Abstract
Aim
Patient Specific Instrumentation (PSI) with 3D bone models have been used to improve the outcomes of Total Knee Arthroplasty (TKA). The PSI, however, needs a CT (Computed tomography)/MRI scan to reproduce a bone-based model. However, CT is not a routine imaging method in the TKA and has challenges such as high radiation exposure and increased investigation cost. Any technology or software which could accurately recreate 3D bone models using X-ray would be a cheaper and safer tool. This study is based on one such technology (XrayTo3D®) using X-ray to 3D as an alternative to other image-based 3D bone models and PSI available in the market. This study compares the accuracy of XrayTo3D® versus a Conventional CT to 3D, in the reconstruction of lower limb bones (femur and tibia).
Method
In an analysis of 45 lower limbs, 11 anatomical parameters were measured [Medial Proximal Tibial Angle—MPTA, Tibial(T)-torsion, T-slope, T-length, Mechanical Lateral Distal Femoral Angle (mLDFA), F-version, F-length, Distal femoral Medio lateral width (F-ML), Distal Femoral Antero Posterior (F-AP), Proximal Tibia Antero Posterior (T-AP), Proximal Tibia Medio Lateral (T-ML) based on landmarks selected by three orthopaedic surgeons(numbers of the authors superscript), on two groups of 3D models, one reconstructed using XrayTo3D® and the other using CT. Mean and standard-deviation values were measured for all the parameters in both the groups. Statistical association between both the groups was measured by Pearson's correlation coefficient. Two-sided t tests of the mean values were calculated to compare the two measurement methods. The interobserver reproducibility within each group was measured by the intraclass correlation coefficient (ICC). Point-to-surface (P2S) error, in the distal femur and proximal tibia regions of the models reconstructed using XrayTo3D®, were also measured.
Results
For all the 11 parameters, no statistically significant difference was found between the 2 groups (p > 0.05). Pearson's correlation coefficients for all the parameters were not significant. The interobserver reproducibility was ranging from 0.90 to 1.00 and 0.90 to 1.00 for the XrayTo3D® and CT groups, respectively. The mean P2S distance was 1.0 mm in distal femur and 1.1 mm in proximal tibia which was within the acceptable limits.
Conclusion
The reconstruction accuracy of the XrayTo3D® is an accurate, safe and cost effective as compared to a CT-based method.
Keywords: X-ray 3D reconstruction, CT, TKA
Introduction
To improve the outcomes of a total knee arthroplasty (TKA), newer modalities for pre-operative and intra-operative planning have been developed over the recent years. These methods include pre-operative 3D surgery planning, design of 3D printable patient-specific instrumentation (PSI), computer navigation and robotic techniques [1–9]. All these methods have proven to improve surgical alignment accuracy and final patient outcomes [2, 5–7]. These methods are based on patient-specific virtual 3D reconstructed models of the lower limb (femur and tibia) based on CT/MRI scans. In the surgical planning, 3D implant templating, patient-specific 3D bone models are used to simulate the surgery [1–4]. In image-based navigation techniques, patient-specific 3D bone models are registered with real bones of the patient during the surgery for intra-operative guidance (like accessing mechanical axis or implant selection and positioning) [5–7]. Future surgical methods such as Augmented-Reality (AR), Virtual Reality (VR) and Robotic surgeries are based on 3D reconstruction of the lower limb that help in the intra-operative surgical plan [8–11]. For all these methods, 3D bone models are developed using computed tomography (CT) or magnetic resonance imaging (MRI) [1–5].
Apart from improving the surgical accuracy, any new method should also address the safety of the patient, improve surgical efficiency, must be cost effective and reduce the time of overall procedures (imaging, preprocessing data, etc.) [2, 4, 6, 12]. CT and MRI-based PSI are not cost effective as they are not a routine imaging method for TKA. CT scans are costly compared to other imaging methods, and also exposes the patients to more radiation [2, 4, 6].
To address the shortcomings of CT-based 3D reconstruction, X-ray-based 3D bone reconstruction was introduced [13–18]. The X-ray imaging method is low cost, widely available, and is a routine imaging technique involving less radiation. However, its suitability for any surgical method depends on its 3D reconstruction accuracy.
As an extension of the concept, the X-ray-based 3D bone reconstruction may be a considered as a cost-effective and safe imaging option for the upcoming surgical methods such as robotic surgery, AR and VR-based guidance systems. This article evaluates an X-ray-based 3D modeling method referred to as XrayTo3D® [18, 19], in terms of the 3D reconstruction accuracy by comparing it with the CT-based 3D reconstruction.
Materials and Methods
This study evaluates 11 anatomical parameters (as mentioned in Table 1) measured in XrayTo3D®-based 3D bone models and compared with the CT-based 3D models for the same 45 lower limbs. Surface reconstruction accuracy around the knee region of XrayTo3D®-based models was also measured by comparing them with the CT-based models.
Table 1.
List of anatomical parameters selected in the study
| Parameters | Full form | Definition |
|---|---|---|
| Femur parameters | ||
| mLDFA | Mechanical Lateral Distal Femoral Angle | Angle between mechanical axis and distal joint line |
| F-torsion | Femoral version angle | Angle between neck axis and posterior condylar axis measured along mechanical axis |
| F-length | Femoral length | Distance between femoral head center and intercondylar notch landmark |
| F-ML Width | Femoral Condyle Medio Lateral Distance | Distance between medial and lateral epicondyle of femur |
| F-AP Width | Femoral Condyle Antero Posterior Distance | Distance between the most anterior and posterior aspects of lateral condyle of femur |
| Tibia parameters | ||
| MPTA | Medial Proximal Tibial Angle | Angle between mechanical axis and proximal joint line |
| T-AP Width | Tibial Antero Posterior Distance | Maximum distance between the most anterior and posterior aspects of medial condyle of tibia |
| T-ML Width | Tibial Medio Lateral Distance | Maximum distance between medial and lateral condyles of tibia |
| T-torsion | Tibial torsion angle | Angle between posterior condylar axis and transmalleolar axis measured along mechanical axis direction |
| T-slope | Tibial slope | Posterior angle between medial plateau axis and mechanical axis |
| T-length | Tibial length | Distance between intercondylar tubercle and ankle center |
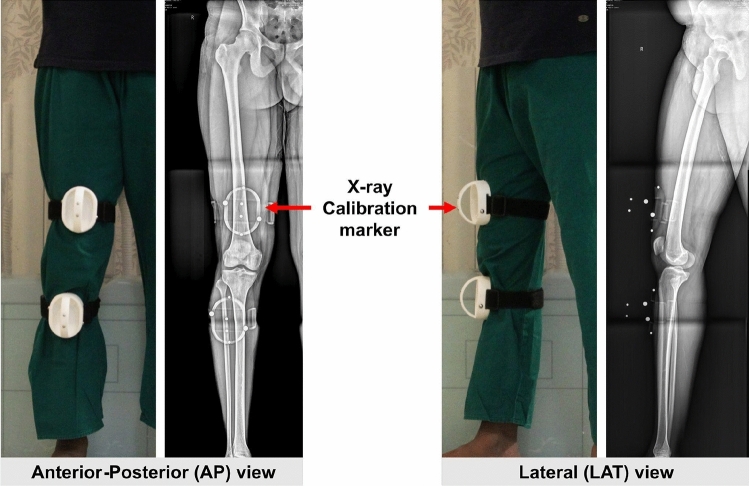
Data including pre-operative CT and two weight-bearing long-leg X-rays (AP and LAT view) of 45 lower limbs 25 patients (7 males, 18 females, age ranging from 49 to 82) who underwent unilateral or bilateral total knee arthroplasty (TKA) between April 2019 and April 2020 was collected. The CT (Company:General Electricals) scans were taken for all 45 limbs with 1 mm slice thickness, 0.6 mm axial resolution, image resolution of 512 × 512 pixels, only around Pelvis, Hip, Knee and Ankle regions. The plain X-ray images were taken with a specialized calibration marker attached to the patient's leg as shown in Fig. 1. These markers are required to calibrate the two images (AP and LAT) to bring them in a virtual 3D coordinate system for the X-ray-based 3D reconstruction method.
Fig. 1.
Plain X-rays taken in anterior–posterior-AP (left) and lateral-LAT (right) view with calibration marker
Data of the 45 patients were collected and entered in a spreadsheet. 3D models of lower limb bones (femur and tibia) for all patients were developed; one group using CT scans and the other group using the plain X-rays. The MIMICs software (v19) was used to reconstruct the 3D models from the CT [20]. For plain X-ray-based 3D model reconstruction, the XrayTo3D technology was used [18, 19]. The 3D models included the bone envelope in a form of 3D surface mesh. The models were exported in STL file (format used to save 3D images) which included the vertices and faces of the surface mesh. This study mainly focuses on the measurements of the anatomical parameters and hence, the description of the detailed methodology of 3D bone model reconstruction is out of the scope of this paper.
For each patient case, the STL mesh files of the corresponding 3D bone models of femur and tibia were imported in specialised cloud-based software named Tabplan3D®. The software is intended for 3D surgery planning of TKA and could also be used for planning of other alignment correction lower limb surgeries. The software allows selection of anatomical landmarks on the surface of the 3D bone models by the surgeon. Based on these landmark positions, various anatomical angles and lengths are automatically calculated. For each case, anatomical landmarks and axes as shown in Fig. 2, were selected and reviewed by three independent observers. The landmarks were then used for calculation of seven anatomical parameters as listed in Table 1 and shown in Fig. 3. The landmark, axes, the anatomical angles and parameters were the established definitions taken from the literature [13–15, 21]. Data for all the 45 bone models in each group were calculated.
Fig. 2.
Software-based reconstruction of the lower limb 3D models with Automated generation of the lower limb measurements
Fig. 3.
Set of anatomical axes and landmarks to measure the anatomical parameters. The black dots are landmark inputs from the surgeon
For measuring the 3D surface reconstruction accuracy, the following method was used. For each patient, the 3D models of femur and tibia extracted from the XrayTo3D® group and were then superimposed or aligned with the corresponding 3D models from the CT group (Fig. 4), using Iterative closest-point (ICP) algorithm [22]. Point-to-surface (P2S) error for the XrayTo3D®-based 3D model was defined as the average of the normal absolute distances of all the mesh vertices of the 3D model from the surface of the corresponding ground-truth, after superimposition [18]. The standard deviation in the distances was also calculated for all the 45 models. The mean values of P2S error and standard deviations across all the 45 bone models was then calculated (see Fig. 5).
Fig. 4.
Eleven anatomical parameters measured in the study
Fig. 5.
XrayTo3D®-based 3D bone models (color coded according to the P2S distances) superimposed over CT-based 3D bone model meshes (shown in wireframe for distinction) for comparison
Data of the 45 patients were collected and entered in a Microsoft Excel spreadsheet. The same software was used to perform all the statistical tests. Pearson's correlation coefficient was calculated to measure the association between the data in the two groups. Two-sided t test (two independent means, two-tailed hypothesis) was performed to check significant differences between the 2 groups for all the 11 anatomical parameters. Based on the power calculation, when there was a correlation of τ = 0.41, with a desired power of 80% and a bilateral significance level of 5%, a necessary sample size of 44 was calculated to obtain a statistically significant result when using the Pearson correlation to discover a correlation coefficient of P ≠ 0.. Intraclass correlation coefficient (ICC) was calculated to measure the interobserver reproducibility of the data for all the 11 parameters in both the groups.
Result
The mean values of all the anatomical parameters for both the groups, averaged over 45 limbs and the 3 sets corresponding to the 3 independent observers (Author 5,6,7) are listed (Table 2). The Pearson's correlation coefficients (r values) for all the seven parameters are listed on the table. The values were ranging from 0.86 to 0.99. The calculation of the correlation of the mean values for matched samples in the two-sided t test are also listed. All the values are less than 0.05. The ICC values for interobserver reproducibility within the group averaged over the three sets are also listed for the seven parameters. This was ranging from 0.90 to 1.00 for the XrayTo3D® group and 0.90 to 1.00 for the CT group. The p values of the student's t test are also listed.
Table 2.
Mean and ICC values of seven anatomical parameters, corresponding p values for student’s t test and Pearson’s correlation coefficients for the XrayTo3D® group and the CT group for 45 patient cases
| Parameters | XrayTo3D® group | CT group | Pearson’s coefficient r values | Two-sided t test | ||
|---|---|---|---|---|---|---|
| Mean | ICC | Mean | ICC | |||
| Femur parameters | ||||||
| mLDFA (°) | 88.85 | 0.99 | 88.28 | 0.99 | 0.97 | p < 0.001 |
| F-version (°) | 11.10 | 0.99 | 11.53 | 0.96 | 0.96 | p < 0.001 |
| F-length (mm) | 389.07 | 1.00 | 391.88 | 1.00 | 0.99 | p < 0.001 |
| F-ML width (mm) | 76.07 | 0.99 | 77.69 | 0.99 | 0.94 | p < 0.001 |
| F-AP width (mm) | 59.45 | 0.99 | 61.29 | 0.99 | 0.92 | p < 0.001 |
| Tibia parameters | ||||||
| MPTA (°) | 84.70 | 0.97 | 84.65 | 0.95 | 0.86 | p < 0.001 |
| T-torsion (°) | 26.76 | 0.90 | 26.47 | 0.90 | 0.96 | p < 0.001 |
| Tibial slope (°) | 7.61 | 0.97 | 8.50 | 0.95 | 0.88 | p < 0.001 |
| T-length (mm) | 345.62 | 0.98 | 350.93 | 1.00 | 0.99 | p < 0.001 |
| T-ML width (mm) | 69.86 | 0.98 | 70.40 | 0.98 | 0.93 | p < 0.001 |
| T-AP width (mm) | 42.47 | 0.98 | 45.46 | 0.98 | 0.87 | p < 0.001 |
Table 3 shows the mean values and the standard deviations of the P2S error, for the distal femur (1 mm, 0.9 mm, respectively) and the proximal tibia (1.1 mm, 1 mm, respectively) averaged over the 45 cases. The range of the P2S error for distal femur and proximal tibia the 45 cases were 0.1–1.7 mm and 0.5–1.7 mm, respectively.
Table 3.
Mean values of P2S errors and their standard deviations in millimeters (mm) for the 3D bone models, averaged over 45 patient cases
| Distal femur | Proximal tibia | |
|---|---|---|
| Mean P2S error (mm) | 1.0 | 1.1 |
| Mean P2S standard deviation (mm) | 0.9 | 1.0 |
| Range of P2S (min–max in mm) | 0.1–1.7 | 0.5–1.7 |
Discussion
There are studies comparing commercially available X-ray-based 3D bone reconstruction methods called the Electro Optical System (EOS) with the CT, for lower limb [14]. However, this existing study only compared the femoral version and tibial torsion parameters. In our study, we chose additional parameters which are important for a TKA. These include the mLDFA and MPTA represent the relationship of articulating bone surfaces of femur and tibia, respectively, with their mechanical axis. Similarly, in the lateral view, tibial slope represents the relationship of articulating bone surfaces of tibia with its mechanical axis. This software helps in the assessment of the deformities in the coronal, sagittal as well as axial planes which. The XrayTo3D® technology also has advantages over the EOS system. The EOS system requires a special biplanar imaging system which adds extra acquisition and maintenance cost, while the XrayTo3D® technology works with existing X-ray machines in a free software.
The results of anatomical parameters measured in the XrayTo3D® group were comparable to those in the CT group. Pearson's coefficient value, the correlation was perfect (r very close to 1) for all the seven anatomical parameters [23]. The calculation of the correlation of the mean values for matched samples in the two-sided t test showed a highly significant correlation for all the parameters (p < 0.05).
The ICC values in the XrayTo3D® group were better (F-version and T-torsion) or similar to those in the CT group and they were above 0.9, showing excellent reproducibility and indicating strong correlation [24]. The P2S errors for the 3D surface reconstruction of distal femur and proximal tibia in this study were comparable or better than many other X-ray-based 3D reconstruction methods in the literature [15–18].
Advantages of the XrayTo3D® in terms of efficiency and cost-effectiveness and the results in this study makes it a safe alternative to CT for the existing and upcoming computer-assisted TKA methods. In addition, the weight-bearing 3D image of the lower limb is advantageous compared to the non-weight-bearing imaging like CT. The weight-bearing imaging reveals the true and accurate deformities of the lower limb [25]. The XrayTo3D® technology can also be used for automated simulation of even complex surgeries like deformity corrections. The automatic simulation can be an input for completely/partially automated future robotic surgeries. Although XrayTo3D® results are comparable to that of CT, its application in computer assisted/robotic surgeries needs further practical evaluation. XrayTo3D requires an extra calibration marker to be placed on the patient limb while acquiring the X-ray images. It is a very low-cost device and is a one-time investment for any medical facility.
The Xrayto3D technology reproduces an accurate 3D reconstruction comparable to a CT reconstruction and can be used for planning the surgery in total knee arthroplasty in an efficient, safe and cost-effective way [13–15]. This software has proven to give comparable results utilizing a simple conventional radiograph.
Conclusion
The XrayTo3D® Indian technology is comparable to CT in terms of anatomical measurements of lower limb and surface reconstruction around the knee region. Hence, the XrayTo3D® technology can be a suitable cost effective and safe alternative to CT-based reconstruction for pre-operative planning and developing Patient Specific Instrumentation for TKA.
Limitation of Study
The use of the PSI which is based on this technology has to be validated in a prospective randomised comparative study with a standard instrumentation to establish its superiority.
Strength of the Study
The strength of the study is that it is an X-ray-based patient-specific instrumentation guide. If found to be effective will be a cheaper and a safer alternative to CT/MRI-based PSI.
Acknowledgements
The authors would like to thank Ms. Lata Chawla, Mr. Manu Sankar and Ms. Parvathy Mohanakumar for helping in the data acquisition and preparation.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. VS, Dr. VK and AM. The first draft of the manuscript was written by Dr. VK and AM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Industry Innovation Programme on Medical Electronics (IIPME) grant [BT/IIPME0004/01/15] by Biotechnology Industry Research Assistance Council (BIRAC), a not-for-profit public sector Enterprise, set up by the Department of Biotechnology (DBT), Government of India.
Declarations
Conflict of Interests
The first author has no conflicts of interest to declare that are relevant to the content of this article. The second author has no conflicts of interest to declare that are relevant to the content of this article. The third author has no conflicts of interest to declare that are relevant to the content of this article. The fourth author has no conflicts of interest to declare that are relevant to the content of this article. The fifth author has no conflicts of interest to declare that are relevant to the content of this article. The sixth author has no conflicts of interest to declare that are relevant to the content of this article. The seventh author has no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
Relevant document attached.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vivek Shetty, Email: Vivshetty7777@gmail.com.
Vikas Karade, Email: vikas@algosurg.com.
References
- 1.Mattei L, Pellegrino P, Calò M, Bistolfi A, Castoldi F. Patient specific instrumentation in total knee arthroplasty: A state of the art. Ann Transl Med. 2016;4(7):126. doi: 10.21037/atm.2016.03.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hafez MA, Moholkar K. Patient-specific instruments: Advantages and pitfalls. SICOT-J. 2017;3:66. doi: 10.1051/sicotj/2017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi J, Sbihi A. 3D templating and patient-specific cutting guides (Knee-Plan) in total knee arthroplasty: Postoperative CT-based assessment of implant positioning. Orthopaedics & Traumatology, Surgery & Research. 2014;100:S281–S286. doi: 10.1016/j.otsr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Connor MI, Kransdorf JM. Knee arthroplasty and the role of preoperative imaging. AJR. American Journal of Roentgenology. 2013;201:W443–W450. doi: 10.2214/AJR.13.10778. [DOI] [PubMed] [Google Scholar]
- 5.Van der List JP, Chawla H, Joskowicz L, Pearle A. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: A systematic review with meta-analysis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016;24:3482–3495. doi: 10.1007/s00167-016-4305-9. [DOI] [PubMed] [Google Scholar]
- 6.Waddell BS, Carroll K, Jerabek S. Technology in arthroplasty: Are we improving value? Current Reviews in Musculoskeletal Medicine. 2017;10(3):378–387. doi: 10.1007/s12178-017-9415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayani B, Konan S, Ayuob A, Onochie E, Al-Jabri T, Haddad FS. Robotic technology in total knee arthroplasty: A systematic review. EFORT Open Reviews. 2019;4:611–617. doi: 10.1302/2058-5241.4.190022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battenberg AK, Netravali NA, Lonner JH. A novel handheld robotic-assisted system for unicompartmental knee arthroplasty: Surgical technique and early survivorship. Journal of Robotic Surgery. 2020;14:55–60. doi: 10.1007/s11701-018-00907-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parratte S, Price AJ, Jeys L, Jackson WF, Clarke HD. Accuracy of a new robotically assisted technique for total knee arthroplasty: A cadaveric study. Journal of Arthroplasty. 2019;34:2799–2803. doi: 10.1016/j.arth.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 10.Chytas D, Malahias MA, Nikolaou VS. Augmented reality in orthopedics: current state and future directions. Frontiers in Surgery. 2019;6:38. doi: 10.3389/fsurg.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walbron P, Common H, Thomazeau H, Hosseini K, Peduzzi L, Bulaid Y, et al. Virtual reality simulator improves the acquisition of basic arthroscopy skills in first-year orthopedic surgery residents. Orthopaedics & Traumatology, Surgery & Research. 2020;106(717):724. doi: 10.1016/j.otsr.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Swank, M. L., Alkire, M., Conditt, M., Lonner, M., Lonner, J. H. (2019). Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. The American Journal of Orthopedics38:32–36. https://www.ncbi.nlm.nih.gov/pubmed/19340382. Accessed Feb 2009 [PubMed]
- 13.Schlatterer B, Suedhoff I, Bonnet X, Catonne Y, Maestro M, Skalli W. Skeletal landmarks for TKR implantations: Evaluation of their accuracy using EOS imaging acquisition system. Orthopaedics & Traumatology, Surgery & Research. 2009;95:2–11. doi: 10.1016/j.otsr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Folinais D, Thelen P, Delin C, Radier C, Catonne Y, Lazennec JY. Measuring femoral and rotational alignment: EOS system versus computed tomography. Orthopaedics & Traumatology, Surgery & Research. 2013;99:509–516. doi: 10.1016/j.otsr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Zheng G, Hommel H, Akcoltekin A, Thelen B, Stifter J, Peersman G. A novel technology for 3D knee prosthesis planning and treatment evaluation using 2D X-ray radiographs: A clinical evaluation. International Journal of CARS. 2018;13:1151–1158. doi: 10.1007/s11548-018-1789-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Lee K, Lee D, Baek N. 3D reconstruction of leg bones from X-ray images using CNN-based feature analysis. International Conference on Information and Communication Technology Convergence. 2019 doi: 10.1109/ictc46691.2019.8939984. [DOI] [Google Scholar]
- 17.Kasten, Y., Doktofsky, D., & Kovler, I. (2020). End-to-end convolutional neural network for 3D reconstruction of knee bones from bi-planar X-ray images. Electrical Engineering and Systems Science. https://arxiv.org/abs/2004.00871.
- 18.Karade V, Ravi B. 3D femur model reconstruction from biplane X-ray images: A novel method based on Laplacian surface deformation. International Journal of CARS. 2015;10:473–485. doi: 10.1007/s11548-014-1097-6. [DOI] [PubMed] [Google Scholar]
- 19.Karade, V., Maurya, A., inventors; Karade, V., assignee. Systems and methods for obtaining 3-d images from x-ray information for deformed elongate bones. WIPO(PCT)-WO2019180745. https://patents.google.com/patent/WO2019180745/en. Accessed 26 Sep 2019
- 20.Raluca M, Comaneanu T, Mihai V, Daniel C, Mihai C. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. International Journal of Nano and Biomaterials. 2012;4:69–77. doi: 10.1504/IJNBM.2012.048212. [DOI] [Google Scholar]
- 21.Paley D. Principles of deformity correction. Springer; 2002. [Google Scholar]
- 22.Zhang Z. Iterative closest point (ICP) In: Ikeuchi K, editor. Computer vision 2014. Springer; 2014. [Google Scholar]
- 23.Ratner B. The correlation coefficient: Its values range between +1/−1, or do they? Journal of Targeting, Measurement and Analysis for Marketing. 2009;17:139–142. doi: 10.1057/jt.2009.5. [DOI] [Google Scholar]
- 24.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna, C., Wade, R., & Faria, R., et al. (2012). EOS 2D/3D X-ray imaging system: A systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2012 Mar. (Health Technology Assessment, No. 16.14.) 1, Background and definition of the decision problem. https://www.ncbi.nlm.nih.gov/books/NBK97738/. Accessed 16 Mar 2012 [DOI] [PMC free article] [PubMed]