Abstract
Objective
To measure the effect of a mobile integrated health community paramedicine (MIH‐CP) transitional care program on hospital utilization, emergency department visits, and charges.
Data Sources
Retrospective secondary data from the electronic health record and regional health information exchange were used to analyze patients discharged from a large academic medical center and an affiliated community hospital in Baltimore, Maryland, May 2018–October 2019.
Study Design
We performed an observational study comparing patients enrolled in an MIH‐CP program to propensity‐matched controls. Propensity scores were calculated using measures of demographics, clinical characteristics, social determinants of health, and prior health care utilization. The primary outcome is inpatient readmission within 30 days of discharge. Secondary outcomes include excess days in acute care 30 days after discharge and emergency department visits, observation hospitalizations, and total health care charges within 30 and 60 days of discharge.
Data Collection
Included patients were over 18 years old, discharged to home from internal/family medicine services, and live in eligible ZIP codes. The intervention group was enrolled in the MIH‐CP program; controls met inclusion criteria but were not enrolled during the study period.
Principal Findings
The adjusted model showed no difference in 30‐day inpatient readmission between 464 enrolled patients and propensity‐matched controls (adjusted incidence rate ratio = 1.19, 95% confidence interval [CI] [0.89, 1.60]). There was a higher rate of observation hospitalizations within 30 days of index discharge for MIH‐CP patients (adjusted incidence rate ratio = 1.78, 95% CI = [1.01, 3.14]). This difference did not persist at 60 days, and there were no differences in other secondary outcomes.
Conclusions
We found no significant difference in short‐term health care utilization or charges between patients enrolled in an MIH‐CP transitional care program and propensity‐matched controls. This highlights the importance of well‐controlled, robust evaluations of effectiveness in novel care‐delivery systems.
Keywords: care management, care transition, community paramedicine, health care utilization, mobile integrated health
What is known on this topic
Mobile integrated health and community paramedicine (MIH‐CP) programs are partnerships between paramedics, emergency medical services systems, and interdisciplinary health care teams that care for patients with complex medical and social needs in their homes and communities.
MIH‐CP programs vary widely in structure, focus, and training requirements.
Literature supporting MIH‐CP's effectiveness in assisting care transitions is limited, drawing mostly from evaluations of pilot programs that lack a control group or use poorly matched controls—techniques that may inaccurately overestimate program effect.
What this study adds
This observational study adds to prior literature by describing a robust and well‐controlled evaluation of an MIH‐CP care transition program in an urban environment.
We found no significant differences in 30‐day inpatient readmission, charges, excess days, emergency department visits, or hospital observations following hospital discharge among program participants compared to controls.
1. INTRODUCTION
The transition between hospital and home has been shown to be a crucial time in the care trajectory of illness. 1 , 2 , 3 There are significant financial incentives for hospital systems to assist with care transitions to avoid unnecessary hospital utilization and rehospitalization penalties. 4 While the optimal structure of care transition programs is uncertain due to mixed evidence, common aspects among effective programs include in‐home evaluation, multidisciplinary teams, and multifaceted intervention. 2 , 5 , 6 , 7
Maryland's Total Cost of Care All‐Payer Model, a capitated payment system for hospitals, incentivizes innovative models of care to reduce per capita costs and improve patient health outcomes. 8 , 9 One such model, mobile integrated health and community paramedicine (MIH‐CP), uses paramedics and emergency medical services systems to support the transition of patients with complex medical conditions from hospital to their homes and communities. MIH‐CP programs vary widely in structure, focus, and training requirements; however, successful programs are consistent in their ability to identify and fill gaps in community services, coordinating interventions to meet patients' medical and social needs. 10 , 11 However, the evidence base for such interventions on care transitions is mixed, and studies frequently use methods that either lack a control group or use poorly matched controls. Such techniques are subject to selection bias and regression to the mean. 7 , 12 , 13
The Baltimore City MIH‐CP program is a unique academic‐governmental partnership between the University of Maryland Medical Center (UMMC) and the Baltimore City Fire Department (BCFD). Patients are enrolled for the 30 days following hospital discharge, during which community paramedicine teams conduct home visits and coordinate interdisciplinary support to assist patients living in West Baltimore in their transition from hospital to home. By addressing complex social and medical needs, the program works toward its goals of reducing health care utilization and cost and improving quality of care. Patients served by the program are likely to have multiple chronic health conditions, lower health literacy, and more frequent social barriers to accessing preventive and acute health care services.
Initial findings from the program are promising. We found anecdotally that partnership with the BCFD, an institution that had a strong preexisting relationship of trust, engagement, and accountability with the community, has enabled more successful uptake of program services by the community compared to other transitional care programs that do not use emergency medical services personnel and do not perform home visits. After the first operational year, the risk‐adjusted readmission rate for enrolled patients is lower than those who are eligible but not enrolled in the program. The hospitalization rate for enrolled patients 30 days after hospital discharge is 72.2% lower than the 30 days prior. These evaluation methods, however, are subject to selection bias and regression to the mean effects. Stakeholders such as the medical center, medical system, and city and state government are interested in continued and more rigorous program evaluation.
The objective of this study is to conduct a rigorous analysis of the effect of the Baltimore City MIH‐CP transitional care program on cost and health care utilization using multiple data sources. We hypothesize the patients enrolled in the MIH‐CP program will have fewer 30‐day inpatient rehospitalizations, emergency department (ED) visits, excess days in acute care, and lower total health care costs compared to propensity‐matched controls.
2. METHODS
2.1. Study design, setting, and participants
This is a retrospective observational study comparing outcomes for patients enrolled in the MIH‐CP program to those of propensity‐matched controls discharged from hospitalization between May 1, 2018 and October 31, 2019. Our initiative targets all adult patients older than 18 years of age living in eligible West Baltimore ZIP codes (21201, 21216, 21217, 21223, 21229, and 21230), who are discharged to home from an inpatient or observation hospitalization on internal medicine or family medicine services at UMMC (a large tertiary care academic medical center) or University of Maryland Midtown Campus (a nearby, closely affiliated community hospital). In addition, patients must have stable housing so the field team can conduct home visits. The intervention group is defined as patients enrolled in the MIH‐CP program during the study period; patients with multiple enrollments in the MIH‐CP program were included in the intervention but only their first enrollment and corresponding index changes were analyzed. All patients enrolled in the program during the study period were excluded from the control population. Patients enrolled in the ED that were not admitted to the hospital were excluded from the study since they are likely different from patients recruited from hospital stays and would therefore require a different pool of controls. Patients are identified and referred to the program during interdisciplinary discharge rounds, during which inpatient physicians, administrators, social workers, case managers, and program community health workers (CHWs) identify eligible patients with complex medical and social needs likely to benefit from MIH‐CP support. Participation in the program is voluntary and patients must consent to enrollment (about 75% of patients approached accept enrollment). Program capacity (30–50 patients per month) may limit the number of eligible patients that can be offered MIH‐CP enrollment. Eligible patients already participating in other longitudinal care programs offering similar services were unlikely to be referred to MIH‐CP in order to prioritize patients that were in most need of support.
For our analysis, we identified patients enrolled in the program using records kept internally by the MIH‐CP program. Propensity‐matched controls were identified from the hospital‐based electronic health record (EHR) as adult patients living in the ZIP codes served by the program who were discharged to home (i.e., not to a nursing home or assisted living or rehabilitation facility) after an inpatient or observation hospitalization with an eligible service and were not enrolled in the MIH‐CP program during the study period. Patients that declined enrollment in the program were not excluded from the pool of potential controls. The unit of analysis is the discharge rather than the patient. There are 464 discharges in the intervention group and 5530 discharges in the control group that we used for final analysis (Figure 1).
FIGURE 1.
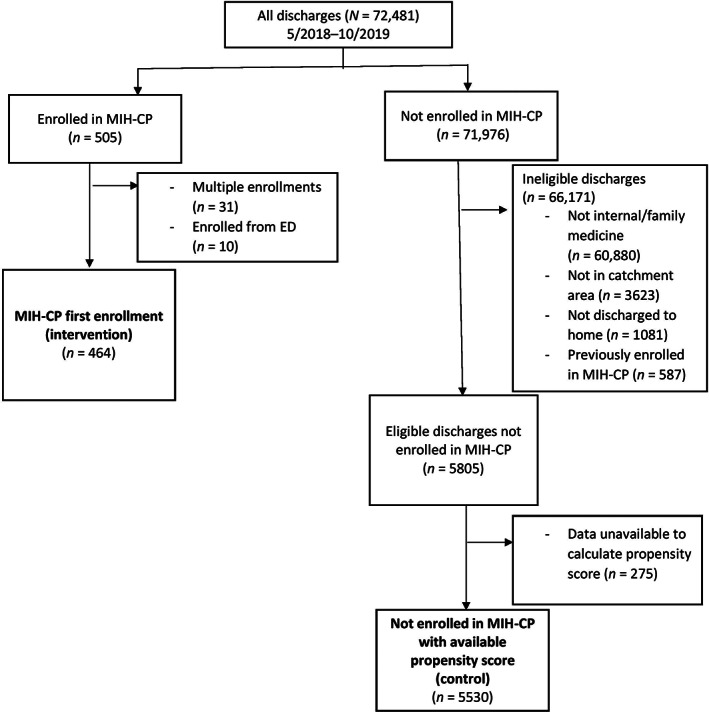
Study flow diagram for identifying intervention and control groups. ED, emergency department; MIH‐CP, Mobile Integrated Health Community Paramedicine program
2.2. Intervention
The Baltimore City MIH‐CP uses a multidisciplinary team of physicians, nurse practitioners, paramedics, nurses, pharmacists, social workers, and CHWs to provide tailored, patient‐centered support to individuals in their homes for 30 days after hospital discharge. CHWs enroll patients into the program, schedule home visits with the field team, and coordinate and execute the wide variety of activities needed to address or mitigate social and environmental needs. CHWs work with the discharging medical team and staff pharmacists to address medication‐related problems and with primary care providers and other disease specialists to schedule both new and follow‐up appointments. Specially trained community paramedics working under novel, state‐approved expanded practice protocols and the program's medical director (a physician) conduct a comprehensive screening of demographic information, self‐reported health, and social and medical needs on the first visit to a patient's home, as well as a real‐time medication reconciliation with the pharmacist over videoconference. Team members, including community paramedic field teams, use cutting‐edge health information technology—such as the hospital‐based EHR and videoconferencing/text messaging compliant with the Health Insurance Portability and Accountability Act—to communicate and to coordinate interventions in order to meet patients' medical and social needs. Community paramedics consult with advanced practice providers either in‐person or via telemedicine while performing in‐home assessments. Also involved in program design, evaluation, and administration are quality assurance professionals, a health economist, epidemiologist, biostatistician, and project managers. Needs are reassessed frequently during repeat home visits and patient contacts via telephone. A discharge visit at the end of the 30‐day program reassesses outstanding needs and self‐reported quality of life. It also provides an opportunity to extend enrollment if necessary or enact a “warm handoff” to the patient's a primary care physician, case manager, or other care teams for ongoing care and support.
2.3. Variables
Variables for building the propensity score model were abstracted for all patients from the EHR (see Appendix S1). Our propensity score model included patient demographics, diagnosis, number and type of comorbidities, calculated risk of readmission, employment, housing, proxies for social support, multiple measures of prior health care utilization and charges, medical service discharging the patient, the number of inpatient discharges by physician, and the relative frequency with which the inpatient discharging physician referred patients to the MIH‐CP program. Codes from the International Statistical Classification of Diseases, Tenth Revision, identifying diagnoses present on index admission were used to identify disease‐specific comorbidities and calculate the Charlson comorbidity index. 14 Risk of 30‐day potentially avoidable readmission was measured by internationally validated HOSPITAL score, which assigns weights to seven clinical variables and has been internationally validated to predict the risk of readmissions. 15 , 16 Outcome data for hospitalizations and ED visits, as well as data about prior health care utilization for the propensity score model, were obtained from our regional health information exchange, the Chesapeake Regional Informational System for Our Patients (CRISP). This data source captures ED and hospital utilization occurring outside our hospital system in Maryland and the District of Columbia. The primary outcome is number of inpatient hospitalizations within 30 days of initial hospital discharge. Secondary outcomes are ED visits, observation hospitalizations, and total inpatient and outpatient charges within 30 and 60 days after discharge, as well as excess days in acute care 30 days after discharge. Excess days in acute care is a weighted summation of days associated with ED visits, observation stays, and unplanned inpatient readmissions 30 days after discharge. 17 , 18 An additional secondary outcome was 30‐day unplanned inpatient readmission within 30 days of an initial inpatient hospitalization, as defined by the Centers for Medicare and Medicaid Services Hospital Readmissions Reduction Program. This outcome is of particular interest to our hospital partners as they face financial penalties associated with such revisits. This outcome only applies to patients whose index admission was inpatient and were identified by a special indicator in CRISP.
2.4. Analysis
Differences in patients' and physicians' characteristics between discharges in the intervention group and discharges in the control group were compared using Mann‐Whitney U test for continuous variables and chi‐square test for categorical variables.
Each index discharge in the intervention group was matched to a discharge in the control group by 1:1 nearest neighbor matching without replacement using propensity scores estimated using the variables described above and shown in the Appendix S1. The balance of individual predictors between the intervention and control groups before and after matching was examined using standardized differences. 19 Average treatment effect for the treated was used to compute the association of MIH‐CP on various outcomes of interest using the propensity‐score‐matched sample; 95% confidence intervals (CIs) and p values of average treatment effects for the treated were computed using a bootstrap method.
In addition, imbalance in some predictors is expected after propensity score matching, and the rule of thumb is that imbalance is acceptable if the absolute value of standardized differences between case and control groups is 10% or less. Covariate adjustment was conducted using the propensity‐score‐matched sample to further adjust for clinically important covariates or covariates with the absolute value of standardized differences greater than 10%. For the primary outcome (i.e., number inpatient readmissions within 30 days of discharge) and other count data (e.g., ED, observation visits), we used mixed‐effects Poisson or negative binomial regression to account for matching pairs and adjust for important covariates (e.g., prior health care utilization and charges). For continuous secondary outcomes (excess days in acute care and charges), mixed‐effects linear model was used. Analysis of the secondary outcome of 30‐day unplanned readmission was performed using the subgroup of enrolled and control patients whose index hospitalization was inpatient status, since only inpatient admissions can result in this outcome; therefore, it required a separate and de novo propensity matching but the same analysis plan was used.
Since our MIH‐CP program is new (began in May 2018), was piloted with a small number of patients, and changed rapidly in scope, structure, and function over its first 6 months of operation, its effectiveness may change over the study period. In order to evaluate for this, we performed a sensitivity analysis by stratifying cohorts by date of index readmission. Using mixed‐effects models similar to those described above, we replaced the binary group variable with a three‐level group variable (intervention enrolled before 2019 vs. intervention enrolled January 1, 2019 and later vs. control). Database creation and cleaning were performed using SAS version 9.4 (SAS Institute Inc). Statistical analysis was performed using Stata/SE version 16 (StataCorp LLC).
3. RESULTS
Out of 72,481 discharges identified during the study period, 505 (0.69%) were for patients enrolled in the MIH‐CP program, 464 (91.8%) of which were retained for analysis after removing duplicate enrollments and patients enrolled from settings besides hospitalization (e.g., the ED). The remaining 71,976 (99.3%) served as potential controls. Of these, 66,171 (91.9%) were excluded because they did not meet inclusion criteria for enrollment in the program. After excluding discharges that lacked the data necessary to calculate a propensity score, we identified 5530 discharges to include as potential matched controls. This process is shown in Figure 1.
Comparison between the 464 intervention discharges and 5530 control discharges is shown in Table 1. Compared to controls, patients enrolled in the MIH‐CP program were more likely to be older, female, and have a steady place to live, more comorbidities, and a higher body mass index. Their index admission was more likely to be at the larger academic center (due to recruitment protocols), be an inpatient admission, and have higher charges, and they were more likely to be taken care of by a physician with higher rates of referral to the program. Intervention discharges were less likely to be high utilizers of inpatient and ED services in the year prior to index hospital discharge, although overall mean total charges across all settings of care (ED, inpatient, and observation) were similar between groups. Common reasons for the index admission were bronchospastic disease, congestive heart failure, chest pain, and substance use (data not shown). Propensity matching of intervention and control discharges was successful. All observable characteristics did not differ significantly between propensity‐score‐matched groups (all p > 0.05); only two covariates had standardized differences slightly greater than 10% (% bias = −10.3 for employment, p = 0.08; % bias = −11.2 for charges in 30 days preceding discharge, p = 0.15), demonstrating effective matching (Appendix S1, Figure A).
TABLE 1.
Summary statistics by group
| Variable | Unenrolled (n = 5530) | MIH‐CP (n = 464) | Total (N = 5994) | p Value |
|---|---|---|---|---|
| Mean age ± SD, y | 56.46 ± 15.83 | 61.37 ± 14.36 | 56.84 ± 15.78 | <0.001 a , * |
| Female gender, # (%) | 2979 (53.87) | 279 (60.13) | 3258 (54.35) | 0.01 b , * |
| Mean hospital length of stay ± SD, d | 3.63 ± 4.43 | 4.95 ± 10.86 | 3.74 ± 5.23 | <0.001 a , * |
| Mean charges for index admission ± SD, $ | 12,706.26 ± 14,510.26 | 15,262.03 ± 18,558.18 | 12,904.1 ± 14,876.85 | <0.001 a , * |
| Inpatient admissions 365 days before discharge, # (%) | <0.001 b , * | |||
| 0–1 | 3817 (69.02) | 321 (69.18) | 4138 (69.04) | |
| 2–5 | 1209 (21.86) | 125 (26.94) | 1334 (22.26) | |
| >5 | 504 (9.11) | 18 (3.88) | 522 (8.71) | |
| ED visits 365 days before discharge, # (%) | 0.02 b , * | |||
| 0 | 1600 (28.93) | 156 (33.62) | 1756 (29.3) | |
| 1–9 | 3402 (61.52) | 278 (59.91) | 3680 (61.39) | |
| >9 | 528 (9.55) | 30 (6.47) | 558 (9.31) | |
| Mean charges 365 days before discharge ± SD, $ | 42,530.1 ± 73,661.22 | 39,423.18 ± 73,817.82 | 42,289.59 ± 73,671.87 | 0.63 a |
| Mean Charlson comorbidity index ± SD | 4.31 ± 3.83 | 4.45 ± 3.42 | 4.32 ± 3.8 | 0.04 a , * |
| Comorbidities, # (%) | ||||
| COPD/asthma | 2052 (37.11) | 176 (37.93) | 2228 (37.17) | 0.76 b |
| CHF | 1558 (28.17) | 173 (37.28) | 1731 (28.88) | <0.001 b , * |
| Diabetes | 2054 (37.14) | 215 (46.34) | 2269 (37.85) | <0.001 b , * |
| Substance abuse | 2905 (52.53) | 215 (46.34) | 3120 (52.05) | 0.01 b , * |
| HIV | 504 (9.11) | 45 (9.7) | 549 (9.16) | 0.74 b |
| Cancer | 713 (12.89) | 86 (18.53) | 799 (13.33) | 0.001 b , * |
| Hypertension | 4036 (72.98) | 366 (78.88) | 4402 (73.44) | 0.01 b , * |
| ESRD | 1210 (21.88) | 115 (24.78) | 1325 (22.11) | 0.16 b |
| Psychiatric disease | 2532 (45.79) | 231 (49.78) | 2763 (46.1) | 0.11 b |
| Cardiovascular disease | 531 (9.6) | 62 (13.36) | 593 (9.89) | 0.01 b , * |
| Race, # (%) | 0.55 b | |||
| Black | 4785 (86.53) | 405 (87.28) | 5190 (86.59) | |
| White | 648 (11.72) | 54 (11.64) | 702 (11.71) | |
| Other | 97 (1.75) | 5 (1.08) | 102 (1.7) | |
| Health insurance payer, # (%) | 0.03 b , * | |||
| Private | 443 (8.01) | 29 (6.25) | 472 (7.87) | |
| Medicaid | 2456 (44.41) | 182 (39.22) | 2638 (44.01) | |
| Medicare | 2547 (46.06) | 247 (53.23) | 2794 (46.61) | |
| Other/none | 84 (1.52) | 6 (1.29) | 90 (1.5) | |
| Housing: “I have a steady place to live,” # (%) | 4244 (76.75) | 414 (89.22) | 4658 (77.71) | <0.001 b , * |
| Index hospitalization status: inpatient (vs. observation), # (%) | 3039 (54.95) | 317 (68.32) | 3356 (55.99) | <0.001 b , * |
| Enrolled at UMMC # (%) | 3125 (56.51) | 411 (88.58) | 3536 (58.99) | <0.001 b , * |
| Mean BMI ± SD | 28.95 ± 8.49 | 30.48 ± 10.14 | 29.07 ± 8.64 | 0.01 a , * |
| Mean physician MIH‐CP referral rate ± SD | 0.07 ± 0.06 | 0.13 ± 0.07 | 0.08 ± 0.07 | <0.001 a , * |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ED, emergency department; ESRD, end‐stage renal disease; HIV, human immunodeficiency virus; MIH‐CP, Mobile Integrated Health Community Paramedicine program; SD, standard deviation; UMMC, University of Maryland Medical Center (large academic center).
Calculated using Mann‐Whitney U test.
Calculated using chi‐square test.
Denotes statistical significance at p < 0.05.
Results from the propensity score matching and mixed‐effects models are shown in Table 2. Prior to matching, the intervention group had a higher rate of inpatient readmission and higher charges, although none of these differences were statistically significant. In the final adjusted model, there was no difference in 30‐day inpatient readmissions between intervention and control groups (adjusted incidence rate ratio = 1.19 [0.89, 1.60]). In general, differences in secondary outcomes between groups narrowed after propensity score matching, and adjusted measures of association moved closer to the null. The adjusted model did show a higher likelihood of observation hospitalizations within 30 days of index discharge for MIH‐CP patients compared to matched controls (adjusted incidence rate ratio = 1.78, 95% CI = [1.01, 3.14]). However, this difference did not persist at 60 days, and there were no statistically significant differences in other secondary outcomes of cost or utilization between groups. This was also true of the subgroup analysis of patient with inpatient index hospitalizations examining the outcome of 30‐day unplanned inpatient readmission (unadjusted incidence rate ratio = 1.04, 95% CI = [0.75, 1.44]; adjusted incidence rate ratio = 0.93, 95% CI = [0.67, 1.30]).
TABLE 2.
Summary of MIH‐CP association with utilization and cost
| Outcome | MIH‐CP (n = 464) | Unenrolled (n = 5530) | Effect size | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference [95% CI] a | IR [95% CI] b | Adjusted IRR [95% CI] c | ||
| Inpatient admission | Before matching | 0.22 (0.54) | 0.20 (0.51) | 0.02 [−0.03, 0.08] | ||
| 30 days postdischarge | After matching | 0.22 (0.54) | 0.20 (0.48) | 0.02 [−0.04, 0.08] | 1.12 [0.85, 1.48] | 1.19 [0.89, 1.60] |
| Mean (SD) | Mean (SD) | Difference [95% CI] d | Adjusted difference [95% CI] | |||
|---|---|---|---|---|---|---|
| Excess days | Before matching | 1.14 (3.7) | 1.10 (3.73) | 0.04 [−0.32, 0.39] | ||
| In acute care e | After matching | 1.14 (3.7) | 1.18 (3.77) | −0.05 [−0.57, 0.48] | 0.01 [−0.44, 0.46] f | |
| Charges 30 days | Before matching | 7601.31 (24,596.2) | 5560.75 (16,027.99) | 2040.56 [−242.51, 4323.63] | ||
| Postdischarge, $ | After matching | 7601.31 (24,596.2) | 5965.34 (15,955.03) | 1635.97 [−1152.83, 4424.77] | 2033.22 [−497.32, 4563.77] g | |
| Charges 60 days | Before matching | 11,190.17 (29,460.81) | 10,177.28 (26,449.12) | 1012.89 [−1763.25, 3789.03] | ||
| Postdischarge, $ | After matching | 11,190.17 (29,460.81) | 10,449.37 (24,395.73) | 740.80 [−3416.54, 4898.25] | 1415.10 [−1823.99, 4654.19] g |
| Mean (SD) | Mean (SD) | Difference [95% CI] d | IRR [95% CI] h | Adjusted IRR [95% CI] | ||
|---|---|---|---|---|---|---|
| ED visits 30 days | Before matching | 0.30 (0.95) | 0.46 (1.91) | −0.16 [−0.26, −0.06]* | ||
| Postdischarge | After matching | 0.30 (0.95) | 0.48 (2.98) | −0.19 [−0.44, 0.06] | 0.79 [0.55, 1.14] | 0.81 [0.60, 1.10] i |
| ED visits 60 days | Before matching | 0.54 (1.29) | 0.83 (3.41) | −0.29 [−0.44, −0.14]* | ||
| Postdischarge | After matching | 0.54 (1.29) | 0.85 (5.89) | −0.31 [−0.80, 0.18] | 0.98 [0.72, 1.33] | 1.02 [0.80, 1.31] i |
| Obs 30 days | Before matching | 0.08 (0.29) | 0.07 (0.31) | 0.01 [−0.02, 0.04] | ||
| Postdischarge | After matching | 0.08 (0.29) | 0.04 (0.21) | 0.04 [−0.002, 0.07] | 1.85 [1.07, 3.19]* | 1.78 [1.01, 3.14] j , * |
| Obs 60 days | Before matching | 0.119 (0.38) | 0.122 (0.47) | −0.004 [−0.04, 0.03] | ||
| Postdischarge | After matching | 0.119 (0.38) | 0.084 (0.35) | 0.034 [−0.01, 0.08] | 1.41 [0.94, 2.13] | 1.35 [0.87, 2.09] j |
Abbreviations: CI, confidence interval; ED, emergency department; IRR, incidence rate ratio; MIH‐CP, Mobile Integrated Health Community Paramedicine program; Obs: hospital observation status; OR, odds ratio; RD, risk difference; SD, standard deviation.
Calculated as MIH‐CP – Unenrolled.
Calculated as MIH‐CP ÷ Unenrolled.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects Poisson regression model to account for matching pairs.
Calculated as MIH‐CP – Unenrolled.
Calculated as [Days of unplanned inpatient hospitalization + # hospital observations + 0.5*ED visits] within 30 days of hospital discharge.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; ED visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects linear model to account for matching pairs.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects linear model to account for matching pairs.
Calculated as MIH‐CP ÷ Unenrolled.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; ED visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects negative binomial regression model to account for matching pairs.
Adjusted for employment status, charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; observation hospitalizations in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects Poisson regression model to account for matching pairs.
Denotes statistical significance at p < 0.05.
Table 3 demonstrates the results from the sensitivity analysis, stratifying the cohorts to early (2018) and mature (2019) program periods. The early period showed higher likelihood of inpatient readmission, higher costs, and more frequent hospital observations for enrolled patients compared to controls, although none of these trends were statistically significant except for observation hospitalizations within 30 days of discharge. Beginning in 2019, there was no longer a trend toward increased 30 ‐day inpatient readmissions, and differences between groups narrowed, although again no association was statistically significant. However, it does imply that patient selection and program effectiveness improved over the study period.
TABLE 3.
Summary of MIH‐CP effect stratified by time of index hospitalization discharge
| Outcome | Effect size | ||
|---|---|---|---|
| IRR [95% CI] a | Adjusted IRR [95% CI] b | ||
| Inpatient admission | MIH‐CP enrolled before 2019 vs. unenrolled | 1.34 [0.92, 1.96] | 1.38 [0.95, 2.02] |
| 30 days postdischarge | MIH‐CP enrolled in 2019 vs. unenrolled | 1.01 [0.73, 1.40] | 1.09 [0.78, 1.53] |
| Difference [95% CI] c | Adjusted difference [95% CI] | ||
|---|---|---|---|
| Excess days | MIH‐CP enrolled before 2019 vs. unenrolled | 0.17 [−0.5, 0.84] | 0.21 [−0.42, 0.84] d |
| In acute care e | MIH‐CP enrolled in 2019 vs. unenrolled | −0.16 [−0.69, 0.37] | −0.09 [−0.59, 0.41] d |
| Charges 30 days | MIH‐CP enrolled before 2019 vs. unenrolled | 2531.45 [−1239.22, 6302.12] | 3036.44 [−533.48, 6606.35] f |
| Postdischarge, $ | MIH‐CP enrolled in 2019 vs. unenrolled | 1186.78 [−1797.57, 4171.13] | 1526.58 [−1305.89, 4359.04] f |
| Charges 60 days | MIH‐CP enrolled before 2019 vs. unenrolled | 1353.71 [−3541.73, 6249.16] | 2045.21 [−2525.43, 6615.86] f |
| Postdischarge, $ | MIH‐CP enrolled in 2019 vs. unenrolled | 433.36 [−3429.87, 4296.58] | 1096.88 [−2529.59, 4723.34] f |
| IRR [95% CI] g | Adjusted IRR [95% CI] | ||
|---|---|---|---|
| ED visits 30 days | MIH‐CP enrolled before 2019 vs. unenrolled | 0.77 [0.45, 1.33] | 0.73 [0.46, 1.15] h |
| Postdischarge | MIH‐CP enrolled in 2019 vs. unenrolled | 0.80 [0.53, 1.21] | 0.86 [0.61, 1.20] h |
| ED visits 60 days | MIH‐CP enrolled before 2019 vs. unenrolled | 1.02 [0.65, 1.59] | 0.98 [0.69, 1.40] h |
| Postdischarge | MIH‐CP enrolled in 2019 vs. unenrolled | 0.96 [0.68, 1.36] | 1.04 [0.79, 1.37] h |
| Obs 30 days | MIH‐CP enrolled before 2019 vs. unenrolled | 2.66 [1.39, 5.08]* | 2.26 [1.15, 4.45] i , * |
| Postdischarge | MIH‐CP enrolled after 2019 vs. unenrolled | 1.44 [0.76, 2.70] | 1.50 [0.78, 2.87] i |
| Obs 60 days | MIH‐CP enrolled before 2019 vs. unenrolled | 1.75 [1.03, 3.00]* | 1.49 [0.85, 2.61] i |
| Postdischarge | MIH‐CP enrolled after 2019 vs. unenrolled | 1.24 [0.77, 1.98] | 1.26 [0.77, 2.08] i |
Abbreviations: CI, confidence interval; ED, emergency department; IRR, incidence rate ratio; MIH‐CP, Mobile Integrated Health Community Paramedicine program; Obs, hospital observation status; OR, odds ratio.
Calculated as MIH‐CP ÷ unenrolled.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects Poisson regression model to account for matching pairs.
Calculated as MIH‐CP − unenrolled.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; ED visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects linear model to account for matching pairs.
Calculated as [Days of unplanned inpatient hospitalization + # hospital observations + 0.5*ED visits] within 30 days of hospital discharge.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; ED visits in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects negative binomial regression model to account for matching pairs.
Calculated as MIH‐CP ÷ unenrolled.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; inpatient visits in the 30 and 365 days preceding discharge; observation hospitalizations in the 30 and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects Poisson regression model to account for matching pairs.
Adjusted for employment status; charges in the 30, 60, 90, and 365 days preceding discharge; and amount charged at the index discharge using mixed‐effects linear model to account for matching pairs.
Denotes statistical significance at p < 0.05.
4. DISCUSSION
In a retrospective analysis, we found that the rate of 30‐day inpatient readmissions were similar between hospitalized patients enrolled in an MIH‐CP transitional care program and hospitalized propensity‐matched controls. We found a trend toward fewer ED visits but increased cost among program participants; these differences were not statistically significant. There was a small increase in hospital observations at 30 days after discharge among MIH‐CP patients, but this association did not persist to 60 days. Sensitivity analysis showed a trend toward improved effectiveness of the program in the latter portion of the study period (2019), although none of these associations were statistically significant.
Actionable conclusions from prior literature evaluating MIH‐CP programs are limited by study quality. 7 Many evaluations are of pilot programs and therefore have small sample sizes. Some studies report large effects on readmissions and/or cost, but frequently these evaluations either lack a control group and compare treatment groups to implausible counterfactuals, or have poorly matched controls that are subject to selection bias and regression to the mean. 7 , 12 , 13 Pre–post studies are the most common and are very susceptible to these effects. Evaluated this way, our program would demonstrate a 72.2% “decrease” in hospitalization rates in 30‐day periods before and after program enrollment. This result is likely in part because the index hospitalization is included in the “pre” period, and because hospitalizations tend to occur during peaks of individual patient health care utilization (patterns of health care utilization at the patient level are commonly cyclical).
Evaluations that have more appropriate control groups or are randomized often estimate smaller effects. This phenomenon was highlighted when the Camden “Hotspotting” project, a comprehensive primary care program that initially reported a large effect on costs using retrospective analysis, published results of a randomized controlled trial that showed no effect on primary outcomes. 20 Similarly, evaluating our program with contemporaneous and matched controls reduces the estimated effect. However, other randomized trials of care management programs do show positive results, highlighting the difficulty in generalizing findings from evaluations of such programs across settings and contexts. 21
Our retrospective study is primarily limited by selection bias due to unobserved confounders that may affect the risk of 30‐day inpatient readmission. Patients are not randomly assigned to the MIH‐CP intervention; there was likely a subjective component to the recruitment of patients by CHWs and interdisciplinary discharge teams. In addition, recruitment was performed only at certain times and days of the week. Therefore, it is possible that factors affecting patient recruitment were incompletely accounted for in our propensity score model, such as health‐related behaviors, social support, other care coordination support, health literacy, or time/day of discharge. We attempted to adjust for observable factors affecting the risk of readmission using a propensity score model that included of over 50 variables. Notably, we were able to incorporate only a few social factors in our model because these data are not routinely collected at our institution. Social factors may be related to risk of readmission as well as recruitment into the MIH‐CP program. Anecdotally, however, our team has found that we frequently identify needs during home visits that went unrecognized during the hospital stay. The lack of effective recognition of these needs in the hospital may reduce the role these factors play in confounding through selection bias unmitigated by propensity modeling.
The structure of our MIH‐CP program did evolve somewhat over time, and changes in utilization, evolving health care delivery models outside MIH‐CP, and in the program's function and efficacy could have resulted in variation in program effect over the study period. Since our sample size was limited by the convenience sample of patients enrolled, our study may have been underpowered to detect clinically significant differences, especially when stratified by early (2018) and mature (2019) program periods. If patient selection and operational effectiveness similar to the mature program period continue, a longer study with more patients may show a statistically significant improvement in our primary outcome. A modest but significant reduction in likelihood of post‐discharge ED visits might be observed with a longer study period of an operationally refined program. Repeat analysis after a longer program duration may allow for increased power and the ability to identify smaller but meaningful associations between program enrollment and decreased health care utilization or cost.
Despite the lack of observed impact on short‐term health care utilization outcomes, it is possible that our MIH‐CP program improves care quality and patient‐reported health outcomes. In general, patients' satisfaction with MIH‐CP programs has been shown to be high. 7 Pilot data from our program show improved patient‐reported health over the program's 30‐day course for enrolled patients, and high average satisfaction scores (9.75/10). 22 In addition to conducting analyses with sufficient power and methods to reduce selection bias, future work should examine the effect of MIH‐CP programs on patient‐reported outcomes including the ability to improve self‐care (patient activation); few studies have measured such outcomes compared to robust control populations. If MIH‐CP were shown to improve patient health and experience without significantly increasing cost or utilization, continuation of the program may be justified. In addition, incorporating more complete data measuring social determinants of health into propensity models may improve matching between recruited patients and controls. Consistent documentation and data collection regarding these determinants would enable further research to determine which social interventions have the greatest effect on health care utilization and individual health outcomes, and would inform more effective program development.
MIH‐CP programs are becoming increasingly common across Maryland and the United States, but the optimal structure and approach is not clear, to date, as evaluation methodology has been of poor quality. A robust, long‐term evaluation of a mature program informs the national discussion on the cost‐effectiveness of MIH‐CP for improving care transitions. The Baltimore City MIH‐CP program is similar to many other MIH‐CP programs in urban areas in the United States. Policy makers should be cautious to assume the cost‐effectiveness of similar programs and should encourage more robust evaluation, including randomized trials, of new and existing programs. Key stakeholders were hesitant to approve a randomized trial of our program due to a perception that there is a lack of clinical equipoise between MIH‐CP‐supported discharges and standard discharge care and an interest in providing services to as many patients as possible. Results from our study provide evidence that it may be reasonable to conduct a randomized trial comparing MIH‐CP to standard discharge care or another care coordination program in order to ascertain a more accurate measure of program effectiveness. We await the findings of randomized trials currently under way 23 , 24 , 25 ; similar trials in multiple sites would be helpful for a comprehensive evaluation of the MIH‐CP strategy across different contexts and perhaps support meta‐analysis.
5. CONCLUSION
This propensity‐matched observational study of an MIH‐CP transitional care program did not show a significant difference in 30‐day inpatient readmissions, charges, excess days in acute care, ED visits, or observations after hospital discharge among program participants compared to controls. However, it does inform a deeper understanding of our program and we plan to use the results to guide performance improvement strategies to optimally benefit patients. While limited power may have obscured small but meaningful associations, this study demonstrates the necessity of realistic comparisons with control groups accurately representing the counterfactual when evaluating care coordination programs to mitigate the pervasive effects of selection bias and regression to the mean. We encourage other MIH‐CP and care transition programs and funders to insist on rigorous evaluation methods, including randomized control trials, to provide evidence‐based guidance that can drive implementation of novel care‐delivery models to meet the Triple Aim and improve population health.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
The authors acknowledge support from the Baltimore City Fire Department (BCFD), particularly Mark Fletcher and Jim Matz, for leadership developing and operating the Mobile Integrated Health Community Paramedicine (MIH‐CP) program, and material support from Baltimore City, BCFD, and the University of Maryland Medical Center. In addition, we acknowledge Deborah M. Stein, ELS, for providing language and technical editing of the manuscript.
Financial support for the MIH‐CP program is provided by the Maryland Health Services Cost Review Commission. This funding provided partial salary support for Dr. Gingold, Dr. Liang, and Dr. Marcozzi.
Gingold DB, Liang Y, Stryckman B, Marcozzi D. The effect of a mobile integrated health program on health care cost and utilization. Health Serv Res. 2021;56(6):1146‐1155. 10.1111/1475-6773.13773
Funding information Maryland Health Services Cost Review Commission
REFERENCES
- 1. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure. Ann Intern Med. 2014;160(11):774‐784. 10.7326/M14-0083 [DOI] [PubMed] [Google Scholar]
- 2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review. BMC Health Serv Res. 2007;7:1‐19. 10.1186/1472-6963-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mistiaen P, Poot E. Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006. 10.1002/14651858.cd004510.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gai Y, Pachamanova D. Impact of the Medicare hospital readmissions reduction program on vulnerable populations. BMC Health Serv Res. 2019;19(1):1‐15. 10.1186/s12913-019-4645-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65(1):471‐485. 10.1146/annurev-med-022613-090415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688‐1698. 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregg A, Tutek J, Leatherwood MD, et al. Systematic review of community paramedicine and EMS Mobile integrated health care interventions in the United States. Popul Health Manag. 2019;22(3):213‐222. 10.1089/pop.2018.0114 [DOI] [PubMed] [Google Scholar]
- 8. Sharfstein JM, Stuart EA, Antos J. Global budgets in Maryland: assessing results to date. JAMA. 2018;319(24):2475‐2476. 10.1001/jama.2018.5871 [DOI] [PubMed] [Google Scholar]
- 9. Sharfstein JM, Kinzer D, Colmers JM. An update on Maryland's all‐payer approach to reforming the delivery of health care. JAMA Intern Med. 2015;175(7):1083‐1084. 10.1001/jamainternmed.2015.1616 [DOI] [PubMed] [Google Scholar]
- 10. Iezzoni LI, Dorner SC, Ajayi T. Community paramedicine – addressing questions as programs expand. N Engl J Med. 2016;374(12):1107‐1109. 10.1056/NEJMp1516100 [DOI] [PubMed] [Google Scholar]
- 11. Choi BY, Blumberg C, Williams K. Mobile integrated health care and community paramedicine: an emerging emergency medical services concept. Ann Emerg Med. 2016;67(3):361‐366. 10.1016/j.annemergmed.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Condino AE. The impact of community paramedicine programs on the health of rural communities in the United States and Canada: a systematic review. Ann Emerg Med. 2016;68(4 suppl 1):S71. 10.1016/j.annemergmed.2016.08.192 [DOI] [Google Scholar]
- 13. Thurman WA, Moczygemba LR, Tormey K, Hudzik A, Welton‐Arndt L, Okoh C. A scoping review of community paramedicine: evidence and implications for interprofessional practice. J Interprof Care. 2020;35(2):229‐239. 10.1080/13561820.2020.1732312 [DOI] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15. Robinson R. The HOSPITAL score as a predictor of 30 day readmission in a retrospective study at a university affiliated community hospital. PeerJ. 2016;4:e2441. 10.7717/peerj.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30‐day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496‐502. 10.1001/jamainternmed.2015.8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horwitz L, Wang C, Altaf FK, et al. Excess Days in Acute Care after Hospitalization for Acute Myocardial Infarction (AMI) (Version 1.0); 2015. https://qualitynet.cms.gov/files/5d0d38a8764be766b0102a14?filename=EDAC_MsrMthdRpt_AMI.pdf
- 18. Horwitz LI, Wang Y, Altaf FK, et al. Hospital characteristics associated with postdischarge hospital readmission, observation, and emergency department utilization. Med Care. 2018;56(4):281‐289. 10.1097/MLR.0000000000000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care Hotspotting ‐ a randomized, controlled trial. N Engl J Med. 2020;382(2):152‐162. 10.1056/NEJMsa1906848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powers BW, Modarai F, Palakodeti S, et al. Impact of complex care management on spending and utilization for high‐need, high‐cost Medicaid patients. Am J Manag Care. 2020;26(2):e57‐e63. 10.37765/ajmc.2020.42402 [DOI] [PubMed] [Google Scholar]
- 22. Seidl KL, Gingold DB, Stryckman B, et al. Development of a logic model to guide implementation and evaluation of a Mobile integrated health transitional care program. Popul Health Manag. 2020;24:275‐281. 10.1089/pop.2020.0038 [DOI] [PubMed] [Google Scholar]
- 23. Mi R, Hollander MM, Jones CMC, et al. A randomized controlled trial testing the effectiveness of a paramedic‐delivered care transitions intervention to reduce emergency department revisits. BMC Geriatr. 2018;18(1):1‐9. 10.1186/s12877-018-0792-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah MN, Hollander MM, Jones CMC, et al. Improving the ED‐to‐home transition: the community paramedic–delivered care transitions intervention—preliminary findings. J Am Geriatr Soc. 2018;66(11):2213‐2220. 10.1111/jgs.15475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drennan IR, Dainty KN, Hoogeveen P, et al. Expanding paramedicine in the community (EPIC): study protocol for a randomized controlled trial. Trials. 2014;15(1):1‐10. 10.1186/1745-6215-15-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


