Abstract
VlsE, the variable surface antigen of Borrelia burgdorferi, contains an immunodominant conserved region named IR6. In the present study, the diagnostic performance of a peptide enzyme-linked immunosorbent assay (ELISA) based on a 26-mer synthetic peptide (C6) with the IR6 sequence was explored. Sensitivity was assessed with serum samples (n = 210) collected from patients with clinically defined Lyme disease at the acute (early localized or early disseminated disease), convalescent, or late disease phase. The sensitivities for acute-, convalescent-, and late-phase specimens were 74% (29 of 39), 85 to 90% (34 of 40 to 35 of 39), and 100% (59 of 59), respectively. Serum specimens from early neuroborreliosis patients were 95% positive (19 of 20), and those from an additional group of patients with posttreatment Lyme disease syndrome yielded a sensitivity of 62% (8 of 13). To assess the specificity of the peptide ELISA, 77 serum samples from patients with other spirochetal or chronic infections, autoimmune diseases, or neurologic diseases and 99 serum specimens from hospitalized patients in an area where Lyme disease is not endemic were examined. Only two potential false positives from the hospitalized patients were found, and the overall specificity was 99% (174 of 176). Precision, which was assessed with a panel of positive and negative serum specimens arranged in blinded duplicates, was 100%. Four serum samples with very high anti-OspA antibody titers obtained from four monkeys given the OspA vaccine did not react with the C6 peptide. This simple, sensitive, specific, and precise ELISA may contribute to alleviate some of the remaining problems in Lyme disease serodiagnosis. Because of its synthetic peptide base, it will be inexpensive to manufacture. It also will be applicable to serum specimens from OspA-vaccinated subjects.
In the United States, Lyme borreliosis continues to be the most frequently reported arthropod-borne infectious disease. Approaches taken towards Lyme disease prevention and control include the recent development of prophylactic vaccines (29, 34), one of which is already commercially available (34), and the exertion of concerted efforts to improve and standardize methods of serologic diagnosis (7). Precise diagnosis of infection at an early phase is of great importance in Lyme disease management, as the timely administration of appropriate antibiotics is usually curative (31). Because of the ambiguities that still bedevil serodiagnosis of Lyme disease (1, 22, 28, 32, 35, 39), patients with nonspecific clinical signs or symptoms of early infection (e.g., absence of erythema migrans, the skin rash that heralds infection) may remain undetected. Long courses of antibiotic therapy may be required if chronic infection ensues, sometimes with a prolonged convalescence or an uncertain outcome (31, 33).
Over- and underdiagnosis (22, 28, 32, 35, 39), as well as interlaboratory discrepancies (1), are current problems of Lyme disease serodiagnosis. To improve specificity, a multiple-band set of criteria was developed by Dressler et al. (10) and Engstrom et al. (11) for a positive immunoblot test, and a two-tiered approach composed of an initial enzyme-linked immunosorbent assay (ELISA) of relatively high sensitivity but low specificity followed by an immunoblot incorporating the Dressler (immunoglobulin G [IgG]) or Engstrom (IgM) band criteria was recommended by the Centers for Disease Control and Prevention (CDC) (7). This approach likely entails several advantages, such as enhanced specificity and the opportunity to estimate duration of infection. However, the need to include the Western blot (WB) technique will increase the cost of Lyme disease diagnosis and possibly further enhance inter- and intralaboratory discrepancies, as the test is itself more difficult to perform than a standard ELISA and its outcome may depend on subjective interpretation of the banding pattern.
In the wake of the recent availability of the OspA (outer surface protein A) vaccine, a new difficulty has been added to the field of Lyme disease serodiagnosis, namely, the possible presence of anti-OspA antibodies in patient serum. Whole-cell antigen-based tests will not be useful in this context, as the antigen extracts currently used include OspA.
A procedure that was intrinsically unable to detect anti-OspA antibodies and which retained, or improved upon, the simplicity and sensitivity of the current ELISA and the specificity of a WB would, in principle, circumvent the shortcomings of the two-tiered approach while preserving some of its advantages. We recently identified, within the variable (cassette) domain of VlsE, the variable surface antigen of Borrelia burgdorferi (40), an invariable 26-amino-acid region, named IR6, which we determined to be antigenically conserved among strains and species of the B. burgdorferi sensu lato complex and immunodominant in both human and nonhuman primate hosts (17a). Based on these initial results, we further investigated the sensitivity, specificity, and precision of an ELISA based on a synthetic peptide (C6) whose sequence is essentially that of IR6. Serial serum samples from nonhuman primates that were tick inoculated with different strains of B. burgdorferi were assessed to ascertain in which phase of Lyme disease antibody to C6 first appeared and the duration of its persistence in the serum. Sensitivity of the C6 ELISA was assessed by using several serum panels with specimens from patients with acute (early localized or early disseminated phase) or late manifestations of Lyme disease or from patients who were either convalescent or had posttreatment Lyme disease syndrome. Specificity of the C6 ELISA was examined with a panel of serum samples from patients living in a region where Lyme disease is not endemic and with serum samples from an array of patients with autoimmune or neurologic diseases, spirochetal diseases other than Lyme borreliosis, or other chronic infections. Precision was assessed with a subgroup of Lyme disease and non-Lyme disease serum specimens arranged in blinded duplicates. Finally, absence of reactivity of the C6 peptide with anti-OspA antibodies was assessed with high-titer anti-OspA serum samples from rhesus monkeys. These animals had been vaccinated with an OspA vaccine formulation and according to a vaccine administration protocol which were similar to those employed in human trials (36). Our results, related here, indicate that the C6-peptide-based ELISA performs extremely well in terms of simplicity, sensitivity, specificity, and precision and that it will be utilizable with serum samples that contain anti-OspA antibodies.
MATERIALS AND METHODS
Serum sources. (i) Monkey serum samples.
Serum specimens from animals that were employed in previous studies were used (25, 26). For the serial study, samples were obtained from rhesus monkeys (2- to 4-year-old Macaca mulatta animals) that had been infected by the bite of Ixodes scapularis nymphal ticks (nine animals) or by needle inoculation (one animal) as previously described (25). Ticks feeding on the animals were themselves infected with spirochetes of either the JD1 (25) or B31 (26) strain of B. burgdorferi sensu stricto. The needle-inoculated animal received JD1 spirochetes. Blood specimens were collected every 1 or 2 weeks postinoculation, and serum samples were stored at −20°C until being tested. To assess cross-reactivity of the C6 peptide with anti-OspA antibodies, serum samples from animals that had been administered an OspA vaccine were utilized (26). Specimens were obtained from four rhesus macaques before and after they were vaccinated with recombinant lipidated OspA adsorbed onto aluminum hydroxide. The vaccine formulation and administration protocol, which also had been used in human trials (36), have been described previously (26).
(ii) Human serum samples.
Human serum samples were obtained from several sources. To assess diagnostic sensitivity, three serum panels from Lyme disease patients were used. One was from the CDC (kindly provided by Martin Schriefer), a second panel was from patients of the Tufts-New England Medical Center, and a third one was from the National Institutes of Health (NIH). The CDC serum panel was composed of 40 samples from patients who were in the convalescent phase of Lyme disease. Twenty-seven of these samples were also culture confirmed. All of the serum specimens in this panel were from patients whose case satisfied the CDC Lyme disease case definition (6). In addition, all of the samples had been tested at the CDC with commercially available kits: ELISA was performed with Lyme Screen II (bioMerieux, St. Louis, Mo.), and IgG and IgM WB was performed with Marblot (MarDx, Carlsbad, Calif.). The panel from Tufts-New England Medical Center was composed of 157 specimens, of which 39 were from patients in the acute phase of Lyme disease. These patients were in the early (localized or disseminated) phase of Lyme disease. Also in this panel were 39 specimens from individuals in the convalescent phase (obtained from the same patients whose sera had been obtained in the acute phase), 20 from patients in the early disseminated phase who had presented with signs and/or symptoms of early neuroborreliosis, and 59 from patients with late Lyme disease (49 with Lyme arthritis and 10 with late neuroborreliosis). All of the patients in the Tufts panel met clinical criteria for Lyme disease diagnosis, as described previously (10). The NIH panel was composed of 13 specimens obtained from patients with posttreatment Lyme disease syndrome, defined as persistent or intermittent symptoms for at least 6 months after appropriate antibiotic therapy for Lyme disease. Usual symptoms include widespread musculoskeletal pain and fatigue, memory and/or concentration impairment, radicular pain, paresthesia, or dysesthesia. The beginning of the symptoms coincides with, or occurs within 6 months of, the initial B. burgdorferi infection. Symptoms are significant enough to interfere with daily life activities, and other causes have been excluded. All of these samples met CDC criteria for seropositivity (7).
To examine the diagnostic specificity of the peptide ELISA, a panel of 56 serum specimens from patients with autoimmune or neurologic diseases, spirochetal diseases other than Lyme borreliosis, or other chronic infections was obtained from the NIH. Sera were from patients with multiple sclerosis (n = 10), positive anticardiolipin antibody (n = 10), positive rheumatoid factor (n = 10), positive rapid plasma reagin (n = 10), positive antinuclear antibody (n = 10), Guillain-Barré syndrome (n = 1), or mycobacterial infection (n = 5). A second panel, obtained from the CDC, was composed of nine serum samples from patients with relapsing fever. A third panel of 12 additional syphilis serum samples, 9 from patients with early latent disease and 3 from patients with late latent disease, were obtained from the syphilis serum bank maintained by one of us (J.N.M.); each serum sample was positive by the Treponema pallidum immobilization test, and the patients satisfied the criteria for early and late syphilis (9), respectively. Finally, a panel of 99 specimens was obtained blindly from hospital patients in Louisiana, where Lyme disease is not known to be endemic.
Definitions.
Sensitivity was defined as true positives/(true positives plus false negatives), specificity was defined as true negatives/(true negatives plus false positives), and precision was defined as the frequency of obtaining the same result on duplicate analysis of a set of positive and negative specimens. Early localized, early disseminated, and late Lyme disease were defined as described in reference 31.
Peptide synthesis and conjugation to biotin.
A 26-amino-acid peptide (C6) was prepared by using the fluorenylmethoxycarbonyl synthesis protocol (4). A cysteine residue was included at the N terminus and used as a biotinylation site. Biotinylation was performed by the N-succinimidyl maleimide carboxylate method. The maleimide reagent was from Molecular Probes (Eugene, Oreg.), and the protocol suggested by the manufacturer was followed. The sequence of the peptide (C6) used in this study is CMKKDDQIAAAMVLRGMAKDGQFALK. Its immunologic properties have been described elsewhere (17a).
Peptide ELISA.
Ninety-six-well ELISA plates (Corning Inc., Corning, N.Y.) were coated with 100 μl of streptavidin (4 μg/ml), (Pierce Chemical Company, Rockford, Ill.) per well in coating buffer (0.1 M carbonate buffer, pH 9.2) and incubated at 4°C overnight. After two 3-min washes with 200 μl of PBST (10 mM sodium phosphate, 150 mM NaCl, and 0.1% Tween 20, pH 7.4) per well at 200 rpm in a rotatory shaker (orbital shaker; Lab-Line Instruments Inc., Melrose Park, Ill.), 200 μl of biotinylated peptide (5 μg/ml) dissolved in blocking buffer (PBST supplemented with 5% nonfat dry milk [Carnation; Nestlé Food Company, Glendale, Calif.]) was applied to each well. The plate was shaken at 150 rpm for 2 h at room temperature (RT). After three washes with PBST as described above, 50 μl of serum (monkey or human) diluted 1:200 with blocking buffer was added to each well. The plate was incubated with shaking at 150 rpm for 1 h at RT and then washed three times with PBST as before. Each well then received 100 μl of goat anti-monkey IgG (0.2 μg/ml) or goat anti-monkey IgM (0.5 μg/ml) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) goat anti-human IgG (0.1 μg/ml) (Pierce), or goat anti-human IgM (0.5 μg/ml) (Sigma), each conjugated to horseradish peroxidase and dissolved in blocking buffer. Incubation was at RT for an additional 1 h with shaking at 150 rpm. After four washes with PBST for 3, 4, 5, and 6 min, respectively, 100 μl of a solution composed of the chromogen 3,3′,5,5′-tetramethylbenzidine at 0.2 mg/ml and 0.01% hydrogen peroxide in the buffer supplied by the manufacturer (Kirkegaard & Perry) was added, and color was allowed to develop for 10 min. The enzyme reaction was stopped by addition of 100 μl of 1 M H3PO4. The optical density (OD) was measured at 450 nm with an ELISA plate spectrophotometer model SLT Spectra (SLT Lab Instruments, Salzburg, Austria). The cutoff OD value was defined as the mean OD plus 3 standard deviations (SDs) for 97 serum samples collected from patients of a hospital in Louisiana (where Lyme disease is not endemic). All of the samples were assessed blindly, in duplicate, at least twice. Mean OD values from duplicate determinations are reported. OD values of individual samples never varied more than 5%.
RESULTS
IgG and IgM antibody responses to C6 in infected rhesus monkeys.
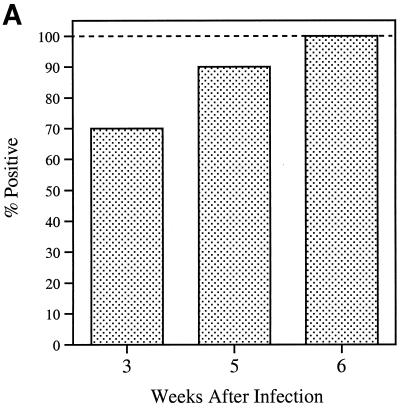
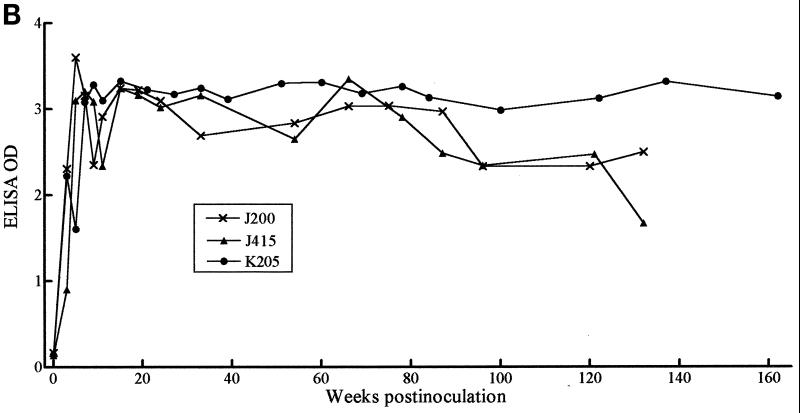
Serum samples serially collected from 10 monkeys that had been inoculated with either the B31 or JD1 strain of B. burgdorferi were tested by ELISA for antibody responses to C6. IgG antibody was detectable in seven animals as early as 3 weeks postinoculation. At week 5 postinoculation, nine animals had responded, and all had responded at week 6 (Fig. 1A). Antibody to C6 persisted at high levels in all animals during the entire study period, which was between 25 and 160 weeks postinoculation. The results for 3 of the 10 animals studied are shown in Fig. 1B. IgM anti-C6 antibody responses were detectable in only some of the animals and did not appear earlier than the corresponding IgG responses (not shown).
FIG. 1.
Early and persisting IgG antibody response to C6 in infected monkeys. After infection, serum samples were serially collected from 10 animals and tested for IgG antibodies with the C6 ELISA. (A) Fraction of the animals that became positive at weeks 3, 5, and 6 postinfection. (B) Persistence of response over time for three animals. The last point of each curve corresponds to the serum specimen collected at the time of sacrifice. All sera were diluted 1:200.
Sensitivity of the C6 ELISA.
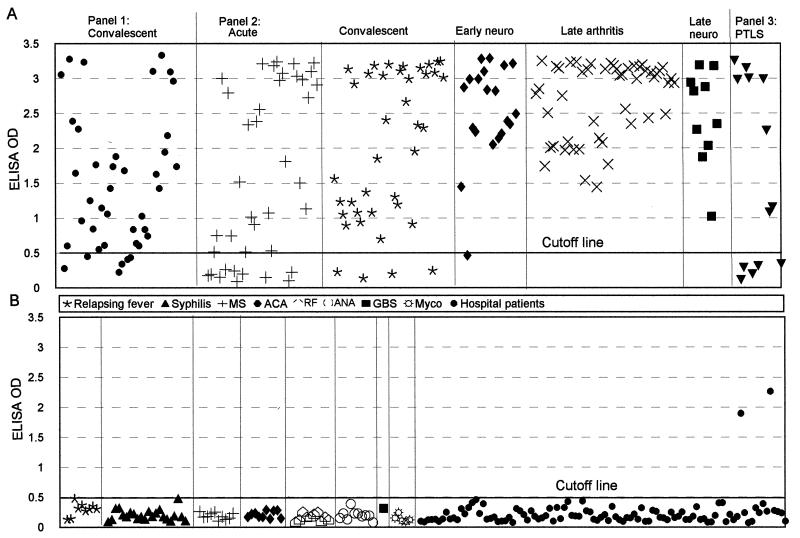
Forty serum samples obtained by the CDC from patients in the convalescent phase of Lyme borreliosis were assessed with the C6 ELISA. Thirty-four were positive (Table 1; Fig. 2A), thus yielding a sensitivity of detection of 85%. An assessment of the same samples performed at the CDC with commercially available assay kits yielded sensitivities of 80% (32 of 40) with a conventional ELISA and 75% (30 of 40) with the combination of both IgG and IgM immunoblots.
TABLE 1.
Sensitivity of the C6 ELISAa
| Serum panel (institution) | Disease phase | No. of specimens | No. positive | No. negatives | Sensitivity (%) |
|---|---|---|---|---|---|
| 1 (CDC) | Convalescent | 40 | 34 | 6 | 85 |
| 2 (Tufts) | Acute | 39 | 29 | 10 | 74 |
| Convalescent | 39 | 35 | 4 | 90 | |
| Early disseminated (neuroborreliosis) | 20 | 19 | 1 | 95 | |
| Late (arthritis) | 49 | 49 | 0 | 100 | |
| Late (neuroborreliosis) | 10 | 10 | 0 | 100 | |
| 3 (NIH) | Posttreatment Lyme disease syndrome | 13 | 8 | 5 | 62 |
The cutoff OD value (0.500) was defined as the mean plus 3 SDs for 97 serum samples obtained from hospitalized patients in an area where Lyme disease is not endemic. C6 ELISA was performed as described in Materials and Methods.
FIG. 2.
Assessment of sensitivity (A) and specificity (B) of the C6 ELISA. (A) Three panels of serum samples from patients with Lyme disease were tested with the C6 ELISA at a serum dilution of 1:200. Panel 1 was composed of specimens collected during the convalescent phase; panel 2 was composed of samples from the acute, convalescent, early disseminated (early neuroborreliosis [neuro]), and late (arthritis and neuroborreliosis [neuro]) phases; and panel 3 was composed of samples from patients with posttreatment Lyme disease syndrome (PTLS). (B) Specimens were from patients with relapsing fever, syphilis, multiple sclerosis (MS), positive anticardiolipin antibody (ACA), positive rheumatoid factor (RF), positive antinuclear antibody (ANA), Guillain-Barré syndrome (GBS), or mycobacterial infection (Myco). In addition, 99 serum specimens from patients of a hospital in Louisiana, where Lyme disease is not endemic, were tested; the cutoff OD value (0.500) was defined as the mean plus 3 SDs for 97 serum samples from this panel.
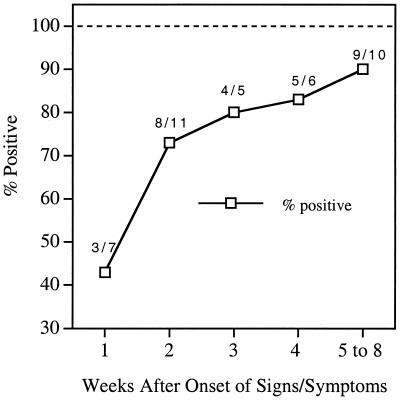
One hundred fifty-seven serum samples collected from patients with different phases of Lyme disease at Tufts-New England Medical Center also were assessed with the C6 ELISA. The sensitivity was 74% (29 of 39) for patients in the acute disease phase, 90% (35 of 39) for convalescent-phase patients, 95% (19 of 20) for patients in the early disseminated phase (neuroboreeliosis), and 100% (59 of 59) for late Lyme disease patients. Of the late Lyme disease patients, 49 had late Lyme arthritis and 10 had late neuroborreliosis (Table 1; Fig. 2A). All of the acute-phase patients in this panel had signs of localized infection (erythema migrans), and some had, in addition, signs or symptoms of disseminated infection. An analysis of the anti-C6 antibody-positive fraction of serum samples taken at consecutive weeks after the onset of signs or symptoms showed that 80 to 90% of the samples taken 3 weeks or later after disease onset were positive (Fig. 3). An additional test of 13 serum specimens collected at the NIH from patients with posttreatment Lyme disease syndrome yielded a sensitivity of 62% (8 of 13) (Table 1; Fig. 2A).
FIG. 3.
Fraction of positive samples as a function of time after disease onset. The anti-C6 antibody-positive fraction of serum samples obtained at consecutive weeks after the onset of signs or symptoms is shown. The acute-phase group of serum specimens from panel 2 was used at a dilution of 1:200.
Acute- and convalescent-phase serum specimens from panel 2 were employed to examine the IgM antibody response to C6. Of the 39 samples in each category, none were only IgM antibody positive, 16 acute-phase and 10 convalescent-phase specimens were both IgG and IgM antibody positive, and 13 and 25 in the acute- and convalescent-phase categories, respectively, were only IgG antibody positive (Table 2).
TABLE 2.
IgM and IgG antibody responses to the C6 peptide in humans in the acute and convalescent phases of Lyme disease
| Disease phasea | No. of specimens | No. only IgM positive | No. only IgG positive | No. both IgG and IgM positive |
|---|---|---|---|---|
| Acute | 39 | 0 | 13 | 16 |
| Convalescent | 39 | 0 | 25 | 10 |
Serum specimens were from panel 2 (Tufts).
Specificity of the C6 ELISA.
The C6 ELISA yielded a specificity of 100% (77 of 77) when serum samples from patients with other chronic infections or autoimmune diseases were tested (Table 3; Fig. 2B). This panel included 9 specimens from relapsing fever patients, 22 specimens from syphilis patients (10 from NIH and 12 from UCLA), and 46 specimens from patients with either multiple sclerosis (n = 10), positive anticardiolipin antibody (n = 10), positive rheumatoid factor (n = 10), positive antinuclear antibody (n = 10), Guillain-Barré syndrome (n = 1), or mycobacterial infection (n = 5). Only two potential false-positive results were obtained when a group of 99 human serum samples randomly collected at a local hospital in Louisiana were tested (Table 3; Fig. 2B). Lyme disease is not endemic in Louisiana, but the identities of the patients and their clinical histories are unknown.
TABLE 3.
Specificity of the C6 ELISAa
| Sample descriptionb | No. of samples | No. positive | No. negative | Specificity (%) |
|---|---|---|---|---|
| Relapsing fever | 9 | 0 | 9 | 100 |
| Syphilis (UCLA) | 12 | 0 | 12 | 100 |
| Syphilis (NIH) | 10 | 0 | 10 | 100 |
| MS | 10 | 0 | 10 | 100 |
| ACA | 10 | 0 | 10 | 100 |
| RF | 10 | 0 | 10 | 100 |
| ANA | 10 | 0 | 10 | 100 |
| GBS | 1 | 0 | 1 | 100 |
| Myco | 5 | 0 | 5 | 100 |
| Hospital patients | 99 | 2 | 97 | 98 |
| Total | 176 | 2 | 174 | 99 |
The cutoff OD value (0.500) was defined as the mean plus 3 SDs for 97 serum samples obtained from hospitalized patients in an area where Lyme disease is not endemic. C6 ELISA was performed as described in Materials and Methods.
MS, multiple sclerosis; ACA, positive anticardiolipin antibody; RF, positive rheumatoid factor; ANA, positive antinuclear antibody; GBS, Guillain-Barré syndrome; Myco, mycobacterial infection.
Precision of the C6 ELISA.
To assess precision, a panel of serum specimens composed of samples from patients with posttreatment Lyme disease syndrome (n = 7), early disseminated Lyme disease (n = 1), mycobacterial infection (n = 5), positive antinuclear antibody (n = 5), positive rheumatoid factor (n = 5), or positive rapid plasma reagin (n = 5) was set up in blinded duplicates and assessed with the C6 ELISA. Precision was 100%. Results for any pair of duplicates derived from true-positive specimens were both positive, and, in addition, their OD values differed by less than 10%. Similarly, duplicates derived from true-negative samples were both negative in that both OD values were below the cutoff line.
Nonreactivity of the C6 peptide with anti-OspA antibodies.
To ascertain whether high-titer anti-OspA antibodies could cross-react with the C6 peptide, serum samples from four rhesus macaques that had been vaccinated with an OspA vaccine formulation also used in humans (26, 36) were tested with the C6 ELISA. The anti-OspA antibody geometric mean titer in these four serum samples was 1:0.9 × 106, as measured by an OspA ELISA (24). No antibody to C6 was detected in any of the four specimens.
DISCUSSION
Current problems of Lyme disease serodiagnosis include over- and underdiagnosis (22, 28, 32, 35, 39) as well as interlaboratory discrepancies (1). A late or feeble antibody response to B. burgdorferi antigens may lead to serologic underdiagnosis, whereas antigenic determinants shared by proteins of B. burgdorferi and other bacteria are likely a contributing factor to overdiagnosis. The 10 or more antigen bands that can be detected on spirochete whole-cell lysate immunoblots when such blots are reacted with Lyme disease patient serum (10, 19) include homologues of the omnipresent bacterial heat shock proteins (74, 66, and 58 kDa) (16, 18); flagellin (41 kDa), which is shared with other spirochetes such as Borrelia hermsii (3), one of the relapsing fever agents, and T. pallidum (19), the cause of syphilis; and a 60-kDa antigen which is expressed by a wide range of bacteria (16). Antiflagellin serum antibody can be detected in 87% of Lyme disease patients, 75% of syphilis patients, and 43% of normal humans (19). The frequencies of detection of antibody to the 60-kDa antigen are 58, 42.9, and 16.6% in borreliosis, syphilis, and normal human serum, respectively (19). Therefore, ELISAs based on detection of antibody to B. burgdorferi whole-cell extracts are not expected to be highly specific and may thus lead to overdiagnosis. In fact, in a recent interlaboratory comparison of results of tests for detection of Lyme disease, in which the methodology used was based on enzyme immunoassay, 516 laboratories each received 28 serum samples from Lyme disease patients and 22 samples from individuals without previous or current Lyme disease. False-positive test results approached 55% with some serum samples from healthy donors (1). A serum sample with anti-T. pallidum antibodies was reported as positive by 70% of the participants (1). Also, in a survey of normal human serum samples, most samples had antibodies to at least one B. burdorferi immunoblot band (12), 64% of serum samples from tick-borne relapsing fever patients were falsely diagnosed as positive by a whole-antigen ELISA (20), and human serum samples collected in a tropical country where Lyme disease is not endemic yielded 98% false-positive results by ELISA and 57% by immunoblotting (5).
The two-tiered ELISA-WB approach that was recently recommended by the CDC (7), although more costly, is almost certain to improve diagnostic specificity (e.g., none of the bands recommended by Dressler et al. [10] are heat shock proteins). However, interlaboratory, and even intralaboratory, variation may persist because of two types of possible shortcomings of the whole-cell antigen immunoblot procedure: first, the procedure is technically involved—more so than the ELISA—and its results are largely qualitative and dependent on subjective interpretation; second, the composition of the whole-cell antigen extract is ill defined. This is so in part because some B. burgdorferi antigens are polymorphic and thus their apparent molecular masses vary from strain to strain (2, 37, 38). Conversely, different antigens may have the same apparent molecular mass, e.g., OspC (outer surface protein C) and decorin binding protein (22 kDa) (15) BmpA (Borrelia membrane protein A) (39 kDa) (30), or flagellin (41 kDa) (3) and VlsE (39 to 45 kDa) (40). In addition, expression of several B. burgdorferi antigens is dependent on the spirochetal growth phase and on the length of time spent in continuous culture by a given spirochetal isolate. Thus, spirochetes harvested in mid-log phase during growth in vitro do not express an array of antigens that are detectable only in late log or stationary growth phase (17, 27), and the expression of several antigens is lost after repeated serial passage of cultured spirochetes (21, 23). Hence, the whole-cell antigen extract composition will depend on how long a particular isolate has been maintained in continuous culture and on whether cells have been harvested at an early or late growth phase.
A procedure with the simplicity of an ELISA and based on synthetic antigens would in principle circumvent the technical complexity and potential antigen variability associated with immunoblot diagnosis. Synthetic peptides used as antigens have the added advantage of avoiding contamination with low-level impurities derived from Escherichia coli or other cloning vectors required to produce recombinant proteins. Such impurities could decrease the specificity of the assay if they were cross-reactive with B. burgdorferi antigens. Indeed, the specificity of diagnostic ELISAs for Lyme disease can be significantly improved by adsorption of the serum samples with soluble E. coli protein (13, 14). The C6 ELISA utilizes a chemically synthesized 26-mer peptide as the diagnostic antigen, thus permitting the uniformity of antigen preparations at relatively low costs and minimizing spurious cross-reactivity.
The sensitivity of the C6 ELISA ranged from 62 to 100%, depending on when in the course of disease the serum samples were collected and on the clinical definition of the patients' signs and symptoms (Table 1). Based on the results of our longitudinal analysis of the anti-C6 antibody response in infected rhesus monkeys (Fig. 1A), it is unlikely that such a response may be detectable with good sensitivity earlier than 1 to 2 weeks postinfection. However, by week 5 postinfection, 90% of the animals (9 of 10) had responded. This result is consistent with that obtained with the acute-phase serum panel (panel 2 [Tufts]), which showed that 80% or more of the patients whose serum had been collected 3 or more weeks after disease onset (5 or more weeks after infection?) were positive by the C6 ELISA (Fig. 3). With the CDC serum panel of 40 specimens from convalescent patients, the sensitivity of the C6 ELISA (85%) was slightly higher than that of the conventional ELISA (80%) and higher than the combination of IgG and IgM WB (75%). A lower sensitivity (74%) was obtained with serum specimens collected during the acute phase of Lyme disease (74%) (39 samples from Tufts-New England Medical Center). However, only three of these patients remained negative in the convalescent phase (Table 1). The patient with the fourth negative sample among the convalescent-phase samples had been positive during the acute phase. We explored the possibility of making an earlier diagnosis by testing IgM responses to C6 both in monkeys and in humans. In either case, no IgM antibody response was seen in the absence of an IgG response, no matter how early after infection or disease onset the serum samples had been collected.
Overall, and as expected from the results obtained with rhesus monkeys as infection progressed in these animals (Fig. 1), the sensitivity of anti-C6 antibody detection was higher in humans with later forms of Lyme disease. For patients in the early disseminated phase (neuroborreliosis), sensitivity was 95%, and for patients with late Lyme disease it was 100% (Table 1, panel 2). The exception was the samples from patients with posttreatment Lyme disease syndrome, which were only 62% positive (Table 1, panel 3). Patients in this group had a history of Lyme disease, and despite having received antibiotic therapy, they had persistent Lyme disease symptoms. The etiology of these symptoms is currently under investigation at the NIH.
The C6 ELISA is highly specific (Table 3; Fig. 2B). It could discriminate between Lyme borreliosis and infections with spirochetes of different species but of the same (B. hermsii) or different (T. pallidum) genera. Moreover, none of the samples from patients with autoimmune diseases, Mycobacterium infections, or diseases that often need to be differentially diagnosed with respect to Lyme disease, such as multiple sclerosis or Guillain-Barré syndrome, were positive with the C6 test. Only 2 of 99 serum samples from a local hospital in Louisiana, where Lyme disease is not endemic, yielded a positive C6 ELISA result. Given that the samples were collected randomly from unknown patients, it is impossible to assess whether these two patients could have been exposed to B. burgdorferi. The specificity results obtained are supported by our finding that, except for the Vls cassette of B. burgdorferi, no other sequences homologous to C6 could be identified (by using the BLAST search algorithm) in the National Center for Biotechnology Information protein sequence database. High diagnostic specificity is extremely critical to improve the positive predictive value of a test, especially when the incidence of a disease is very low. The incidence of Lyme disease in most areas of endemicity is less than 0.01% (8, 28); the highest incidence in the United States is 0.09% in Connecticut (8).
The cassette portion of VlsE contains six invariable regions (IRs) interspersed with an equal number of variable regions (40). The latter, by their very nature, have no diagnostic value. The additional five IRs are not as conserved as IR6 (17a). Nonetheless, we assessed whether the remaining IRs (IR1 to IR5) could contribute to improve the diagnostic performance of C6, whose sequence is based on that of IR6. No antibody responses to peptides reproducing the sequences of IR1 to IR5 were detected in humans or monkeys, infected with B. burgdorferi, in the absence of a response to C6 (17b). On this basis, we concluded that no improvement would be accrued from incorporating any of these peptides into the C6 ELISA. We did not test the diagnostic potential of other conserved regions of VlsE, which are located outside the cassette region (40). The C6 ELISA was very precise. This suggests that intralaboratory variations with this test may not be a problem. As yet, the assay has not been tested by other laboratories to evaluate interlaboratory performance.
As expected, monkey serum samples which contained high-titer anti-OspA antibody did not react with C6. Our test, therefore, will be suitable in the OspA vaccine era. Moreover, because of its simplicity and high sensitivity, specificity, and precision, it may contribute to alleviate some of the remaining problems in Lyme disease serodiagnosis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI35027 and RR00164 and by a grant from SmithKline Beecham Biologicals.
REFERENCES
- 1.Bakken L L, Callister S M, Wand P J, Schell R F. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists proficiency testing program. J Clin Microbiol. 1997;35:537–543. doi: 10.1128/jcm.35.3.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Tessier S L, Hayes S F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984;45:94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S F. A Borrelia genus-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barony G, Merrifield R B. The peptides: analysis, synthesis, & biology. New York, N.Y: Academic Press; 1980. pp. 3–285. [Google Scholar]
- 5.Burkot T R, Schriefer M E, Larsen S A. Cross-reactivity to Borrelia burgdorferi proteins in serum samples from residents of a tropical country nonendemic for Lyme disease. J Infect Dis. 1997;175:466–469. doi: 10.1093/infdis/175.2.466. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Case definition for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46:20–21. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Guidelines for treatment of sexually transmitted diseases. Morbid Mortal Weekly Rep. 1998;47:33. [Google Scholar]
- 10.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fawcett P T, Gibney K M, Rose C D, Dubbs S B, Doughty R A. Frequency and specificity of antibodies that crossreact with Borrelia burgdorferi antigens. J Rheumatol. 1992;19:582–587. [PubMed] [Google Scholar]
- 13.Fawcett P T, Gibney K M, Rose C D, Klein J D, Doughty R A. Adsorption with a soluble E. coli antigen fraction improves the specificity of ELISA tests for Lyme disease. J Rheumatol. 1991;18:705–708. [PubMed] [Google Scholar]
- 14.Fawcett P T, Rose C D, Gibney K M. Comparative evaluation of adsorption with E. coli on ELISA tests for Lyme borreliosis. J Rheumatol. 1995;22:684–688. [PubMed] [Google Scholar]
- 15.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen K, Bangsborg J M, Fjordvang H, Pederson N S, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen component to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indest K J, Ramamoorthy R, Sole M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol., in press. [PubMed]
- 17b.Liang F T, Philipp M T. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun. 1999;67:6702–6706. doi: 10.1128/iai.67.12.6702-6706.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luft B J, Gorevic P D, Jiang W, Munoz P, Dattwyler R J. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J Immunol. 1991;146:2776–2783. [PubMed] [Google Scholar]
- 19.Ma B, Christen B, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnarelli L A, Anderson J F, Johnson R C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infection. J Infect Dis. 1987;156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 21.Margolis N, Rosa P A. Regulation of expression of major surface proteins in Borrelia burgdorferi. Infect Immun. 1993;61:2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadelman R B, Wormser G P. Lyme borreliosis. Lancet. 1998;352:557–564. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 23.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philipp M T, Lobet Y, Bohm R P, Jr, Conway M D, Dennis V A, Desmons P, Gu Y, Hauser P, Lowrie R C, Jr, Roberts E D. Safety and immunogenicity of recombinant outer surface protein a (OspA) vaccine formulations in the rhesus monkey. J Spirochetal Tick-borne Dis. 1996;3:67–79. [Google Scholar]
- 25.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philipp M T, Lobet Y, Bohm R P, Jr, Roberts E D, Dennis V A, Gu Y, Lowrie R C, Jr, Desmons P, Duray P H, England J, Hauser P, Piesman J, Xu K. The outer surface protein A (OspA) vaccine against Lyme disease: efficacy in the rhesus monkey. Vaccine. 1997;15:1872–1887. doi: 10.1016/s0264-410x(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 27.Ramamoorthy R, Philipp M T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigal L H. Pitfalls in the diagnosis and management of Lyme disease. Arthritis Rheum. 1997;41:195–204. doi: 10.1002/1529-0131(199802)41:2<195::AID-ART3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Molloy P J, Seidner A L, Sabetta J R, Simon H J, Klempner M S, Mays J, Marks D, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 30.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 32.Steere A C, Taylor E, McHugh G L, Logigian E L. The overdiagnosis of Lyme disease. JAMA. 1993;269:1812–1816. [PubMed] [Google Scholar]
- 33.Steere A C, Levin R E, Molloy P J, Kalish R A, Abraham J H, 3rd, Liu N Y, Schmid C H. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 34.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 35.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Clinical guideline, part 2: laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 36.van Hoecke C, Comberbach M, de Grave D, Desmons P, Fu D, Hauser P, Lebacq E, Lobet Y, Voet P. Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) Lyme vaccines in healthy adults. Vaccine. 1996;14:1620–1626. doi: 10.1016/s0264-410x(96)00146-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang I N, Dykhuizen D E, Qiu W, Dunn J J, Bosler E M, Luft B J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wormser G P, Horowitz H W, Dumler I S, Schwartz I, Agüero-Rosenfeld N E. False-positive Lyme disease serology in human granulocytic ehrlichiosis. Lancet. 1996;347:981. doi: 10.1016/s0140-6736(96)91475-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]