Abstract
With the interdisciplinary convergence of biology, medicine and materials science, both research and clinical translation of biomaterials are progressing at a rapid pace. However, there is still a huge gap between applied basic research on biomaterials and their translational products - medical devices, where two significantly different perspectives and mindsets often work independently and non-synergistically, which in turn significantly increases financial costs and research effort. Although this gap is well-known and often criticized in the biopharmaceutical industry, it is gradually widening. In this article, we critically examine the developmental pipeline of biodegradable biomaterials and biomaterial-based medical device products. Then based on clinical needs, market analysis, and relevant regulations, some ideas are proposed to integrate the two different mindsets to guide applied basic research and translation of biomaterial-based products, from the material and technical perspectives. Cartilage repair substitutes are discussed here as an example. Hopefully, this will lay a strong foundation for biomaterial research and clinical translation, while reducing the amount of extra research effort and funding required due to the dissonance between innovative basic research and commercialization pipeline.
Keywords: Biomaterials, Polymer, Translation, Medical devices, Regulation, Clinical needs
Graphical abstract
Highlights
-
•
To elucidate the chain of medical devices development and basic research process.
-
•
To propose rationales of biomaterial research with mindset of clinical translation.
-
•
To elaborate with established medical devices for cartilage repairs as examples.
1. Introduction
Biomaterials are materials designed to take a form that can direct the course of any therapeutic or diagnostic procedure, through interactions with living systems [1]. Biomaterials are important components (if it does not constitute the entirety) of medical devices [2]. With the progress of regenerative medicine, biodegradable biomaterials-based medical devices have great potential to replace traditional tissue substitutes. Medical devices with new materials or technology must undergo a rigorous and systematic design and verification process. Furthermore, for verified products based on biodegradable biomaterials with high risk, they have to undergo a series of evaluation as medical devices by relevant authorities, such as Food & Drug Administration (FDA, United States of America), Notified Body (NB, European Union), National Medical Products Administration (NMPA, China), before clinical applications are permitted [3].
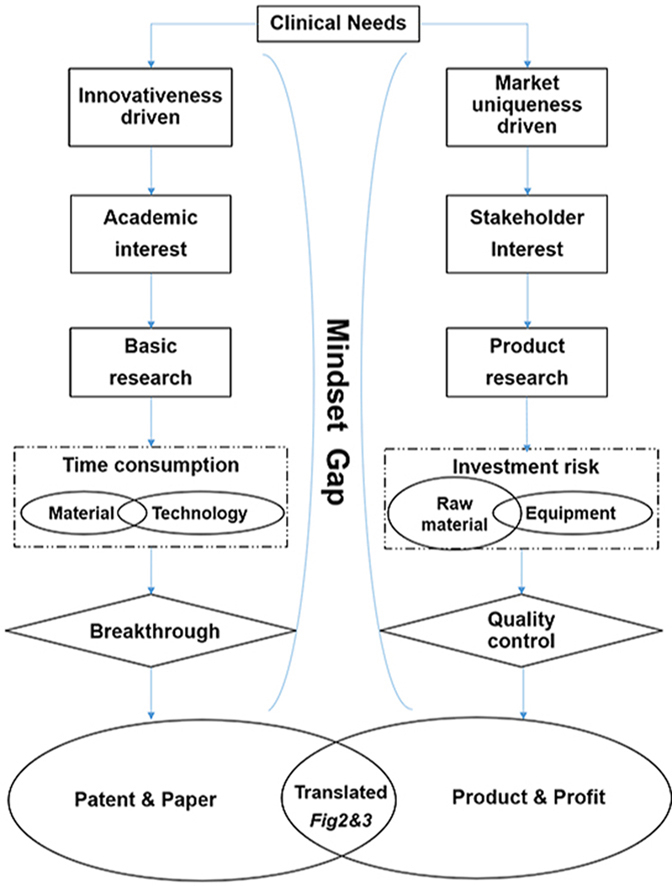
Applied basic research and product research & development are often carried out separately with two different mindsets, i.e., innovation-driven and clinic or commerce-driven, respectively, both of which are integral parts of the upstream and downstream development of biomaterials. Applied basic research of biomaterials aims to develop novel materials and technology without full consideration for clinical translation, while product research and development aims to fulfill unmet clinical needs with suitable biomaterials to go through regulatory approval and succeed in market competition. These two mindsets need to be integrated, as clinical translation still need a certain degree of novelty, such as new components or technology, to upgrade their functionalities, while applied basic research has to build a library of methodologies to contribute to clinical translation directly or indirectly to fulfill their potential. Though some countries and competent scholars begin to attach importance to the integration of industry, market and research, the gap between these two different mindsets still poses a major challenge, which has led to a majority of applied basic scientific research studies on biomaterials to just end with either article publication or patent application, without significant impetus to move forward as medical devices. If post-graduates or even new PIs are inspired to enter a specific biomaterial research area by examining previous publications that are biased without a commerce-driven translation mindset, this gap may become increasingly widened.
There exist some reviews focusing on the clinical translation of biomaterials that summarize how to design biomaterials [4], and what it takes to turn a promising biomaterial into an actual product from the aspect of regulatory approval [5] so as to maximize the possibility of commercialization. However, when only a part of this long procedure is discussed without delving deeper to explore fundamental differences in mindset, it is far from practical. In this article, we would like to discuss the facts that need to be known and understood before biodegradable biomaterials are designed and fabricated, from the clinical, market and regulatory perspectives and based on principles in selection of materials as well as technology platform utilized, with the aim of increasing chances of success in commercialization.
2. Moving beyond scientific publications
Ideally, a biomaterial study would start off with comprehensive understanding of both science as well as clinical needs. However, this hardly happens for early-stage scientists or engineers. The overwhelming majority of biomaterial studies end up with poor industrialization potential. Meanwhile, commerce-driven product research and development are more intensive and strive more than innovation-driven applied basic research, due to financial and time pressures. For example, COLTRIX® CartiRegen (Ubiosis Co., Ltd) entered the National Medical Products Administration (NMPA, P.R. China) special review and approval procedures for innovative medical devices in March 2017, but it is still conducting clinical trials to date, in order to fully validate that the product design can meet clinical application needs [6]. Exceeding a four-year product validation stage is a big concern for investors. Based on these two different mindsets, they present two different routes and stages in research and development. Some of the differences between these two routes may be overlooked by scientific research, resulting in a low clinical translation rate.
2.1. Huge gap between publications and products
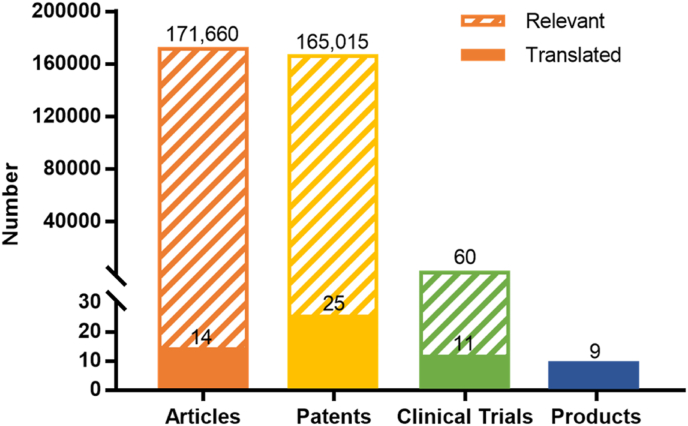
The gap between scientific publications and commercial products can be seen by tracing commercial products for cartilage regeneration as well as relevant clinical trials, patents and papers. According to the International Cartilage Regeneration & Joint Preservation Society (ICRS) and relevant publicly-available information, there are 21 listed biomaterial-based products for cartilage repair or regeneration worldwide [7,8]. Among these, there are 9 collagen-based products (Table 1). Clinical trials of the 9 products were carried out from 2003 to 2019, with relevant patents and articles being published during 1995–2017. In terms of numbers, the 9 products are linked to 11 clinical trials (clinicaltrials.gov, the clinical trials of some products cannot be found, and the number of clinical trials of the corresponding product is recorded as 1), as compared with 60 similar trials over the same year that the study was completed. The number of patents and articles relevant to these 9 products are 25 and 14, respectively (based on the data from the official website of the products and google patents. However, the articles relating to some products cannot be found, and the number of articles of the corresponding product is recorded as 1). Before clinical trials were carried out, there were 165,015 similar patents and 171,660 similar articles being published, respectively, with the key words of collagen and cartilage repair (Fig. 1). As some articles and patents might only deal with a certain part or certain specific procedure of the entire process of product development, such as manufacture, evaluation, verification, some certain biological effect, or clinical output, the ratio of patents (or articles) to products is not 1:1. Due to the small number of products, it is difficult to give a meaningful ratio. But even so, the gap between products and patents or articles is still significant.
Table 1.
List of biomaterial-based products for cartilage repair or regeneration.
| Cell-free products | |||
|---|---|---|---|
| Product | Company | Material | Reference |
| Agili-C™ | CartiHeal | Aragonite-based bi-phasic scaffold | [9] |
| BioCartilage® | Arthrex | Micronized cartilage matrix | [10] |
| Cartiva® SCI | Cartiva | Polyvinyl alcohol cryogel | [11] |
| aChondro-Gide® | Geistlich Pharma | Bilayer collagen I/III membrane | [12] |
| aChondroFiller® | Meidrix Biomedicals GmbH | Collagen solutions | [13] |
| aChondroMimetic® | Collagen Solutions | Collagen, glycosaminoglycans, and calcium phosphate in a dual-layer porous implant | [14] |
| Chondrotissue® | Biotissue | Polyglycolic acid felt and hyaluronic acid-based scaffold | [15] |
| CHONDROVEIL™ | Swissbiomed Orthopaedics | Poly glycolic acid (PGA)-based scaffold | [16] |
| Hyalofast® | Anika Therapeutics, Inc | Esterified hyaluronic acid fibers | [17] |
| JOINTREP™ | Oligo Medic Inc | Chitosan-based injectable implant | [18] |
| aMaioRegen | Finceramica | Multi-layered matrix composed by collagen and hydroxyapatite enriched with magnesium | [19] |
| aNovocart® Basic | TETEC AG | Collagen-based matrix | [20] |
| TruFit® CB |
Smith & Nephew |
Porous bilayer PLGA scaffold reinforced with polyglycolic acid fibers and calcium sulfate mineral |
[21] |
|
Cell-based products | |||
|
Product |
Company |
Material |
Reference |
| BioSeed®-C | Biotissue | Polyglycolic/polylactic acid and polydioxane- based material | [22] |
| BioCart™ II | Histogenics | Human fibrin and recombinant hyaluronic acid-based scaffold | [8] |
| BST-Cargel® | Smith & Nephew | Chitosan-based liquid scaffold | [23] |
| aCaReS® | Arthro Kinetics | Type I collagen hydrogel | [24] |
| INSTRUCT | CellCoTec | Poly (ethylene oxide-terephtalate)/poly (butylene terephtalate) (PEOT/PBT) scaffold | [8] |
| aJACC® | Japan Tissue Engineering Co., Ltd. | Type I collagen gel | [25] |
| aMACI | Vericel Corporation | Collagen membrane-based matrix | [26] |
| aNOVOCART ® 3D | TETEC AG | Type I collagen scaffold | [27] |
Collagen-based product.
Fig. 1.
Number of published articles, patents, clinical trials and products for cartilage engineering, with listed collagen-based products as an example.
The number of clinical trials, patents, and articles associated with listed collagen-based products for the treatment of cartilage defect were traced, namely translated groups. The number of similar clinical trials, patents and articles, namely similar groups, were retrieved in ClinicalTrials.gov (search term: cartilage implant | Completed Studies | Start date on or after 2003 | Primary completion on or before 12/31/2019), Google Patents (search term: ((cartilage) OR (osteochondral) OR (chondrogenic) OR (chondrocyte)) (collagen) before:publication:20171231 type: PATENT), Web of Science (Databases = WOS, BIOSIS, CSCD, DRCI, DIIDW, INSPEC, KJD, MEDLINE, RSCI, SCIELO, search term: TS = cartilage OR TS = osteochondral OR TS = chondrogenic OR TS = chondrocyte* AND TS = collagen, Timespan = 1900–2017), respectively.
There is no denying that every material and technology developed are potentially useful and each research study has its own meaning that lays the foundation for clinical translation, but it is often ignored that only some routes are of significant value.
2.2. Distinct perspectives on research routes
Product research and development requires systematic consideration, including the product's performance, stability, effectiveness and safety, on the basis of medical regulations, and usually embodies integrated innovation. On the other hand, applied basic research focuses more on the performance of biomaterials, with concrete innovation in materials science or methodology.
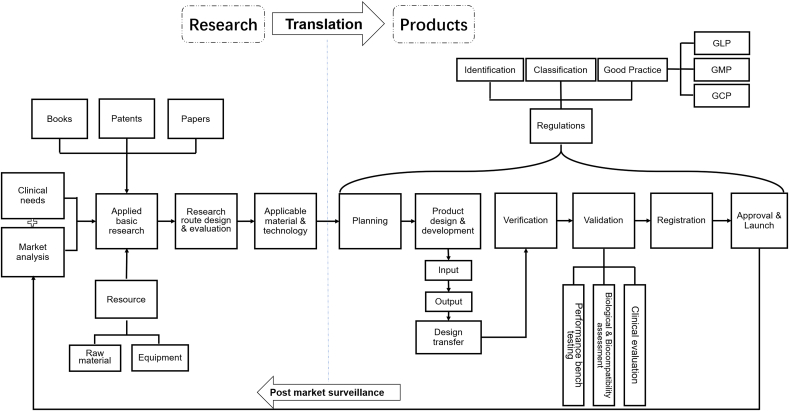
The product development pipeline can be divided into several stages: clinical needs research, market analysis, regulations analysis, planning, product design, verification, validation and registration (Fig. 2). The clinical needs, especially the priority of unmet needs are identified, accompanied with market analysis to clarify the market capacity and to fully analyze similar competitive products on the marketplace. Additionally, feasibility analysis is also carried out, including difficulty in market access, technical and manufacturing feasibility, resource allocation requirements, cost of regulatory compliance for the product, and other possible risks. Next, according to planning and new product definition, would come the product design and verification stage, where the process and performance based on safety, effectiveness and stability are designed, evaluated, and determined. Then, following the fabrication of the product prototype, it is mandatory for implantable medical devices to go through validation of safety and effectiveness through clinical evaluation and registration review, in addition to performance testing and biological assessment. Finally, the product is launched with the permission of the regulatory authority.
Fig. 2.
Routes of product research & development and applied basic research.
For medical devices, the early stage of product research and development mainly consists of planning and product design, the former of which is based on applied basic research, clinical needs, market analysis, etc. After product design, the prototype is produced and verified, and then may be validated, registered and become a product. For biomaterials research, planning of applied basic research are more based on literature search, with little attention being payed to verification and validation after design.
In contrast, applied basic research is often more limited to literature research, performance improvement and innovative design. Though a major portion of research are often aimed at solving unmet clinical needs in the biomedical engineering field, there are still plenty of research without too much consideration of clinical needs investigation, market and risk evaluation. For example, in the field of cartilage repair, there are a lot of studies involving biodegradable biomaterials that cooperate with drugs (i.e. intra-articular injected thrombin and fibrinogen) that may be compatible with future clinical needs, but not with immediate clinical needs, which have no translational potential in the short term, since the drugs has not yet been registered in China [28]. A scientific literature search usually kick-starts applied basic research, and involves seeking for scientific problems to be solved from previous research work. Based on problems found, the biomaterial design in applied basic research is more focused on supplementing or improving one or several aspects of the biomaterial's performance and rarely considers the whole picture, which represents just an intersection of the product design in product research and development. Similarly, the evaluation of biomaterial mainly focuses on certain performance characteristics and simple biosafety tests, without enough attention being paid to the feasibility and stability of the process. Most of this kind of research ultimately ends with journal publications, while some drop out or fail during design validation and registration. Research on using collagen-based biomaterials to repair cartilage is an obvious example, with many articles and patents filed, with few progressing to clinical trials and products, as shown in Fig. 1.
Optimal product design requires the input of applied basic research all the time to become a product prototype for product research and development. In the same way, applied basic research needs to focus more on key clinical challenges, feasibility and stability of design, and be more conducive to the clinical translation of biomaterials. Roughly speaking, the difference between the two routes, which is neglected by applied basic research, is often a hindrance to the clinical translation of biomaterials. Thus, it is essential to familiarize with the product research and development route to facilitate the clinical translation of biomaterials, which in the “ivory towers” of academic institutions may not be able to fit clinical needs, or stand up to the challenges of regulatory and market demands.
MACI can serve as a successfully commercialized example of upgrading from the first generation of autologous chondrocyte implantation (ACI), during which surgeons have to spend significant time to harvest and suture periosteum onto cartilage, in order to keep the chondrocyte suspension in place. To deal with these problems, collagen membrane has become the core material of MACI to replace periosteum, which is not a novel biomaterial but a mature product with good biocompatibility, easy sterilization method and stable supply. Even so, MACI has also incorporated some technological innovations, such as pre-seeding cells on membranes and implanting them to replace direct coverage, and using fibrin glue to replace suture surgery. In summary, MACI delivers the perfect combination of clinical needs, material advantages, mature product technology and innovation.
3. Key factors for translation
Clinical needs research provides a framework for product performance design, while competitive product analysis is to modify this framework, while the study of regulations provides the basic requirements for designing medical devices.
3.1. Clinical needs
Clinical translation of biomaterials requires a deep understanding of clinical needs. A comprehensive study of clinical needs is essential before each biomaterial study with the aim of clinical translation is initiated. Sometimes, the clinical needs to be addressed already have solutions at present. Therefore, it is necessary to have a deep understanding of the clinical needs. Is there no good clinical treatment for a certain disease so far? From which perspective can the treatment of this disease be further improved to guide the design of biological materials and the overall experiment?
In addition, human factor engineering is important for medical devices with goals to minimize user-related hazards and risks and ensure that users can apply the device safely and effectively. Human factor engineering is defined as the application of knowledge on human behavior, abilities, limitations, and other characteristics of medical device users to the design of medical devices, with the goal of reducing human error, increasing productivity, and enhancing safety and comfort with a specific focus on the interaction between humans and the object of interest [29]. Evolution of ACI to Matrix-enhanced ACI could be a good example, which shortens surgery time and make surgery more easily performed. So, an in-depth understanding of the convenience and ease of clinical application of biomaterials is required. Biomaterials designed by scientists and the subsequent products developed by engineers need to be simpler, easier, and more convenient to operate in clinical applications, so as to maximize the therapeutic effects of biomaterials and reduce tissue damage. This requires familiarity with clinical procedures. To take cartilage tissue engineering as an example, most biomaterials-based applied basic research focused on bioactivity [30], mimetic structures [31], as well as mechanical properties [32,33] of the biomaterials. Clinical compliance requires biomaterials to be safe, simple and conducive for the production of hyaline cartilage. Broadly-used, simple procedure microfracture can lead to fibrocartilage regeneration, while theoretically-advanced autologous chondrocyte implantation (ACI) requires two surgical procedures as well as additional resources [34]. Biomaterial research and products design should be well-balanced with regards to effectiveness, simplicity, feasibility of clinical practice and scientific innovation.
Furthermore, in-depth communication with clinicians is helpful to prevent cognitive discrepancies caused by common-sense understanding of errors or differences in professional opinions and some other unforeseen factors that designers have not considered, such as standard operating procedure and surgeon's habits, both of which may result in some difficult situation during the operation or go against the requirements of human factors. For example, different implantation methods of cartilage implants (open suture or arthroscopic injection) will affect the design of biomaterial structure and other performance indicators, such as viscoelasticity, strength, viscosity, injectability, etc. In order to be compatible with practical clinical needs, researchers should more frequently consult clinicians, and seek solutions together. It is quite important to understand the key requirements of clinical practice, and get feedbacks of doctors about the products under development. Another way to identify clinical needs is to go into the hospital and observe diagnostic and therapeutic procedures. It is only through careful observation of real-world clinical practice that one can identify the key requirements of clinical translation.
3.2. Market competition
Competition with similar products significantly affects the commercial success of a potential product. It is essential to comprehensively evaluate the products based on all factors, including degree of being needed, advantages over similar products, easy operation, and cost-income ratio. Furthermore, analyses of products that failed in clinical trials and were recalled or updated products with post-market surveillance, can give cues on their existing problems [35]. Therefore, systematic scientific literature research, patent research, adverse event reporting, market research and customer (doctors in most situation) survey are essential when designing the technical route of biomaterial research, but in-depth analysis of competing products is also of utmost important at the same time. Taking cartilage repair as an example, MACI® (Vericel Corporation) [26], the first product approved by FDA, requires two surgical procedures and is classified as a drug but not a medical device, while Cartiva® SCI (Cartiva, Inc) [11], the other FDA-approved product, is approved for treatment of cartilage damage only in the big toe joint (low weight-bearing joints) but not in knee joint. There are as yet no FDA-approved implant products for the repair of knee cartilage defect. But several implant products have received the Conformité Européenne (CE) marking in Europe, such as Chondro-Gide® (Geistlich Pharma), a bilayer collagen I/III membrane that was granted with FDA Breakthrough Device Designation [36]; ChondroMimetic® (Collagen Solution), a dual-layer porous implant composed of collagen, glycosaminoglycans and calcium phosphate [37]; and Trufit CB® (Smith & Nephew), a porous bilayer poly (lactic-co-glycolic acid) scaffold that is reinforced with polyglycolic acid fibers, and calcium sulfate mineral [38]. A case of dislocation after long-term implantation of SaluCartilage™ (SaluMedica) showed that securely anchoring the implant to the host tissue is challenging [35].
Unlike product research and development, which focuses on practical feasibility (materials, packaging, sterilization, cost, etc.), applied basic research focuses more on theoretical feasibility. However, applied basic research activities should also be based on competitive product thinking.
3.3. Regulations
Obtaining regulatory approval is a critical and challenging process during commercialization of biomaterial products. For instance, Good Manufacturing Practice (GMP) is the basic norm for the production and quality control of medical devices, which should be strictly observed in product research and development but not required in applied basic research. It is one of the gaps that needs to be bridged during the translation process. Neglect and misunderstanding of regulations often lead to early failure in the clinical translation of biomaterials, with all resources input being in vain. Therefore, considering the regulatory review requirements at the beginning of biomaterial product design can be of great benefit to future clinical translation.
For regulatory compliance, identification and classification of biomaterial products should be the first step. For example, hyaluronic acid, a mucopolysaccharide found in various tissues of the body, shows excellent performance and great potential in cartilage repair. Its administration identification is according to the molecular size and the clinical application site of the body. It is identified as a medical device when utilized as joint lubricants in the US and used for subcutaneous filling in China [39], but identified as a drug instead when used as joint lubricant and eye drops in China [40]. In addition to medical devices, some biomaterial products may be identified as a combination of drug and medical device, which have more stringent pre-market clinical data requirements than medical devices, and should be further identified as drug-led or device-led combination products during registration in China. Taking implantable medical devices used for cartilage regeneration as an example, it should be clear whether the biomaterials are first identified as a medical device [41]. Medical devices are classified into three classes—Class I, II, or III—according to the degree of their risks in China. Class I medical devices generally pose low risk to the patient and/or user and are under routine management to assure their safety and effectiveness, such as surgical knives, while Class III medical devices, including passive orthopedic implants, pose high risk and are under strict control management. Class II medical devices are in between with moderate risk. Clinical evaluation is required for all medical devices. For clinical evaluation, clinical trial is needed for medical devices without sufficient clinical evidence to be able to ensure conformity with relevant Essential Principles of safety and performance of medical devices (considering risk level and/or novelty of device, and benefit/risk analysis) [42]. Specifically, clinical trials are not required for clinical evaluation of Class I devices in China. High-risk Class II and Class III devices need to undergo clinical trials compliant with the requirements of Good Clinical Practice (GCP) and ISO 14155 [43], with 3 exemption conditions: 1) The devices have clear working mechanisms, confirmed design, mature production process, with equivalent medical devices with same route of usage having been on the market for a long time without serious adverse events; 2) The safety and effectiveness can be proven via non-clinical assessment; 3) The clinical data from clinical trial or practice of equivalent devices can prove the safety and effectiveness of the devices [44]. So, it is essential to define the classification of the product according to the Medical Device Classification Catalogue (2017) [45] published by NMPA. If the product is a new type, the classification will be made by the committee (Center for Medical Device Standardization Administration NMPA). So far, cartilage repair implants are classified as Class III medical devices.
Pre-market technical review is of great importance, according to the guidance documents and standards of medical device technical review. During technical review, it is required to define standards and technical requirements of the products which are suitable for the product based on national standards, industrial standards and the requirements of relevant regulations. Specifically, the technical indexes such as testing methods and limit values should be defined in product technical requirements. These standards and technical requirements are formulated to guide the requirement of materials, process and final products to ensure the safety and effectiveness of the products. So, the researcher has to find out relevant guidance documents and seek out what standards should be met in the product technical requirements. For cartilage repair products, Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage [46] can be one of the references, and the standards, Non-active surgical implants—General requirements [47] (ISO 14630: 2012 or YY/T 0640 in China) and Tissue engineered medical product Part 10: In vivo assessment of implantable devices intended to repair or regenerate articular cartilage [48] (YY/T 0606.10 in China), should be considered, in addition to biological evaluation of medical devices (ISO 10993). Besides, materials of different types, sources, morphologies, etc. also have corresponding standards to be considered. For example, when animal-derived materials are used, the standard, Medical devices utilizing animal tissues and their derivatives – Part 3: Validation of the elimination and/or inactivation of viruses and transmissible spongiform encephalopathy (TSE) agents [49] (ISO 22442-3: 2007 or YY/T 0771 in China), must be considered. As the regulation of medical devices is being constantly modified, it is important to take note of the newest regulations on the relevant website of NMPA, as well as maintain communication with administrators all the time. It is also desirable to follow up the review process for materials in the relevant field to gain a deeper understanding of the impact of regulations on the clinical translation of biomaterials.
Understanding clinical needs, competitive products in the market and relevant regulations is conducive to the establishment of a sense of direction for applied basic research topics. On this basis, the clinical critical needs, product shortcomings and applied basic research innovation points are seamlessly connected under the requirement of regulations, which is conducive for achieving the clinical translation of biomaterials.
4. Material overview
In applied basic research, most researchers may be more concerned on which materials are more likely to achieve the designated properties, and less concerned on the issues that these materials may pose in their clinical translation process. Obviously, thinking about these potential problems in advance facilitates their clinical translation. Hence, based on the perspective of product research and development, the safety, novelty of materials, raw material supply and other aspects should be kept in mind when selecting materials.
4.1. Safety
Safety and effectiveness are indispensable parameters and are basic requirements for translating biomaterials into medical devices. The evaluation of safety encompasses many aspects, including physical, chemical and biological properties, microbial infection, sterilization, usage, and clinical evaluation [50]. When it comes to practice, researchers often simply use cytotoxicity, inflammation, interactions with blood, local effects after implantation as indicators of safety or so called biocompatibility, but for permanent implant devices contacted with tissue/bone, the FDA guidance document on the use of standard ISO-10993-1 also recommends biological effect testing of sensitization, irritation or intracutaneous reactivity, acute systemic toxicity, material-mediated pyrogenicity, subacute/subchronic toxicity, genotoxicity, implantation, chronic toxicity and carcinogenicity [51]. It can be expected that most of the materials used in current studies seem to meet safety requirements, but the situation is more challenging in clinical translation. Therefore, in order to minimize the risks of encountering problems with subsequent safety evaluation, safety issues such as sensitization, degradation and metabolism of materials should also be considered. Furthermore, the different requirements according to biomaterial sources should not be ignored. For natural biomaterials, especially for animal-derived materials, allergens, viruses, other pathogens and immunogenic substances should also be considered [50]. For example, chitosan [52] extracted from shellfish should be used with caution for safety in people with shellfish allergies. For synthetic materials, especially biodegradable synthetic materials, such as poly-lactic acid (PLA) [53] and polyethylene glycol (PEG) [54], the main safety issue is the effect of degradation products, which are mainly acidic substances that may lower the pH of the local tissue microenvironment and potentially cause inflammation [55].
4.2. Novel or mature biomaterials
Though novel materials may overcome the shortage of existing materials in research or clinical practice, they have to undergo complex clinical verification and more rigorous evaluation to ensure safety once in the clinical translational process. In practice, as rigorous criteria have been applied, FDA, EMA and NMPA-approved materials have been widely recognized and referenced by regulators in various countries, which may have higher clinical translation potential [56]. Silk fibroin is one such new material in tissue engineering, where there is neither referable similar products nor relevant criteria for comparison evaluation, and its safety in clinical medicine is not guaranteed [57]. Developers have to provide comprehensive and detailed information since reviewers lack experience and previous references in product clinical translation [58]. From the perspective of national supervision, reviewers are responsible for their supervised products for life, resulting in increased difficulty in obtaining approval for a new material product, while prolonging the whole development process. Of course, material innovations in biomaterials research are to be encouraged, but in terms of clinical translation, unless the new material/design solves the clinical problem, it is just superfluous performance.
4.3. Raw materials supply
Raw materials supply is of great significance. The raw materials used in the laboratory are usually chemically pure and in small quantities, but it is critical to have a sustainable large quantity of medical-grade raw materials for clinical translation. Chitosan is obtained from the shells of shrimps and crabs, but trace amounts of remnant protein may cause inflammatory reaction and anaphylaxis [59]. Annual incidence of adverse reactions to chitosan products ranges from 0.1 to 1%, mainly manifested as intestinal adhesion, anaphylaxis and skin pruritus according to the appendix of document No. 455 [2016] issued by the food and drug administration [60]. In order to get pure and protein-free chitosan, a large amount of strong acids and bases are needed which often leads to unqualified environmental assessment results for factories. Previously, we have put in much effort in attempting to develop chitosan-based biomaterials in cartilage regeneration [[61], [62], [63], [64], [65]]. Nevertheless, translational potentials are blocked partly, whenever a consistent supply of medical grade raw materials are unavailable. Additionally, it is not enough to have only one supplier of a raw material, and multiple supply sources are necessary to ensure a stable and consistent supply.
Obviously, the most important concern should still be the safety of materials in applied basic research for translation, and the issue of raw material supply appears to be premature and more commercially-based. Moreover, when a material shows strong clinical application value, there will naturally be market supply and demand to facilitate the emergence of relevant raw material suppliers. However, with a good supply of raw materials, using these materials in advance for basic research can better reflect the final results of the product, and the clinical translation is more likely to be successful.
5. Technology overview
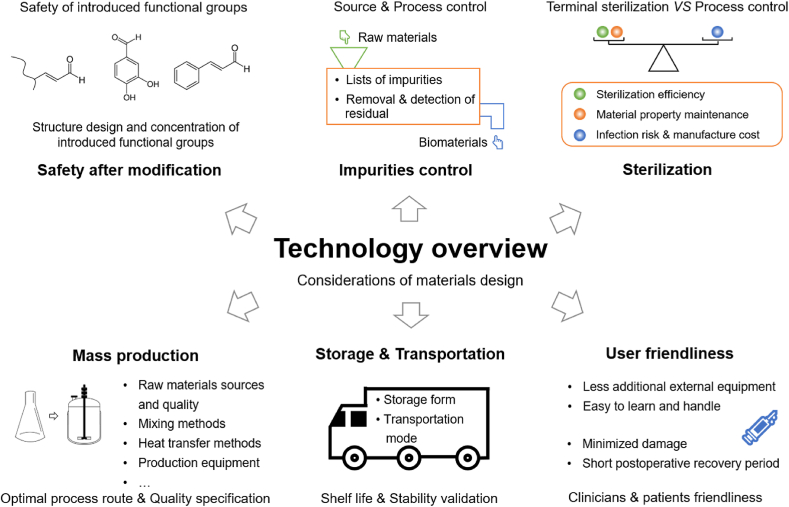
The design and processing of materials are important processes in transforming raw materials into biomaterials. The evaluation of safety requires that the product should be designed, produced, packaged, transported, stored and used to minimize residual exposure to contaminants or microorganisms, so as to reduce the risk to patients [50]. In the process of designing medical devices, the selected materials are often modified to improve their performance, which may change the structure and properties of the materials, and introduce new components and impurities. The issues encountered in the process of clinical translation have garnered relatively little attention, which also have crucial roles in biomaterial clinical translation (Fig. 3).
Fig. 3.
Technology overview of considerations of materials design.
From the technology aspect, materials design should properly consider safety issues after modification, impurities control, sterilization, mass production, storage & transportation and user friendliness, which often impede the process of clinical translation of biomaterials.
5.1. Safety after modification
Some functional groups are introduced to enhance mechanical and other properties of biomaterials [66], which are sometimes accompanied by safety issues. For instance, Schiff's base reaction based on amine and aldehyde groups is often introduced to materials to form reversible chemical bonds and enhance the toughness and adhesive strength of materials in cartilage regeneration [33,67]. Toxicity of aldehyde groups depends on their structure and concentration. Most of the α,β-unsaturated aldehydes have been associated with increased aging [68], cardiovascular diseases [69], inflammation [70], neurological disorders [71] and cancer [69], such as 4-hydroxynonenal (4-HNE), acrolein, malondialdehyde (MDA) and crotonaldehyde (Cr). But other types of aldehydes could suppress inflammation, such as protocatechuic aldehyde [72], cinnamaldehyde [2] and coniferyl aldehyde [73]. Nevertheless, a large number of experiments are necessary to verify the safety of the various aldehyde groups. Hence, the structural design and concentration control of aldehyde groups should be performed with more caution.
5.2. Impurities control
Impurities in biomaterial-based medical devices should be minimized as per regulatory requirement in most jurisdictions [50]. There are two main concerns for impurity control, source control (impurity removal during acquisition of raw materials) and process control (impurity removal during product fabrication).
Source control is key for impurities control. Collagen used in cartilage implants are usually extracted from animals (mouse or bovine) or human tissues, as it is difficult to produce collagen through microorganisms and the yield is very low [74]. Due to the triple-helix structure of the collagen macromolecule and the imprecise quantitative methods (no standard reference) used in structure type determination, it is difficult to determine the collagen type and molecular weight, which can be affected by different extraction methods, resulting in difficulty in establishing a general control method. Batch to batch variation is a big challenge, as collagen is harvested from tissues with varied composition utilizing different processes. However, it is necessary for clinical translation to make clear how raw materials are obtained, how viruses, other pathogens and immune-derived substances are removed or inactivated, and their precise composition to assure the purity of raw materials [50]. Genetically engineered microbial fermentation may be the solution to the source control of collagen in the future.
Process control of impurities is also critical. To minimize residual impurities in the biomaterial synthesis process, it is necessary to clarify the list of impurities, as well as the removal and detection methods in the design of the synthesis route. In the list of impurities, it should be noted that except for the solvents, auxiliaries and raw materials that do not fully react, it is inevitable that some chemical reactions are accompanied with byproducts. Then, the appropriate method with high removal efficiency should be determined based on the source and properties of the impurity. Ultra-filtration, dialysis, rotary evaporation, and extraction are commonly used methods in the laboratory, and one method is often sufficient for removing multifold impurities in the synthetic product [75,76], but impurities that are not suitable for these methods may be ignored. The detection of residual impurities is often one of the most neglected issues in scientific research, but it is often a strict requirement during clinical translation to identify biological hazards and estimate and control biological risks [77,78]. Take 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride/N-Hydroxy succinimide (EDC/NHS) as an example, which is usually used for collagen chemical crosslinking by catalyzing amidation reactions [79]; the use of it may lead to byproduct formation, because it also can catalyze esterification reactions [80]. In another example, hyaluronic acid is usually cross-linked by divinylsulfone (SYNVISC® Hylan G-F 20, Genzyme) [81], which is toxic, and more attention should be given to its removal and detection. Besides, materials such as gelatin and hyaluronic acid can be modified with methacrylate to form chemical cross-linked networks and attain stable mechanical properties, which is another strategy used in biomaterials for cartilage regeneration [82,83]. The molecule used for modification is often methacrylic anhydride [84], which is also toxic.
It can be said that, every added chemical agent will increase the cost significantly, as it costs huge amount of energy to remove and monitor its final concentration. Hence, it is helpful for clinical translation to give adequate consideration to the control of impurities during the design and fabrication of biomaterials, reduce the additives and simplify the synthesis of biomaterials as much as possible. For those unavoidable impurities, the removal and detection methods and safe concentrations should be well-defined.
5.3. Sterilization
Sterilization is essential before clinical utilization of medical devices, while effects of sterilization on medical products are often ignored. The sterilization process in applied basic research is often less rigorous, without clear process and parameters, and certain sterility assurance level (SAL), while strict procedures of sterilization are required for clinical translation [85]. The methods for terminal sterilization of medical devices are categorized by FDA as established sterilization methods, which include dry heat, ethylene oxide, moist heat or steam, radiation (e.g., gamma, electron beam), all of which have a long history of safe and effective use, as demonstrated by multiple sources of information [86]. In research projects of cartilage regeneration, some biomaterials are disinfected with ultraviolet rays [87], but it is sometimes ineffective and not ideal for opaque materials due to its limited penetrability [88]. Besides, it is categorized as a novel sterilization method, and additional information is required to be provided, as compared to established methods, including a comprehensive description of the sterilization process, the validation protocol, and the sterilization validation data [86]. Anyway, a standard sterilization method should be established to meet sterility requirements when designing biomaterials for medical devices.
The sterilized biomaterials should still maintain their target properties to meet the requirements of effectiveness at time of use, in addition to obtaining sterile products. The properties of biomaterials are obtained through precise design, and different materials have different corresponding sterilization methods [89], which may have different side-effects on the material properties. There is research showing that moist heat and steam is the optimal method to ensure sterilization efficiency, but sometimes it will result in structural alterations for biomaterials used for cartilage regeneration [87]. Different sterilization methods have their corresponding applications. For radiation sterilization, the photocrosslinked material has better stability to radiation than the photodegradable material, and the lower the density in a given polymer species, the better the stability to radiation [90]. Ethylene oxide sterilization is the most effective sterilization method for most materials, which involves utilizing its ring structure fracture to alkylate the active groups of amino, carboxy, hydroxy and mercapto on the bacterial protein [91] Nevertheless, this will also alkylate the active groups on the materials and affect the biological properties of the materials [92]. On the other hand, different preparations of the same material being sterilized by the same method may yield different performance results. For example, after ethylene oxide sterilization, fibrin microfibrous scaffolds crosslinked in acidic buffers show a significant reduction in myoblast attachment due to the alkylation of microthreads, while the microthreads crosslinked in neutral buffers do not appear to be affected by the sterilization [93].
When all terminal sterilization methods are not applicable for the designed biomaterial, sterilization process controls can be used as a compromised solution such as filtration sterilization. These are often not recommended but tolerated by the regulatory administration. Compared to terminal sterilization, sterilization process controls require extremely controlled environment, validated, controlled, monitored sterilization procedures and appropriately qualified and trained personnel, which is accompanied by rising infection risk and manufacturing costs [94].
Thus, it can be seen that, the choice of sterilization method may affect the final performance of the designed biomaterial, and the smart design of the biomaterial can avoid the side effects of some sterilization methods on biomaterial performance. So, it is significant to consider the relationship between sterilization methods and material design when designing biomaterials.
5.4. Other processes
The mass production, storage, transportation, and use of materials are closely related to material design. Commercial production often reduces costs via high yield, while some production processes in the laboratory are complicated, expensive and small scale. When it comes to mass production, this requires an optimal process route with short synthesis steps, high total yield, simple equipment technical conditions and process flow, abundant and affordable raw materials including solvents and additives. In addition, the enlarged scale of production is accompanied by different raw materials sources and quality, mixing methods, heat transfer methods and production equipment from the laboratory, which may result in instability in quality and low rate of conforming products or exorbitant cost. For example, stirring is a common procedure in the process of mixing the cross-linking agent with collagen or other materials that need to be cross-linked. When it comes to mass production, the main problem is how to achieve the originally designed parameters and indicators, such as the dispersion of the cross-linking agent. Once the mixing system has a higher viscosity, it will be very difficult to achieve the ideal dispersion of cross-linking agent, and easy to cause product quality substandard, or require more special equipment, more mixing time, etc., resulting in rising costs. Thus, during materials design, consideration of process optimization to adapt to mass production in advance is conducive to translation. Materials can be designed to simplify the operation and have stable quality control, which is also conducive to the feasibility of production.
Shelf life should be as long as at least two years or above, as a long process from manufacturer to hospital is required, usually taking years instead of months. For example, the shelf life of a liquid product can be extended by designing storage form as powder or solid, because the liquid environment is more likely to provide a reactive system for the active substances than either the powder or solid form. Transportation verification is critical for biomaterial-based medical devices, especially those sensitive to time, temperature and shock. Though cold-chain transportation could effectively maintain key properties of biomaterials, it however increases cost significantly. Hence, it is better to take the final storage and transportation form into account when designing biomaterials, which is beneficial to increasing their clinical translation potential.
User friendliness of a product is of great advantage, as it reduces time, energy and cost in training. It is almost impossible for surgeons to spare much time (2–3 min are the upper limit) to prepare the biomaterials during operation. In the meantime, it is very difficult to take additional external equipment into the operating theatre, which potentially increase contamination risk. Platelet-rich plasma needs to be obtained through centrifugation with some specially designed equipment before it can be used [95]. Photosensitive hydrogels also need to be equipped with an additional ultraviolet light source that sometime limit their usage with arthroscope [96]. Also, the products should be beneficial for patients to minimize the damage caused by surgery or other treatments. Biomaterials that can be injected through arthroscopy are better than implant biomaterials that need surgery and a longer recovery time in the case of cartilage injury [34].
Biomaterials designed in innovation-driven research and commercial-driven translation are normally difficult to apply well in both areas, if there is no holistic design that take into account both perspectives in advance. Biomaterials, used in research, are normally considered on the basis of novelty of certain properties, often involving new materials and new complex modification methods. However, due to safety, efficiency and cost, biomaterials used for clinical translation are more inclined to safe materials with less modification, which had already undergone systematic safety evaluation, consideration of impurity control, sterilization, storage, transportation, operation, costs and regulations. Thus, not only should the biological effects of biomaterials be considered, including, but not limited to safety and impurity control, but also the stability of biomaterial properties should be maintained during the process from production to use, such as sterilization and storage, when designing the properties of biomaterials. To some extent, the simpler the biomaterial design is, the more beneficial it is to the clinical translation process.
6. Future perspectives
Scientifically, with deeper understanding of biomaterials, the relationship between human bodies with biomaterials-based medical devices will move forward. Advances of Materials Genome Initiative could provide more information and guidance to biomaterials, while single-cell sequencing and other advanced technology platforms deepen our understanding of human bodies. All these cutting-edge technology platforms push forward biomaterial science. But most of the biomaterials that perform well in the laboratory do not make it into approved medical devices. The widening gap between newly-developed biomaterials and medical devices is what we have to confront and overcome together. Therefore, it is crucial to have a more comprehensive selection and design of materials, so that biomaterials can be more easily translated into clinical products.
Frankly speaking, understanding of the human body itself is still puzzling. Materials, might be a tool to stimulate or trigger cells, but the mechanisms are still unclear, or at least, not fully understood. That is to say, more comprehensive studies about the effects of biomaterials on the body are still needed, especially the effects and mechanisms of biomaterials on cells, tissues and organs during the later stage of implantation with the degradation of materials, which are almost blank.
Besides, how many researchers are truly interested in clinical translational work is still unclear. A complicated and novel structure is conducive for publication, but may increase the difficulty in becoming a product, because it tends to increase the difficulty of quality control during fabrication and also increases the risks of failure upon evaluation by agents. One way to improve the clinical translational potential of scientific research results, would be to cooperate with enterprises during the early stages of research, and try to simplify the process and reduce costs of the research process, so as to achieve compatibility between scientific research and clinical translation. Thus, the integration of industry, market and research is very important, but this is still in its infancy and requires the joint efforts of all parties to create a more suitable research environment for translation. Also, rather important but seldom mentioned is that financial investment and long-term confidence are critical for the development of medical devices, which often take many years and consume huge amounts of resources.
The global education system aims to train the people how to think critically and resolve pertinent problems based on scientific principles, but commercialization is conducted by industrial organization, which at least involves people with a scientific background, as well as others with technology, clinical, legal, business and administration expertise. As a result, a team with interdisciplinary knowledge is the first step towards success. Science and patent may be the first step to work on, but it must be kept in mind that the product depends on standards, and that innovation may only give a chance to make a difference, but whether successful or not may be decided by quite a number of factors.
In conclusion, there are still many issues that have not been discussed in depth. For example, the discussion about intellectual properties, selection of preclinical animal models, effectiveness of biomaterials, the latest development in regulations, etc. is fading out in this article, but they are also important for medical devices translation of biomaterials. Besides, a comprehensive analysis of FDA-approved products is limited in this article. Specifically, the advantages and disadvantages are need to be analyzed. From the perspective of translation, the analysis of the material source, processing method, quality control method, preclinical and clinical trial situation, approval situation, etc. of the products will be needed for reference. In the future, we hope to write further articles to go through all the steps from research and development to translation, with one or more successfully approved cartilage repair products as demonstration.
CRediT authorship contribution statement
Li Wang: Conceptualization, Investigation, Writing – original draft, Visualization. Xiaolei Guo: Investigation, Writing – review & editing, Visualization. Jiaqing Chen: Investigation. Zhen Zhen: Investigation. Bin Cao: Investigation. Wenqian Wan: Investigation. Yuandong Dou: Writing – review & editing. Haobo Pan: Writing – review & editing. Feng Xu: Writing – review & editing. Zepu Zhang: Writing – review & editing. Jianmei Wang: Writing – review & editing. Daisong Li: Writing – review & editing. Quanyi Guo: Writing – review & editing. Qing Jiang: Writing – review & editing. Yanan Du: Writing – review & editing. Jiakuo Yu: Writing – review & editing. Boon Chin Heng: Writing – review & editing. Qianqian Han: Writing – review & editing. Zigang Ge: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgement
This work was supported by the National Natural Science Foundation of China grant (81772334).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Qianqian Han, Email: hanqianqian@nifdc.org.cn.
Zigang Ge, Email: gez@pku.edu.cn.
References
- 1.Williams D., Zhang X., editors. Definitions of Biomaterials for the Twenty-First Century. Elsevier; 2019. II - biomaterials and biomedical materials; pp. 15–23. [Google Scholar]
- 2.Khare P., Jagtap S., Jain Y., Baboota R.K., Mangal P., Boparai R.K., Bhutani K.K., Sharma S.S., Premkumar L.S., Kondepudi K.K., Chopra K., Bishnoi M. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. Biofactors. 2016;42(2):201–211. doi: 10.1002/biof.1265. [DOI] [PubMed] [Google Scholar]
- 3.Cui F., Liu B., Tan R. Science Press; Beijing: 2019. Medical Device Translation of Biomaterials. [Google Scholar]
- 4.Darnell M., Mooney D.J. Leveraging advances in biology to design biomaterials. Nat. Mater. 2017;16(12):1178–1185. doi: 10.1038/nmat4991. [DOI] [PubMed] [Google Scholar]
- 5.Serban M.A. Translational biomaterials—the journey from the bench to the market—think ‘product’. Curr. Opin. Biotechnol. 2016;40:31–34. doi: 10.1016/j.copbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 6.NMPA . 2017. Announcement of the Review Results of Special Approval Applications for Innovative Medical Devices.https://www.cmde.org.cn/CL0050/5998.html [Google Scholar]
- 7.ICRS . 2018. Search for Information on Cartilage Repair-Related Medical Devices & Techniques.https://cartilage.org/patient/find/medical-devices-and-techniques/ [Google Scholar]
- 8.Huang B.J., Hu J.C., Athanasiou K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CartiHeal Agili-C™. 2017. https://www.cartiheal.com/agili-c/
- 10.Arthrex BioCartilage®. 2013. https://www.arthrex.com/resources/presentation/fJ3kA93jWki3BQE9fyQCXw/biocartilage
- 11.Cartiva Cartiva® synthetic cartilage implant. https://www.cartiva.net/for-physicians/
- 12.Pharma G. Chondro-Gide®. 2021. https://www.geistlich-pharma.com/en/orthopaedic/products/chondro-gide/
- 13.GmbH M.B. ChondroFiller®. 2018. https://www.bioregio-stern.de/en/database/company/meidrix-biomedicals-gmbh
- 14.Enterprise U.o.C. 2015. Collagen Solutions Acquires Assets for ChondroMimetic and Related Products.https://www.enterprise.cam.ac.uk/news/collagen-solutions-acquires-assets-for-chondromimetic-and-related-products/ [Google Scholar]
- 15.Biotissue Chondrotissue®. 2021. http://www.biotissue.de/chondrotissue/health-professionals/chondrotissue/product-features/
- 16.AG S.O. CHONDROVEIL™. 2011. http://www.swissbiomedortho.com/chondroveil/
- 17.Anika Therapeutics I. Hyalofast®. 2021. https://hyalofast.anikatherapeutics.com/
- 18.Inc O.M. JOINTREP™. 2017. http://medicwave.com.my/jointrep/
- 19.Finceramica MaioRegen. 2020. https://jri-ltd.com/our-products/orthobiologics/maioregen
- 20.AG T. 2020. Novocart® Basic.https://www.tetec-ag.com/en/products/b/novocart-basic.html [Google Scholar]
- 21.Kang R.W., Ma R., Pais D.A., Williams R.J. In: Techniques in Cartilage Repair Surgery. Shetty A.A., Kim S.-J., Nakamura N., Brittberg M., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. TruFit®; pp. 69–80. [Google Scholar]
- 22.Biotissue BioSeed®-C. 2021. http://www.biotissue.de/bioseed/health-professionals/bioseed-c/product-features/
- 23.Nephew S. BST-Cargel®. https://www.smith-nephew.com/key-products/sports-medicine/bst-cargel/
- 24.Nehrer S., Halbwirth F., Luksch T. In: Techniques in Cartilage Repair Surgery. Shetty A.A., Kim S.-J., Nakamura N., Brittberg M., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. CaReS®, cartilage regeneration system: autologous chondrocyte transplantation in a collagen gel; pp. 245–250. [Google Scholar]
- 25.Engineering J.T. 2014. JACC®.https://discovery.lifemapsc.com/stem-cell-differentiation/in-vitro-cells/cartilage-homo-sapiens-cultured-cartilage-jacc-japan-tissue-engineering-co-ltd [Google Scholar]
- 26.Vericel MACI®. https://www.maci.com/patients/
- 27.Biologics A. 2020. NOVOCART ® 3D.https://www.aesculapbiologics.com/en/healthcare-professionals/novocart-3d.html [Google Scholar]
- 28.Lam J., Lu S., Kasper F.K., Mikos A.G. Strategies for controlled delivery of biologics for cartilage repair. Adv. Drug Deliv. Rev. 2015;84:123–134. doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA . 2018. Human Factors and Medical Devices.https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/human-factors-and-medical-devices [Google Scholar]
- 30.Li J., Huang Y., Song J., Li X., Zhang X., Zhou Z., Chen D., Ma P.X., Peng W., Wang W., Zhou G. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018;79:202–215. doi: 10.1016/j.actbio.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radhakrishnan J., Manigandan A., Chinnaswamy P., Subramanian A., Sethuraman S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials. 2018;162:82–98. doi: 10.1016/j.biomaterials.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 32.Means A.K., Shrode C.S., Whitney L.V., Ehrhardt D.A., Grunlan M.A. Double network hydrogels that mimic the modulus, strength, and lubricity of cartilage. Biomacromolecules. 2019;20(5):2034–2042. doi: 10.1021/acs.biomac.9b00237. [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Yang J., Wang L., Zhang X., Heng B.C., Wang D.-A., Ge Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioactive Materials. 2021;6(6):1689–1698. doi: 10.1016/j.bioactmat.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer C., Horas U., Hörbelt R., Schnettler R. Implantatdislokation bei künstlichem Knorpelersatz (SaluCartialge™) Unfallchirurg. 2005;108(2):163–166. doi: 10.1007/s00113-004-0798-7. [DOI] [PubMed] [Google Scholar]
- 36.Pharma G. 2021. Chondro-Gide® Articular Cartilage Cover: FDA Approval as a Breakthrough Device.https://www.geistlich-pharma.com/en/about-us/newsroom/corporate-news/news-details/news/detail/News/chondro-gider-articular-cartilage-cover-fda-approval-as-a-breakthrough-device/ [Google Scholar]
- 37.Getgood A., Henson F., Skelton C., Herrera E., Brooks R., Fortier L.A., Rushton N. The augmentation of a collagen/glycosaminoglycan biphasic osteochondral scaffold with platelet-rich plasma and concentrated bone marrow aspirate for osteochondral defect repair in sheep: a pilot study. Cartilage. 2012;3(4):351–363. doi: 10.1177/1947603512444597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melton J.T., Wilson A.J., Chapman-Sheath P., Cossey A.J. TruFit CB bone plug: chondral repair, scaffold design, surgical technique and early experiences. Expet Rev. Med. Dev. 2010;7(3):333–341. doi: 10.1586/erd.10.15. [DOI] [PubMed] [Google Scholar]
- 39.FDA TRILURON™ - P180040. 2019. https://www.fda.gov/medical-devices/recently-approved-devices/trilurontm-p180040
- 40.NMPA . 2009. Announcement on the Management Category of Sodium Hyaluronate Products for Medical Use.http://www.nmpa.gov.cn/WS04/CL2138/299895.html [Google Scholar]
- 41.NIFDC Summary of the classification and definition results of the first batch of medical devices in 2020. 2020. https://www.nifdc.org.cn/nifdc/bshff/ylqxbzhgl/qxxxgk/fljd/20200327160800941.html
- 42.IMDRF . 2019. IMDRF/MDCE WG/N57FINAL:2019–"Clinical Investigation".http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-191010-mdce-n57.pdf [Google Scholar]
- 43.ISO . 2020. Clinical Investigation of Medical Devices for Human Subjects — Good Clinical Practice. [Google Scholar]
- 44.IMDRF . 2019. IMDRF/MDCE WG/N56FINAL:2019– “Clinical Evaluation”.http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-191010-mdce-n56.pdf [Google Scholar]
- 45.NMPA . 2017. Announcement of the General Administration on the Release of the Classification Catalogue of Medical Devices. [Google Scholar]
- 46.FDA . 2019. Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preparation-ides-and-inds-products-intended-repair-or-replace-knee-cartilage [Google Scholar]
- 47.ISO . 2012. Non-active Surgical Implants — General Requirements. [Google Scholar]
- 48.NMPA . 2008. Tissue Engineered Medical Product. Part 10: in Vivo Assessment of Implantable Devices Intended Torepair or Regenerate Articular Cartilage. [Google Scholar]
- 49.ISO . 2007. Medical Devices Utilizing Animal Tissues and Their Derivatives — Part 3: Validation of the Elimination And/or Inactivation of Viruses and Transmissible Spongiform Encephalopathy (TSE) Agents. [Google Scholar]
- 50.NMPA . 2014. The Announcement on the Requirements for the Registration of Medical Devices and the Format of the Approval Documents.http://www.nmpa.gov.cn/WS04/CL2138/299985.html [Google Scholar]
- 51.FDA . 2020. Use of International Standard ISO 10993-1, "Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a Risk Management Process".https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and [Google Scholar]
- 52.Amaral L., Silva D., Couto M., Nunes C., Rocha S.M., Coimbra M.A., Coimbra A., Moreira A. Safety of chitosan processed wine in shrimp allergic patients. Ann. Allergy Asthma Immunol. : official publication of the American College of Allergy, Asthma, & Immunology. 2016;116(5):462–463. doi: 10.1016/j.anai.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Ramot Y., Haim-Zada M., Domb A.J., Nyska A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016;107:153–162. doi: 10.1016/j.addr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Knop K., Hoogenboom R., Fischer D., Schubert U.S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 55.Elsawy M.A., Kim K.H., Park J.W., Deep A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017;79:1346–1352. doi: 10.1016/j.rser.2017.05.143. [DOI] [Google Scholar]
- 56.NMPA . 2007. Notice on Issuing the Guidelines for Biological Evaluation and Review of Medical Devices.https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjylqx/20070615010101796.html [Google Scholar]
- 57.Zhang W., Chen L., Chen J., Wang L., Gui X., Ran J., Xu G., Zhao H., Zeng M., Ji J., Qian L., Zhou J., Ouyang H., Zou X. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv Healthc Mater. 2017;6(10) doi: 10.1002/adhm.201700121. [DOI] [PubMed] [Google Scholar]
- 58.Xingyuebio Fibroin protein membrane dressing (Class III) http://www.xingyuebio.com/Product/ShowProduct37.html
- 59.Patel S., Goyal A. Chitin and chitinase: role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017;97:331–338. doi: 10.1016/j.ijbiomac.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 60.NMPA . 2016. Letter of the General Office of National Medical Products Administration on Notification of the Reevaluation of Chitosan Medical Device Products. [Google Scholar]
- 61.Ge Z., Baguenard S., Lim L.Y., Wee A., Khor E. Hydroxyapatite-chitin materials as potential tissue engineered bone substitutes. Biomaterials. 2004;25(6):1049–1058. doi: 10.1016/s0142-9612(03)00612-4. [DOI] [PubMed] [Google Scholar]
- 62.Li C., Wang L., Yang Z., Kim G., Chen H., Ge Z. A viscoelastic chitosan-modified three-dimensional porous poly(L-lactide-co-epsilon-caprolactone) scaffold for cartilage tissue engineering, Journal of biomaterials science. Polymer edition. 2012;23(1–4):405–424. doi: 10.1163/092050610X551970. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Yang Z., Li C., Dou Y., Li Y., Thote T., Wang D.A., Ge Z. Cells behave distinctly within sponges and hydrogels due to differences of internal structure. Tissue Eng. 2013;19(19–20):2166–2175. doi: 10.1089/ten.TEA.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J., Wu Y., Thote T., Lee E.H., Ge Z., Yang Z. The influence of scaffold microstructure on chondrogenic differentiation of mesenchymal stem cells. Biomed. Mater. 2014;9(3) doi: 10.1088/1748-6041/9/3/035011. 035011. [DOI] [PubMed] [Google Scholar]
- 65.Chen J.Q., Li Y.J., Wang B., Yang J.B., Heng B.C., Yang Z., Ge Z.G., Lin J.H. TGF-beta 1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. J. Mater. Chem. B. 2018;6(4):675–687. doi: 10.1039/c7tb02132a. [DOI] [PubMed] [Google Scholar]
- 66.Klotz B.J., Gawlitta D., Rosenberg A.J.W.P., Malda J., Melchels F.P.W. Gelatin-Methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34(5):394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 68.Dmitriev L.F., Titov V.N. Lipid peroxidation in relation to ageing and the role of endogenous aldehydes in diabetes and other age-related diseases. Ageing Res. Rev. 2010;9(2):200–210. doi: 10.1016/j.arr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Voulgaridou G.P., Anestopoulos I., Franco R., Panayiotidis M.I., Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res-Fund Mol M. 2011;711(1–2):13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Barrera G., Pizzimenti S., Daga M., Dianzani C., Arcaro A., Cetrangolo G.P., Giordano G., Cucci M.A., Graf M., Gentile F. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants-Basel. 2018;7(8) doi: 10.3390/antiox7080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trevisani M., Siemens J., Materazzi S., Bautista D.M., Nassini R., Campi B., Imamachi N., Andre E., Patacchini R., Cottrell G.S., Gatti R., Basbaum A.I., Bunnett N.W., Julius D., Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. P Natl Acad Sci USA. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao L., Wu W.F., Dong L., Ren G.L., Li H.D., Yang Q., Li X.F., Xu T., Li Z., Wu B.M., Ma T.T., Huang C., Huang Y., Zhang L., Lv X.W., Li J., Meng X.M. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akram M., Kim K.A., Kim E.S., Shin Y.J., Noh D., Kim E., Kim J.H., Majid A., Chang S.Y., Kim J.K., Bae O.N. Selective inhibition of JAK2/STAT1 signaling and iNOS expression mediates the anti-inflammatory effects of coniferyl aldehyde. Chem. Biol. Interact. 2016;256:102–110. doi: 10.1016/j.cbi.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 74.Rutschmann C., Baumann S., Cabalzar J., Luther K.B., Hennet T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014;98(10):4445–4455. doi: 10.1007/s00253-013-5447-z. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Li Y., Wang B., Yang J., Heng B.C., Yang Z., Ge Z., Lin J. TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. J. Mater. Chem. B. 2018;6(4):675–687. doi: 10.1039/c7tb02132a. [DOI] [PubMed] [Google Scholar]
- 76.Xu J., Feng Q., Lin S., Yuan W., Li R., Li J., Wei K., Chen X., Zhang K., Yang Y., Wu T., Wang B., Zhu M., Guo R., Li G., Bian L. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 77.ISO . 2018. Biological Evaluation of Medical Devices — Part 1: Evaluation and Testing within a Risk Management Process. [Google Scholar]
- 78.ISO . 2020. Biological Evaluation of Medical Devices — Part 18: Chemical Characterization of Medical Device Materials within a Risk Management Process. [Google Scholar]
- 79.Nair M., Johal R.K., Hamaia S.W., Best S.M., Cameron R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: a comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials. 2020;254:120109. doi: 10.1016/j.biomaterials.2020.120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Everaerts F., Torrianni M., Hendriks M., Feijen J. Biomechanical properties of carbodiimide crosslinked collagen: influence of the formation of ester crosslinks. J. Biomed. Mater. Res. 2008;85(2):547–555. doi: 10.1002/jbm.a.31524. [DOI] [PubMed] [Google Scholar]
- 81.Lai J.Y. Relationship between structure and cytocompatibility of divinyl sulfone cross-linked hyaluronic acid. Carbohydr. Polym. 2014;101:203–212. doi: 10.1016/j.carbpol.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 82.Bian L., Hou C., Tous E., Rai R., Mauck R.L., Burdick J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34(2):413–421. doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 84.Gan D., Xu T., Xing W., Wang M., Fang J., Wang K., Ge X., Chan C.W., Ren F., Tan H., Lu X. Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J. Mater. Chem. B. 2019;7(10):1716–1725. doi: 10.1039/c8tb01664j. [DOI] [PubMed] [Google Scholar]
- 85.ISO . 2018. Sterilization of Health Care Products — Vocabulary of Terms Used in Sterilization and Related Equipment and Process Standards. [Google Scholar]
- 86.FDA . 2016. Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submission-and-review-sterility-information-premarket-notification-510k-submissions-devices-labeled [Google Scholar]
- 87.Al-Sabah A., Burnell S.E.A., Simoes I.N., Jessop Z., Badiei N., Blain E., Whitaker I.S. Structural and mechanical characterization of crosslinked and sterilised nanocellulose-based hydrogels for cartilage tissue engineering. Carbohydr. Polym. 2019;212:242–251. doi: 10.1016/j.carbpol.2019.02.057. [DOI] [PubMed] [Google Scholar]
- 88.Lerouge S. In: Sterilisation of Biomaterials and Medical Devices. Lerouge S., Simmons A., editors. Woodhead Publishing; 2012. 5 - non-traditional sterilization techniques for biomaterials and medical devices; pp. 97–116. [Google Scholar]
- 89.Tipnis N.P., Burgess D.J. Sterilization of implantable polymer-based medical devices: a review. International journal of pharmaceutics. 2018;544(2):455–460. doi: 10.1016/j.ijpharm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 90.ISO . 2006. Sterilization of Health Care Products — Radiation — Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. [Google Scholar]
- 91.Mendes G.C., Brandão T.R., Silva C.L. Ethylene oxide sterilization of medical devices: a review. American journal of infection control. 2007;35(9):574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Rediguieri C.F., Sassonia R.C., Dua K., Kikuchi I.S., de Jesus Andreoli Pinto T. Impact of sterilization methods on electrospun scaffolds for tissue engineering. Eur. Polym. J. 2016;82:181–195. doi: 10.1016/j.eurpolymj.2016.07.016. [DOI] [Google Scholar]
- 93.Grasman J.M., O'Brien M.P., Ackerman K., Gagnon K.A., Wong G.M., Pins G.D. The effect of sterilization methods on the structural and chemical properties of fibrin microthread scaffolds. Macromol. Biosci. 2016;16(6):836–846. doi: 10.1002/mabi.201500410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.FDA . 2014. Sterilization Process Controls.https://www.fda.gov/sterilization-process-controls [Google Scholar]
- 95.Oryan A., Alidadi S., Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expet Opin. Biol. Ther. 2016;16(2):213–232. doi: 10.1517/14712598.2016.1118458. [DOI] [PubMed] [Google Scholar]
- 96.Qi C., Liu J., Jin Y., Xu L., Wang G., Wang Z., Wang L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104. doi: 10.1016/j.biomaterials.2018.02.016. [DOI] [PubMed] [Google Scholar]