Abstract
Drought stress is the major abiotic factor limiting crop production. Co-inoculating crops with nitrogen fixing bacteria and plant growth-promoting rhizobacteria (PGPR) improves plant growth and increases drought tolerance in arid or semiarid areas. Soybean is a major source of high-quality protein and oil for humans. It is susceptible to drought stress conditions. The co-inoculation of drought-stressed soybean with nodulating rhizobia and root-colonizing, PGPR improves the root and the shoot growth, formation of nodules, and nitrogen fixation capacity in soybean. The present study was aimed to observe if the co-inoculation of soybean (Glycine max L. (Merr.) nodulating with Bradyrhizobium japonicum USDA110 and PGPR Pseudomonas putida NUU8 can enhance drought tolerance, nodulation, plant growth, and nutrient uptake under drought conditions. The results of the study showed that co-inoculation with B. japonicum USDA110 and P. putida NUU8 gave more benefits in nodulation and growth of soybean compared to plants inoculated with B. japonicum USDA110 alone and uninoculated control. Under drought conditions, co-inoculation of B. japonicum USDA 110 and P. putida NUU8 significantly enhanced the root length by 56%, shoot length by 33%, root dry weight by 47%, shoot dry weight by 48%, and nodule number 17% compared to the control under drought-stressed. Co-inoculation with B. japonicum, USDA 110 and P. putida NUU8 significantly enhanced plant and soil nutrients and soil enzymes compared to control under normal and drought stress conditions. The synergistic use of B. japonicum USDA110 and P. putida NUU8 improves plant growth and nodulation of soybean under drought stress conditions. The results suggested that these strains could be used to formulate a consortium of biofertilizers for sustainable production of soybean under drought-stressed field conditions.
Subject terms: Microbiology, Plant sciences
Introduction
Soybean (Glycine max L.) is a principal oilseed crop grown throughout the world, accounting for about 200 million tons/annum. It is the major source of vegetable oil and vegetable protein food for humans and a high-quality animal feed. About 50–60% more soybean production will be needed by 20501. This global crop is affected by several biotic and abiotic stresses. Drought is one of the crucial abiotic stresses that affect the growth and yield of soybean and many other crops in dry and semiarid regions2. Drought stress causes a series of adverse effects on germination, plant growth, development, and yield of different crops, including soybean3. Drought negatively impacts the seed germination rate4, leaf area5,6, flowers7, pods8, seed9, and yield of soybean10.
Physiological properties and availability of plant nutrients play essential roles in plant growth, plant development, and yield11,12. Drought impacts adverse effects on plant physiological properties such as chlorophyll content13, photosynthesis rate14, stomatal conductance15,16, and transpiration rate17–19. Furthermore, water stress significantly reduced plants' nitrogen (N) content20,21.
Drought stress affects soil nutrient availability, microbiological parameters, nutrient adsorption, and soil enzyme such as protease, acid, and alkaline phosphomonoesterase22. These enzymes mediate protein and phosphate (P) hydrolysis into bioavailable amino acids, organic nitrogen, and soluble P22. However, the activities of these enzymes are governed by many factors, such as soil properties, soil organic matter contents, and the availability of organic compounds23. This warrants the need to search for sustainable strategies to manage drought stress. Among the various approaches, inoculation of plant growth-promoting rhizobacteria (PGPR) in the rhizosphere of crops has been seen as one of the most suitable strategies to promote plant growth under drought stress conditions24. PGPRs are known for their beneficial effects on plant growth, development25,26, nutrient uptake27,28, and yield under drought stress conditions24,29. These inoculants in single or co-inoculation form enhance nodulation30,31, nodule weight30, nitrogen fixation32, plant biomass33,34, dry matter and grain yield35,36. Moreover, PGPR also help in controlling phytopathogens37–40. However, co-inoculation is more effective than single inoculum31,35,36. Thus there is a need to search for a good combination of co-inoculations for good growth and yield in soybean. The present study was aimed to evaluate the effect of co-inoculation with B. japonicum, USDA 110 and P. putida NUU8 significantly enhanced plant and soil nutrients and soil enzymes compared to control under normal and drought stress conditions.
Methods
Bacterial culture, soybean seeds, and soil
Bacterial cultures, namely B. japonicum USDA 110 and P. putida NUU8, were obtained from the culture repository of the Microbiology and Biotechnology Department of the National University of Uzbekistan Tashkent, Uzbekistan. The soybean seeds (Glycine max L. Merr.) were collected from Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany. The soil for pot assay was collected from Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany.
Preparation of B. japonicum USDA 110 and P. putida NUU8 inoculum
For inoculum preparation, B. japonicum USDA110 and P. putida NUU8 were grown in Yeast extract mannitol broth and nutrient broth respectively at 30 °C and 120 rpm for 48 h. This inoculum was used for seed bacterization.
Surface sterilization, germination, and bacterization of seeds
Soybean seeds were surface sterilized in 10% sodium hypochlorite solution for 5 min, followed by three washings with sterile distilled water. Sterilized seeds were germinated in Petri dishes (85 mm × 15 mm). For bacterization, germinated seeds were immersed in culture broth (5 × 106 CFU g−1) B. japonicum USDA 110 and P. putida NUU8 for 10 min, air-dried, and then planted in 1. Kg capacity plastic pots containing 400 g sandy loamy soil.
Experimental design
The effect of rhizobacteria on soybean growth was studied in pot experiments in a greenhouse at ZALF, Müncheberg, Germany, during July 2019. The experiments were carried out. All the experiments were carried out in a randomized block design with five replications. Experimental treatments included:
T1-Control under normal water conditions
T2-Control under drought stress conditions
T3-Inoculation with B. japonicum USDA 110 under normal water conditions
T4-Inoculation with B. japonicum USDA 110 drought stress conditions
T5-Inoculation with P. putida NUU8 under normal water conditions
T6-Inoculation with P. putida NUU8 under drought stress conditions
T7-Co-inoculation with B. japonicum USDA 110 and P. putida NUU8 strains under normal water conditions
T8-Co-inoculation with B. japonicum USDA 110 and P. putida NUU8 strains under drought stress conditions.
Plants were grown in pots under greenhouse conditions at 24 °C during the day and 16 °C at night for 30 days. Normal water conditions (70% of the pot capacity) and drought stress conditions (40% of pot capacity) were maintained.
Measurement of plant growth parameters and plant nutrients
Soybean plants were harvested from pots after 30 days of germination. The measurement of seed germination rate (%), root length (cm), shoot length (cm), root dry weight (mg/g), shoot dry weight (mg/g), and the number of nodules per plant was measured.
For the estimation of plant nutrients, such as nitrogen, phosphorus, potassium, magnesium, sodium, and calcium, 1 g of crushed plant tissue was added in phosphate buffer (pH 6.7), and these nutrients were measured spectrophotometrically (iCAP 6300 Duo, Thermo Fischer Scientific Inc., Waltham, MA, USA)41,42. The nitrogen and phosphorus contents of root and shoot were determined from dried plant biomass. For nitrogen estimation, one g of dried leaf biomass was digested in 10 mL concentrated H2SO4 and 5.0 g catalyst mixture in a digestion tube. The digested and cooled mixture distillate and the distillate was titrated with H2SO4. The mixture that did not contain leaf biomass served as a control. Total nitrogen was calculated from the blank and sample titer reading43.
The P content of plant biomass was first extracted with 0.5 N NaHCO3 buffer (pH 8.5) followed by treatment with ascorbic acid. The intensity of the blue color produced was measured at 540 nm. The amount of P from plant biomass was calculated from the standard curve of P44. For the estimation of potassium content of the plant, 5 g of the plant biomass was added in 25 mL of ammonium acetate, shaken for 5 min, and filtered. The amount of potassium from the filtrate was measured according to Upadhyay and Sahu45. To estimate Na, Mg, and Ca, one g of plant extract was mixed with 80 mL of 0.5 N HCl and incubated for 5 min at 25 °C and filtered. The amount of Na, Mg, and Ca from the filtrate was estimated according to Sahawat46.
Analysis of soil nutrient
The root soil (10 g) of each experimental pot was air-dried and shaken with 100 mL ammonium acetate buffer (0.5 M) for 30 min to displace the adhered nutrients and minerals. Soil organic carbon (SOC), nitrogen, phosphorus, and potassium contents of soil were determined according to the method of Sims34. This method mixed 1.0 g of soil with 10 mL of 1 N K2Cr2O7 and 20 mL of concentrated H2SO4. This suspension was mixed thoroughly and diluted to 200 mL of distilled water, followed by the addition of 10 mL each of H3PO4 and sodium fluoride. The resulting solution was used to estimate N, P, and K47. Blank (without soil) served as control.
Estimation of soil enzymes
The acid and alkaline phosphomonoesterase activities of soil were assayed according to the method of Tabatabai and Bremner48. For this, 0.5 g of moist soil was mixed in 2 mL of modified universal buffer (pH 6.5 for the acid phosphatase and pH 11 for the alkaline phosphatase) and 0.5 mL of p-nitrophenyl phosphate (PNP) substrate solution (0.05 M). The change in the color of solution due to p-nitrophenol (p-NP) production due to acid and alkaline phosphomonoesterase activities was measured at 400 nm, and the amount of p-NP was calculated from a p-NP calibration curve. Solution without soil served as the control. One unit of phosphomonoesterase activity was defined as the amount of enzyme required to liberate 1 mM of p-NP (product) from 1 kg of dried soil at 37 °C per 1 h48.
Protease activity was assayed according to the method of Ladd and Butler49. For this, 0.5 g of soil was added in 2.5 mL of 0.2 M phosphate buffer (pH of 7.0) and 0.5 mL of 0.03 M N-benzoyl-l-arginine amide (BAA) substrate solution. The amount of ammonium released during the reaction was measured at 690 nm. One unit of protease activity was defined as the amount of enzyme ammonium equivalents released from BAA per minute.
Statistical analyses
All the experiments were performed in five replicates, and the mean values of five replicates were considered. The data were statistically analyzed by one-way analysis of variance (ANOVA) and multiple comparisons of HSD employing the Tukey test with Stat View Software (SAS Institute, Cary, NC, USA, 1998). The significance of the effect of various treatments on plant growth parameters, plant nutrients, and soil nutrients was determined by the magnitude of the p-value (p < 0.05 < 0.001).
Results
Measurement of plant growth parameters
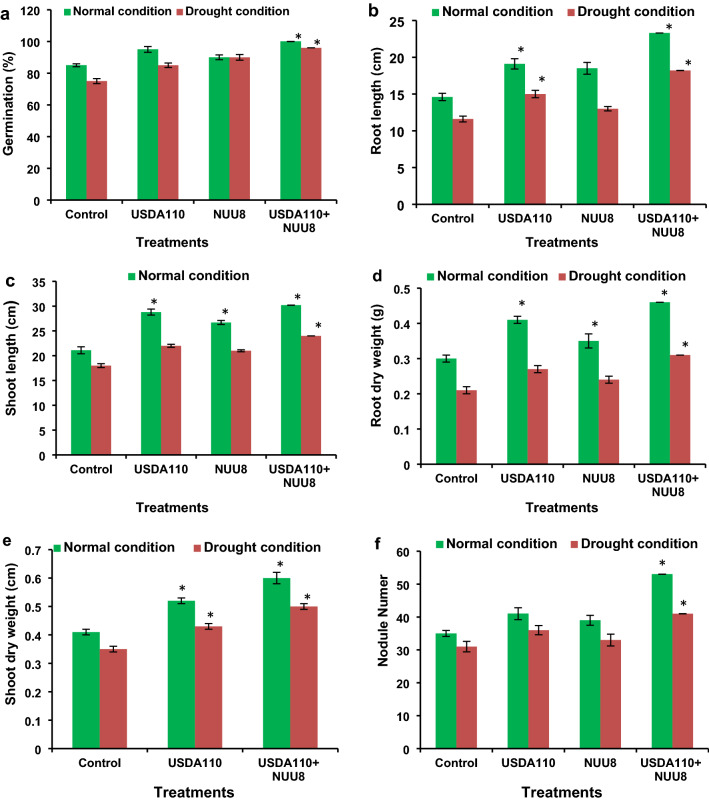
Drought stress conditions affected the seed germination in soybean (Fig. 1) compared to the normal water conditions. Application of rhizobacteria enhanced seed germination under drought conditions and normal conditions compared to control under drought and normal conditions, respectively. Inoculation of B. japonicum USDA 110 alone increased the seed germination by 12.5% under drought conditions and by 10.0% under normal conditions over the control. Co-inoculation of B. japonicum USDA 110 and P. putida strain NUU 8 significantly improved the seed germination under drought stress and normal water conditions. Under drought conditions, co-inoculation of B. japonicum USDA 110 and P. putida strain NUU 8 enhanced the seed germination by 16.2% and 13% under drought and normal conditions compared to the control under drought and normal conditions, respectively (Fig. 1a).
Figure 1.
Effect of single inoculation of B. japonicum USDA 110, and P. putida NUU8 and co-inoculation of B. japonicum USDA 110, and P. putida NUU8 strains: (a) germination, (b) root length, (c) shoot length, (d) root dry weight of soybean under normal and drought conditions, (e) shoot dry weight, and (f) nodule number per plant. Data are presented as mean + standard deviation of five replicates. *Significant differences (p ≤ 0.05).
The rhizobacterial inoculation significantly improved the growth of the soybean plant under normal and drought stress conditions. Inoculation of B. japonicum USDA 110 alone significantly enhanced the root length by 30% (Fig. 1b), shoot length by 36% (Fig. 1c), root dry weight by 33% (Fig. 1d), and shoot dry weight by 26% (Fig. 1e), as compared to the control under normal water conditions. Inoculation of B. japonicum USDA 110 under drought stress conditions significantly increased the root length by 29% (Fig. 1b), shoot length by 22% (Fig. 1c), root dry weight by 28% (Fig. 1d), and shoot dry weight by 22% (Fig. 1e) over the control under drought and normal conditions, respectively. Whereas the co-inoculation with B. japonicum USDA 110 and P. putida strains NUU8 significantly increased the root length, shoot length, root dry weight, shoot dry weight, and nodule number compared to the control under normal and drought conditions.
A 59% and 56% increase in root length (Fig. 1b), 43% and 33% increase in shoot length (Fig. 1c), 53% and 47% rise in root dry weight (Fig. 1d), 48% and 46% improvement in shoot dry weight (Fig. 1e) and 29% and 27% rise in nodule number (Fig. 1f) was evident over the control under normal condition and drought stress, respectively.
Measurement of plant nutrient contents
Analysis of nutrients in a soybean plant revealed that single inoculation of B. japonicum USDA 110 significantly increased N content by 29% and 28%, P content by 15% and 12%, K content by 32 and 28%, Mg content by 12%, and 9.0%, Na content by 50% and 43% and Ca content by 13% and 11% respectively as compared to control under normal condition and drought stress conditions respectively. A single inoculation of P. putida NUU8 also exhibited a substantial increase in the nutrient contents. It increases nitrogen by 21% and 17%, P content by 14% and 11%, K content by 30 and 26%, Mg content by 10% and 8.0%, Na content by 45% and 38%, and Ca content by 10% and 8.0% respectively as compared to control under normal condition and drought stress conditions respectively. However, co-inoculation of B. japonicum USDA 110 and P.putida NNU8 resulted in a significant improvement in N content by 44%, and 35% P content by 34% and 31%, K content by 41% and 28, Mg content by 19% and 15%, Na content by 83% and 50% and Ca content by 41% and 35% over the control under normal condition and drought stress conditions respectively (Table 1).
Table 1.
Effect of coinoculation with B. japonicum USDA 110 and P. putida NUU8 and single inoculation B. japonicum USDA 110 strains on plant nutrients under normal and drought conditions.
| Conditions | Treatments | N (%) | P (%) | K (%) | Mg (%) | Na (%) | Ca (%) |
|---|---|---|---|---|---|---|---|
| Normal | Control | 1.99 + 0.02 | 0.24 + 0.02 | 1.45 + 0.01 | 0.58 + 0.01 | 0.18 + 0.02 | 1.20 + 0.01 |
| USDA 110 | 2.57 + 0.01* | 0.29 + 0.01 | 1.62 + 0.02 | 0.61 + 0.01 | 0.27 + 0.01* | 1.22 + 0.01 | |
| NUU8 | 2.37 + 0.01* | 0.21 + 0.01 | 1.43 + 0.02 | 0.52 + 0.01 | 0.21 + 0.01* | 1.01 + 0.01 | |
| USDA + NUU8 | 2.87 + 0.01* | 0.33 + 0.01* | 2.05 + 0.02* | 0.69 + 0.01* | 0.33 + 0.03* | 1.69 + 0.01 | |
| Drought | Control | 1.76 + 0.01 | 0.20 + 0.01 | 1.34 + 0.01 | 0.54 + 0.01 | 0.04 + 0.01 | 1.01 + 0.01 |
| USDA 110 | 2.26 + 0.02* | 0.25 + 0.01 | 1.55* + 0.01 | 0.56 + 0.01 | 0.05 + 0.02 | 1.15 + 0.01 | |
| NUU8 | 2.01 + 0.02* | 0.18 + 0.01 | 1.21* + 0.01 | 0.45 + 0.01 | 0.05 + 0.02 | 1.01 + 0.01 | |
| USDA + NUU8 | 2.38 + 0.02* | 0.29 + 0.02 | 1.71 + 0.02* | 0.58 + 0.02 | 0.06 + 0.02* | 1.53 + 0.02* |
Values are the average of three replicates ± values are standard deviations. Plant nutrient contents were measured after 30 days of plant growth under greenhouse conditions.
*Values significant at p 0.01.
Analysis of soil nutrient contents
Analysis of soil nutrient contents revealed significant improvement in soil N, P, and K content due to rhizobacterial inoculation compared to control (Table 2). Inoculation with B. japonicum USDA 110 alone significantly increased total N content by 16% and 12%, P content by 18% and 16%, and K content by 16% and 14%, respectively, compared to the control under normal conditions and drought conditions respectively. In comparison, single inoculation with P. Putida NUU8 increased total N content by 13% and 11%, P content by 16% and 13%, and K content by 13% and 11% compared to the control under normal conditions and drought conditions, respectively. However, the highest N, P, and K values were observed in soil amended co-inoculation with B. japonicum USDA 110 and P. putida NUU8 treatment under normal and drought stress conditions. The co-inoculation significantly increased the total N content by 20% and 23%, P content by 14% and 12%, and K content by 48% and 30%, respectively, compared to the control under conditions and drought stress conditions, respectively (Table 2).
Table 2.
Effect of coinoculation with B. japonicum USDA 110 and P. putida NUU8 and single inoculation B. japonicum USDA 110 strains on soil nutrients under normal and drought conditions.
| Conditions | Treatments | Total N (%) | P (mg) | K (mg) |
|---|---|---|---|---|
| Normal | Control | 0.080 ± 0.01 | 4.61 ± 0.02 | 4.25 ± 0.02 |
| USDA 110 | 0.093 ± 0.03* | 4.84 ± 0.02 | 4.88 ± 0.03* | |
| NUU8 | 0.087 ± 0.03* | 4.11 ± 0.02 | 4.13 ± 0.03* | |
| USDA + NUU8 | 0.096 ± 0.02* | 5.24 ± 0.02* | 6.30 ± 0.03* | |
| Drought | Control | 0.075 ± 0.02 | 4.02 ± 0.01 | 3.66 ± 0.01 |
| USDA 110 | 0.084 ± 0.01* | 4.50 ± 0.01 | 4.20 ± 0.02* | |
| NUU8 | 0.077 ± 0.01* | 4.19 ± 0.01 | 4.16 ± 0.02* | |
| USDA + NUU8 | 0.092 ± 0.03* | 4.52 ± 0.02* | 4.75 ± 0.02* |
Values are the average of three replicates. ± values are standard deviations.
*Values significant at p 0.01. Soil nutrient contents were measured after 30 days of growth of the plant under greenhouse conditions.
Analysis of soil enzyme activities
Data regarding soil enzymes showed that rhizobacteria treatments improved the protease, acid, and alkaline phosphomonoesterase activities in both conditions (Table 3). A single inoculation of B. japonicum USDA 110 significantly increased the protease, acid, and alkaline phosphomonoesterase compared to the control under both conditions. However, the co-inoculation of B. japonicum USDA 110 and P. Putida NUU8 significantly improved the activities of these enzymes under both conditions (Table 3). Co-inoculation of soybean with B. japonicum USDA 110 and P. putida NUU8 strains significantly enhanced protease activity by 19%, acid phosphomonoesterase activity by 10%, and acid phosphomonoesterase and alkaline phosphomonoesterase activity by 27% over the control under normal conditions. Co-inoculation with B. japonicum USDA 110 and P. putida NUU8 under drought stress conditions significantly increased the protease activity by 32%, acid phosphomonoesterase by 27%, and alkaline phosphomonoesterase by 19% over the control (Table 3).
Table 3.
Effect of coinoculation with B. japonicum USDA 110 and P. putida NUU8 and single inoculation B. japonicum USDA 110 strains on soil enzymes under normal and drought conditions.
| Conditions | Treatments | Protease activity (µg NH4+-N g−1 h−1) | Acid phosphomonoesterase activity (µg pNPg−1 h−1) | Alkaline phosphomonoesterase activity (µg pNPg−1 r−1) |
|---|---|---|---|---|
| Normal | Control | 25.8 ± 0.08 | 725.1 ± 21.3 | 315.1 ± 10.1 |
| USDA 110 | 27.2 ± 0.12* | 783.0 ± 22.4* | 385.6 ± 16.4 | |
| NUU8 | 24.2 ± 0.11 | 731.0 ± 19.3* | 338.2 ± 13.4 | |
| USDA + NUU8 | 30.6 ± 0.11* | 799.6 ± 28.6* | 399.2 ± 18.1* | |
| Drought | Control | 20.6 ± 0.05 | 683.2 ± 20.5 | 312.5 ± 11.2 |
| USDA 110 | 25.1 ± 0.07* | 730.9 ± 23.1* | 346.6 ± 17.3* | |
| NUU8 | 23.2 ± 0.06* | 701.2 ± 21.2* | 3176 ± 15.6* | |
| USDA + NUU8 | 27.3 ± 0.08* | 750.4 ± 31.3* | 372.2 ± 18.4* |
Values are the average of three replicates. ± values are standard deviations.
*Values significant at p 0.01. Soil nutrient contents were measured after 30 days of growth of the plant under greenhouse conditions.
Discussion
Drought stress has adverse effects on seed germination and growth in various plants. Several researchers2,3,29 reported a decrease in the germination rate in legumes crops by drought stress. The negative impacts of drought on seed germination, plant growth, nodulation, and soybean yield have been reported2,3. Mafakheri et al.17 reported a 73% decrease in soybean yield under drought stress conditions. PGPR strains like Bradyrhizobium sp. and Pseudomonas sp. improve drought tolerance and plant growth by modifying root architecture and the secretion of siderophore, phytohormones, and EPS20,21,24. Gholami et al.50 reported improved germination and growth in soybean due to the synergistic effect of co-inoculation of B. japonicum and P. putida. Inoculation with PGPR improves plant growth, development, nodulation, and yield of different crops24,30,33,35. Co-inoculation of Rhizobium sp. and other PGPR in bean and chickpea enhance nodulation, plant growth, and nutrient uptake36,43. Co-inoculation of Rhizobium tropici CIAT 899 and P. polymyxa DSM36 significantly increase plant growth and nodulation in common bean compared to inoculation with Rhizobium sp. alone under drought-stressed conditions51. Tewari and Arora52 reported a 50% increase in germination due to the inoculation with EPS producing Pseudomonas aeruginosa PF23 under stress. A wide variety of PGPR have been reported to produce EPS53, and they help crop plants in better root colonization52, better seed germination, and stress tolerance55. They enhance water retention by maintaining the diffusion of organic carbon sources54. Vardharajula et al.56 observed that Bacillus sp. synthesized osmolytes and antioxidants that facilitate plant growth under drought stress conditions. The synthesis of phytohormones by bacterial strains is another mechanism that imparts stress tolerance in plants29,57.
Drought stress also adversely affects plant nutrient uptake such as N, P, K, Ca, and Mg20. Several studies reported that drought stress reduces the concentration of N, K, and P in plant tissue and declines nutrient uptake from soil58,59. Drought stress is known to significantly decrease N content in cowpea60. He and Dijkstra58 reported that drought stress conditions significantly decline N and P in plant tissues. Results of the present study shows that co-inoculation with B. japonicum USDA 110 and P. putida strains NUU8 significantly increased the N content, P content, and K content compared to the control under drought conditions. PGPR is known to colonize the plant's rhizosphere, adhere to the root surface, and maintain moisture content25,61–63. This makes stable aggregates that help in nutrient absorption in plants52.
Drought stress exhibits adverse effects on soil nutrient availability, soil nutrient adsorption, and soil enzyme activities. Hinsinger et al.64 reported that drought-stressed conditions significantly decrease the soil nutrients such as N, P, K, and microelements such as B, Fe, Mn, and Zn. Drought-stressed in the soil is known to decrease enzyme activities65. The decrease in soil enzyme activities observed in this study is in agreement with the decrease in P available forms in the drought-stressed conditions41. The enhancement in soil enzymes such as protease, acid phosphomonoesterase, and alkaline phosphomonoesterase due to rhizobial inoculation has been observed by Fall et al.66 and Jabborova et al.67. Nitrogen fixing symbionts, alone or in combination with other rhizobacteria have been reported to improve growth, nutrient uptake and root architecture in soybean as well as to improve the resistance in soybean and other plants46,47,68–76.
Conclusions
The application of PGPR exerts beneficial effects on plant growth and nodulation in soybean through increased uptake of nutrients such as N, P, and K in soil under normal and drought stress conditions. Inoculation with single strains of PGPR, i.e., B. japonicum USDA 110, improve soybean growth; however, co-inoculation of B. japonicum USDA 110 and P. putida NUU8 improves more growth, nutrient contents in soybean and soil, and activities of soil protease and acid and alkaline monophosphoeserase, as compared to the single inoculation and control under drought condition. Thus the combination of B. japonicum USDA 110 and P. putida NUU8 can serve as an effective and sustainable approach for improving the growth, nutrient contents, and enzyme activities in soybean and soil under drought-stressed conditions.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/15), King Saud University, Riyadh, Saudi Arabia, German Academic Exchange Service for funding this research work, and Research fellowship granted by the Alexander von Humboldt Foundation, Bonn, Germany, to AG.
Author contributions
D.J. wrote the original draft of the manuscript and performed the methodology part. K.D., Y E, A.N., performed the formal analysis. D.J., A. K., A.S, A.M.E., A.H.B., S. W., R.Z.S., and A.G. edited and reviewed the manuscript.
Funding
This work was funded by The German Academic Exchange Service DAAD 2019, 57440916 and Researchers Supporting Project number (RSP-2021/15), King Saud University, Riyadh, Saudi Arabia and German Academic Exchange Service.
Data availability
Permissions were obtained to collect the Soybean (Glycine max L. Merr.) seeds from Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany. Experimental research and field studies on plants were in accordance with the guidelines of ZALF, Müncheberg, Germany.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dilfuza Jabborova, Email: dilfuzajabborova@yahoo.com.
R. Z. Sayyed, Email: sayyedrz@gmail.com
Abdul Gafur, Email: gafur@uwalumni.com.
References
- 1.Carciochi WD, Rosso LHM, Secchi MA. Soybean yield, biological N2 fixation, and seed composition responses to additional inoculation in the United States. Sci. Rep. 2019;9:19908. doi: 10.1038/s41598-019-56465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y, Jin J, Jiang S, Ning S, Liu L. Quantitative response of soybean development and yield to drought stress during different growth stages in the Huaibei Plain, China. Agronomy. 2018;8:97. [Google Scholar]
- 3.Demirta C, Yazgan S, Candogan BN, Sincik M. Quality and yield response of soybean (Glycine max L. Merrill) to drought stress in the sub-humid environment. Afr. J. Biotechnol. 2010;9:6873–6881. [Google Scholar]
- 4.Awari VR, Mate SN. Effect of drought stress on early seedling growth of chickpea (Cicer arietinum L.) genotypes. Life Sci. Int. Res. J. 2015;2:356–361. [Google Scholar]
- 5.Atti S, Bonnell R, Smith D, Prasher S. Response of an indeterminate soybean (Glycine max (L.) Merrill) to chronic water deficit during reproductive development under greenhouse conditions. Can. Water Resour. J. 2004;29(4):209–222. [Google Scholar]
- 6.Gavili E, Moosavi AA, Kamgar Haghighi AA. Does biochar mitigate the adverse effects of drought on the agronomic traits and yield components of soybean? Ind. Crop Prod. 2019;128:445–454. [Google Scholar]
- 7.De Souza PI, Egli DB, Bruening WP. Water stress during seed filling and leaf senescence in soybean. Agron. J. 1997;89:807–812. [Google Scholar]
- 8.Li D, Liu H, Qiao Y, Wang Y, Cai Z, Dong B, Shi C, Liu Y, Li X, Liu M. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max L.) under drought stress. Agric. Water Manag. 2013;129:105–112. [Google Scholar]
- 9.Desclaux D, Roumet P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000;40:716–722. [Google Scholar]
- 10.Pushpavalli R, Zaman-Allah M, Turner NC, Baddam R, Rao MV, Vadez V. Higher flower and seed number leads to higher yield under water stress conditions imposed during reproduction in chickpea. Funct. Plant Biol. 2015;42:162–174. doi: 10.1071/FP14135. [DOI] [PubMed] [Google Scholar]
- 11.Anjum S, Xie X, Wang L, Saleem M, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. J. Afr. Agric. Res. 2011;6:2026–2032. [Google Scholar]
- 12.Makbul S, Güler NS, Durmus N, Güven S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Botany. 2011;35:369–377. [Google Scholar]
- 13.Abid G, Mahmoud M, Mingeot D, Aouida M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.) Arch. Agron. Soil Sci. 2016;63:536–552. [Google Scholar]
- 14.Ohashi Y, Nakayama N, Saneoka H, Fujita K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006;50:138–141. [Google Scholar]
- 15.Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture: Not by affecting ATP synthesis. Trend Plant Sci. 2000;5:187–188. [Google Scholar]
- 16.Wang W, Wang C, Pan D, Zhang Y, Luo B, Ji J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max L.) seedlings. Int. J. Agric. Biol. Eng. 2018;11:196–201. [Google Scholar]
- 17.Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y. Effect of drought stress on yield, proline, and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010;4:580–585. [Google Scholar]
- 18.Mak M, Babla M, Xu S, Carrigan AO, Liu X, Gong Y, Holford P, Chen Z. Leaf mesophyll K+, H+, and Ca2+ fluxes are involved in the drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014;98:1–12. [Google Scholar]
- 19.Mutava RN, Jebakumar S, Prince K, Hasan N, Song L, Valliyodan B, Chen W, Nguyen HT. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2014;86:109–120. doi: 10.1016/j.plaphy.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Lozano JM, Azcon R. Mycorrhizal colonization and drought stress as factors affecting nitrate reductase activity in lettuce plants. Agric. Ecosyst. Environ. 1996;60:175–181. [Google Scholar]
- 21.Silveira JAG, Costa RCL, Oliveira JTA. Drought-induced effects and recovery of nitrate assimilation and nodule activity in cowpea plants inoculated with Bradyrhizobium spp. under moderate nitrate level. Braz. J. Microbiol. 2001;32:187–194. [Google Scholar]
- 22.Buckley S, Allen D, Brackin R, Jämtgård S, Näsholm T, Schmidt S. Microdialysis as an in situ technique for sampling soil enzymes. Soil Biol. Biochem. 2019;135:20–27. [Google Scholar]
- 23.Holík L, Hlisnikovský L, Honzík R, Trögl J, Burdová H, Popelka J. Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability. 2019;11(12):3251. [Google Scholar]
- 24.Ilyas N, Komal M, Nosheen A, Humaira Y, Sayyed RZ, Wajiha K, Hesham A, El Enshasy H, Daniel JD, Elsayed AE, Zeshan A. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability. 2020;12:8876. doi: 10.3390/su12218876. [DOI] [Google Scholar]
- 25.Babu AN, Jogaiah S, Ito S-I, Nagaraj AK, Tran L-S. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015;231:62–73. doi: 10.1016/j.plantsci.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Jogaiah S, Shivanna RK, Gnanaprakash PH, Hunthrike SS. Evaluation of plant growth-promoting Rhizobacteria for their efficiency to promote growth and induce systemic resistance in pearl millet against downy mildew disease. Arch. Phytopathol. Plant Prot. 2010;43(4):368–378. [Google Scholar]
- 27.Hamid B, Zaman M, Farooq S, Fatima S, Sayyed RZ, Baba ZA, Sheikh TA, Reddy MS, El Enshasy H, Gafur A, Suriani NL. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability. 2021;21(13):2856. doi: 10.3390/su13052856. [DOI] [Google Scholar]
- 28.Kusale SP, Attar YC, Sayyed RZ, Malek RA, Ilyas N, Suriani NL, Khan N, El Enshasy H. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules. 2021;26:1894. doi: 10.3390/molecules26071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan I, Awan SA, Ikram R, Rizwan M, Akhtar N, Humaira Yasmin RZ, Sayyed SA, Ilyas N. 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiol. Planta. 2020 doi: 10.1111/ppl.13237. [DOI] [PubMed] [Google Scholar]
- 30.Graham PH, Vance CP. Legumes: Importance and constraints to greater use. Plant Physiol. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vafa ZN, Sohrabi Y, Sayyed RZ, Luh Suriani N, Datta R. Effects of combinations of Rhizobacteria, mycorrhizae, and seaweeds on growth and yields in wheat cultivars under the influence of supplementary irrigation. Plants. 2021;10:811. doi: 10.3390/plants10040811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter JM, Gardne WK, Gibson AH. Improved growth and yield of faba beans (Vicia faba cv. fiord) by inoculation with strains of Rhizobium leguminosarum biovar. viciaein acid soils in southwest Victoria. Aust. J. Agric. Res. 1994;94:613–623. [Google Scholar]
- 33.Daba S, Haile M. Effects of rhizobial inoculant and nitrogen fertilizer on yield and nodulation of common bean. J. Plant Nutr. 2000;23:581–591. [Google Scholar]
- 34.Sharma P, Khurana AS. Effect of single and multi-strain Rhizobium inoculants on biological nitrogen fixation in summer mungbean, Vigna radiata (L.) Wilczek. Res. Dev. Rep. 1997;14:8–11. [Google Scholar]
- 35.Jabborova D, Baboev S, Davranov K. Enhancement of plant growth, nodulation, and yield of mungbean (Vigna radiate L.) by microbial preparations. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:2382–2388. [Google Scholar]
- 36.Jabborova D, Baboev S, Davranov K, Jabbarov Z. Improvement of plant growth, nodulation, and yield of common bean (Phaseolus vulgaris L.) by microbiological preparations. J. Biol. Chem. Res. 2019;36:52–57. [Google Scholar]
- 37.Jogaiah S, Sharathchandra RG, Raj N, Vedamurthy AB, Shetty HS. Development of SCAR marker associated with downy mildew disease resistance in pearl millet (Pennisetum glaucum L.) Mol. Biol. Rep. 2014;2014(41):7815–7824. doi: 10.1007/s11033-014-3675-7. [DOI] [PubMed] [Google Scholar]
- 38.Jogaiah S, Abdelrahman M, Tran LS, Shin-ichi I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J. Exp. Bot. 2013;64(12):3829–3842. doi: 10.1093/jxb/ert212. [DOI] [PubMed] [Google Scholar]
- 39.Murali M, Sudisha J, Amruthesh KN, Ito S-I, Shetty HS. Rhizosphere fungus Penicillium chrysogenum promotes growth and induces defence-related genes and downy mildew disease resistance in pearl millet. Plant Biol. 2013;15:111–118. doi: 10.1111/j.1438-8677.2012.00617.x. [DOI] [PubMed] [Google Scholar]
- 40.Sukmawati D, Family N, Hidayat I, Sayyed RZ, Elsayed EA, Dailin DJ, Hanapi SZ, Wadaan MA, Enshasy HE. Biocontrol activity of Aureubasidium pullulans and Candida orthopsilosis isolated from Tectona grandis L. Phylloplane against Aspergillus sp. in post-harvested citrus fruit. Sustainability. 2021;13:7479. doi: 10.3390/su13137479. [DOI] [Google Scholar]
- 41.Sardans J, Penuelas J. Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil. 2004;267:367–377. [Google Scholar]
- 42.Sharma MK, Kumawat DM. Co-inoculation study of Bradyrhizobium japonicum and Aspergillus niger in soybean for nitrogen fixation. J. Microbiol. Biotechnol. Food Sci. 2020;9(4):383–394. [Google Scholar]
- 43.Daramola DS, Danso SKA, Hardarson G. Nodulation, N2 fixation and dry matter yield of soybean [Glycine max (L.) Merrill] inoculated with effective and ineffective Bradyrhizobium japonicum strains. Soil Biol. Biochem. 1994;26:883–889. [Google Scholar]
- 44.Sims J. Soil test phosphorus: Principles and methods. Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern Coop. Ser. Bull. 2009;408:9–19. [Google Scholar]
- 45.Upadhyay, A. & Sahu, R. Determination of potassium in soil and plant. In Laboratory Manual on Advances in Agro-Technologies for Improving. Soil, Plant and Atmosphere Systems, 23–35. (CAFT, 2012).
- 46.Sahrawat K. Determination of calcium, magnesium, zinc, and manganese in plant tissue using a dilute HCl extraction method. Commun. Soil Sci. Plant Anal. 1987;18:947–962. [Google Scholar]
- 47.Labconco C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus. Labconco Corporation; 1998. [Google Scholar]
- 48.Tabatabai M, Bremner J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1:301–307. [Google Scholar]
- 49.Ladd J, Butler J. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972;4:19–30. [Google Scholar]
- 50.Gholami A, Shahsavani S, Nezarat S. The effect of plant growth-promoting rhizobacteria (PGPR) on germination, seedling growth, and yield of maize. Proc. Word Acad. Sci. Eng. Technol. 2009;37:2070–3740. doi: 10.3923/pjbs.2009.26.32. [DOI] [PubMed] [Google Scholar]
- 51.Márcia VB, Figueiredo H, Burity A, Cosme R, Martínez C, Chanway P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;40(1):182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 52.Tewari S, Arora NK. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol, and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 2014;69(4):484–494. doi: 10.1007/s00284-014-0612-x. [DOI] [PubMed] [Google Scholar]
- 53.Sayyed RZ, Patel PR, Shaikh SS. Plant growth promotion and root colonization by EPS producing Enterobacter sp. RZS5 under heavy metal contaminated soil. Indian J. Exp. Biol. 2015;53:116–1233. [PubMed] [Google Scholar]
- 54.Yasmin F, Othman R, Maziz MNH. Yield and nutrient content of sweet potato in response of plant growth-promoting rhizobacteria (PGPR) inoculation and N fertilization. Jordan J. Biol. Sci. 2020;13:117–122. [Google Scholar]
- 55.Etesami H, Adl SM. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In: Kumar M, Kumar V, Prasad R, editors. Phyto-Microbiome in Stress Regulation. Springer; 2018. pp. 147–203. [Google Scholar]
- 56.Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V. Drought-tolerant plant growth-promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6(1):1–14. [Google Scholar]
- 57.Ghosh D, Gupta A, Mohapatra SA. Comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. and Biotechnol. 2019;35:6–90. doi: 10.1007/s11274-019-2659-0. [DOI] [PubMed] [Google Scholar]
- 58.He M, Dijkstra FA. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014;204:924–931. doi: 10.1111/nph.12952. [DOI] [PubMed] [Google Scholar]
- 59.Waraich EA, Rashid A, Ashraf MY. Role of mineral nutrition in the alleviation of drought stress in plants. Aust. J. Crop Sci. 2011;5:764–777. [Google Scholar]
- 60.Chowdhury JA, Karim MA, Khaliq QA, Ahmed AU, Khan MSA. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh. J. Agric. Res. 2016;41:195–205. [Google Scholar]
- 61.Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ, Reddy MS, El El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- 62.Kusale SP, Attar YC, Sayyed RZ, El Enshasy H, Hanapi SZ, Ilyas N, Elgorban AM, Bahkali AH, Marraiki N. Inoculation of Klebsiella variicola alleviated slat stress salinity and improved growth and nutrients in wheat and maize. Agronomy. 2021;11:927. doi: 10.3390/agronomy11050927. [DOI] [Google Scholar]
- 63.Kalam S, Basu A, Ahmad I, Sayyed RZ, El Enshasy HA, Dailin DJ, Suriani N. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020;11:580024. doi: 10.3389/fmicb.2020.580024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinsinger P, Bengough AG, Vetterlein D, Young IM. Rhizosphere: Biophysics, biogeochemistry, and ecological relevance. Plant Soil. 2009;321:117–152. [Google Scholar]
- 65.Henry HAL. Soil extracellular enzyme dynamics in a changing climate. Soil Biol. Biochem. 2012;47:53–59. [Google Scholar]
- 66.Fall D, Bakhoum N, NourouSall S, Zoubeirou A, Sylla S, Diouf D. Rhizobial inoculation increases soil microbial functioning and gum arabic production of 13-year-old Senegalia senegal (L.) britton, trees in the north part of Senegal. Front. Plant Sci. 2016;7:1355. doi: 10.3389/fpls.2016.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jabborova D, Wirth S, Kannepalli A, Narimanov A, Desouky S, Davranov K, Sayyed RZ, El Enshasy H, Malek RA, Syed A, Bahkali AH. Co-inoculation of rhizobacteria and biochar application improves growth and nutrientsin soybean and enriches soil nutrients and enzymes. Agronomy. 2020;10:1142. [Google Scholar]
- 68.Egamberdieva D, Jabborova D, Berg G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth and nodulation of soybean under salt stress. Plant Soil. 2016;405:35–45. [Google Scholar]
- 69.Egamberdieva D, Jabborova D, Wirth SJ, Alam P, Alyemeni MN, Ahmad P. Interactive effects of nutrients and Bradyrhizobium japonicum on the growth and root architecture of soybean (Glycine max L.) Front. Microbiol. 2018;9:1000. doi: 10.3389/fmicb.2018.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Egamberdieva D, et al. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 2017;12:100–107. [Google Scholar]
- 71.Holik L, Vranová V. Proteolytic activity in meadow soil after the application of phytohormones. Biomolecules. 2019;9:507. doi: 10.3390/biom9090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jabborova DP, Enakiev YI, Davranov KD, Begmatov SA. Effect of co-inoculation with Bradyrhizobium japonicum and Pseudomonas putida on root morph-architecture traits, nodulation, and growth of soybean in response to phosphorus supply under hydroponic conditions. Bulgarian J. Agric. Sci. 2018;24(6):1004–1011. [Google Scholar]
- 73.Jabborova DP, Narimanov AA, Enakiev YI, Davranov KD. Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulgarian J. Agric. Sci. 2020;26(4):744–747. [Google Scholar]
- 74.Kashem MA, Mian MH, Rahman MF. Effect of Bradyrhizobium on the yield of mungbean (Vigna radiata L.) grown in Ganges Tidal floodplain soil. J. Agric. Res. 2000;33:407. [Google Scholar]
- 75.Jogaiah S, Shetty HS, Ito S-I, Tran L-S. Enhancement of downy mildew disease resistance in pearl millet by the G_app7 bioactive compound produced by Ganoderma applanatum. Plant Physiol. Biochem. 2016;105:109–117. doi: 10.1016/j.plaphy.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Reshma P, Naik MK, Aiyaz M, Niranjana SR, Chennappa G, Shaikh SS, Sayyed RZ. Induced systemic resistance by 2,4diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J. Exp. Biol. 2018;56(3):207–212. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Permissions were obtained to collect the Soybean (Glycine max L. Merr.) seeds from Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany. Experimental research and field studies on plants were in accordance with the guidelines of ZALF, Müncheberg, Germany.