co-treatment group (P < 0.01, Figure 3c). Thus, DHA can increase miR-130b-3p expression.
Figure 3.
DHA upregulates miR-130b-3p expression in LSCC. (a) miR-130b-3p expression in LSCC cells following DHA treatment. (b) miR-130b-3p expression level in LSCC cells following IL-6 treatment. (c) miR-130b-3p expression in LSCC cells following IL-6/DHA co-treatment. *P < 0.05 and ***P < 0.001 versus the control group.
DHA, dihydroartemisinin; IL-6, interleukin 6; LSCC, laryngeal squamous cell carcinoma.
Upregulating miR-130b-3p inhibits IL-6-induced EMT and invasion in LSCC cells
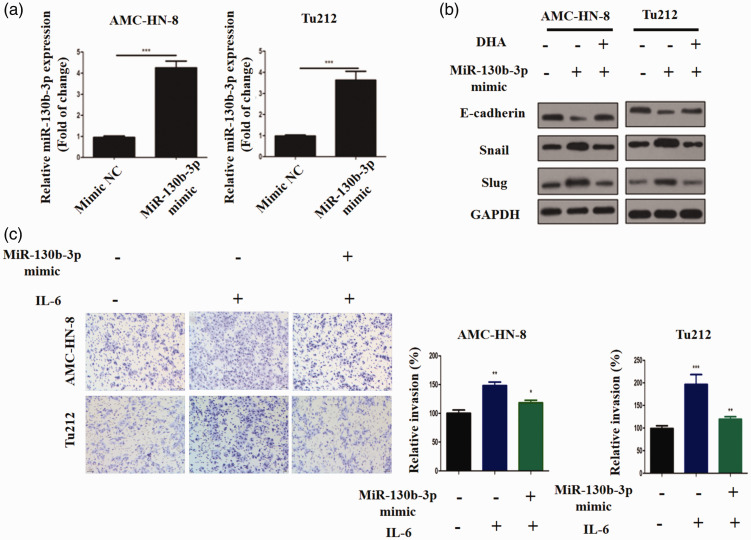
To determine whether miR-130b-3p can regulate EMT and invasion in LSCC, AMC-HN-8 and Tu212 cells were transfected with miR-130b-3p mimics, which resulted in increased miR-130b-3p expression (P < 0.001, Figure 4a). miR-130b-3p overexpression decreased SLUG protein expression in IL-6-treated cells but increased the protein expression of E-cadherin (Figure 4b). Moreover, miR-130b-3p-overexpressing AMC-HN-8 and Tu212 cells treated with IL-6 were less invasive than their respective NC counterparts (P < 0.05, Figure 4c). Collectively, these results suggest that DHA may inhibit IL-6-triggered EMT and invasion in LSCC via miR-130b-3p.
Figure 4.
Upregulation of miR-130b-3p inhibits IL-6-induced EMT and invasion in LSCC. (a) miR-130b-3p expression was quantified by RT-qPCR after transfection with miR-130b-3p or NC mimics. (b) The protein expression of E-cadherin, Snail, and SLUG in LSCC cells after transfection with miR-130b-3p or NC mimics and treatment with DHA. (c) The Transwell assay was performed to evaluate the invasiveness of LSCC cells. **P < 0.01 and ***P < 0.001 versus the NC mimic group. NS, non-significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control group.
DHA, dihydroartemisinin; EMT, epithelial–mesenchymal transition; NC, negative control; LSCC, laryngeal squamous cell carcinoma; RT-qPCR, real time quantitative polymerase chain reaction.
DHA inhibits the IL-6/STAT3 signaling pathway
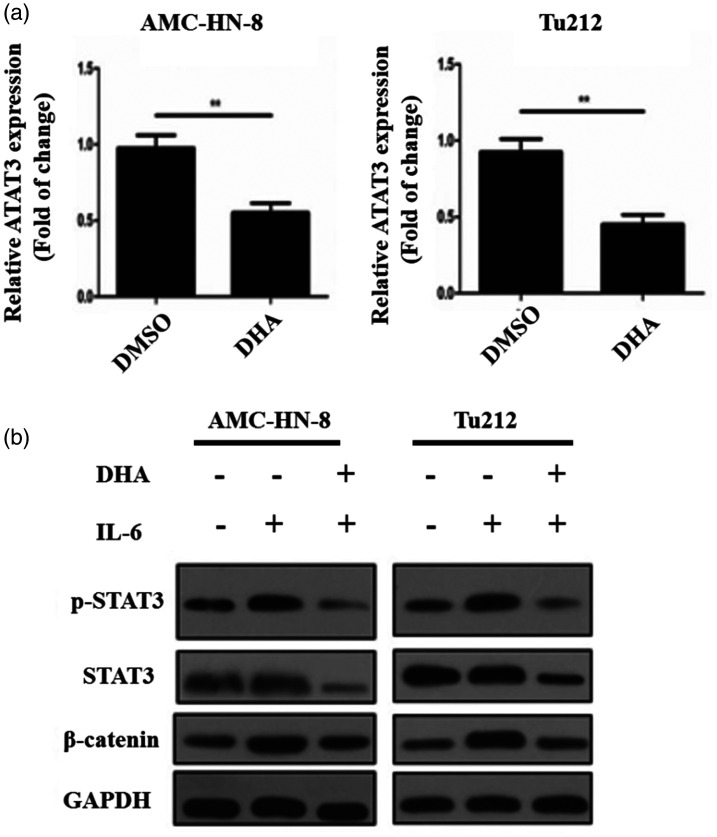
IL-6 has been found to activate STAT3, a primary downstream effector that mediates IL-6-induced EMT, to exert its biologic effects. 21 As illustrated in Figure 5a, STAT3 mRNA expression was downregulated by DHA exposure (P < 0.01). Then, we explored whether DHA influences this signaling pathway. When LSCC cells were exposed to IL-6, the activity of STAT3 and β-catenin was increased (Figure 5b). However, in the IL-6/DHA co-treatment group, the protein expression of STAT3 and β-catenin was considerably decreased, and p-STAT3 protein expression was much lower (Figure 5b). Therefore, we conclude that DHA downregulates STAT3 expression.
Figure 5.
DHA inhibits the IL-6/STAT3 signaling pathway. (a) The mRNA expression of STAT3 in AMC-HN-8 and Tu212 cells following DHA treatment. (b) The protein expression of STAT3, p-STAT3, and β-catenin in AMC-HN-8 and Tu212 cells. **P < 0.01 versus the control group.
DHA, dihydroartemisinin, IL-6, interleukin 6; p-STAT3, phosphorylated STAT3; STAT3, signal transducer and activator of transcription 3.
miR-130b-3p downregulates STAT3
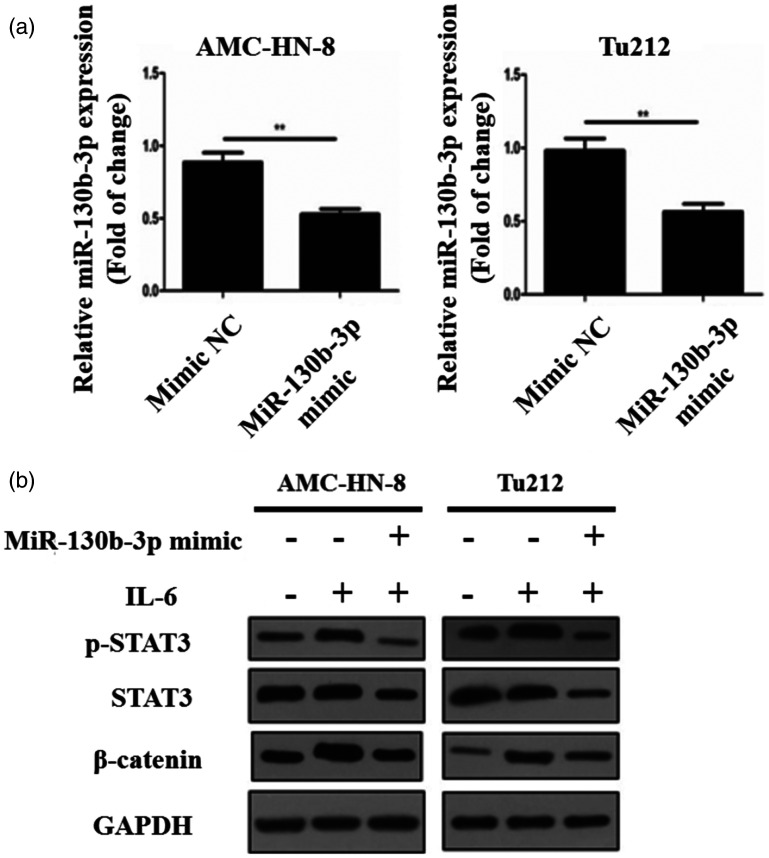
To increase our understanding of the interaction between miR-130b-3p and STAT3, miR-130b-3p-overexpressing AMC-HN-8 and Tu212 cells were treated with IL-6. Using RT-qPCR, we discovered that miR-130b-3p overexpression in these two cell lines reduced STAT3 mRNA expression (P < 0.01, Figure 6a). Next, when miR-130b-3p-overexpressing cells were treated with IL-6, these two cell lines displayed lower STAT3, p-STAT3, and β-catenin protein expression than their respective NC counterparts (Figure 6b). Thus, miR-130b-3p appears to negatively modulate the activity of STAT3 and even inhibit the activation of IL-6/STAT3 signaling.
Figure 6.
miR-130b-3p downregulates STAT3. (a) STAT3 mRNA expression in AMC-HN-8 and Tu212 cells following miR-130b-3p mimic transfection. (b) Protein expression of STAT3, p-STAT3, and β-catenin after the cells were transfected with miR-130b-3p mimics or treated with IL-6. **P < 0.01 and ***P < 0.001 versus the NC mimic group.
DHA, dihydroartemisinin, IL-6, interleukin 6; NC, negative control; p-STAT3, phosphorylated STAT3; STAT3, signal transducer and activator of transcription 3.
miR-130b-3p targets STAT3 directly
Our previous experiments demonstrated that miR-130b-3p could negatively regulate STAT3, and thus, we assumed that miR-130b-3p might target STAT3 directly. To confirm our assumption, we used starBase. The complementary sequence between miR-130b-3p and STAT3 is presented in Figure 7a. In the two cell lines, transfection with miR-130b-3p mimics suppressed luciferase activity compared with the effects of the NC mimic (P < 0.05, Figure 7b). Furthermore, transfection with miR-130b-3p inhibitors increased luciferase activity relative to the findings in the NC inhibitor group. Second, the two cell lines were co-transfected with pGL3-STAT3-MUT as well as NC mimics, miR-130b-3p mimics, NC inhibitors, or miR-130b-3p inhibitors. Transfection with miR-130b-3p mimics or miR-130b-3p inhibitors did not affect luciferase activity compared with the findings in the respective NC groups (Figure 7c). These results imply that miR-130b-3p binds STAT3 directly.
Figure 7.
miR-130b-3p targets STAT3 directly. (a) The complementary sequence between miR-130b-3p and STAT3. (b) The dual-luciferase reporter assay was performed in cells that were transfected with pGL3-STAT3-WT luciferase constructs as well as NC mimics, miR-130b-3p mimics, NC inhibitors, or miR-130b-3p inhibitors. (c) Luciferase activity was assessed in cells transfected with pGL3-STAT3-MUT luciferase constructs as well as NC mimics, miR-130b-3p mimics, NC inhibitors, or miR-130b-3p inhibitors. NS, non-significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the NC mimic group. NS, non-significant; *P < 0.05 and ***P < 0.001 versus the NC inhibitor group.
MUT, mutant; NC, negative control; STAT3, signal transducer and activator of transcription 3, WT, wild-type.
Discussion
Some patients with laryngeal cancer may present with distant metastasis. There are many hypotheses regarding the causes of distant metastasis of cancer, including cancer stem cells, EMT, and tumor niche formation. 22 It is believed that the loss of polarity and cell–cell adhesion in EMT may be the initial steps of metastasis. To prevent metastasis, numerous studies have focused on EMT regulation. The concentration of IL-6, which is correlated with poorer survival, has been found to be abnormally high in many patients with cancer. IL-6 promotes progression, proliferation, tumorigenesis, metastasis, and inflammation in cancer; thus, targeting the IL-6/STAT3 axis may be a promising direction for future cancer treatment. Siltuximab, an antibody against IL-6, has demonstrated efficacy against smoldering multiple myeloma and Castleman’s disease.23,24 In these studies, LSCC cells underwent EMT following IL-6 stimulation, implying that IL-6/STAT3 signaling participates in the metastasis of laryngeal cancer. Therefore, targeting the IL-6/STAT3 signaling pathway represents a novel potential pharmaceutical strategy against LSCC.
In addition, our study demonstrated that DHA suppresses IL-6-triggered EMT in LSCC cells by downregulating STAT3 expression and decreasing STAT3 phosphorylation. This discovery carries significance for cancer pharmacotherapy because STAT3 signaling has been revealed to promote chemoresistance, angiogenesis, invasion, and tumorigenesis and maintain the stemness of cancer stem cells. 25 Moreover, it was discovered that STAT3 phosphorylation is correlated with the progression of head and neck cancer and that high cytoplasmic p-STAT3 expression is associated with increased tumor size and metastasis in head and neck cancer. 26 In addition, non-small cell lung carcinoma with high STAT3 and p-STAT3 expression carries a poor prognosis. 27 Thus, DHA may be effective in preventing the metastasis of LSCC and prolonging patient survival.
Another finding from this research was that DHA can upregulate miR-130b-3p to reduce STAT3 protein expression and phosphorylation. Several studies indicated that miR-130b-3p can suppress invasion and migration in malignant tumors, but its interaction with STAT3 has not been reported. Our findings demonstrated that miR-130b-3p can bind to STAT3 directly and reduce its protein expression. In addition, the overexpression of miR-130b-3p can block EMT and invasion in LSCC cells. Collectively, these findings suggest that miR-130b-3p agonists may be effective against LSCC.
This study had several limitations. First, other miRNAs that are regulated by DHA to suppress STAT3 activity might exist; therefore, transcriptomic analysis should be conducted as a screening method. Second, a laryngeal cancer metastasis animal model was not established in this research. Ideally, IL-6 and DHA could be administered to rats with laryngeal cancer, and metastatic foci could be collected for further analysis. Third, although the existing medical literature implies an association between STAT3 and patient prognosis in laryngeal cancer, clinical specimens from patients and healthy individuals should be collected and analyzed to further support this result.
In conclusion, this study discovered that DHA can suppress the IL-6-induced EMT and migration in LSCC cells via the miR-130b-3p/STAT3/β-catenin pathway. Thus, DHA has the potential to prevent the metastasis of laryngeal cancer and prolong patient survival.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the Hebei province graduate student innovation fund project (No. CXZZBS2017115).
ORCID iD: Xiaoming Li https://orcid.org/0000-0002-0931-727X
References
- 1.Marur S, Forastiere AA. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc 2016; 91: 386–396. DOI: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Baird BJ, Sung CK, Beadle BM, et al. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral Oncol 2018; 87: 8–16. DOI: 10.1016/j.oraloncology.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Fong PY, Tan SH, Lim DWT, et al. Association of clinical factors with survival outcomes in laryngeal squamous cell carcinoma (LSCC). PLoS One 2019; 14: e0224665. DOI: 10.1371/journal.pone.0224665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y, Hong Y, Liang Z, et al. Survival analysis of distant metastasis of laryngeal carcinoma: analysis based on SEER database. Eur Arch Otorhinolaryngol 2019; 276: 193–201. DOI: 10.1007/s00405-018-5244-5. [DOI] [PubMed] [Google Scholar]
- 5.Pearson GW. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J Clin Med 2019; 8: 646. DOI: 10.3390/jcm8050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, You Y, Jiang H, et al. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol 2017; 232: 3261–3272. DOI: 10.1002/jcp.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Liu X, Lu Y, et al. PIM1 is responsible for IL-6-induced breast cancer cell EMT and stemness via c-myc activation. Breast Cancer 2019; 26: 663–671. DOI: 10.1007/s12282-019-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotirović J, Perić A, Vojvodić D, et al. Serum cytokine profile of laryngeal squamous cell carcinoma patients. J Laryngol Otol 2017; 131: 455–461. DOI: 10.1017/s0022215117000573. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Hou Y, Cai L, et al. The Evaluation of (68)Ga-Citrate PET/CT Imaging for Dihydroartemisinin in the Treatment of Rheumatoid Arthritis. Mol Imaging Biol 2020. DOI: 10.1007/s11307-020-01534-4. [DOI] [PubMed] [Google Scholar]
- 10.Fan M, Li Y, Yao C, et al. Dihydroartemisinin derivative DC32 attenuates collagen-induced arthritis in mice by restoring the Treg/Th17 balance and inhibiting synovitis through down-regulation of IL-6. Int Immunopharmacol 2018; 65: 233–243. DOI: 10.1016/j.intimp.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Zhong H, Wang R, et al. Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 2015; 6: 5582–5596. DOI: 10.18632/oncotarget.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Meng X, Han J, et al. Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour Biol 2013; 34: 3791–3800. DOI: 10.1007/s13277-013-0963-0. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Gao X, Sun Y, et al. Dihydroartemisinin alleviates high glucose-induced vascular smooth muscle cells proliferation and inflammation by depressing the miR-376b-3p/KLF15 pathway. Biochem Biophys Res Commun 2020; 530: 574–580. DOI: 10.1016/j.bbrc.2020.07.095. [DOI] [PubMed] [Google Scholar]
- 14.Paccez JD, Duncan K, Sekar D, et al. Dihydroartemisinin inhibits prostate cancer via JARID2/miR-7/miR-34a-dependent downregulation of Axl. Oncogenesis 2019; 8: 14. DOI: 10.1038/s41389-019-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao S, Mao X, Zhao S, et al. miR-217 inhibits laryngeal cancer metastasis by repressing AEG-1 and PD-L1 expression. Oncotarget 2017; 8: 62143–62153. DOI: 10.18632/oncotarget.19121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Sun J, Wang B, et al. MicroRNA-10b Triggers the Epithelial-Mesenchymal Transition (EMT) of Laryngeal Carcinoma Hep-2 Cells by Directly Targeting the E-cadherin. Appl Biochem Biotechnol 2015; 176: 33–44. DOI: 10.1007/s12010-015-1505-6. [DOI] [PubMed] [Google Scholar]
- 17.Shui Y, Yu X, Duan R, et al. miR-130b-3p inhibits cell invasion and migration by targeting the Notch ligand Delta-like 1 in breast carcinoma. Gene 2017; 609: 80–87. DOI: 10.1016/j.gene.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Liao Y, Wang C, Yang Z, et al. Dysregulated Sp1/miR-130b-3p/HOXA5 axis contributes to tumor angiogenesis and progression of hepatocellular carcinoma. Theranostics 2020; 10: 5209–5224. DOI: 10.7150/thno.43640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Chen Y, An T, et al. Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway. J Exp Clin Cancer Res 2019; 38: 139. DOI: 10.1186/s13046-019-1134-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Lin JC, Tsai JT, Chao TY, et al. The STAT3/Slug Axis Enhances Radiation-Induced Tumor Invasion and Cancer Stem-like Properties in Radioresistant Glioblastoma. Cancers (Basel) 2018; 10: 512. DOI: 10.3390/cancers10120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taher MY, Davies DM, Maher J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc Trans 2018; 46: 1449–1462. DOI: 10.1042/bst20180136. [DOI] [PubMed] [Google Scholar]
- 22.Doak GR, Schwertfeger KL, Wood DK. Distant Relations: Macrophage Functions in the Metastatic Niche. Trends Cancer 2018; 4: 445–459. DOI: 10.1016/j.trecan.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brighton TA, Khot A, Harrison SJ, et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin Cancer Res 2019; 25: 3772–3775. DOI: 10.1158/1078-0432.Ccr-18-3470. [DOI] [PubMed] [Google Scholar]
- 24.Deisseroth A, Ko CW, Nie L, et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res 2015; 21: 950–954. DOI: 10.1158/1078-0432.Ccr-14-1678. [DOI] [PubMed] [Google Scholar]
- 25.Galoczova M, Coates P, Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett 2018; 23: 12. DOI: 10.1186/s11658-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen-Kaplan V, Jrbashyan J, Yanir Y, et al. Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer. J Biol Chem 2012; 287: 6668–6678. DOI: 10.1074/jbc.M111.271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol 2014; 40: 311–317. DOI: 10.1016/j.ejso.2013.11.012. [DOI] [PubMed] [Google Scholar]