Abstract
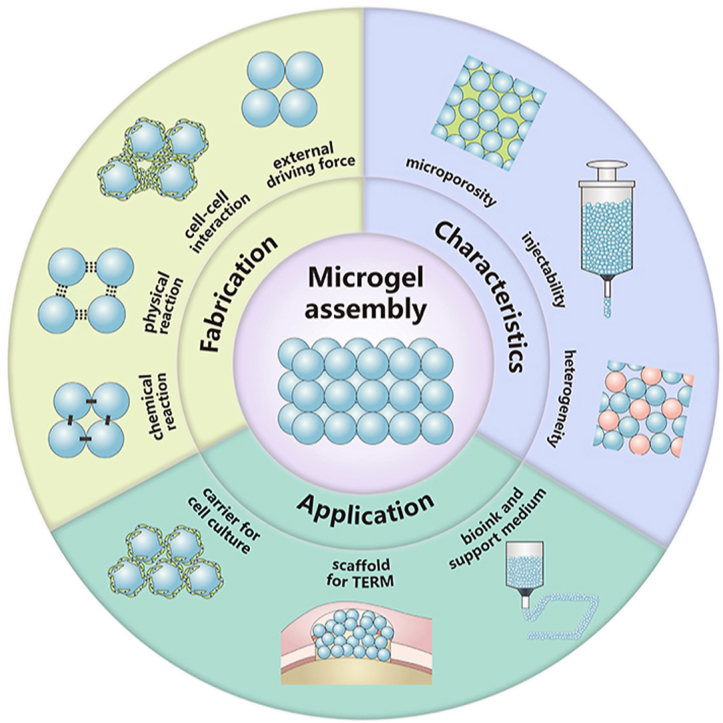
Microgel assembly, a macroscopic aggregate formed by bottom-up assembly of microgels, is now emerging as prospective biomaterials for applications in tissue engineering and regenerative medicine (TERM). This mini-review first summarizes the fabrication strategies available for microgel assembly, including chemical reaction, physical reaction, cell-cell interaction and external driving force, then highlights its unique characteristics, such as microporosity, injectability and heterogeneity, and finally itemizes its applications in the fields of cell culture, tissue regeneration and biofabrication, especially 3D printing. The problems to be addressed for further applications of microgel assembly are also discussed.
Keywords: Microgel assembly, Tissue engineering, Regenerative medicine
Graphical abstract
Highlights
-
•
Review the recent advances of microgel assembly.
-
•
Elaborate the state-of-the-art fabrication strategies of microgel assembly under various driving forces.
-
•
Highlight the unique characteristics of microgel assembly including microporosity, injectability and heterogeneity.
-
•
Summary the current applications of microgel assembly in tissue engineering and regenerative medicine.
-
•
Discuss the challenges and feasible solutions for future clinical application.
1. Introduction
Most natural tissues are composed largely of multi-type cells and related extracellular matrix (ECM). Due to their high similarity to ECM, hydrogels, a class of crosslinking polymers with an insoluble network that can absorb amount of water, have attracted worldwide attention [1]. To date, numerous natural and synthetic polymers have been explored to form hydrogels that can serve as vessels for cell culture, carriers for drug delivery and scaffolds for tissue engineering (TE) [2]. The biophysical and biochemical requirements of hydrogels for these applications are diverse, and they can be met in various ways, either to resemble particular natural tissues or to trigger desired biological responses [3]. For example, biophysical properties of a hydrogel, such as water content, mechanical strength and viscoelasticity, can be tailored by controlling the macromer composition, concentration and crosslinking density [4,5]. Biochemical cues, such as active peptides, growth factors, proteoglycans and small-molecule drugs can be incorporated into hydrogels through covalent linkages or noncovalent interactions [6,7]. However, traditional hydrogels are often processed into a relatively large size with low surface/volume ratio, leading to slow degradation kinetics and thus poor cell infiltration and weak vascularization. Besides, such bulk hydrogels have merely nanoporous meshes inside the crosslinked network and lack of micropores, indicative of insufficient nutrient exchange and low cell viability inside hydrogels [8,9]. A promising solution to this problem is the replacement of bulk hydrogel with microporous microgel assembly (also called as microporous annealed particle, MAP), whose large surface/volume ratio and short diffusion distance enhance the mass transport of nutrients and thus promote the long-term survival of cells [[10], [11], [12]]. Moreover, its interconnected pores can also guide cell ingrowth and tissue formation before hydrogel degradation.
In addition, microgel assembly also shows unique superiority in mimicking the natural tissue structures. As well known, many tissues comprise repeated 3D cellular microstructures (or namely, basic functional modules), such as lobule in the liver, nephron in the kidney and islet in the pancreas [13]. Tissue functionality arises from the ordered spatial alignment and distribution of these microstructures. TERM that aims to restore, regenerate and repair damaged tissues, should recreate and reassemble these basic microstructures, so as to rebuild the complex tissue functions. Unfortunately, traditional top-down approaches to engineer artificial tissues offer weak control over these 3D microstructures and their assembly, such as precisely positioning multi-type cells, achieving tissue-specific geometries and cell densities, incorporating vasculature throughout the 3D space [14]. In comparison, bottom-up methods, which involve high-throughput generation of microstructures (for examples, microgels) as building blocks and their precise assembly into a macroscale construct, have the potential to overcome these limitations. By designing and adjusting the composition, mechanical strength, size and shape of individual microgels, as well as incorporating diverse cells and/or bioactive factors, it is feasible to achieve microgel assembly with microscale homogeneous or heterogeneous composition, localized mechanical properties and tunable microporosity through appropriate assembly technologies [15].
In view of the remarkable features that microgels present and their promising applications in TERM, tremendous efforts have been exerted in the past few years on their preparation, bottom-up assembly and possible applications (Fig. 1). Since the synthesis and functionality of monodisperse microgels have been already reviewed in several reported works [[16], [17], [18]], the current mini-review mainly focuses on the advances of microgel assembly and its applications in TERM. Specifically, we first introduce the state-of-the-art fabrication strategies of microgel assembly under various driving forces, including chemical reaction, physical reaction, cell-cell interaction and external driving force, then describe its unique characteristics, like microporosity, injectability and heterogeneity, and finally highlight its applications as carrier for cell culture and cell behavior regulation, scaffold for tissue repair and regeneration, bioink and support medium for 3D printing. Our purpose is to provide a fundamental guide for those who are interested in microgel assembly but may not be sufficiently familiar with this new technology.
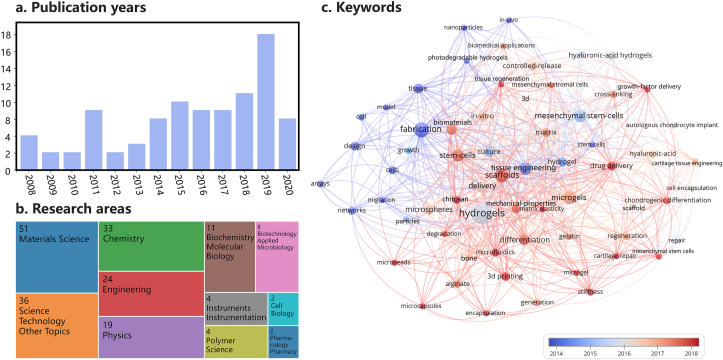
Fig. 1.
Summary of the published articles (2008–2020) on microgel preparation, bottom-up assembly and possible applications in TERM: (a) number of articles in each year; (b) research scopes of the articles; (c) keywords evolution over time. Keywords for data searching are microgels (microsphere, microbead, particle hydrogel, microstructure, microscale hydrogels, granular gel, granular hydrogel), tissue engineering, cell, 3D printing (bioinks, support medium) and searching date is April 2021. It can be seen that the number of articles is increasing year by year and reaching its peak in 2019. The decline of articles in 2020 may be possibly caused by COVID-19. Microgel assembly involves many fields, including material science, science technology, chemistry, engineering, physics, biochemistry molecular biology, biotechnology applied microbiology etc, and its research focus (keywords of articles) changes from fabrication of materials and microgels in the earlier period (<2016) to applications such as tissue engineering, drug delivery, scaffolds etc in recent years (>2016).
2. Fabrication of microgel assembly
In comparison to monodisperse microgels that have been successfully applied as individual cell culture platforms and drug carriers, microgel assembly can create macroscopic scaffolds as tissue substitute both in vitro and in vivo, and hence has become another hot research topic in the past few years [19]. To fabricate microgel assembly, microgels should be first generated via several techniques, such as batch emulsion [20], microfluidic emulsion [21], lithography [22], electrohydrodynamic spraying [23] and mechanical fragmentation [24]. The as-prepared microgels are then assembled together under certain conditions. At present, there are many assembling strategies available for microgel assembly [25]. Depending on the assembly driving forces, these assembling strategies can be roughly divided into four categories, namely, chemical reaction, physical reaction, cell-cell interaction and external driving force. Fig. 2 shows examples of these microgel assembly strategies and Table 1 summarizes their merits and limitations. Prior to detailed description of specific assembly driving force, several things should be noted. Firstly, only assembly driving forces for microgel assembly (or namely, inter-microgel crosslinking) are discussed in this section. The intra-microgel crosslinking and applications of the as-formed microgel assembly for TERM are listed in Table 2 without further discussion. Some important applications are emphasized in Section 4. Secondly, random microgel assembly can be also formed by microgel jamming, where microgels are packed in a limited space without any assembly driving forces [26]. However, it lacks regularity and, as such, will not be discussed in this section but their applications will be mentioned in section 4.3.
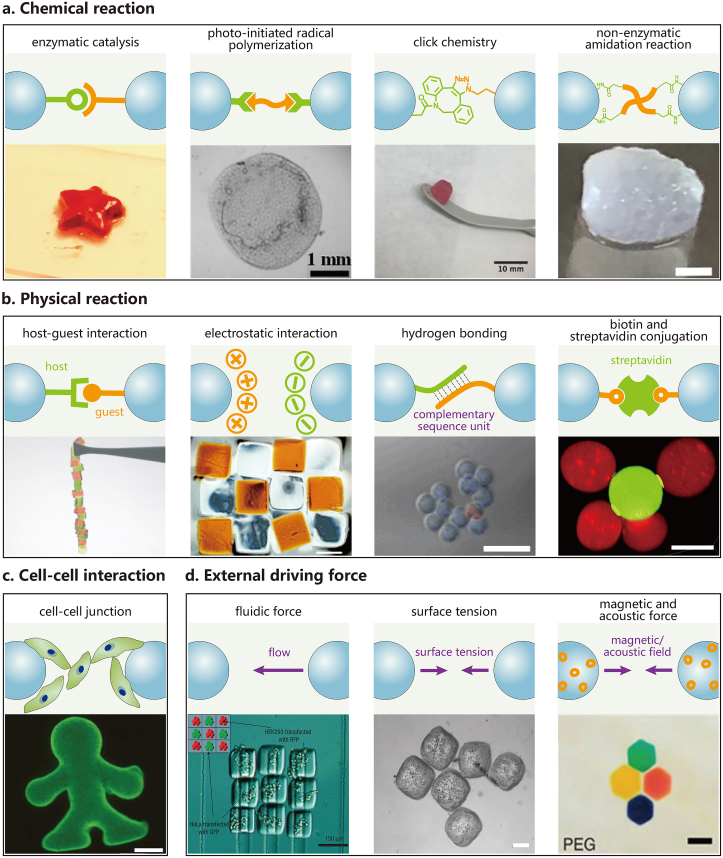
Fig. 2.
Assembling strategies for microgel assembly: (a) chemical reaction, including enzymatic catalysis (scale bar: N/A) [19], photo-induced radical polymerization (scale bar: 1 mm) [31], click chemistry (scale bar: 10 mm) [39] and non-enzymatic amidation reaction (scale bar: 1 mm) [43]; (b) physical reaction, including host-guest interaction (scale bar: N/A) [48], electrostatic interaction (scale bar: 500 μm) [50], hydrogen bonding (scale bar: 250 μm) [55] and biotin-streptavidin conjugation (scale bar: 20 μm) [40]; (c) cell-cell interaction (scale bar: 1 mm) [60]; and (d) external driving force, including fluidic force (scale bar: 150 μm) [63], surface tension (scale bar: 200 μm) [67], magnetic and acoustic force (scale bar: 1 mm) [70]). All the cited literatures are discussed in section 2 where readers can find more details. N/A: not available. Reprinted with the permission from Springer Nature, John Wiley and Sons, American Chemical Society, Elsevier, IOP Publishing, The Royal Society of Chemistry, National Academy of Sciences.
Table 1.
Merits and limitations of microgel assembly strategies.
| Driving forces for microgel assembly | Merits | Limitations | Ref. |
|---|---|---|---|
| 1.Chemical reaction | Chemically and mechanically stable microgel assembly; Irreversible assembling process in most cases; |
Complicated functional group modification on monomer or microgel surface; Possible damage to cells; |
|
|
Mild reaction conditions (neutral pH, moderate temperature); Cytocompatible; |
Fragile enzyme activity; Possible unexpected side reactions induced by enzyme in the microgel system; |
19, 21, 28, 29 |
|
Mild reaction conditions (normally at room temperature); Short reaction time; High spatiotemporal resolution; |
Possible damage to cells by released active free radicals; Incomplete crosslinking inside microgel assembly especially for microgels with deep color; |
21, 30-36 |
|
Mild reaction conditions; Fast and efficient; |
Complicated synthesis steps for functional group modification; | 37–42 |
|
No additional modification to microgels containing peptides and proteins; Mature reaction condition and crosslinker; |
Possible damage to cells by reacting membrane proteins with crosslinker or other proteins in microgels; | 43 |
| 2.Physical reaction | Reversible and spontaneous; Special properties of microgel assembly (except biotin and streptavidin conjugation) like dynamical bonding, self-healing, shear-thinning and injectability; |
Relatively weaker mechanical strength of microgel assembly compared with chemical reaction; | |
|
Biocompatible; Fast and spontaneous; No toxic crosslinker; |
Complicated functional group modification; Weak and unstable binding force especially in aqueous solution; |
47–49 |
|
Easy-going assembly process; Fast and spontaneous; No toxic crosslinker; |
Easily destroyed electrostatic interaction among microgels especially in electrolyte solution; | 9, 50-53 |
|
Biocompatible; Tunable bonding intensity; No toxic crosslinker; |
Special treatment (freezing-thawing cycle) or complicated high-cost fabrication of DNA strands and polypeptides to enhance hydrogen bonding; | 24, 55-57 |
|
Biocompatible, fast and spontaneous; No toxic crosslinker; |
Irreversible interaction; | 40 |
| 3.Cell-cell interaction | No additional chemicals or external stimuli (e.g. UV); No cytotoxicity; |
Relatively slow assembly process with the rate depending on cell seeding density and cell adhesion/migration/proliferation rates; cells may possibly occupy pores on microgel surface and thereby reduce diffusion efficiency. | 10, 60-62 |
| 4.External driving force | Simple, fast and cost-effective; No special requirements for microgel materials; |
Unstable microgel assembly; A subsequent second crosslinking is necessary; |
|
|
Precise 1D or 2D structures of microgel assembly; | Complex fabrication of individual microgel; Difficult in achieving 3D constructs composed of multilayer microgels; |
63–66 |
|
No complicated design for microgel materials; Easy to operate; Biocompatible; |
Secondary cross-linking is required to stabilize the assembly; Difficult in achieving 3D constructs composed of multilayer microgels; |
67, 68 |
|
Fast and efficient; Suitable for various microgel materials; |
Potential cytotoxicity of magnetic nanoparticles; Fast decay in magnetic and acoustic energy along microgel thickness, limiting the final size assembled 3D constructs; |
69–72 |
Table 2.
Intra-microgel crosslinking strategties and applications of various microgel assembly driven by chemical reaction, physical reaction, cell-cell interaction and external driving forces.
| Driving forces for microgel assembly (inter-microgel crosslinking) | Materials of microgel | Intra-microgel crosslinking | Application | Ref. |
|---|---|---|---|---|
| 1.Chemical reaction | ||||
|
PEG | Thiol-ene click reaction | Skin tissue engineering | [19] |
| Hyaluronic acid | Thiol-ene click reaction | Carrier for cell culture and cell behavior regulation | [21] | |
| PEG | Thiol-ene click reaction | Carrier for cell culture and cell behavior regulation | [28] | |
| Hyaluronic acid | Thiol-ene click reaction | Neuronal tissue engineering | [29] | |
|
Hyaluronic acid | Thiol-ene click reaction | Carrier for cell culture and cell behavior regulation | [21] |
| Poly(MMA/MAA/EGD)-GM | Free-radical chemistry | Intervertebral discs tissue engineering | [30] | |
| Gelatin | UV crosslinking | Carrier for cell culture and cell behavior regulation | [31] | |
| Hyaluronic acid | UV crosslinking | Bio-ink for 3D printing | [32] | |
| Alginate | Ionic crosslinking | Bio-ink for 3D printing | [33] | |
| Alginate | Ionic crosslinking | Support medium for 3D printing | [34] | |
| PEG | Click reaction | Neuronal tissue engineering | [35] | |
| PAMPS | Free-radical chemistry | Bio-ink for 3D printing | [36] | |
|
PEG | UV crosslinking | Carrier for cell culture and cell behavior regulation | [37] |
| PEG | UV crosslinking | Bio-ink for 3D printing | [38] | |
| PEG | Azide-Alkyne Cycloaddition | Carrier for cell culture and cell behavior regulation | [39] | |
| Alginate | Ionic crosslinking | Carrier for cell culture and cell behavior regulation | [40] | |
| Hyaluronic acid | UV crosslinking | Carrier for cell culture and cell behavior regulation | [41] | |
| PEG | Click reaction | Neuronal tissue engineering | [42] | |
|
Gelatin, PEG | UV crosslinking | Cartilage tissue engineering | [43] |
| 2.Physical reaction | ||||
|
Hyaluronic acid | UV crosslinking | Cardiac tissue engineering: | [47] |
| Polyacrylamide | Free-radical chemistry | Not available | [48] | |
| Polyrotaxanes, Hydroxyethyl cellulose |
Host-guest interaction | Not available | [49] | |
|
Gelatin, Chitosan | UV crosslinking | Neuronal tissue engineering | [9] |
| PEG | UV crosslinking | Carrier for cell culture and cell behavior regulation | [50] | |
| Methacrylic acid, 2-hydroxyethyl methacrylate |
Free-radical chemistry | Not available | [51] | |
| Chitosan | Schiff base | Bone tissue engineering | [52] | |
| Gelatin | Schiff base | Carrier for cell culture and cell behavior regulation | [53] | |
|
Chitosan, Polyvinyl alcohol | UV crosslinking | Bio-ink for 3D printing | [54] |
| PEG | UV crosslinking | Carrier for cell culture and cell behavior regulation | [[55], [57]] | |
| PEG | UV crosslinking | Not available | [56] | |
|
Alginate | Ionic crosslinking | Carrier for cell culture | [40] |
| 3.Cell-cell interaction | Hyaluronic acid, Gelatin | Thiol-Ene click reaction | Cartilage tissue engineering | [10] |
| Collagen | Hydrogen bond | Carrier for cell culture and cell behavior regulation | [60] | |
| Chitosan | Schiff base | Carrier for cell culture and cell behavior regulation | [61] | |
| Gelatin | Schiff base | Skin tissue engineering | [62] | |
| 4.External driving force | ||||
|
PEG | UV crosslinking | Not available | [[63], [65], [66]] |
|
PEG | UV crosslinking | Carrier for cell culture and cell behavior regulation | [[67], [68]] |
|
PEG, Gelatin | UV crosslinking | Carrier for cell culture and cell behavior regulation | [[69], [70], [71], [72]] |
2.1. Chemical reaction
As the name implies, microgel assembly driven by chemical reaction relies on the occurrence of chemical reactions and the formation of covalent bonding between adjacent microgels [27], which are irreversible assembling processes in most cases and eventually result in chemically and mechanically stable microgel assembly. Chemical reaction-driven assembly usually involves complicated functional group modification on monomer or microgel surface and thus maybe possibly harmful for the encapsulated cells. At present, four types of chemical reactions have been developed for microgel assembly, including enzymatic catalysis, photo-initiated radical polymerization, click chemistry and non-enzymatic amidation reaction.
Enzymatic catalysis: Enzymatic catalysis refers to a kind of chemical reactions using enzyme as a catalyst. Currently, the enzyme reported for microgel assembly is mainly transglutaminase factor XIIIa (FXIIIa), a naturally occurring enzyme responsible for stabilizing blood clots. Griffin et al. [19] and Rutte et al. [28] assembled two different peptide-grafted poly(ethylene glycol)-vinyl sulphone (PEG-VS) microgels via a non-canonical amide linkage between the peptides mediated by FXIIIa. Dehydration of the microgel assembly after addition of FXIIIa exhibited a highly stretched but interconnected mesh whilst microgel aggregate without FXIIIa separated into individual spherical beads, indicating the successful formation of covalent bonding between adjacent microgels. Later, the same research group used a similar enzymatic reaction to fabricate hyaluronic acid (HA) based microgel assembly [21,29]. The enzyme-mediated assembly process is normally performed under mild condition (neutral pH and moderate temperature), and thus allows incorporation of living cells into a dynamically forming microgel assembly. Despite of its obvious merits, this assembly method is hard to control due to the fragile enzyme activity and possible unexpected catalytic side reactions in the complex systems.
Photo-initiated radical polymerization: Photo-initiated radical polymerization contains two steps, namely, the generation of free radicals by decomposition of an initiator under light and the successive polymerization reaction induced by free radicals. Milani et al. [30] prepared vinyl-functionalized microgels consisting of methyl methacrylate, methacrylic acid, ethyleneglycol dimethacrylate and glycidyl methacrylate, and realized their assembly via free-radical chemistry. Sheikhi et al. [31] first stabilized gelatin methacrylate (GelMA) microgels through the low-temperature induced physical crosslinking to form triple helices that can serve as network junctions of polymer chains, and then adopted photo-initiated radical polymerization to fabricate 3D GelMA scaffolds with high mechanical resilience. After UV radiation and incubation at 37 °C, such scaffolds still show excellent stability without dissolution although physical crosslinking inside microgels disappeared. Highley and colleagues [32] synthesized norbornene-modified HA microgels, jammed them together and realized their assembly by UV exposure after addition of dithiothreitol (crosslinker) and photoinitiator. Jeon et al. [33,34] prepared Ca2+-crosslinked methacrylated alginate microgels and assembled them via photo-initiated radical polymerization. Dumont et al. [35] used 8-arm PEG maleimide (PEG-MAL) as raw materials to fabricate microgels by crosslinking part of the maleimide functional groups with crosslinking peptide. The remaining maleimide functional groups were then used to assemble microgels into bridge and tube shapes by photo-initiated radical polymerization. Hirsch et al. [36] fabricated 2-acrylamido-2-methylpropane sulfonic acid (AMPS) microgels, immersed them in acrylamide monomer solution, and realized microgel assembly though photo-initiated radical polymerization to form a second covalently crosslinked percolating network among microgels. Sideris et al. [21] mixed HA microgels with eosin Y and cysteine and then exposed to white light to promote radical formation and microgel assembly. Interestingly, after comparing the pore interconnectivity, Young's modulus in compression, void fraction, and pore size of HA microgel assemblies fabricated both by enzymatic catalysis and by radical polymerization, it was found that their properties were quite similar, except for the higher void fraction of microgel assembly via radical polymerization, which could be attributed to the shorter reaction time of radical polymerization and shorter settling time for microgels. In brief, photo-initiated radical polymerization only requires mild reaction conditions (normally at room temperature) and short reaction time. Despite of its high spatiotemporal resolution, this method can cause possible damage to cells by released active free radicals and incomplete crosslinking inside microgel assembly especially for microgels with deep color.
Click chemistry: In addition to enzymatic catalysis and photo-initiated radical polymerization, click chemistry was also utilized for microgel assembly. Click chemistry is a group of highly-selective and high-yield chemical reactions that connect molecules together with heteroatom linkages. Xin et al. [37,38] used PEG-dithiol to link norbornene-grafted PEG microgels via thiol-ene click chemistry. Caldwell and colleagues [39] prepared two types of PEG microgels containing excess azide or alkyne functional groups and realized their assembly via strain-promoted azide/alkyne click reaction. Hu et al. [40] assembled alginate microgels via inverse electron demand Diels–Alder reaction, i.e., tetrazine/trans-cyclooctene and tetrazine/norbornene after grafting these functional groups onto alginate chains. Based on similar assembling mechanism, Truong et al. [41] synthesized norbornene-grafted HA microgels and the macroscopic 3D construct was obtained via the click reaction between norbornene groups and 4-arm PEG-tetrazine. Roam et al. [42] mixed 8-arm PEG-VS and 8-arm poly(ethylene glycol)-amine (PEG-NH2) together to fabricate PEG-based microgels. The unreacted vinylsulfone and amine groups present on the microgel surfaces induced the aggregation and assembly of microgels via vinylsulfone-amine click chemistry, which could be further enhanced by addition of serum, since serum proteins crosslinked the microgels via reaction of amines on the proteins with vinylsulfone groups on the microgels. Click chemistry is fast, mild, efficient and cytocompatible, but usually involves complicated synthesis steps for functional group modification.
Non-enzymatic amidation reaction: Once peptides or proteins are present in microgels, amidation reaction, a process where the hydrogen atom on an amino group is replaced by amide group, is an ideal choice for assembling since no additional modifications are required. Li et al. [43] applied 4-arm poly(ethylene glycol)-N-hydroxysuccinimide (PEG-NHS) as a crosslinker to assemble PEG/Gel hybrid microgels via the formation of amide bonds between primary amines on the gelatin backbone and PEG-NHS. However, amidation reaction is not very beneficial for cell-encapsulated microgels because membrane proteins on cell surface may react with crosslinker or other proteins in microgel, resulting in decrease in cell viability.
2.2. Physical reaction
Inspired by traditional gelation methods for bulk hydrogels and microgels, physical reactions, such as host-guest interaction, electrostatic interaction, hydrogen bonding and biotin-strepavidin conjugation, can also be used to assemble microgels [44]. Compared with chemical reaction, most physical reactions (except biotin-strepavidin conjugation) are weak, spontaneous, reversible and biocompatible. As a result, the as-formed microgel assemblies show some particular properties, such as dynamic bonding, self-healing, shear-thinning and injectability (or printability) [24,[45], [46], [47]], and the mechanical strength of microgel assembly is relatively weaker compared with that driven by chemical reaction.
Host-guest interaction: Host-guest interaction for microgel assembly is often carried out through mutual molecular recognition between host moieties (such as α-, β-, γ-cyclodextrin) and guest moieties (such as linear alkyl chains, adamantane and ferrocene) located on different microgel surfaces. For example, Harada's team [48] proved that acrylamide-based microgels functionalized with either cyclodextrin rings (host) or small hydrocarbon-groups like adamantyl (guest) can adhere to one another. By changing the size and shape of the host and guest moieties, different microgels can be selectively assembled and sorted into larger aggregated structures that are on the order of millimeters to centimeters in size. Mealy et al. [47] fabricated HA microgel assembly through the use of cyclodextrin and adamantane host-guest interparticle crosslinking. More interestingly, Yu et al. [49] used a macrocyclic host molecule (cucurbit [8]uril) capable of simultaneously encapsulating two guests (viologen and naphthyl) for the fabrication of individual microgel and microgel assembly. The threading of cucurbit [8]uril host molecules into highly branched polyrotaxane main chains with a first guest of viologen provided multiple host units in each macromolecular chain. By mixing with naphthyl-functionalized hydroxyethyl cellulose, the two polymers are joined into a supramolecular copolymer owing to the formation of the cucurbit [8]uril/viologen/naphthyl heteroternary host-guest complexes. In general, host-guest interaction is fast, biocompatible and reversible, but often needs complicated functional group modification and the binding force is weak and unstable.
Electrostatic interaction: Electrostatic interaction-driven microgel assembly arises from the attraction force exerting on oppositely charged microgels. Han et al. [50] created electrostatic potential on the surface of PEG microgels by copolymerizing PEG with positive poly(2-(methacryloyloxy) ethyltrimethylammonium chloride) and negative poly(2-acrylamido- 2-methyl-propane sulfonic acid sodium salt), respectively. These microgels were allowed to self-assemble into pre-specified 2D and 3D multilayer constructs in an organized manner. Tomme's team [51] incorporated methacrylic acid or dimethylaminoethyl methacrylate into methacrylate-derivatized dextran, resulting in negatively or positively charged microgels at physiological pH. Hsu et al. [9] employed GelMA and chitosan methacrylate to fabricate negatively and positively charged microgels. It was found that Schwann cells, human adipose-derived stem cells (hADSCs) and fibroblasts in the as-prepared microgel assembly all show good viability, proliferation and migration. Cai et al. [52] synthesized chitosan microgels with positive charge (-NH3+) and O-carboxymethyl chitosan microgels with negative charge (-COO-) for assembling. In another study, Nair et al. [53] showed that electrostatic interaction-mediated microgel assembly could be obtained using the same substance from different sources (e.g. cationic gelatin from porcine skin and anionic gelatin from bovine skin). Since the positive and negative charges of microgels are mainly originated from charged functional groups whilst the backbones of polymer chains are usually electroneutral, there is no strong charges in between microgel assembly. As a result, electrostatic interaction is generally biocompatible. Similar to host-guest interaction, electrostatic interaction between microgels is fast and easy-going, but may be weakened especially in electrolyte solutions.
Hydrogen bonding: Hydrogen bonding is prone to form when the atoms on both sides of hydrogen atom (one atom is covalently bonded to hydrogen atom and the other is not) are strong electronegative atoms [5,54]. Although a single hydrogen bond is weak, it can form an assembly with high mechanical strength if there are many hydrogen bonds in the microgel system. For example, Fu's group [24] blended chitosan methacrylate and polyvinyl alcohol (PVA) to produce microgels and then assembled these microgels by repeating freezing thawing procedure to build strong hydrogen bonding between chitosan methacrylate and PVA molecules. The resulting construct has not collapsed for months at room conditions. More importantly, DNA base complementation and polypeptide self-assembly, as ubiquitous hydrogen bonding interaction in living organisms, are sequence-specific and highly programmable, which makes them very useful in microgel assembly [16]. DNA contains four different nucleotide bases, each of which forms a base pair with another complementary base via hydrogen bonding, i.e., adenine with thymine and guanine with cytosine. By programming the sequence of these four nucleotide bases and the length of complementary DNA strands on different microgel surface, the hydrogen bonding interaction between microgels can be tuned precisely. Li et al. [55] connected single-stranded DNA to the polymer network of cell-laden poly(ethylene glycol) diacrylate (PEGDA) microgels, and assembled these microgels by sequence-specific hybridization onto spotted DNA microarrays. Qi et al. [56] decorated DNA strands onto the prescribed surfaces of PEGDA microgel cubes to produce an asymmetric glue pattern, which induced the assembly of microgel cubes with diverse structures, such as linear chains, open networks, T-junctions and square structures in aqueous or in interfacial systems. Similar to DNA base complementation, the amphiphilic polypeptide chain can spontaneously form an ordered structure by hydrogen bonds, van der Waals forces and hydrophobic interaction. The amino acid sequence of polypeptide chain can significantly change the morphology of aggregates. Yao et al. [57] created large porous cell-laden constructs through self-assembly of the coiled-coil polypeptide in PEG-based microgels. Both the pores and extracellular microenvironments within assembled microgels could be tailored simultaneously by adjusting the polypeptide and morphological features of microgels. In a word, hydrogen bonding is often biocompatible and the bonding intensity can be tuned by several methods like freezing-thawing cycles. However, sometimes this process involves complicated high-cost fabrication of DNA strands and polypeptides.
Biotin-streptavidin conjugation: As one of the strongest non-covalent interaction, biotin and streptavidin conjugation (namely, the tenacious interaction between the vitamin biotin and the glycoprotein avidin) is very stable (KD = 10−14 mol L−1) in the presence of pH changes, organic solvents and denaturing agents [58]. Mooney's team [40] first synthesized biotin-functionalized alginate microgels using EDC/NHS carbodiimide crosslinker and then fabricated streptavidin microgels by incubating biotin-functionalized alginate microgels with soluble streptavidin protein. The self-assembly capabilities of biotin and streptavidin microgels were evaluated in detail by varying the ratio, size and concentration of these two microgels. Their results indicated that the ratio between biotin and streptavidin microgels controlled the number of microgels per assemblage (Nm), and a high ratio led to a more uniform distribution. Reducing the concentration of microgels in suspension and size of streptavidin microgel (from 25 μm to 10 μm) could produce assemblages with lower Nm. Different from other physical reactions, biotin-streptavidin conjugation is a very stable and irreversible process. In fact, it is much more like a chemical reaction although no covalent bonding exists between biotin and streptavidin conjugate.
2.3. Cell-cell interaction
Microgel assembly driven by cell-cell interaction can be realized by either seeding cells onto microgel surface or, alternatively, allowing cell migration/proliferation to the surface from within cell-laden microgels, and finally obtaining macroscopic 3D constructs via a molding approach [59]. For example, Matsunaga et al. [60] cultured cells over the surface of monodisperse collagen microgels and then stacked cell-coated microgels in a mold to trigger cell-cell interaction. Cruz and colleagues [61] discovered that interparticulate cellular bridges and small cell-microgel aggregates could be formed after seeding cells on surfaces of chitosan microgels for 7 days. Similarly, Imparato et al. [62] packed gelatin microgels in disc shaped chambers and seeded fibroblasts onto them. The results showed that after 2 weeks of culture the assembled microgels were compact and could retain the disc shape of the mold. In contrast, Feng et al. [10] encapsulated cells into gelatin/hyaluronic acid hybrid microgels, injected them into a silicone cavity mold and cultured for a certain period (allowing cells to proliferate and grow onto the microgel surface) for microgel assembly. During these above assembly processes, the cells located on different microgel surfaces formed cell-cell connections and the cell medium diffused across the cavities between the microgels to supply nutrients. In short, such an assembly happens spontaneously by cells and no additional chemicals or external stimuli (such as UV or crosslinker) are needed, indicating no potential risk of biological toxicity. However, its shortcomings are also obvious, i.e., the assembly process requires adequate cell adhesion sites on microgel surface for cell adhesion and proliferation, and the assembling rate depends dramatically on the cell seeding density and proliferation rate. If cells grow too slowly, it may take a long time to complete the whole assembly process. In addition, cells may block many pores on the microgel surface with the increase of cell number and the secretion of extracellular matrix, thereby reduce nutrient exchange efficiency.
2.4. External driving force
Compared with the above-mentioned methods, microgel assembly driven by external driving forces, such as fluidic force, surface tension, magnetic and acoustic forces usually has no special requirements for microgel materials and thus avoids complicated synthesis steps, such as grafting the reactive functional groups onto polymer chains through multi-step reactions to achieve microgel assembly [25]. Although these assembly processes are simple, fast and cost-effective, they are usually unstable and the constructs are prone to collapse after withdrawing these external driving forces. Therefore, second crosslinking is usually needed to stabilize the structure after the formation of microgel assembly.
Fluidic force: In this assembly system, microgels were often fabricated with a structure complementary to microgrooves. These microgels were then guided by fluidic force in 1D and 2D railed microfluidic channels. Complex geometries such as Eiffel Tower and human skeleton shapes could be created using this method, and microgels made of different polymeric materials were assembled heterogeneously via cross-solution movement [[63], [64], [65], [66]]. This approach eliminates multiple alignment and material patterning steps, which must be performed by conventional lithography and is highly deterministic compared to other assembly technologies. However, it requires production of many complex microgels.
Surface tension: Owing to the surface tension on the liquid-liquid and liquid–air interface, hydrophilic microgels can move towards each other and induce aggregation to minimize the system free energy. Du et al. [67] demonstrated that the directed assembly of cell-laden PEGDA microgels could be driven by the surface tension on the mineral oil-water interface. After removing the mineral oil, NIH 3T3 mouse fibroblast cells can maintain good viability in microgel assembly. Addition of surfactant decreased the surface tension of the oil/water interface and thus weakened the microgel assembly. Tightly packed microgel sheets with much greater overall structure size could be further harvested by replacing mineral oil with a more hydrophobic and dense organic solvent (e.g. perflourodecalin) at liquid-air interface [68]. Although this assembly process is quite simple and straightforward, the assembled structures are unstable outside of the oil phase due to the disappearance of surface tension and a subsequent secondary crosslinking step is required to stabilize the assembled constructs [25].
Magnetic and acoustic forces:Microgel assembly in magnetic field has been reported to undergo in three distinct manners, loading magnetic nanoparticles in microgels, inducing the paramagnetic properties of hydrogels and exploiting magnetic micro-robot. Pioneering works mainly come from Demirci's research group. In their earliest works [69], magnetic nanoparticles were mixed into cell-encapsulated PEGDA and GelMA microgels, and these microgels were assembled into multiple rows and spheroid multilayers geometry using parallel sheet magnets and a magnetic rod, respectively. Although this approach is easy-to-operate, the cytotoxicity of magnetic nanoparticles restricts its application in tissue engineering. To solve this problem, the authors [70] used strong permanent magnets to induce the paramagnetic properties of PEGDA and GelMA microgels through the addition of stable radicals instead of magnetic nanoparticles. To switch off their magnetization and thus minimize potential negative effects of free radicals on cell proliferation for tissue engineering applications, the assembled constructs were submerged into an antioxidant solution (vitamin E) after a secondary crosslinking. However, the assembling efficiency was low and only several microgels could be assembled each time. Later on, they improved the assembling accuracy and efficiency by using a magnetic micro-robot (amplitude: 0–24.4 mT) and more complicated 3D microarchitecture were successfully obtained [71]. Similar to magnetic force, acoustic force was also tried by Demirci's team [72] for microgel assembly. The PEG microgels dispersed in a droplet of liquid, when exposed to acoustic excitation (pulse frequency: 0.8–7.0 kHz; amplitude: 1–16 V), could move to the center area of the transducer and form a packed assembly. Independently if magnetic or acoustic forces are exploited, a second crosslinking is necessary to stabilize the assembled construct.
3. Characteristics of microgel assembly
3.1. Microporosity
As confirmed by optical and fluorescent images, the majority of microgel assembly show abundant void space and interconnected micropores (Fig. 3a), which can be modulated easily via the size, shape and deformability of individual microgels as well as the packing density [26]. If microgels are flat-faced and perfectly aligned side by side, or if microgels are soft, deformable and compressible, the void space and microporosity of microgel assembly can be quite low or even close to zero. In contrast, if microgels are round and rigid, the microgel assembly would show much higher porosity and void space. Besides, microgel size can also alter the micropore diameter. For example, Griffin et al. [19] modified the microgels with fluorescent molecules and proved via laser confocal scanning microscope that the average pore diameter in assembly networks ranged from ~10 to ~35 μm when microgel size changed from 30 to 150 μm. Compared with bulk hydrogel with a dense matrix of polymer chains and a mesh size on the order of ~10 nm, microgel assembly provided abundant inner microscale pores and thus improved the rate of molecular diffusion and convectional fluid flow of nutrients and soluble signaling mediators. As a result, cells could easily infiltrate and traverse across the microgel assembly without the need to degrade the microgel first, whereas hydrogel degradation is often required for cells to infiltrate a bulk hydrogel.
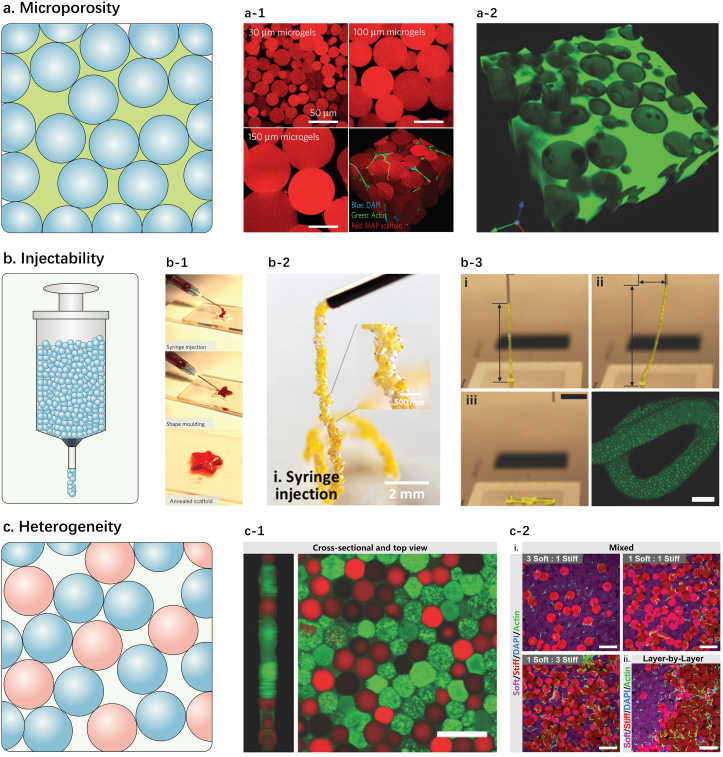
Fig. 3.
Characteristics of microgel assembly: (a) microporosity. a-1: images from Ref. [19] show the porous structures inside microgel assembly using different sizes of PEG microgels (scale bar: 50 μm); a-2: image from Ref. [39] shows a 3D PEG microgel assembly filled with a fluorescently labeled high-molecular-weight dextran solution, demonstrating the interconnectivity of pores within the construct (scale bar: N/A); (b) injectability. b-1: images from Ref. [19] show the formation process of pentagram by needle-injection of PEG microgel assembly (scale bar: N/A); b-2: image from Ref. [9] shows injection of GelMA/chitosan methacrylate microgel assembly from a 26 G needle (scale bar: 2 mm); b-3: images from Ref. [32] show the extrudability of HA microgels on a 3D printer (scale bar: 5 mm); (c) heterogeneity. c-1: images from Ref. [47] show confocal images (cross-sectional and top views) of two-component HA microgel assembly containing cleavable microgels with encapsulated FITC-BSA (green) and stable microgels containing RHO-DEX (red) (scale bar: 100 μm); c-2: images from Ref. [28] show mechanically heterogeneous scaffolds formed by two kinds of cell-laden microgels in situ through various schemes and different ratios of microgel components (scale bar: 200 μm). Reprinted with the permission from Springer Nature, John Wiley and Sons. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Injectability
Injection of traditional hydrogels through a needle or catheter is usually challenging. Most bulk hydrogels are only injectable as a homogeneous precursor solution that exhibits liquid-like rheological properties until gelation is induced using in situ crosslinking approaches [14]. Similarly, microgel assembly can also promote injectability by injecting individual microgels via minimally invasive techniques and then assembling in situ [29]. More interestingly, microgel assembly with reversible dynamic bonding, such as host-guest interaction, electrostatic interaction and hydrogen bonding presents shear-thinning behavior, indicating that such microgel assembly can flow as a fluid under certain injection force and return to a viscoelastic solid state with gel-like rheological properties once the applied force is attenuated (Fig. 3b). This feature minimizes the possible toxicity effects of in situ polymerization chemistry such as ultraviolet light, radicals, and reactive nucleophiles, and is also valuable given the lengthy waiting times for slow crosslinking reaction [26,73]. Microgel jamming, which regularly happens under syringe pressure, reduces turbulent flow and allows microgels to move as a plug during injection. When injected into a cavity, microgel assembly readily fills the space and takes the shape of the cavity, similar to a precursor hydrogel solution, whilst its viscoelasticity limits dispersion of microgels upon injection. The additional self-healing properties further enhance the stability and practicality of microgel assembly for minimally invasive therapy [52].
3.3. Heterogeneity
The bottom-up aggregate nature of microgel assembly makes it an optimal platform to incorporate interparticle heterogeneity [15,16]. Herein interparticle heterogeneity refers to microgels with different materials, composition, structure, mechanical stiffness, encapsulated cells, etc. (Fig. 3c). The simplest heterogeneous microgel assembly can be produced by randomly mixing and assembling multi-type microgel populations together, while the microgel assembly with gradient characteristics can be realized by pre-controlling the layer-by-layer distribution of microgels with different properties. Proof of concept has already been established in the literature for certain heterogeneous combinations. For example, Mealy et al. [47] fabricated microgel assembly with spatial variation in hydrogel degradability and drug release by mixing equal amounts of stable and cleavable HA microgels containing rhodamine labeled dextran or FITC-BSA, respectively. Also, Rutte et al. [28] created mechanically heterogeneous microgel assembly composed of both stiff and soft PEG microgels via a layer-by-layer method.
4. Applications of microgel assembly in tissue engineering and regenerative medicine
TERM is concerned with the replacement or regeneration of cells, tissues or organs to restore normal biological function. Microgel assembly is attractive in this field due to their remarkable features, such as well-defined shapes, good biocompatibility and biodegradability, resemblance to the natural extracellular matrix (ECM), capability to entrap cells, regulation and control over porosity of the system, and tailorable mechanical properties to fit the injury site [16]. Herein we summarize three main applications of microgel assembly, i.e., carrier for cell culture and cell behavior regulation, scaffold for tissue repair and regeneration, bioink and support medium for 3D printing.
4.1. Carrier for cell culture and cell behavior regulation
As carrier for cell culture, microgel assembly can be classified into two general categories depending on the spatial locations of the cells, namely, cells can be encapsulated in microgels or seeded on their surface (Fig. 4). For the first category, cells are often pre-mixed evenly with hydrogel precursor solution, then the mixture is either photo-patterned or emulsified into microgels, and finally the crosslinked cell-laden microgels are assembled into macroscale constructs [13,40]. Due to the presence of live cells, the microgel fabrication and assembling process should be fast, cytocompatible and nontoxic, indicating the limited choice of microgel materials and gelation/assembly methods [74]. However, encapsulation of cells in microgel assembly mimics the native 3D cellular microenvironment and thus is especially useful for in vivo applications in which cell-laden microgels can be delivered via a minimally invasive means to the defect area and assembled locally without suffering from the fatal shearing force and host immune system attack [75]. In contrast, the second category (culturing cells on the surface of microgel assembly) provides an alternative method for in vitro cell culture that shows several advantages over the conventional 2D culture [13]. Owing to the significantly higher surface-to-volume ratio of microgels, a greater number of cells can be cultured on the surface of microgel assembly using a small volume of culture media. Compared to plastic or glass petri dish, the mechanical and chemical properties of microgel assembly can be tailored to replicate physiological conditions more precisely. In addition, microgel assembly possesses continuous microporous structure, which not only provides sufficient growth space for the cells, but also facilitates rapid nutrient exchange to maintain the high viability of the cells. Since cells are only cultured on the microgel surface, microgel assembly can be generated prior to cell culture, thereby offering more flexibility in the type of precursor and gelation/assembly conditions [28].
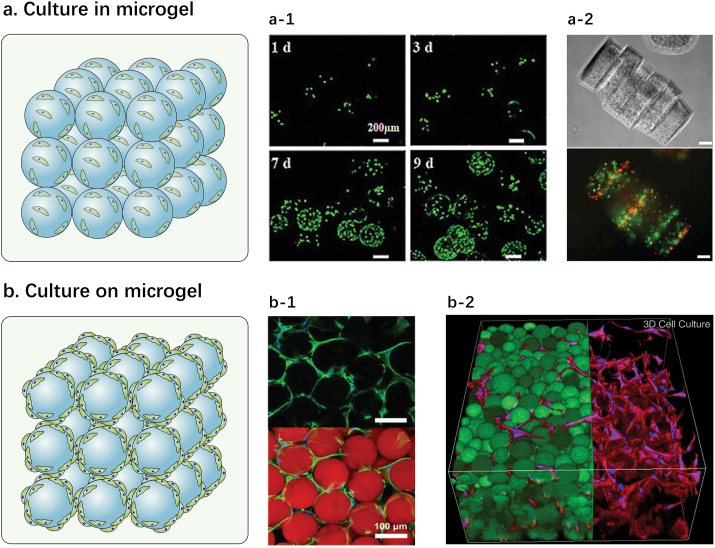
Fig. 4.
Applications of microgel assembly as carrier for cell culture: (a) encapsulation of cells in microgels. a-1: images from Ref. [10] show live/dead staining of BMSC‐laden Gel‐HA microgels under incubation for 1, 3, 7 and 9 d (scale bar: 200 μm); a-2: images from Ref. [67] show a phase-contrast and fluorescence image of cell-laden (NIH 3T3) PEG microgel assembly (scale bar: 100 μm); (b) culturing cells on the surface of microgel assembly. b-1: images from Ref. [28] show cell proliferation on human dermal fibroblasts -laden microporous PEG microgel scaffold after incubation for 3 d (scale bar: 100 μm); b-2: image from Ref. [21] shows a 3-D rendering of z-stack of human dermal fibroblasts cultured on HA microgel assembly surface (scale bar: N/A). Reprinted with the permission from John Wiley and Sons, National Academy of Sciences, American Chemical Society.
Relative to bulk hydrogel, microgel assembly allows for rapid adjustment of its physiological properties (chemical composition, microporosity, mechanical stiffness, etc.) to achieve both spatial and temporal control over cellular behaviors, such as cell morphology, spreading, infiltration, proliferation, differentiation and ECM deposition [60]. For example, Caldwell et al. [39] demonstrated that morphologies of mesenchymal stem cells (MSCs) could be controlled via the size of microgel and porosity of microgel assembly. Imparato et al. [62] proved that gelatin microgel assembly could promote rapid cell spreading, collagen deposition and maturation, and the rate of matrix deposition could be controlled by altering the degradation rate of the microgel assembly. Griffin et al. [19] cultured MSCs on porous PEG microgel assembly, and the cells exhibited rapid cell infiltration, proliferation and lower rates of inflammation compared to nonporous hydrogel. In another work from Segura's group [41], human dermal fibroblasts cultured on different microgel assembly comprising spherical HA microgels that ranged from small (20–60 μm), medium (60–100 μm) to large (100–200 μm) diameters, showed significant difference in cell spreading and proliferation depending on microgel size. In the large microgel group, cells wrapped around microgels and adopted a more flattened shape. In comparison, the fibroblasts showed less spreading, lower levels of proliferation, and decreased transgene expression in small microgel group. Because cellular morphology, genetic and phenotypic expressions were closely interconnected, manipulation of microgel assembly (porosity, microgel size, etc.) could affect genetic transfection of cells and thus was a unique tool to direct cell fate and function.
4.2. Scaffold for tissue repair and regeneration
Tissue and organ damage is one of the major reasons of death worldwide [1]. The gold standard therapy is autografts or allo-grafts. However, the number of damaged tissues/organs that need transplantation is significantly higher than that of available healthy counterparts. Tissue engineering offers an alternative solution by use of biological and engineering methods to maintain, repair, regenerate, or improve damaged tissue and organ functions [2]. As one of the most important elements in tissue engineering, scaffolds fill defects, offer mechanical support, promote cell infiltration, enable desired cell-cell interactions, release encapsulated factors (such as growth factors, chemokines and cytokines) and match timescales relevant for tissue development and repair [76]. Different from traditional polymer scaffolds, such as hydrogel, sponge and electrospun scaffold, microgel assembly combines the merits of these above-mentioned polymer scaffolds including the high water content and injectability of hydrogel, the interconnected porous structure of sponge, and the designable micro-nano structural unit of electrospun scaffolds. Specifically, injectable and porous microgel assembly can provide rapid tissue repair and regeneration without being limited by the degradation rate of bulk hydrogel. Scaffold properties (e.g., stiffness, degradability, etc.) can be tuned in micro-scale through the functionalization of individual microgel to provide guidance and control of the biological responses, such as designing the gradient structure of growth factor to guide the regeneration of axons, setting the micropore structure and enzyme degradation sequence to treat myocardial infarction, establishing the chiral structure of peptide crosslinker to activate the special immune response. In addition, a microgel assembly implant could be an off-the-shelf product that does not need to be fabricated on an individual patient basis, but can still conform to an injury site without knowing the wound geometries and maintain close apposition with intact tissue while supporting regeneration. Currently, microgel assembly is being explored in vitro and in vivo for various tissue engineering applications, including neuronal, cardiac and vascular, cartilage and skin tissues, as shown in Fig. 5.
Fig. 5.
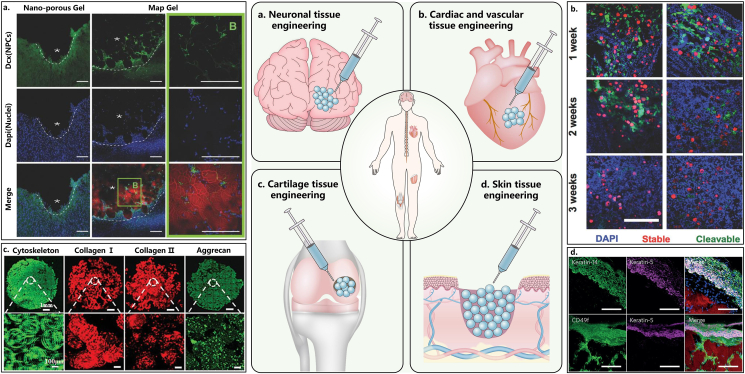
Applications of microgel assembly as scaffolds for tissue repair and regeneration: (a) neuronal tissue engineering. Images from Ref. [29] show neural progenitor cells reached not only the peri-infarct area but also the infarct area filled by peptide-grafted HA microgel assembly (scale bar: 100 μm); (b) cardiac and vascular tissue engineering. Images from Ref. [47] show that two-component HA microgels labeled with FITC for cleavable microgels and rhodamine for stable microgels were injected into rat hearts either without myocardial infarction (no MI) or with MI (scale bar: 500 μm); (c) cartilage tissue engineering. Images from Ref. [10] show cytoskeleton and immunostaining of cartilage biomarkers (col I, col II, and aggrecan) in BMSC-laden Gel/HA hybrid microgels (top scale bar: 1 mm, bottom scale bar: 100 μm); (d) skin tissue engineering. Images from Ref. [19] show the high stratified expression of the epithelial markers (keratin-5, keratin-14 and CD49f) above the PEG-VS microgel assembly, and large-scale tissue structures within the construct (scale bar: 50 μm). Reprinted with the permission from John Wiley and Sons, Springer Nature.
Neuronal tissue engineering: Stroke is the leading cause of long-term disability. Following stroke, a large influx of astrocytes and microglia releasing proinflammatory cytokines leads to dramatic inflammation and glial scar formation, affecting brain tissue's ability to repair itself. Nih et al. [29] demonstrated that the injection and in situ enzyme-mediated assembly of peptide-grafted HA microgels in the stroke cavity of C57BL/6 male mice not only reduced the poststroke inflammatory response by promoting astrocyte infiltration into the stroke cavity rather than scar formation and reducing the total number of reactive microglia within the infarct, but also increased peri-infarct vascularization and neural progenitor cell migration compared to nonporous hydrogel controls and stroke only controls. Besides, the spinal cord and peripheral nerve are highly organized structures, requiring a directional repair and regeneration. Dumont et al. [35] packed PEG-MAL microgels in molds to generate tubes or bridges via free radical photopolymerization. The as-prepared bridge/tubes were implanted into a spinal cord injury model of C57BL/6J female mice. The implanted bridge/tubes had good apposition and were integrated with the host tissue due to the infiltration of immune cells and support cells. The glial scar was significantly reduced relative to control while the lumen provided an orientation to guide axon regeneration. As a result, axon density within the hydrogel tubes was significantly increased more than 3-fold compared to the control, with approximately 30 % of axons within the tube myelinated. Hsu et al. [9] loaded high, medium and low concentrations of neuron growth factor into negatively charged GelMA microgels and then mixed with equal volumes of positively charged chitosan methacrylate microgels to construct interconnected injectable porous scaffold via electrostatic interaction. The propagated gradient of the nerve growth factor, combined with the cell-penetrative connected pores in microgel assembly scaffold, effectively promoted Schwann cell migration, induced dramatic bridging effects (axon outgrowth of up to 4.7 mm) on peripheral nerve defects of SD rats.
Cardiac and vascular tissue engineering: Myocardial infarction can cause expansion of the left ventricle, thinning of the myocardial wall, loss of efficient ventricular mechanics, and eventual heart failure. Mealy et al. [47] developed a two-component HA microgel assembly containing the protease-cleavable and stable microgels via guest–host interactions. This microgel assembly displayed shear-thinning and self-healing properties that rendered it amenable for injection. More importantly, after injection into myocardial wall of male Wistar rats having undergone myocardial infarction, the cleavable microgels showed significantly accelerated degradation due to the higher protease activity in myocardial infarction condition, while stable microgels remained present with non-degraded spherical morphologies, resulting in accelerated cellularization at earlier time points compared to no myocardial infarction condition. Besides, to attain clinically significant viable cell densities for large tissues, laboratory-constructed tissue scaffolds must provide an internal vascular supply. Scott et al. [20] assembled three types of PEG-based microgels with diverse properties via click chemistry, among which the first type provided mechanical support, the second type provided controlled delivery of the angiogenesis-promoting molecule (sphingosine 1-phosphate, S1P) and the third type served as a slowly dissolving non-cytotoxic porogen. In vitro experiments revealed that the RGD peptides conjugated to microgels and S1P delivery promoted endothelial cell infiltration through macropores in the scaffolds. McGuigan et al. [77] encapsulated HepG2 cells in short cylindrical collagen microrods, seeded endothelial cells on their outer surface, assembled these microrods by particle clogging and finally perfused the construct in vitro with whole blood. The endothelial cells retained their nonthrombogenic phenotype, delayed clotting times and inhibited the loss of platelets associated with perfusion of whole blood through the construct, indicative of their promising application as scalable (micro) vascularized tissue-engineered scaffolds containing multiple cell types.
Cartilage tissue engineering: The repair and regeneration of cartilage defect is very challenging because of its avascular and aneural nature [78]. Li and colleagues [43] designed a tissue-like 3D structure by assembling PEG/gelatin hybrid microgels via covalent bonding (4-arm PEG-NHS as crosslinker) to preserve the viability and cellular functions of the encapsulated human bone marrow mesenchymal stem cells (hBMSCs). This assembled microgel construct encouraged upregulation of chondrogenic markers in both gene (Sox-9, Aggrecan, COMP, Col2a1, Col1a1) and glycosaminoglycan (GAG) expression levels. In addition, the regenerated tissue in the assembled microgels showed positive staining with alcian blue and safranin O, and a favorable distribution and significantly higher content of collagen II when compared to both the bulk hydrogel and pellet cultures, exhibiting unique hyaline-like cartilage features. Feng et al. [10] prepared BMSC-laden gelatin/hyaluronic acid hybrid microgels using droplet-based microfluidics, then injected them subcutaneously into nude mice by a minimally invasive way, and finally realized in situ self-assembly of microgels via cell-cell interaction. During this process, the encapsulated BMSCs showed high viability, proliferation, and chondrogenic differentiation potential in the microgels. In vitro immunohistochemical staining (collagen I, collagen II) and mRNA expression (Sox 9, Aggrecan, col2a1, col1a1) of chondrogenic markers showed significant upregulation compared to control group (pure BMSCs), and in vivo experiments confirmed that the self-assembled microgels can effectively inhibit vascularization and hypertrophy after implantation for 8 weeks, indicative of promising applications in cartilage tissue engineering.
Skin tissue engineering: Imparato et al. [62] constructed human dermal tissue equivalent in vitro by assembling Gel microgels via cell-cell interaction, which fully replicated the composition and organization of native dermis both in terms of cellular and extracellular components. It was also demonstrated that the degradation rate of microgel assembly played an important role in the collagen maturation and thus offered the possibility to tune the properties of the final 3D tissue. Specifically, when the less degradable microgels were used, the dermis-like construct retained its shape and thickness up to 6 weeks of culture and residues of microgels were present. By using the less stiff and faster degradable microgels, the dermis-like construct underwent consistent contraction of both shape and thickness even before 2 weeks of culture. In contrast, intermediate degradable microgels were completely made up by endogenous ECM just after 2 weeks of culture and the dermis-like construct were stable in retaining its shape for longer time. In another study, Griffin et al. [19] reported the enzyme-mediated assembly of peptide-grafted PEG-vinyl sulfone microgels and its application in skin tissue engineering. In vitro experiments showed that such microgel assembly enabled both cell proliferation and expedient network formation. Meanwhile, in vivo test in Balb/c mice proved that the microgel assembly scaffold exhibited significantly more conducive to tissue growth, better integration with tissue, more rapid scaffold degradation, faster wound closure, a sustained lower inflammatory response and higher expression of the epithelial markers than the non-porous bulk hydrogel, mainly due to the unique combination of microporosity and injectability of the microgel assembly. In order to promote more extensive tissue ingrowth before scaffold degradation, the same group [79] switched the chirality of the peptides from L-to d -amino acids so that the degradation of microgel assembly scaffold could be slowed down. The resultant microgel assembly significantly promoted cutaneous wound healing and hair neogenesis, mainly by recruiting IL-33 type 2 myeloid cells and activating the adaptive immune system in C57BL/6 (B6) mice.
4.3. Bioink and support medium for 3D printing
3D printing of biopolymers (also referred to as bioinks) has exhibited the powerful ability to create a precisely-designed 3D architecture for customized or personalized TERM applications [80]. Compared with other 3D printing strategies, such as stereolithography or fused deposition modeling, extrusion bioprinting is more suitable for hydrogels due to its low cost and mild operation conditions. Most hydrogel bioinks for extrusion bioprinting are precursor solutions with very high viscosity in order to maintain structural integrity after extrusion, but these materials require large extrusion pressure during printing, which may negatively impact cell viability [38]. Unlike traditional crosslinked hydrogels, when microgels are packed closely together in a jammed state, they look like a solid, but when external forces are applied, they display fluidic collective movement, i.e., shear-thinning behavior, ensuring high viability of the encapsulated cells (Fig. 6a). Moreover, microgels are modular and thus can be used to construct heterogeneous structures. In most cases, the jammed microgels (or microgel assembly) only have physical interactions between microgels, such as cohesive force, host-guest interaction, electrostatic interaction and hydrogen bonding, and therefore the printed constructs lack long-term stability. To this end, a secondary crosslinking is often required.
Fig. 6.
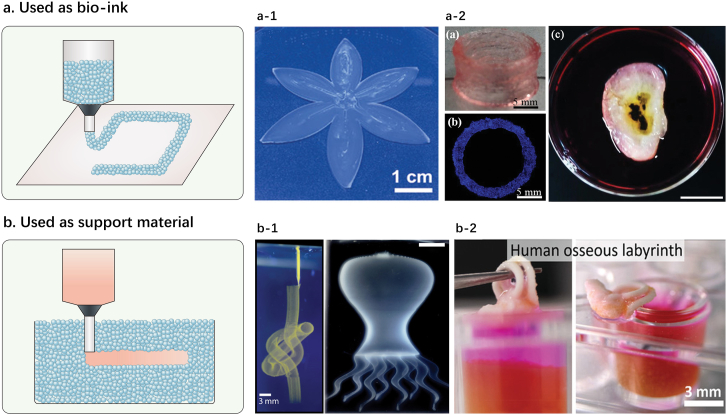
Applications of microgel assembly as bioink and support medium for 3D printing: (a) bioink for extrusion bioprinting. a-1: image from Ref. [36] shows a shape-morphing flower by printing two layers of inks with different swelling behaviors containing AMPS microgels and acrylamide monomer solution (scale bar: 1 cm); a-2: images from Ref. [24] show a 35-layer cylinder and a human-size ear model by 3D printing self-healable pre-crosslinked chitosan methacrylate/polyvinyl alcohol hybrid microgels (left scale bar: 5 mm, right scale bar: 2 cm); (b) support medium for 3D printing. b-1: images from Ref. [81] show a continuous knot and a thin-shell model octopus by writing polystyrene microspheres (dispersed in a photocrosslinkable polyvinyl alcohol solution) in carbopol microgel medium (left scale bar: 3 mm, right scale bar: 5 mm); b-2: images from Ref. [87] show human osseous labyrinth printed by ceramic omnidirectional bioprinting in cell-suspensions (COBICS) which used α-tricalcium phosphate (α-TCP) suspension as ceramic-based ink and a gelatin microgel suspension containing live cells as support medium (scale bar: 3 mm). Reprinted with the permission from John Wiley and Sons, AAAS.
Highley et al. [32] proved that the jammed norbornene-modified HA, or PEGDA, or agarose microgels could be printed either by a layer-by-layer extrusion method or a gel-in-gel printing method (i.e., printing bioink into a support hydrogel). Their results indicated that the printing of microgel inks was feasible, as the jammed microgels supported printing and short-term stability, while the post-crosslinking (photo-initiated radical polymerization) can be used to introduce long-term stability. Similarly, Xin et al. [38] used norbornene-grafted PEG microgels as bioink to print 1D filament, 2D honeycomb, 3D cylinder, anatomically sized ear and nose. The extruded microgels formed a continuous line from the nozzle, indicative of their excellent printability. Because the PEG microgels contained unreacted residual norbornene groups, bis-thiol crosslinker or photoinitiator solutions were added onto the printed structure to assemble microgels for long-term stability via photo-initiated click chemistry or radical polymerization. hMSC viability in the printed structure (cell density: 5 × 106/mL) was assessed at 1 h, 1, 5, and 10 days after bioprinting, and the high cell viability proved the cytocompatibility of the process. Jeon et al. [33] encapsulated hMSCs in ion-crosslinked oxidized and methacrylated alginate microgels, cultured them in osteogenic or chondrogenic differentiation medium, then used as bioinks to print femur, skull, ear structures in gelatin microparticle slurry support bath and finally stabilized these structures by photocrosslinking under UV light. Fu's team [24] developed a novel self-healable bioink containing pre-crosslinked chitosan methacrylate/PVA microgels. A series of biomimetic structures with very high aspect ratio and delicate fine structures were printed and their mechanical properties could be further strengthened via cyclic freezing-thawing procedure to build strong hydrogen bonding among microgels. The biocompatibility of the microgel bioink was tested by growing BMSCs and cell spheroids on top of the constructs. Recently, Hirsch et al. [36] designed an ink composed of methacrylate-modified chitosan and polyvinyl alcohol. After the ink was printed, the monomer-loaded microgels were converted into a percolating network, resulting in the formation of macroscopic objects with high mechanical strength and high shape fidelity.
In addition to bioink, jammed microgels (namely, microgel assembly by jamming) can also serve as support medium for 3D printing (Fig. 6b) [[81], [82], [83], [84], [85], [86], [87]]. Microgel-based support medium is fluidized under low shear stress, permitting easy insertion and rapid motion of needles deep within the bulk. After removing shear stress caused by needle movement and ejection of printing material, the locally fluidized microgel bath rapidly ‘self-heals’ and forms a stable medium that firmly holds the printed constructs [34]. Bhattacharjee et al. [81] used silicones, hydrogels, colloids, and living cells to create complex large aspect ratio 3D objects, thin closed shells, and hierarchically branched tubular networks in carbopol microgel (the authors called them as granular gel) medium. More interestingly, uncrosslinked structures, printed in the microgel medium, were also incredibly stable in time, with the oldest retained model exhibiting no visible changes over more than 6 months. Hinton et al. [82] reported a novel 3D bioprinting technique termed as freeform reversible embedding of suspended hydrogels (FRESH). This method enabled the printing of soft protein and polysaccharide hydrogels (alginate, fibrin, collagen type I, and matrigel) within a second gelatin microgel support medium that maintained the intended structure during the print process and significantly improved print fidelity. The gelatin microgel support medium at low temperature behaved as a rigid body at low shear stresses but flowed as a viscous fluid at high shear stresses. Thus, soft materials that would collapse if printed in air were easy to maintain the intended 3D geometry in gelatin microgel support medium. Complex biological structures like femur, arterial tree, embryonic chick heart and human brain were successfully printed via FRESH. Later, Jeon et al. [34] expanded this technology by directly printing individual cell-only bioink into another photocurable liquid-like support medium comprised of oxidized and methacrylated alginate microgels. The low yield stress of the alginate microgel support medium in its solid state and its rapid self-healing behavior allowed the high-resolution deposition, placement and structuring of hMSCs within the support medium without creating crevasses. Moreover, the microgel support medium could also provide mechanical stability to the printed constructs after additional photo-crosslinking, which permitted culture of the constructs with stable structural maintenance and long-term differentiation in differentiation medium. Dissociation of the photo-crosslinked microgel support medium by gentle agitation can facilitate acquisition of matured 3D tissue constructs. Recently, Romanazzo et al. [87] reported a novel 3D printing technique labeled as ceramic omnidirectional bioprinting in cell-suspensions (COBICS), which enables freeform writing of a novel ceramic-based ink (α-tricalcium phosphate, α-TCP) within a gelatin microgel suspension containing live cells. Complex bone-mimetic architectures were successfully printed via COBICS and the live cells showed robust adhesion and proliferation behavior during printing, with greater than 95 % viability after several weeks in culture.
5. Conclusions and outlook
Microgel assembly, prepared from microgels under proper driving forces, such as chemical reaction, physical reaction, cell-cell interaction and external driving force, is now emerging as promising candidates for TERM. Compared with traditional bulk hydrogels, microgel assembly shows three unique characteristics, namely, microporosity, injectability and heterogeneity. The interconnected micropores and void space inside microgel assembly not only accelerate the mass transfer of nutrients and thus improve the cell viability, but also facilitate cell infiltration and vascularization without the need to wait for degradation of hydrogel. The small size of individual microgel or reversible dynamic bonding of microgel assembly enable the injectability of either microgels or microgel assembly, indicative of their promising usage in minimally invasive therapy. Owing to the bottom-up fabrication of microgel assembly, heterogeneity can be easily achieved inside microgel assembly by mixing microgels with diverse properties in a random or ordered manner. The as-formed macroscopic microgel assembly show broad application prospects as carrier for cell culture and cell behavior regulation, scaffold for tissue repair and regeneration, bioink and support medium for 3D printing.
Despite of the significant progress in scientific research, there are still several challenging issues that should be addressed before microgel assembly can be finally used for clinical applications: (1) The large-scale assembly of microgels into macroscopic 3D construct requires a large number of microgels. However, the current fabrication methods of individual microgels are difficult to assure both the high yield and the stability/uniformity of microgels at the same time. It is necessary to either develop new methods or improve the current available technologies (such as batch emulsion, microfluidic emulsion, electrohydrodynamic spraying, etc.) for the high-throughput manufacture of various pure or cell-encapsulated microgels; (2) Considering the time-consuming steps of microgel fabrication, especially for clinicians and others whose expertise is not material science and engineering, microgels or microgel assembly products (sometimes referred as bioinks) for various TERM purposes as well as their long-term storage and transportation should be explored in detail, which are almost ignored so far; (3) Currently, bottom-up assembly of microgels is mainly isotropic, and directional assembly of microgels into 3D constructs with arbitrary structure is still in its infancy. Integration of various biophysical/biochemical cues into microgels or utilization of signaling factors secreted by encapsulated cells or peripheral tissue cells may help to promote microgel assembly spatio-temporally in vivo. In addition, there is few researches on the combination of microgels with other subunits, such as bioactive inorganic microspheres, solid polymer microspheres, cell spheres and decellularized ECM microspheres, which may address the disadvantages of pure microgel assembly [[88], [89], [90], [91]]; (4) Most of the present studies are focusing on the fabrication and primary biological characterization of proof-of-concept microgels and microgel assembly, the inherent connections between the properties of individual microgel (e.g., size, crosslinking density, stiffness, etc.) and those of as-formed microgel assembly (e.g., porosity, viscoelasticity, mechanical strength, etc.) are still unclear and lack of investigation. Besides, their possible application scopes in TERM still need to be further confirmed and clarified especially by long-term preclinical animal test and clinical test. Scientists and engineers should keep it in mind that fabrication and application of microgel assembly is a comprehensive system project, involving multi-disciplinary work in varied areas, such as chemistry, materials, physics, biology, biomedical engineering and clinical medicine. Cooperation of multidisciplinary scientists and engineers is the key to the successful commercialization of microgels and microgel assembly.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially sponsored by the National Natural Science Foundation of China (Grant No. 51873071, 32071321, 51873069, 52073103) and the National Key R&D Program of China (2018YFC1106300).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xiaodong Cao, Email: caoxd@scut.edu.cn.
Hua Dong, Email: donghua@scut.edu.cn.
References
- 1.Green J., Elisseeff J. Mimicking biological functionality with polymers for biomedical applications. Nature. 2016;540:386–394. doi: 10.1038/nature21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedde R., Mirani B., Navaei A., Styan T., Wong S., Mehrali M. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017;29:1606061. doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- 3.Huang B., Hu J., Athanasiou K. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu F., Cao X., Li Y., Chen X. Diels–Alder click-based hydrogels for direct spatiotemporal postpatterning via photoclick chemistry. ACS Macro Lett. 2015;4:289–292. doi: 10.1021/mz5007427. [DOI] [PubMed] [Google Scholar]
- 5.He H., Cao X., Dong H., Ma T., Payne G. Reversible programing of soft matter with reconfigurable mechanical properties. Adv. Funct. Mater. 2017;27:1605665. [Google Scholar]
- 6.Caliari S., Burdick J. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slaughter B., Khurshid S., Fisher O., Khademhosseini A., Peppas N. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allazetta S., Lutolf M. Stem cell niche engineering through droplet microfluidics. Curr. Opin. Biotechnol. 2015;35:86–93. doi: 10.1016/j.copbio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Hsu R., Chen P., Fang J., Chen Y., Chang C., Lu Y., Hu S. Adaptable microporous hydrogels of propagating NGF‐gradient by injectable building blocks for accelerated axonal outgrowth. Adv. Sci. 2019;6:1900520. doi: 10.1002/advs.201900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Q., Li Q., Wen H., Chen J., Liang M., Huang H., Lan D., Dong H., Cao X. Injection and self‐assembly of bioinspired stem cell‐Laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019;29:1906690. [Google Scholar]
- 11.Feng Q., Gao H., Wen H., Huang H., Li Q., Liang M., Liu Y., Dong H., Cao X. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: insights from a microgel model. Acta Biomater. 2020;113:393–406. doi: 10.1016/j.actbio.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Ma T., Gao X., Dong H., He H., Cao X. High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or Diels–Alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today. 2017;9:49–59. [Google Scholar]
- 13.He Q., Zhang J., Liao Y., Alakpa E., Bunpetch V., Zhang J., Ouyang H. Current advances in microsphere based cell culture and tissue engineering. Biotechnol. Adv. 2019;45:21051–21061. doi: 10.1016/j.biotechadv.2019.107459. [DOI] [PubMed] [Google Scholar]
- 14.Tong X., Yang F. Recent progress in developing injectable matrices for enhancing cell delivery and tissue regeneration. Adv. Healthc. Mater. 2018;7:1701065. doi: 10.1002/adhm.201701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly A., Riley L., Segura T., Burdick J. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2019;5:20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell A., Aguado B., Anseth K. Designing microgels for cell culture and controlled assembly of tissue microenvironments. Adv. Funct. Mater. 2019;30:1907670. doi: 10.1002/adfm.201907670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal G., Agrawal R. Functional microgels: recent advances in their biomedical applications. Small. 2018;14:1801724. doi: 10.1002/smll.201801724. [DOI] [PubMed] [Google Scholar]
- 18.Newsom J., Payne K., Krebs M. Microgels: modular, tunable constructs for tissue regeneration. Acta Biomater. 2019;88:32–41. doi: 10.1016/j.actbio.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin D., Weaver W., Scumpia P., Di Carlo D., Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott E., Nichols M., Kuntz-Willits R., Elbert D. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater. 2010;6:29–38. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sideris E., Griffin D.R., Ding Y., Li S., Weaver W.M., Di Carlo D., Hsiai T., Segura T. Particle hydrogels based on hyaluronic acid building blocks. ACS Biomater. Sci. Eng. 2016;2:2034–2041. doi: 10.1021/acsbiomaterials.6b00444. [DOI] [PubMed] [Google Scholar]
- 22.Li B., Wang L., Xu F., Gang X., Demirci U., Wei D., Li Y., Feng Y., Jia D., Zhou Y. Hydrosoluble, uv-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015;22:59–69. doi: 10.1016/j.actbio.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Gansau J., Kelly L., Buckley C. Influence of key processing parameters and seeding density effects of microencapsulated chondrocytes fabricated using electrohydrodynamic spraying. Biofabrication. 2018;10 doi: 10.1088/1758-5090/aacb95. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H., Cong Y., Osi A., Zhou Y., Huang F., Zaccaria R., Chen J., Wang R., Fu J. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv. Funct. Mater. 2020;30:1910573. [Google Scholar]
- 25.Gurkan U., Tasoglu S., Kavaz D., Demirel M., Demirci U. Emerging technologies for assembly of microscale hydrogels. Adv. Healthc. Mater. 2012;1:149–158. doi: 10.1002/adhm.201200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riley L., Schirmer L., Segura T. Granular hydrogels: emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 2019;60:1–8. doi: 10.1016/j.copbio.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Zhang Y., Yue K., Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutte J., Koh J., Di Carlo D. Scalable high‐throughput production of modular microgels for in situ assembly of microporous tissue scaffolds. Adv. Funct. Mater. 2019;29:1900071. [Google Scholar]
- 29.Nih L., Sideris E., Carmichael S., Segura T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater. 2017;29:1606471. doi: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milani A., Freemont A., Hoyland J., Adlam D., Saunders B. Injectable doubly cross-linked microgels for improving the mechanical properties of degenerated intervertebral discs. Biomacromolecules. 2012;13:2793–2801. doi: 10.1021/bm3007727. [DOI] [PubMed] [Google Scholar]
- 31.Sheikhi A., de Rutte J., Haghniaz R., Akouissi O., Sohrabi A., Di Carlo D., Khademhosseini A. Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads. Biomaterials. 2019;192:560–568. doi: 10.1016/j.biomaterials.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Highley C., Song K., Daly A., Burdick J. Jammed microgel inks for 3D printing applications. Adv. Sci. 2019;6:1801076. doi: 10.1002/advs.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon O., Bin L., Hinton T., Feinberg A., Alsberg E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019;12:61–70. doi: 10.1016/j.mtchem.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon O., Lee Y., Jeong H., Lee S., Wells D., Alsberg E. Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater. Horiz. 2019;6:1625–1631. doi: 10.1039/c9mh00375d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumont C., Carlson M., Munsell M., Ciciriello A., Strnadova K., Park J., Cummings B., Anderson A., Shea L. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019;86:312–322. doi: 10.1016/j.actbio.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch M., Charlet A., Amstad E. 3D printing of strong and tough double network granular hydrogels. Adv. Funct. Mater. 2020:2005929. [Google Scholar]
- 37.Xin S., Wyman O., Alge D. Assembly of PEG microgels into porous cell-instructive 3D scaffolds via thiol-ene click chemistry. Adv. Healthc. Mater. 2018;7:1800160. doi: 10.1002/adhm.201800160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin S., Chimene D., Garza J., Gaharwar A., Alge D. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019;7:1179–1187. doi: 10.1039/c8bm01286e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldwell A., Campbell G., Shekiro K., Anseth K. Clickable microgel scaffolds as platforms for 3D cell encapsulation. Adv. Healthc. Mater. 2017;6:1700254. doi: 10.1002/adhm.201700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y., Mao A., Desai R., Wang H., Weitz D., Mooney D. Controlled self-assembly of alginate microgels by rapidly binding molecule pairs. Lab Chip. 2017;17:2481–2490. doi: 10.1039/c7lc00500h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truong N., Kurt E., Tahmizyan N., Lesher-Perez S., Chen M., Darling N., Xi W., Segura T. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater. 2019;94:160–172. doi: 10.1016/j.actbio.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roam J., Yan Y., Nguyen P., Kinstlinger I., Leuchter M., Hunter D., Wood M., Elbert D. A modular, plasmin-sensitive, clickable poly(ethylene glycol)-heparin-laminin microsphere system for establishing growth factor gradients in nerve guidance conduits. Biomaterials. 2015;72:112–124. doi: 10.1016/j.biomaterials.2015.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F., Truong V., Fisch P., Levinson C., Glattauer V., Zenobi-Wong M., Thissen H., Forsythe J., Frith J. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;77:48–62. doi: 10.1016/j.actbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W., Li M., Chen Z., Leong K. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip. 2016;16:4482–4506. doi: 10.1039/c6lc01193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J., Feng Q., Lin S., Yuan W., Li R., Li J., Wei K., Chen X., Zhang K., Yang Y., Wu T., Wang B., Zhug M., Guo R., Li G., Bian L. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Levato R., Visser J., Planell J., Engel E., Malda J., Mateos-Timoneda M. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 47.Mealy J., Chung J., Jeong H., Issadore D., Lee D., Atluri P., Burdick J. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 2018;30:1705912. doi: 10.1002/adma.201705912. [DOI] [PubMed] [Google Scholar]
- 48.Harada A., Kobayashi R., Takashima Y., Hashidzume A., Yamaguchi H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2011;3:34–37. doi: 10.1038/nchem.893. [DOI] [PubMed] [Google Scholar]
- 49.Yu Z., Liu J., Tan C., Scherman O., Abell C. Supramolecular nested microbeads as building blocks for macroscopic self-healing scaffolds. Angew. Chem. Int. Ed. 2018;57:3079–3083. doi: 10.1002/anie.201711522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han Y., Yang Y., Liu S., Wu J., Chen Y., Lu T., Xu F. Directed self-assembly of microscale hydrogels by electrostatic interaction. Biofabrication. 2013;5 doi: 10.1088/1758-5082/5/3/035004. [DOI] [PubMed] [Google Scholar]
- 51.Van Tomme S., Nostrum C., Dijkstra M., Smedt S., Hennink W. Effect of particle size and charge on the network properties of microsphere-based hydrogels. Eur. J. Pharm. Biopharm. 2008;70:522–530. doi: 10.1016/j.ejpb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Cai B., Zou Q., Zuo Y., Mei Q., Ma J., Lin L., Chen L., Li Y. Injectable gel constructs with regenerative and anti-Infective dual effects based on assembled chitosan microspheres. ACS Appl. Mater. Interfaces. 2018;10:25099–25112. doi: 10.1021/acsami.8b06648. [DOI] [PubMed] [Google Scholar]
- 53.Nair S., Basu S., Sen B., Lin M., Kumar A., Yuan Y., Cullen P., Sarkar D. Colloidal gels with tunable mechanomorphology regulate endothelial morphogenesis. Sci. Rep. 2019;9:1072–1089. doi: 10.1038/s41598-018-37788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo Y., Lee D. Biopolymer microparticles prepared by microfluidics for biomedical applications. Small. 2020;16:1903736. doi: 10.1002/smll.201903736. [DOI] [PubMed] [Google Scholar]
- 55.Li C., Wood D., Hsu C., Bhatia S. DNA-templated assembly of droplet-derived PEG microtissues. Lab Chip. 2011;11:2967–2975. doi: 10.1039/c1lc20318e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi H., Ghodousi M., Du Y., Grun C., Bae H., Yin P., Khademhosseini A. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 2013;4:2275–2285. doi: 10.1038/ncomms3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao M., Yang J., Song J., Zhao D., Du M., Zhao Y., Liu B. Directed self-assembly of polypeptide-engineered physical microgels for building porous cell-laden hydrogels. Chem. Commun. 2014;50:9405–9408. doi: 10.1039/c4cc04018j. [DOI] [PubMed] [Google Scholar]
- 58.Polyak B., Geresh S., Marks R. Synthesis and characterization of a biotin-alginate conjugate and its application in a biosensor construction. Biomacromolecules. 2004;5:389–396. doi: 10.1021/bm034454a. [DOI] [PubMed] [Google Scholar]
- 59.Lee J., Hu J., Yamada S., Athanasiou K. Initiation of chondrocyte self-Assembly requires an intact cytoskeletal network. Tissue Eng. Part A. 2016;22:318–325. doi: 10.1089/ten.tea.2015.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsunaga Y., Morimoto Y., Takeuchi S. Molding cell beads for rapid construction of macroscopic 3D tissue architecture. Adv. Mater. 2011;23:90–94. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- 61.Cruz D., Ivirico J., Gomes M., Ribelles J., Sanchez M., Reis R., Mano J. Chitosan microparticles as injectable scaffolds for tissue engineering. J Tissue Eng. Regen. Med. 2008;2:378–380. doi: 10.1002/term.106. [DOI] [PubMed] [Google Scholar]
- 62.Imparato G., Urciuolo F., Casale C., Netti P. The role of microscaffold properties in controlling the collagen assembly in 3D dermis equivalent using modular tissue engineering. Biomaterials. 2013;34:7851–7861. doi: 10.1016/j.biomaterials.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 63.Chung S., Park W., Shin S., Lee S., Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat. Mater. 2008;7:581–587. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 64.Inamdar N., Borenstein J. Microfluidic cell culture models for tissue engineering. Curr. Opin. Biotechnol. 2011;22:681–689. doi: 10.1016/j.copbio.2011.05.512. [DOI] [PubMed] [Google Scholar]
- 65.Park W., Lee H., Park H., Kwon S. Sorting directionally oriented microstructures using railed microfluidics. Lab Chip. 2009;9:2169–2175. doi: 10.1039/b904153b. [DOI] [PubMed] [Google Scholar]
- 66.Chung S., Jung Y., Kwon S. Three-dimensional fluidic self-assembly by axis translation of two-dimensionally fabricated microcomponents in railed microfluidics. Small. 2011;7:796–803. doi: 10.1002/smll.201001806. [DOI] [PubMed] [Google Scholar]
- 67.Du Y., Lo E., Ali S., Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamanian B., Masaeli M., Nichol J., Khabiry M., Hancock M., Bae H., Khademhosseini A. Interface-directed self-assembly of cell-laden microgels. Small. 2010;6:937–944. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu F., Wu C., Rengarajan V., Finley T., Keles H., Sung Y., Li B., Gurkan U., Demirci U. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011;23:4254–4260. doi: 10.1002/adma.201101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tasoglu S., Yu C., Gungordu H., Guven S., Vural T., Demirci U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 2014;5:4702–4713. doi: 10.1038/ncomms5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tasoglu S., Diller E., Guven S., Sitti M., Demirci U. Untethered micro-robotic coding of three-dimensional material composition. Nat. Commun. 2014;5:3124–3133. doi: 10.1038/ncomms4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu F., Finley T., Turkaydin M., Sung Y., Gurkan U., Yavuz A., Guldiken R., Demirci U. The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials. 2011;32:7847–7855. doi: 10.1016/j.biomaterials.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jivan F., Yegappan R., Pearce H., Carrow J., McShane M., Gaharwar A., Alge D. Sequential thiol-ene and tetrazine click reactions for the polymerization and functionalization of hydrogel microparticles. Biomacromolecules. 2016;17:3516–3523. doi: 10.1021/acs.biomac.6b00990. [DOI] [PubMed] [Google Scholar]
- 74.Jiang Y., Chen J., Deng C., Suuronen E., Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Kamperman T., Karperien M., Le Gac S., Leijten J. Single-cell microgels: technology, challenges, and applications. Trends Biotechnol. 2018;36:850–865. doi: 10.1016/j.tibtech.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharjee M., Coburn J., Centola M., Murab S., Barbero A., Kaplan D., Martin I., Ghosh S. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv. Drug Deliv. Rev. 2015;84:107–122. doi: 10.1016/j.addr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 77.McGuigan A., Sefton M. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madeira C., Santhagunam A., Salgueiro J., Cabral J. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015;33:35–42. doi: 10.1016/j.tibtech.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Griffin D., Archang M., Kuan C., Weaver W., Weinstein J., Feng A., Ruccia A., Sideris E., Ragkousis V., Koh J., Plikus M., Di Carlo D., Segura T., Scumpia P. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat. Mater. 2021;20:560–569. doi: 10.1038/s41563-020-00844-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy S., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharjee T., Zehnder S.M., Rowe K.G., Jain S., Nixon R.M., Sawyer W.G., Angelini T.E. Writing in the granular gel medium. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hinton T., Jallerat Q., Palchesko R., Park J., Grodzicki M., Shue H., Ramadan M., Hudson A., Feinberg A. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015;1:1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin Y., Compaan A., Bhattacharjee T., Huang Y. Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/2/025016. [DOI] [PubMed] [Google Scholar]
- 84.Spencer A.R., Sani E.S., Soucy J.R., Corbet C.C., Primbetova A., Koppes R.A., Annabi N. Bioprinting of a cell-laden conductive hydrogel composite. ACS Appl. Mater. Interfaces. 2019;11:30518–30533. doi: 10.1021/acsami.9b07353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan W.S., Shi Q., Chen S., Juhari M.A.B., Song J. Recyclable and biocompatible microgel-based supporting system for positive 3D freeform printing of silicone rubber. Biomedical Engineering Letters. 2020;10:517–532. doi: 10.1007/s13534-020-00173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LeBlanc K.J., Niemi S.R., Bennett A.I., Harris K.L., Schulze K.D., Sawyer W.G., Taylor C., Angelin T.E. Stability of high speed 3D printing in liquid-like solid. ACS Biomater. Sci. Eng. 2016;2:1796–1799. doi: 10.1021/acsbiomaterials.6b00184. [DOI] [PubMed] [Google Scholar]
- 87.Romanazzo S., Molley T.G., Nemec S., Lin K., Sheikh R., Gooding J.J., Wan B., Li Q., Kilian K.A., Roohani I. Synthetic bone-like structures through omnidirectional ceramic bioprinting in cell suspensions. Adv. Funct. Mater. 2021;31:2008216. [Google Scholar]
- 88.Xie W., Fu X., Tang F., Mo Y., Cheng J., Wang H., Chen X. Dose-dependent modulation effects of bioactive glass particles on macrophages and diabetic wound healing. J. Mater. Chem. B. 2019;7:940–952. doi: 10.1039/c8tb02938e. [DOI] [PubMed] [Google Scholar]
- 89.Zhang F., Li Q., Lin Z., Ma L., Xu S., Feng Q., Dong H., Zhang Y., Cao X. Engineered Fe(OH)3 nanoparticle-coated and rhBMP-2-releasing PLGA microsphere scaffolds for promoting bone regeneration by facilitating cell homing and osteogenic differentiation. J. Mater. Chem. B. 2018;6:2831–2842. doi: 10.1039/c8tb00569a. [DOI] [PubMed] [Google Scholar]
- 90.Vrij E., Rouwkema J., LaPointe V., Blitterswijk C., Truckenmüller R., Rivron N. Directed assembly and development of material-free tissues with complex architectures. Adv. Mater. 2016;28:4032–4039. doi: 10.1002/adma.201505723. [DOI] [PubMed] [Google Scholar]
- 91.Chiang C., Fang Y., Ho C., Assunção M., Lin S., Wang Y., Blocki A., Huang C. Bioactive decellularized extracellular matrix derived from 3D stem cell spheroids under macromolecular crowding serves as a scaffold for tissue engineering. Adv. Healthcare Mater. 2021;10:2100024. doi: 10.1002/adhm.202100024. [DOI] [PubMed] [Google Scholar]