Abstract
Introduction
To compare the cost-effectiveness of flash glucose monitoring versus self-monitoring of blood glucose/point of care testing (SMBG/POCT) in both patients with type 1 and patients with type 2 diabetes (T1D/T2D) receiving insulin therapy.
Methods
The IQVIA CORE Diabetes Model (version 9.5) was used to project the lifetime costs and health outcomes of flash glucose monitoring and SMBG/POCT from a Chinese societal perspective. We considered both hospital and individual version flash glucose monitoring to reflect the clinical practice in China. The clinical inputs leveraged the outcomes from both clinical trials and real-world studies. Cohort characteristics, intervention costs, treatment-related disutility and mortality were extracted from the literature. We also conducted scenario analyses and probabilistic sensitivity analyses to test the robustness of results.
Results
Compared with SMBG/POCT using efficacy results from clinical trial, flash glucose monitoring brought the incremental costs of Chinese yuan (CNY) 58,021 and CNY 90,997 and additional quality-adjusted life years (QALYs) of 1.22 and 0.65 for patients with T1D and patients with T2D, respectively. According to the “WHO-CHOICE threshold” of three times the gross domestic product per capita in China (CNY 217,341 in 2020) as cost-effectiveness threshold, flash glucose monitoring was cost-effective for both patients with T1D and patients with T2D with incremental cost-effectiveness ratios (ICER) of CNY 47,636 and CNY 140,297 per QALY gained, respectively. According to the real-world effectiveness data, flash glucose monitoring was dominant for patients with T1D (lower costs and better effectiveness) and cost-effective for patients with T2D with an ICER of CNY 124,169 per QALY gained compared with SMBG/POCT. Scenario analyses and probabilistic sensitivity analyses confirmed the robustness of the results.
Conclusion
Flash glucose monitoring is likely to be considered as a cost-effective strategy compared to SMBG/POCT for Chinese patients with T1D and patients with T2D receiving insulin therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01166-z.
Keywords: Cost-effectiveness, Flash glucose monitoring, Self-monitoring of blood glucose/point of care testing (SMBG/POCT), Type 1 diabetes, Type 2 diabetes
Key Summary Points
| Continuous glucose monitoring systems are increasingly being adopted as an alternative to self-monitoring of blood glucose/point of care testing (SMBG/POCT) by persons with diabetes receiving insulin therapy. |
| Flash glucose monitoring is demonstrated to facilitate better glucose monitoring, reduce the event rate of hypoglycemia, and improve quality of life of diabetes patients. |
| The IQVIA CORE Diabetes Model v9.5 was used to compare the cost-effectiveness of flash glucose monitoring versus SMBG/POCT for both patients with type 1 and patients with type 2 diabetes from a Chinese societal perspective. |
| The analyses showed that flash glucose monitoring was cost-effective compared with SMBG/POCT, with an incremental cost-effectiveness ratio of Chinese yuan (CNY) 47,636 per quality-adjusted life years gained in patients with type 1 diabetes and CNY 140,297 per quality-adjusted life years gained in patients with type 2 diabetes. |
| Scenario analyses further demonstrated that flash glucose monitoring was likely to be an optimal option from both health outcomes and economic considerations compared to SMBG/POCT. |
Introduction
Diabetes is a major public health concern worldwide [1]. The aging of the diabetic population has raised dramatic needs for better healthcare service [2, 3]. A recent study indicated that the prevalence of diabetes in mainland China has increased from 10.9% in 2013 to 12.8% in 2017 [4]. This climb in prevalence is mostly ascribed to type 2 diabetes (T2D) [5], but the incidence of type 1 diabetes (T1D) has also witnessed a rapid increase, especially among children with an approximately fourfold growth in the last 20 years [6–8].
Insulin therapy is the mainstream treatment for diabetes. At the disease onset, 93.9% of patients with T1D would immediately initiate insulin treatment, while some patients with T2D may also receive insulin treatment but with a lower daily dose [8–10]. Insulin is effective in lowering glucose level, but too much insulin or other diabetes medications may cause the blood sugar level to drop too low, causing hypoglycemia, which can lead to coma and even death [11]. Furthermore, severe hypoglycemia episodes have led to a great economic burden on both global and Chinese healthcare systems [12, 13].
Glucose monitoring has been recognized as one of the most important components in diabetes management. Knowing patients’ current glucose reading is often the first step to further adjust patients’ diets, activities, and medications to achieve their glycemic goals [14, 15]. The self-monitoring of blood glucose (SMBG/POCT) is the mainstream and standard of blood glucose monitoring approach in China [15]. It is universally considered to be an integral part of T1D management and crucial for optimizing the safety of patients with T2D. Patients with diabetes can receive real-time glucose level through SMBG/POCT and adjust their diet and treatment dose accordingly [16]. However, SMBG/POCT cannot detect nocturnal hypoglycemia and asymptomatic hypoglycemia, and it needs multiple finger-stick blood samples throughout the day. Therefore, SMBG/POCT may cause pain, discomfort, and compliance issues. In addition, SMBG/POCT cannot provide any information on glucose trends, which may miss important glucose fluctuations [17].
Continuous glucose monitoring (CGM) systems are increasingly being adopted as an alternative to SMBG/POCT by persons with diabetes receiving insulin therapy. Unlike SMBG/POCT which can only provide a single instant glucose value for each test, CGM can offer continuous glucose data as a reference to adjust insulin therapy and monitor lifestyle intervention [17]. Nevertheless, conventional CGM still requires calibration via SMBG/POCT measurements to ensure accuracy [18].
A flash glucose monitoring system (hereinafter referred to as “flash monitoring”) measures glucose levels in the interstitial fluid every minute, allowing for continuous glucose measurement. Thus, flash monitoring is sometimes referred to as intermittently scanned CGM (isCGM). Compared with SMBG/POCT and conventional CGM, it can be particularly beneficial to patients with diabetes who need long-term glucose monitoring and accurate trends of glucose [19]. In flash monitoring, patients have a sensor worn on the back of their upper arm to measure the glucose, and a separate reader to swipe over the sensor to get the glucose data. In China, there are an individual version (FreeStyle Libre; Abbott Diabetes Care, Witney, UK) mainly for out-of-hospital use, and a hospital version (FreeStyle Libre H; Abbott Diabetes Care, Witney, UK) of flash monitoring for hospital settings. Each sensor for flash monitoring (no matter whether the hospital version or individual version) can be used for up to 14 days without calibration via SMBG/POCT measurements. Each sensor could be matched with one reader for the individual version, while the hospital version enables one reader to connect multiple sensors and monitor the glucose level of multiple patients. Results from several randomized controlled trials (RCTs) and real-world evidence (RWE) studies suggested that flash monitoring can facilitate better glucose monitoring, reduce time in hypoglycemia, increase time in normal glucose range, and improve quality of life and patient satisfaction compared with SMBG/POCT [20, 21].
Previous studies indicated that flash monitoring is cost-effective in Sweden, Greece, and the UK [22–24]. However, no studies addressed its cost-effectiveness in China, and none of the previous studies considered the use of a combination of individual version and hospital version of flash monitoring. To fill this gap, our study aims to evaluate the cost-effectiveness of using flash monitoring (hospital version and individual version) versus SMBG/POCT for insulin-treated patients with T1D and patients with T2D in China.
Methods
CORE Diabetes Model
IQVIA CORE Diabetes Model (CDM) 9.5 version was used for the analyses. The CDM is an internet-based model, simulating the long-term health outcomes and economic consequences of interventions in T1D and T2D. There are 17 interdependent sub-models with the combination of Monte Carlo simulation and Markov modelling designed as the underlying mathematical engine to capture the long-term progression of diabetes complications. The CDM allows the estimation in life expectancy, quality-adjusted life years (QALYs), direct costs, and indirect costs. Past validation studies have confirmed the IQVIA CDM as a credible tool to support policy decisions related to disease management in T1D and T2D [25].
The study was performed from the Chinese societal perspective (indirect costs were not included for patients with T2D as the start age of T2D in this analysis was older than the retirement age). Costs and health outcomes were evaluated over a lifetime horizon. Both costs and health outcomes were discounted at an annual rate of 5% [26]. As there is no documentation on the willingness-to-pay (WTP) threshold for a QALY gained in China, three times the annual gross domestic product (GDP) per capita, Chinese yuan (CNY) 217,341 in 2020 as recommended by the World Health Organizations Commission on Macroeconomics and Health, was used as threshold in this study.
This study is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Collection
Pragmatic reviews were conducted in PubMed, CNKI, and WANFANG database to obtain the best available model inputs. The reviews focused on patient baseline characteristics and healthcare costs of diabetes-related complications. For inputs with multiple sources, we selected the data from the latest and best representative source for Chinese patients with diabetes. Then, in-depth clinical expert interviews were conducted to validate model inputs. The five experts were selected considering their experience in diabetes management and glucose monitoring.
Model Inputs
Cohort
Cohort baseline characteristics were identified from published literature. For those unavailable data inputs, we used the median of interview results. Detailed cohort baseline characteristics are listed in Table 1 and Supplementary Table S1.
Table 1.
Cohort characteristics
| Baseline characteristics | T1D cohort | T2D cohort | ||
|---|---|---|---|---|
| Mean ± SD | Source | Mean ± SD | Source | |
| Demographics | ||||
| Start age (years) | 33.3 ± 13.2 | Ref. [9] | 59.4 ± 10.4 | Ref. [40] |
| Duration of diabetes (years) | 0 ± 13.2 | Ref. [9] | 9 ± 6.7 | Ref. [40] |
| Male (%) | 49.0 | Ref. [9] | 55.6 | Ref. [40] |
| Baseline risk factors | ||||
| HbA1c (%) | 10.3 ± 0.5 | Ref. [9] | 8.4 ± 2.0 | Ref. [40] |
| Systolic blood pressure (mmHg) | 119.8 ± 16.1 | Ref. [6] | 132.0 ± 18.7 | Ref. [40] |
| Diastolic blood pressure (mmHg) | 75.6 ± 11.1 | Ref. [6] | 79.0 ± 10.6 | Ref. [40] |
| Total cholesterol (mg/dL) | 166.3 ± 34.8 | Ref. [9] | 191.9 ± 29.8 | Ref. [40] |
| HDL (mg/dL) | 48.0 ± 16.2 | Ref. [6] | 50.8 ± 13.8 | Ref. [46] |
| LDL (mg/dL) | 102.1 ± 41.0 | Ref. [6] | 106.7 ± 22.0 | Ref. [40] |
| Triglycerides (mg/dL) | 119.0 ± 0.0 | Ref. [47] | 147.0 ± 79.3 | Ref. [40] |
| Body mass index | 21.4 ± 3.5 | Ref. [6] | 25.2 ± 6.5 | Ref. [40] |
T1D type 1 diabetes, T2D type 2 diabetes, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
Intervention Effects
In this study, the intervention is flash monitoring versus SMBG/POCT among insulin-treated patients with T1D and patients with T2D. The intervention effects of flash monitoring were retrieved from two RCTs of flash monitoring, the IMPACT trial for patients with T1D and the REPLACE trial for patients with T2D [27, 28].
Although no significant differences were seen in the effect on HbA1c, the values of HbA1c changes by the end of the studies were still used as the change from baseline HbA1c at the end of year 1. For patients with T1D, an increase of 0.15% in the flash monitoring arm and 0.17% in the SMBG/POCT arm were implemented in the analyses [27]; for patients with T2D, a reduction of 0.29% in the flash monitoring arm and 0.31% in the SMBG/POCT arm were used [28].
Hypoglycemia was defined as three levels in CDM: (1) non-severe hypoglycemia event (NSHE); (2) severe hypoglycemia event grade 1 (SHE1), requiring non-medical assistance; and (3) severe hypoglycemia event grade 2 (SHE2), requiring medical assistance. Event rates of non-severe and severe hypoglycemia in the SMBG/POCT arm were retrieved from a long-term observational study for patients with T1D and a meta-analysis for patients with T2D [29, 30]. We used the CDM default proportion to separate SHE1 and SHE2 [31]. The event rates of the flash monitoring arm were calculated on the basis of the relative differences compared to the SMBG/POCT arm. The relative differences were extracted from the IMPACT trial and REPLACE trial for patients with T1D and patients with T2D, respectively [27, 28].
In addition to the hypoglycemia events, we also considered the impact of ketoacidosis events for patients with T1D. Data from a recent real-world study that published the ketoacidosis event rates in patients with T1D were used [32]. Our analyses for T2D did not consider ketoacidosis events as they rarely occur in patients with T2D [33]. Detailed treatment effects data are presented in Table 2.
Table 2.
Treatment effects
| Flash monitoring | SMBG/POCT | |
|---|---|---|
| Patients with T1D | ||
| Physiological parameters | ||
| Change from baseline HbA1c at the end of year 1 (%) | 0.15 | 0.17 |
| Acute event rates (per 100 patient years) | ||
| NSHE | 2634 | 3550 |
| SHE1 | 35.7 | 79.4 |
| SHE2 | 13.8 | 30.6 |
| Ketoacidosis | 1.0 | 1.4 |
| Patients with T2D | ||
| Physiological parameters | ||
| Change from baseline HbA1c at the end of year 1 (%) | − 0.3 | − 0.3 |
| Acute event rates (per 100 patient years) | ||
| NSHE | 1685 | 2331 |
| SHE1 | 43.9 | 92.6 |
| SHE2 | 5.9 | 12.4 |
SMBG/POCT self-monitoring of blood glucose/point of care testing, T1D type 1 diabetes, T2D type 2 diabetes, NSHE non-severe hypoglycemia event, SHE1 severe hypoglycemia event grade 1 (requiring non-medical assistance), SHE2 severe hypoglycemia event grade 2 (requiring medical assistance), HbA1c glycated hemoglobin
Cost Inputs
Costs included glucose monitoring costs, insulin therapy costs, diabetes-related chronic complication treatment costs, acute event treatment cost, screening costs, and indirect costs (only for the T1D cohort). All costs data were inflated to 2021 Chinese currency using consumer price index from the National Bureau of Statistics of China. Details can be seen in Table 3 and Supplementary Tables S2 and S3.
Table 3.
Intervention costs and acute event treatment costs
| T1D | T2D | |
|---|---|---|
| Intervention: unit costs (CNY) | ||
| Sensor: individual version | 475 | |
| Sensor: hospital version | 850 | |
| SMBG/POCT: out-of-hospital (test strip and lancet) | 1.64 | |
| SMBG/POCT: charge of SMBG/POCT test in hospitals, including test strip and lancet | 6 | |
| Insulin (per unit) | 0.43 | |
| Intervention: daily dosage of insulin (unit) | ||
| Flash monitoring | 37.1 | 34.8 |
| SMBG/POCT | 36.8 | 34.9 |
| Annual intervention costs: glucose monitoring (CNY) | ||
| Flash monitoring | 13,372 | 13,248 |
| SMBG/POCT | 3675 | 2488 |
| Annual intervention costs: insulin therapy (CNY) | ||
| Flash monitoring | 5827 | 5466 |
| SMBG/POCT | 5780 | 5482 |
| Acute event treatment: unit costs (CNY) | ||
| NSHE | 186 | 262 |
| SHE 1 | 75 | 75 |
| SHE 2 | 24,609 | 24,609 |
| Diabetic ketoacidosis (T1D only) | 14,281 | – |
CNY Chinese yuan, SMBG/POCT self-monitoring of blood glucose/point of care testing, T1D type 1 diabetes, T2D type 2 diabetes, NSHE non-severe hypoglycemia event, SHE1 severe hypoglycemia event grade 1 (requiring non-medical assistance), SHE2 severe hypoglycemia event grade 2 (requiring medical assistance)
The monitoring frequency of SMBG/POCT was derived from the IMPACT and REPLACE trials [27, 28]. It is worth noting that for patients using flash monitoring, SMBG/POCT was also administered. The monitoring frequency of SMBG/POCT was 0.5 tests/day and 0.3 tests/day for patients with T1D and patients with T2D using flash monitoring, and 5.6 tests/day and 3.8 tests/day for patients using SMBG/POCT.
According to the clinical interview results, the average numbers of inpatient visit for Chinese patients with T1D and patients with T2D were 1.3 times/year and 1 time/year, and the average lengths of stay were 8.1 days and 7 days. As there are both a hospital version and individual version of flash monitoring in China, our analyses assumed that patients with T1D and patients with T2D would use 1.3 hospital version sensors and 1 hospital version sensor each year, respectively. Except for the inpatient stay, patients would use the individual version of flash monitoring. Similarly, different unit costs of SMBG/POCT test were used for hospital and out-of-hospital settings.
The baseline insulin dosages of Chinese patients with T1D and patients with T2D were obtained from two retrospective studies [34, 35], and further calculated considering the change observed in the IMPACT and REPLACE trials after 6 months of glucose monitoring using different approaches [27, 28].
Diabetes-related chronic complication frequencies were simulated by CDM. The cardiovascular (CV) disease risks were calculated by using China-PAR (prediction for atherosclerotic cardiovascular disease risk in China) CV risk equations to better reflect the correlation between patient characteristics and CV outcomes in the Chinese population. Acute event treatment costs in patients with T1D included treatment costs for severe hypoglycemia and ketoacidosis, while only severe hypoglycemia treatment costs were included for patients with T2D. These acute events were considered without long-term component, and the costs occur only once and are applied in the same year as the corresponding event. The indirect costs calculated the productivity loss resulting from morbidity and mortality associated with the disease for patients of working age. Costs per day absent from work are calculated separately for male and female patients on the basis of their average annual salary and the number of working days per year respectively. Each ongoing complication and acute event is associated with a certain number of days absent from work. As the baseline age of patients with T2D was older than the retirement age in China, no indirect costs were calculated for T2D.
Utilities, Disutility, and Mortality
A treatment-related disutility of − 0.03 was applied to the SMBG/POCT arm in accordance with published research [36]. The mortality of severe hypoglycemia events, 10.77%, retrieved from a recent study in Chinese diabetes population was also implemented into the model to better calibrate the results [37]. In addition, as ketoacidosis events were considered in the analyses, the mortality of ketoacidosis events in T1D, 2.7%, was also included [38].
Supplementary Table S4 shows other utilities and disutilities for diabetes complications. Supplementary Table S5 shows the non-specific mortality for the Chinese population.
Scenario and Sensitivity Analyses
RWE Scenario Analysis
To better reflect the potential benefits of flash monitoring in real-world settings, we conducted a scenario analysis with HbA1c treatment effect from study population whose baseline HbA1c was the closest to Chinese patients with diabetes. For T1D, the change in HbA1c using flash monitoring was retrieved from a real-world study whose baseline HbA1c was most similar to Chinese patients with T1D (10.28% in the real-world study vs.10.3% in Chinese patients with T1D) [34]. The − 0.88% change from baseline HbA1c using flash monitoring was implemented in this scenario analysis. Similarly, a − 0.5% change from the baseline HbA1c was adopted for patients with T2D in the scenario analysis [35].
Sensitivity Analysis
One-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) were performed to test the robustness of results in an RCT scenario concerning variable uncertainties and modeling assumptions. The following OWSAs were also conducted for both T1D and T2D: event rate of NSHE and SHE (from published Chinese literature [13]), discount rate (0% or 8%), time horizon (5 years), disutility of NSHE and SHE (the upper and lower limits of 95% CI), SMBG/POCT treatment disutility (the upper and lower limits of 95% CI), flash monitoring sensor cost (− 20%), insulin cost (± 20%), complication treatment cost (± 20%), and SMBG/POCT frequency (7 times per day recommended by Chinese guidelines [15]).
PSA was conducted to test the composite parameter uncertainty using Monte Carlo simulation with 1000 expected values of flash monitoring and SMBG/POCT.
Results
RCT Scenario
According to the intervention effects from RCTs, flash monitoring was associated with an incremental benefit of 1.22 QALYs and an increased cost of CNY 58,021 compared with SMBG/POCT for patients with T1D. As for patients with T2D, flash monitoring was associated with an incremental benefit of 0.65 QALYs and an increased cost of CNY 90,997 compared with SMBG/POCT. Given a WTP threshold of three times the GDP per capita in China (CNY 217,341 in 2020), flash monitoring was a cost-effective option for both patients with T1D and patients with T2D, resulting in an incremental cost-effectiveness ratio (ICER) of CNY 47,636 per QALY gained and CNY 140,297 per QALY gained, respectively. The full results are presented in Table 4.
Table 4.
Results from RCT and RWE scenarios
| T1D | T2D | |||||
|---|---|---|---|---|---|---|
| Flash monitoring | SMBG/POCT | Incremental | Flash monitoring | SMBG/POCT | Incremental | |
| RCT scenario results | ||||||
| Costs (CNY) | 1,098,593 | 1,040,572 | 58,021 | 497,054 | 406,057 | 90,997 |
| Direct costs | 901,253 | 791,555 | 109,698 | 497,054 | 406,057 | 90,997 |
| Intervention | 288,159 | 134,341 | 153,818 | 209,996 | 88,028 | 121,968 |
| Disease monitoring | 3605 | 3407 | 198 | 3422 | 3359 | 63 |
| Chronic complications | 482,569 | 450,040 | 32,529 | 217,520 | 213,000 | 4520 |
| Acute events | 126,919 | 203,765 | − 76,846 | 66,116 | 101,669 | − 35,553 |
| Indirect costs* | 197,340 | 249,017 | − 51,677 | – | – | – |
| LYs | 14.76 | 13.92 | 0.84 | 10.78 | 10.60 | 0.18 |
| QALYs | 9.20 | 7.98 | 1.22 | 6.87 | 6.22 | 0.65 |
| ICER (CNY/QALY) | – | – | 47,636 | – | – | 140,297 |
| RWE scenario results | ||||||
| Costs (CNY) | 1,040,864 | 1,042,582 | − 1718 | 495,167 | 408,907 | 86,261 |
| Direct costs | 847,675 | 780,404 | 67,271 | 495,167 | 408,907 | 86,261 |
| Intervention | 290,631 | 134,776 | 155,855 | 210,319 | 87,819 | 122,500 |
| Disease monitoring | 3736 | 3443 | 293 | 3426 | 3350 | 76 |
| Chronic complications | 425,419 | 437,663 | − 12,244 | 215,283 | 216,269 | − 986 |
| Acute events | 127,890 | 204,523 | − 76,633 | 66,140 | 101,469 | − 35,329 |
| Indirect costs* | 193,189 | 262,177 | − 68,989 | – | – | – |
| LYs | 14.90 | 13.97 | 0.93 | 10.80 | 10.57 | 0.23 |
| QALYs | 9.31 | 8.00 | 1.32 | 6.89 | 6.20 | 0.70 |
| ICER (CNY/QALY) | – | – | Dominant | – | – | 124,169 |
CNY Chinese yuan, SMBG/POCT self-monitoring of blood glucose, T1D type 1 diabetes, T2D type 2 diabetes, LY life year, QALY quality-adjusted life year, ICER incremental cost-effectiveness ratio
*As the baseline age of patients with T2D was older than the retirement age in China, no indirect costs were calculated for T2D from the societal perspective
RWE Scenario
The results of using treatment effect of HbA1c change from real-world studies are reported in Table 4. In this scenario analysis, flash monitoring dominated SMBG/POCT in patients with T1D, and yield ICER of CNY 124,169 per QALY gained compared with SMBG/POCT in patients with T2D.
Sensitivity Analyses
Sensitivity analyses showed that the results were robust to variable changes. Flash monitoring remained cost-effective compared with SMBG/POCT in all the scenarios investigated (results are presented in Table 5).
Table 5.
Sensitivity analysis results (CNY/QALY)
| Scenario analysis | ICER: flash monitoring vs. SMBG/POCT | |
|---|---|---|
| T1D | T2D | |
| Non-severe and severe hypoglycemia event rate from a Chinese study | 31,150 | 102,664 |
| 0% discount rates for costs and health outcomes | 66,289 | 138,868 |
| 8% discount rates for costs and health outcomes | 41,657 | 141,198 |
| 5-year time horizon | 45,315 | 146,958 |
| Upper limit of 95% CI of non-severe and severe hypoglycemia event disutility | 48,262 | 142,897 |
| Lower limit of 95% CI of non-severe and severe hypoglycemia event disutility | 47,022 | 137,790 |
| Upper limit of 95% CI of SMBG/POCT treatment disutility | 51,874 | 159,280 |
| Lower limit of 95% CI of SMBG/POCT treatment disutility | 43,569 | 123,469 |
| Sensor cost of flash glucose monitoring decreased by 20% | 15,892 | 95,298 |
| Insulin costs increased by 20% | 48,512 | 140,524 |
| Insulin costs decreased by 20% | 46,760 | 140,053 |
| Complication treatment costs increased by 20% | 40,359 | 130,728 |
| Complication treatment costs decreased by 20% | 54,914 | 149,867 |
| SMBG/POCT frequency of 7 times per day | 37,106 | 105,179 |
CNY Chinese yuan, QALY quality-adjusted life year, ICER incremental cost-effectiveness ratio, SMBG/POCT self-monitoring of blood glucose, T1D type 1 diabetes, T2D type 2 diabetes
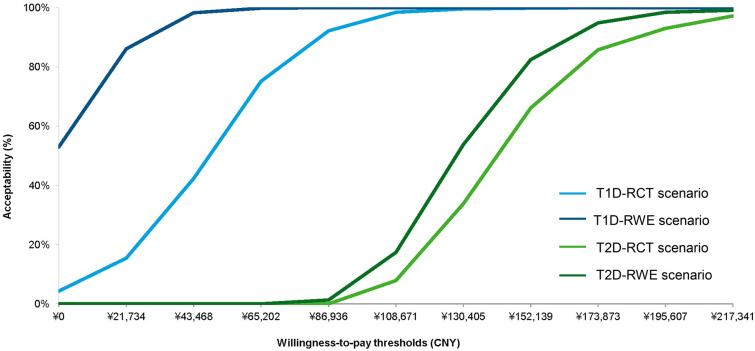
Figure 1 presents the probability of being cost-effective at different WTP values in the form of cost-effectiveness acceptability curves. When the WTP threshold was set to CNY 217,341 per QALY gained, the probability of flash monitoring being a cost-effective strategy compared with SMBG/POCT is 100% for T1D under both scenarios, and 97.2% and 99.1% for T2D under the RCT scenario and RWE scenario, respectively. Other PSA results presented in scatter plots can be found in Supplementary Figs. S1 and S2.
Fig. 1.
Cost-effectiveness acceptability curves under different scenarios. T1D type 1 diabetes, T2D type 2 diabetes, RCT randomized clinical trial, RWE real-world evidence
Discussion
As the first cost-effectiveness study of flash monitoring in China, our study found that flash monitoring is likely to be a cost-effective option compared with SMBG/POCT, with favorable ICERs at CNY 47,636 and CNY 140,297 for patients with T1D and patients with T2D, considering the treatment effect in IMPACT/REPLACE trials. Although flash monitoring is more expensive than SMBG/POCT in glucose monitoring under current prices and SMBG/POCT test frequency, flash monitoring can offset the cost in acute events (ketoacidosis and hypoglycemia in T1D; hypoglycemia in T2D), and reduce indirect cost (productivity loss in T1D only) with additional QALYs. Flash monitoring per se may not directly alter the glucose level of patients with diabetes, but it is considered as part of diabetes management strategy. Improvements in acute events can be achieved through flash monitoring owing to more convenient access to real-time sensor glucose results with trend arrows, which inform the lifestyle modification. Meanwhile, flash monitoring provides more fluent glucose data to guide the insulin adjustment by physicians [28].
IMPACT and REPLACE studies suggested no significant differences in HbA1c changes comparing flash monitoring with SMBG/POCT. Nevertheless, the results could be partially explained by the good glycemic control of the study population. According to a meta-analysis including 25 studies, flash monitoring could result in a significant and sustained reduction in HbA1c and the degree of change in HbA1c was correlated to the baseline HbA1c level of the study population [39]. Given the different baseline HbA1c level between IMPACT/REPLACE trial and Chinese patients with diabetes, a comprehensive cost-effectiveness evaluation from both RCT and RWE scenarios could shed light on future decision-making [9, 27, 28, 40].
The results from RWE scenario analysis also showed promising cost-effectiveness of flash monitoring compared to SMBG/POCT. Flash monitoring dominates SMBG/POCT in Chinese patients with T1D with reduced costs and better health outcomes. Similarly, the result of implementing treatment effect from real-world studies in T2D strengthened flash monitoring’s cost-effectiveness, with an ICER of CNY 124,169. Results under the RWE scenario demonstrated the potential of flash monitoring in reducing chronic complication costs in both patients with T1D and patients with T2D owing to its favorable effect in HbA1c reduction.
As an innovative glucose monitoring approach, flash monitoring is increasingly used in diabetes management in both hospital and out-of-hospital settings. A review of 17 health technology assessment (HTA) reports on flash monitoring has reached a conclusion on its efficacy in reducing events, time in hypoglycemia, improving patient satisfaction, as well as its potential to be cost-effective or cost saving in certain patient populations [20]. Our study results further demonstrate the potential of flash monitoring to be a cost-effective option compared with SMBG/POCT in the Chinese setting.
Apart from this analysis, there are some published abstracts, articles, and HTA reports comparing the cost-effectiveness of flash monitoring and SMBG/POCT in western populations [22–24]. Overall, results of our study were consistent with previous studies. One study found that flash monitoring led to 0.56 additional QALYs in patients with T2D from the Swedish societal perspective [22]. Another study using CDM conducted from a Greek payer perspective reached a similar conclusion that flash monitoring may create 0.567 and 0.317 additional QALYs compared to SMBG/POCT for T1D and T2D, respectively [24]. Even using different modelling approaches, an HTA conducted in Scotland arrived at the same conclusion [41].
Some limitations of the study should be acknowledged. First, health outcome simulation and prediction in this model were mainly conducted on the basis of efficacy data from clinical trials and real-world studies in western populations. Although there are some Chinese-specific clinical studies of flash monitoring, considering the study design (small sample size and short follow-up period), Chinese-specific clinical efficacy data were not used in our analyses. Nevertheless, clinical studies for Chinese patients with T1D and patients with T2D yielded similar treatment effect, such as the reduction of hypoglycemia events and the changes in HbA1c from baseline [42–45].
Some Chinese population baseline clinical data were also missing in our analyses, e.g., the hypoglycemia event rates in Chinese patients with T1D and patients with T2D receiving insulin treatment. To understand the potential impact on results, a scenario analysis using hypoglycemia event rates from the Chinese study was also conducted (no specified data for patients with T1D or T2D, assuming equal event rates of hypoglycemia in T1D and T2D) [13]. Results showed that flash monitoring is still cost-effective compared with SMBG/POCT in both patients with T1D and patients with T2D, demonstrating the robustness of base case results. Future studies are expected with more Chinese population-specific data.
In addition, similar to previous cost-effectiveness studies, the treatment pathway was simplified for both patients with T1D and patients with T2D under the assumption of unchanged use of glucose monitoring and insulin, as the change would not alter the conclusion of the study [22]. However, the frequency of glucose monitoring and the dosage of insulin use are likely to change over time in the real-world clinical setting.
Despite limitations, our study used a well-validated model with best available data inputs and adopted scenarios and sensitivity analyses to disclose comprehensive cost-effectiveness findings. We used different levels of hypoglycemia and nocturnal hypoglycemia, and combining hospital and individual version flash monitoring, to reflect the current clinical reality. Scenarios of HbA1c treatment effect from real-world studies were also analyzed. In comparison with medicines, the cost-effectiveness of medical devices is generally less examined because of the potential challenges of insufficient clinical evidence, shorter product lifecycle, inexplicit target population, etc. This study is valuable in providing implications for future cost-effectiveness studies of medical devices in China. Meanwhile, the study results may provide important references for diabetes disease management and reimbursement decision-making in China.
Conclusion
Compared with SMBG/POCT, flash monitoring enables better glucose control, further reducing expenditures in acute event treatments. Our analyses suggested that flash monitoring (combination use of hospital version and individual version) is likely to be a more cost-effective strategy for both T1D and T2D insulin users compare to SMBG/POCT from the Chinese societal perspective.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Abbott Diabetes Care China. Funding was not contingent upon publication of the manuscript. The study sponsor is also funding the journal’s Rapid Service Fee.
Other Assistance
The authors gratefully acknowledge the participation of Weijia Lu and Ningrui Zhang (resigned employees of IQVIA) in assisting literature review, data collection and data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conceptualization and methodology; X Zhao, J Ming and H Li contributed to the data collection and data analysis; X Zhao and H Li contributed to the original draft preparation; J Ming, S Qu, J Wu, Y Chen and L Ji contributed to the review and editing.
Disclosures
Xinran Zhao, Jian Ming, Shuli Qu and Hsing Jung Li are current employees of IQVIA, which received funds from Abbott Diabetes Care China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Jing Wu, Linong Ji and Yingyao Chen declare that they have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article.
Footnotes
Xinran Zhao and Jian Ming contributed to the work equally and should be regarded as co-first authors.
Contributor Information
Linong Ji, Email: jiln@bjmu.edu.cn.
Yingyao Chen, Email: yychen@shmu.edu.cn.
References
- 1.Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3:1–8. doi: 10.1186/s40842-016-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roglic G. WHO Global report on diabetes: a summary. Int J Noncommunicable Dis. 2016;1:3. doi: 10.4103/2468-8827.184853. [DOI] [Google Scholar]
- 3.Chinese Diabetes Society. A national 10-year retrospective analysis of chronic complications and related risk factors in hospitalized diabetes patients (Chinese). 2003(4):232–237.
- 4.Li Y, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369. [DOI] [PMC free article] [PubMed]
- 5.Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 2018;61:1249–1260. doi: 10.1007/s00125-018-4557-7. [DOI] [PubMed] [Google Scholar]
- 6.Tang X, et al. Prevalence and identification of type 1 diabetes in Chinese adults with newly diagnosed diabetes. Diabetes Metab Syndr Obes. 2019;12:1527–1541. doi: 10.2147/DMSO.S202193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care. 1998;21: 525–9. [DOI] [PubMed]
- 8.Weng J, et al. Incidence of type 1 diabetes in China, 2010–13: population based study. BMJ. 2018;360: j5295. [DOI] [PMC free article] [PubMed]
- 9.Zhou X, Wang X, An Y, Su Q, Li B, Chen H. Characteristics of type 1 diabetes patients aged 60 and older in Shanghai. China. J Endocr Discord. 2020;6:1039. [Google Scholar]
- 10.Xu L, et al. Hypoglycemia during short-term intensive insulin therapy and its association with long-term glycemic remission in patients with newly diagnosed type 2 diabetes. J Diabetes Res. 2020;2020:4097469. doi: 10.1155/2020/4097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17:819–834. doi: 10.4103/2230-8210.117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue X, Wu J, Ruan Z, Wolden ML, Li L, Lin Y. The burden of hypoglycemia in patients with insulin-treated diabetes mellitus in China: analysis of electronic medical records from 4 tertiary hospitals. Value Health Reg Issues. 2020;21:17–21. doi: 10.1016/j.vhri.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Guan X, Li H, Shi L, Han S, Li W. The incidence and economic burden of hypoglycemia episode in patients on insulin therapy. China J Pharma Econ. 2016;8:7–29. [Google Scholar]
- 14.Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China (2020 Edition). Chin J Diabetes. 2021;13(4):315–409.
- 15.Chinese Diabetes Society. Guidelines for clinical application of blood glucose monitoring in China (2015 Edition, Chinese). Chin J Diabetes. 2015;7:603–13.
- 16.Wang X, Luo JF, Qi L, Long Q, Guo J, Wang HH. Adherence to self-monitoring of blood glucose in Chinese patients with type 2 diabetes: current status and influential factors based on electronic questionnaires. Patient Prefer Adherence. 2019;13:1269–82. doi: 10.2147/PPA.S211668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. doi: 10.1186/1758-5996-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staal OM, et al. Differences between flash glucose monitor and fingerprick measurements. Biosensors (Basel). 2018;8(4):93. doi: 10.3390/bios8040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabet Med. 2018;35:472–482. doi: 10.1111/dme.13584. [DOI] [PubMed] [Google Scholar]
- 20.Levrat-Guillen F, Blissett D, Beckerman R, Saborido CM, Benhamou P. The role of health technology assessment in the adoption of new technologies in diabetes care: a review of HTA reports on Freestyle Libre. Pharmacoeconomics. 2019;4:1–12. [Google Scholar]
- 21.Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2019;11:83–95. doi: 10.1007/s13300-019-00720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilir SP, Hellmund R, Wehler E, Li H, Munakata J, Lamotte M. The cost-effectiveness of a flash glucose monitoring system for management of patients with type 2 diabetes receiving intensive insulin treatment in Sweden. Eur Endocrinol. 2018;14:80–5. doi: 10.17925/EE.2018.14.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehler E, Li H, Bilir SP, Hellmund R, Munakata J. Cost effectiveness analysis of a flash continuous glucose monitoring system for type 2 diabetes (T2DM) patients receiving intensive insulin treatment in the UK. Value Health. 2017;20:A-246. [Google Scholar]
- 24.Vellopoulou K, Kourlaba G, Doupis J, Maniadakis N. Economic evaluation of flash glucose monitoring compared to self-monitoring of blood glucose for the management of patients receiving intensive insulin with diabetes type 1 and type 2 in Greece. Value Health. 2017;20:A585. doi: 10.1016/j.jval.2017.08.1056. [DOI] [Google Scholar]
- 25.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17:714–24. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, et al. 2020 China guideline for pharmacoeconomic evaluations (Chinese-English version) Beijing (CHN): China Market Press; 2020. [Google Scholar]
- 27.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–63. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 28.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8:573–86. doi: 10.1007/s13300-017-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 30.Edridge CL, et al. Prevalence and incidence of hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PLoS ONE. 2015;10:e0126427. doi: 10.1371/journal.pone.0126427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29:186–192. [Google Scholar]
- 32.Charleer S, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (future): a prospective observational real-world cohort study. Diabetes Care. 2020;43:389–397. doi: 10.2337/dc19-1610. [DOI] [PubMed] [Google Scholar]
- 33.Tan H, Zhou Y, Yu Y. Characteristics of diabetic ketoacidosis in Chinese adults and adolescents – a teaching hospital-based analysis. Diabetes Res Clin Pract. 2012;97:306–312. doi: 10.1016/j.diabres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell K, McDougall C. Trial of FreeStyle Libre flash glucose monitoring (FGM) in patients with poorly controlled type 1 diabetes. Diabetes Med. 2018;35:P398. [Google Scholar]
- 35.Miller E, Brandner L, Wright E. HbA1c reduction after initiation of the FreeStyle Libre system in type 2 diabetes patients on long-acting insulin or noninsulin therapy. Diabetes. 2020;69:84-LB. doi: 10.2337/db20-84-LB. [DOI] [Google Scholar]
- 36.Matza LS, Boye KS, Stewart KD, Davies EW, Paczkowski R. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17:774. doi: 10.1186/s12913-017-2648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, He X, Zhang L, Wang K, Chen H, Wu J. Association of severe hypoglycemia with all-cause mortality and complication risks among patients with type 2 diabetes mellitus in China. Diabetes Res Clin Pract. 2020;170:108493. doi: 10.1016/j.diabres.2020.108493. [DOI] [PubMed] [Google Scholar]
- 38.MacIsaac RJ, Lee LY, McNeil KJ, Tsalamandris C, Jerums G. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32:379–385. doi: 10.1046/j.1445-5994.2002.00255.x. [DOI] [PubMed] [Google Scholar]
- 39.Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2020;11:83–95. doi: 10.1007/s13300-019-00720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An L, et al. Screening cardiovascular risk factors of diabetes patients in the primary diabetes clinics. Medicine (Baltimore). 2021;100(30):e26722. doi: 10.1097/MD.0000000000026722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scottish Health Technologies Group. What is the clinical and cost effectiveness of Freestyle Libre® flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin therapy? Advice Statement 009-18 [Internet] Scotland; 2018 [updated 2018 July 13; cited 2021 Oct 14]. Available from https://shtg.scot/our-advice/freestyle-libre-flash-glucose-monitoring/.
- 42.Liu Y, et al. Hemoglobin A1c modifies the association between triglyceride and time in hypoglycemia determined by flash glucose monitoring in adults with type 1 diabetes: implications for individualized therapy and decision-making. Ann Transl Med. 2021;9:537. doi: 10.21037/atm-20-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, et al. Thresholds of glycemia and the outcomes of COVID-19 complicated with diabetes: a retrospective exploratory study using continuous glucose monitoring. Diabetes Care. 2021;44:976–982. doi: 10.2337/dc20-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan R, et al. The accuracy and precision of the continuously stored data from flash glucose monitoring system in type 2 diabetes patients during standard meal tolerance test. Int J Endocrinol. 2020;2020:5947680. doi: 10.1155/2020/5947680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y, et al. Relationship between estimated glycosylated hemoglobin using flash glucose monitoring and actual measured glycosylated hemoglobin in a Chinese population. Diabetes Ther. 2020;11:2019–2027. doi: 10.1007/s13300-020-00879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang G, et al. Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med. 2020;17:e1003209. doi: 10.1371/journal.pmed.1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YB, et al. Risk of early mortality and cardiovascular disease in type 1 diabetes: a comparison with type 2 diabetes, a nationwide study. Cardiovasc Diabetol. 2019;18:1–17. doi: 10.1186/s12933-019-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article.