Abstract
Objective
To assess the impact of interventions for improving the management of chronic obstructive pulmonary disease (COPD), specifically increased use of pulmonary rehabilitation (PR) on patient outcomes and cost‐benefit analysis.
Data Sources
We used the national Hospital Episode Statistics (HES) datasets in England, local data and experts from the hospital setting, National Prices and National Tariffs, reports and the literature around the effectiveness of PR programs.
Study Design
The COPD pathway was modeled using discrete event simulation (DES) to capture the patient pathway to an adequate level of detail as well as randomness in the real world. DES was further enhanced by the integration of a health economic model to calculate the net benefit and cost of treating COPD patients based on key sets of interventions.
Data Collection/Extraction Methods
A total of 150 input parameters and 75 distributions were established to power the model using the HES dataset, outpatient activity data from the hospital and community services, and the literature.
Principal Findings
The simulation model showed that increasing referral to PR (by 10%, 20%, or 30%) would be cost‐effective (with a benefit‐cost ratio of 5.81, 5.95, and 5.91, respectively) by having a positive impact on patient outcomes and operational metrics. Number of deaths, admissions, and bed days decreased (ie, by 3.56 patients, 4.90 admissions, and 137.31 bed days for a 30% increase in PR referrals) as well as quality of life increased (ie, by 5.53 QALY among 1540 patients for the 30% increase).
Conclusions
No operational model, either statistical or simulation, has previously been developed to capture the COPD patient pathway within a hospital setting. To date, no model has investigated the impact of PR on COPD services, such as operations, key performance, patient outcomes, and cost‐benefit analysis. The study will support policies around extending availability of PR as a major intervention.
Keywords: COPD, cost‐benefit analysis, decision support toolkit, discrete event simulation, health economics, patient flow modelling, pulmonary rehabilitation
What Is Known on This Topic
Prior studies showed the effectiveness of pulmonary rehabilitation, comparing patient outcomes, and costs.
The quantifiable impact of re‐designing COPD (chronic obstructive pulmonary disease) care has never been previously investigated.
No economic evaluation incorporating the lifetime earning approach (to monetize mortality) has been conducted using an operational model.
What This Study Adds
Practical application of discrete event simulation within COPD study as a novel methodology for improving activity, cost‐saving, and patient outcomes.
A unique comprehensive model to support practitioners for a better management of COPD services.
The study provides a guidance for key decision makers to implement the model to other services or adopt for other chronic diseases for economic evaluation purposes.
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the world's third deadliest disease after ischemic heart disease and stroke. 1 In England, COPD deaths increased by about 20% between 2001 and 2017. 2 Also, only a third of the United Kingdom (UK) COPD population (about 3.5 million people) are diagnosed. 3 The cost (direct and indirect) of COPD is about £1.9 billion/year, the figure rises to £48 billion when intangible costs (the monetization of pain, suffering, and mortality) are also considered. 4
COPD is classified as an ambulatory care sensitive condition (ACSC), where hospitalization due to exacerbation can, at least in part, be prevented by providing effective primary care, such as outpatient or community services. 5 While the UK has the lowest rate of avoidable admissions for other chronic conditions, such as congestive heart failure (CHF) and diabetes, the rate for asthma and COPD is about 25% higher than the EU average. 6
Hospital performance in preventing emergency hospitalization and reducing length of stay (LOS) is a priority for the National Health System (NHS) in the UK. 7 Pulmonary Rehabilitation (PR) is known to have a positive impact on patient outcomes in COPD, including exacerbation, mortality rate, and admissions 8 as well as on anxiety and depression. 9 Outpatient PR considerably decreases the usage of health resources in COPD patients 10 and thus is prioritized in the current NHS long‐term plan. 11
2. BACKGROUND AND OBJECTIVES
Discrete event simulation (DES) is widely applied in health care tackling key issues around scheduling, 12 patient flow, 13 planning of hospital departments, 14 , 15 resource/capacity allocations, 16 economic evaluation, 17 and screening. 18 Moreover, DES has been used to improve patient pathways or disease management, for example, stroke, 19 cataract, 20 depression, 21 diabetes, 22 HIV, 23 Parkinson's disease, 24 and prostate cancer. 25 The method provided a safe environment to increase service quality, better understand disease and reduce waiting times.
Also, it enabled services and clinicians to capture the health care setting at a certain degree of accuracy and track patients individually. Thus, the heterogeneity in patients (eg, age, disease stage, lifestyle) can be reflected which influences probability of the events. 26 Various what‐if scenarios can be tested for the use of decision making with more reliable and comprehensive outputs.
Studies have showed the effectiveness of PR in different delivery forms on patient outcomes and costs. 27 Outpatient PR program was found to be cost‐effective in terms of cost per quality‐adjusted life year (QALY) and providing cost‐savings in hospital activities. 28 Golmohammadi and colleagues showed that community‐based PR improves the health status of patients and reduces total direct costs via comparing real data before and after the program. 29 Similarly, an inexpensive PR program was found to be effective to improve parameters related to quality of life. 30
Gillespie et al 31 demonstrated that PR is cost‐effective considering the Chronic Respiratory Questionnaire scores, but no evidence was found when considering QALYs gained. Their analysis included the costs of the intervention (PR), health care services, and patient. Moreover, Atsou et al 32 found PR to be cost‐effective using a multi‐state Markov model considering QALY and disease costs.
On the other hand, a novel tele‐rehabilitation project in Denmark, which aims to enable COPD patients to do exercises at home and carry out self‐monitoring, was found as more cost‐effective than the traditional way via the cost‐utility analysis. 33 A decision‐tree model evaluated different delivery methods of early PR in the UK. 34 The combination of home and hospital PR had the highest cost‐effectiveness compared to hospital PR or home PR. Also, all these interventions had better results against the usual care in terms of QALYs and costs.
Another trial in the UK demonstrated that community PR had similar effectivity as hospital PR, where phone follow‐up was found to be beneficial (with a moderate cost) in the community only. 35 Recently, a cost‐utility study showed home PR is a cost‐effective alternative for those who are not able to attend PR centers. 36
2.1. The need for an operational model
Most health care modeling studies carried out deterministic cost‐revenue calculations. The calculations do not vary depending on length of stay, admission type (first or recurrent), treatment procedure, etc. Moreover, there are a limited number of innovative applications of economic evaluation to simulation technique, whereas most applied classic methods for the assessment, cost per QALY gained (or per saved lives, averted cases), or cost‐revenue calculations. In addition, a modeling method or scenario for the readmission issue have not proposed or considered.
On the other hand, there are very few modeling studies considering PR. The quantifiable impact of re‐designing COPD services, for example, in the form of increasing the number of patients referred to PR, has never been previously studied. There are no known models that capture individual patient pathways within COPD services that track the movement of COPD patients in hospital and evaluate the operational and economic aspect of the disease. Also, current studies have not tested the practical impact of innovative strategies or policies, such as workforce planning, reducing patient readmissions, and integration of COPD care. As we simulate the patient pathway across a hospital setting, we can estimate and evaluate the impact of intervention(s) comprehensively on many metrics, including patient outcomes, resource utilization, and financial implications of change.
We, therefore, developed a DES model, that captures COPD treatment pathways and service configurations within a hospital setting, in conjunction with health economic modeling. For the first time, our model will enable key decision makers to assess, (a) the operational impact of changes and policies in COPD care, such as increased use of PR (eg, bed usage, staff hours, readmission), (b) the effectiveness of interventions on patient outcomes (eg, quality of life (QoL), emergency admission, mortality), and (c) evaluate the cost‐effectiveness of PR using a health economic model known as lifetime earnings approach.
3. METHODS
3.1. Conceptualization of COPD patient pathway
In collaboration with the COPD team at Royal Free London (RFH) NHS Foundation Trust (a major specialist provider in London) and the local community COPD services, run by Central and North West London (CNWL) NHS Foundation Trust, the COPD patient pathway was conceptualized. The hospital pathway includes outpatient, inpatient and accident and emergency (A&E) departments along with disease progression (see Figure 1). The team was made up of specialist consultants, nurses, physiotherapists, service manager, and experts in health care analytics and simulation programmers. Numerous interviews and meetings were organized to better understand the patient pathway and operational processes and resources consumed within all services.
FIGURE 1.
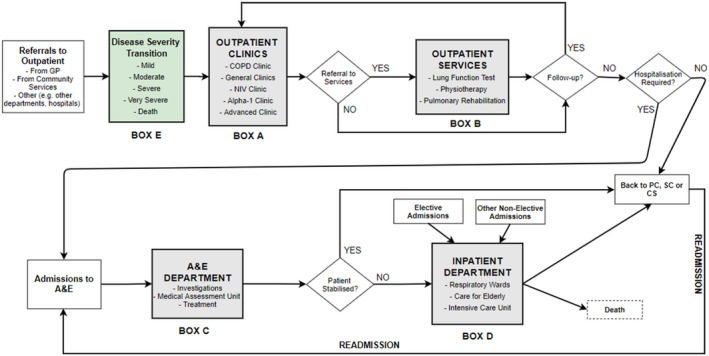
High‐level conceptualization of COPD patient pathway
In outpatient services (see box A in Figure 1), patients are initially first seen by a health care assistant or nurse. Then, they are typically seen by a physician in one of the clinics, be that general or specialist. Patients are mostly given a follow‐up appointment for a time typically six months.
Patients can also be referred to further outpatient services (see box B), that is, physiotherapy, PR, and lung function testing. The PR program includes 16 sessions taking place twice a week for 8 weeks, where patients are assessed before and after the program. However, other outpatient services take place generally once.
Some patients frequently attend A&E due to acute exacerbations or other reasons (see Box C). They are pre‐assessed by a nurse, then seen by a doctor. After the required assessments and examinations in A&E, patients who are not stabilized are admitted for inpatient care. According to our analysis of the national dataset, around 80% of COPD‐related admissions to inpatient departments are made through A&E. 37
COPD‐related inpatient admissions can be elective (very few cases, around 5%) and nonelective (ie, unplanned, through A&E or other). Patients are mostly bedded in a respiratory or Care of the Elderly ward (if they are 80 years or more; see Box D). Also, they may be transferred to the intensive care unit in case of being more critically unwell. Patients may move between units and wards while they stay within inpatient services.
COPD patients stay in wards for a period (a median LOS of 4 days and interquartile range between 2 and 7 days at the national level 38 ). After the inpatient stay, patients may return to community (eg, primary or secondary service) or leave the pathway due to death. Note that in some cases, patients are readmitted back to inpatient departments (through A&E) after a period of time after discharge. The pathway has many stochastics events and decisions. Therefore, distributions were employed to reflect the variation in the reality (eg, referral to outpatient services, LOS in the wards, probability of readmission).
3.2. Patient readmissions
Patients can be admitted to hospital on multiple occasions over a period of time (LOS in the community) as COPD is a chronic condition. According to the national dataset, 63% of all COPD emergency inpatient admissions in England are caused by 33% of COPD patients. 37 The 30‐day readmission rate in COPD admissions is approximately 15% in the hospital.
Therefore, a dynamic approach is adopted in the modeling of inpatient stays to track patients’ journeys (see the arrow named as Readmission in Figure 1). Depending on the reason of admission (ie, primary diagnosis), patients are assigned a probability for readmission. For readmitted patients, a time value for LOS in the community (in days) is assigned using the distribution. Thus, the impact of interventions on emergency readmissions can be tested accurately via this approach.
3.3. Disease severity progression
The pathway includes the course of the disease, namely mild, moderate, severe, and very severe, based on Global Initiative for Obstructive Lung Disease (GOLD) classification. 39 Disease severity transition happens one stmoderate or severe to very severe (see box E in Figure 1). Death can occur at any type of severity.
A multidimensional array was formed for each age group to capture the disease progression. The probability ratios for moving to the next disease stage in the input matrices of transition (see Table A2 in Appendix S1) were derived from Atsou et al's study. 40 Cycle time is assumed as 1 year. 40
3.4. Data sources and input parameters
Some input parameter values were established using the national Hospital Episodes Statistics (HES) dataset, and the rest were obtained from the literature, local data, and local experts (ie, the COPD team at RFH/CNWL). The HES dataset contains detailed records of all patients (more than 250 columns of data) admitted to care providers in England and is released each year by the Department of Health (DoH). The records contain information about patient characteristics (eg, age, sex, and location of residence), clinical details (such as diagnoses, operative procedure codes, and specialty), and administrative details (dates and methods of admission and discharge, and referrer). 41
An extensive data analysis (ie, cleaning, preparation, extraction) were carried out using Microsoft SQL Server. Patient ID and characteristics as well as clinical and administrative details were the variables of our interest. After cleaning the data, COPD‐related records at RFH were retrieved using International Classification of Disease (ICD‐10) codes J40‐J44, and the records were matched in each separate data file. ICD coding from COPD and COPD admissions is complex, and the approach using all J40‐J44 codes is imperfect but consistent with previous studies in the field. 42 , 43 , 44 , 45 , 46
A total of 150 input parameters were needed to run the model, as the model simulates all aspects of the COPD patient pathway. Inputs for the model include demand, pathway‐related parameters (eg, mix of resources, treatment times, number of follow‐ups), the course of the disease, costs, and revenues. Input details are given in Appendix S1 in Table A1. Note that the end‐users of the model, such as service providers, can alter the input parameters according to their hospital settings and patient demographics.
Around 75 distributions, which are the crucial part of stochastic simulations, were established using the available data to ensure variability and uncertainty is captured to a sufficient level of detail (in operational research this is known as a stochastic simulation). Time‐related activities are known to have a significant impact on day‐to‐day operations of systems and services (particularly around capturing variation), such as waiting time for appointments, LOS in hospital, and treatment times. There is also a huge variation in LOS in the community. For instance, a patient could be readmitted after 10 days of being discharged from hospital, whereas another after 60 days. Therefore, statistical distributions must be determined using real data to reflect reality and stochasticity of the service. Other essential variables include referrals to services, follow‐up/discharge for outpatient department, which were varied depending on the clinic and patient type.
Some distributions were estimated for different groups (clinic type, primary diagnosis, admission type) separately to get more accurate outputs and increase reality. For instance, distributions for financial tariff codes, length of stay, risk of readmission, and length of stay in the community were differentiated for each primary diagnosis codes (ie, J40‐J44).
Cost and revenue inputs were mainly taken from the data published by NHS England (National Prices and National Tariffs) and Personal Social Services Research Unit (PSSRU). PSSRU is used to calculate the cost of health care professionals (eg, consultant, nurse, physiotherapist).
3.5. Health economic model with lifetime earnings approach
Alternative strategies and interventions have various effects on performance indicators, for example, change in hospitalization, LOS, QoL, mortality rates, costs, and staff hours. A comprehensive health economic model that compares costs (direct and indirect) and benefits of treatments is needed to assess the impact of scenarios. Unlike previous studies in the literature, our study considers mortality costs using a lifetime earning approach and morbidity costs (monetary value of QALY) of the disease in the analysis. Here, calculation of mortality costs (known as lifetime earnings and value of life), direct and indirect cost of COPD, new intervention cost, and morbidity cost (the monetary value of QALY) are explained in greater detail.
Valuing of a statistical life is used in the cost‐benefit analysis of policies affecting health and safety of individuals. 47 The human capital approach and the willingness to pay approach are the main methods used for estimating mortality costs. 48 In the literature, the lifetime earnings were used to calculate the loss of lifetime earnings by comparing the number of deaths with and without the intervention. 49 , 50 , 51
For COPD, we adapted the model developed by Hussey et al 50 to estimate the potential lifetime earnings of individuals with COPD (who died prematurely). Thus, we calculated the loss of lifetime earnings by comparing the mortality cost in instances with and without the intervention (eg, increased use of PR). The formula with its variables (Table A3) for calculating lifetime earnings is presented in Appendix S2.
Moreover, Thomas and Hussey et al used a generic survival rate in the equation without considering patient types. 49 , 50 However, COPD increases the risk of mortality and reduces life expectancy, 52 and the ratio varies for each disease severity group (mild, moderate, severe, very severe). Therefore, the probabilities of survival (see Table A4 in Appendix S2) were calculated for each patient type (disease severity and age) separately using the data from Atsou et al. 40
Secondly, the COPD annual cost includes direct costs (A&E admission, hospitalization, outpatient care, services, drugs, equipment, therapies) and indirect costs (disability pensions and absence from work). The annual drug and indirect costs (for each disease severity) in Jansonn et al's study were adjusted to the simulation run year. 53 Thirdly, the cost of new intervention was calculated, which arises from more referral to PR as the tested scenario in the study. The value for PR cost by Griffiths et al 28 was adjusted for inflation as the cost input.
Patients lose QALY due to worsening of health condition, for example, exacerbation. 54 On the other hand, interventions like PR increase the QALY value of patients. 32 Therefore, patients’ QoL was transformed into capital as cost of morbidity (the monetary value of QALY) for cost‐benefit analysis. The estimated monetary value of a QALY in terms of the willingness to pay (WTP) is 63 668 GBP. 55
3.6. The simulation model
A DES model representing the conceptual model was developed using Simul8 simulation software. As this is an operational level simulation, the model was run for a period of 3.5 years (including a warm‐up period of 0.5 years). The COPD patient pathway was verified by the COPD team via meetings and validated by comparing the known data in the actual care system with the simulation results. Detailed information about the model development is provided in Appendix S3 with the model snapshot.
4. RESULTS
4.1. Experimentation
In this section, the tested policy (referring more patients to PR) is described. The PR program offers education about living with COPD and physical exercises. It has a real potential of reducing admission, mortality, length of stay and positive patient outcomes, such as exercise capacity, QoL, and disease symptoms. 56
The simulation model was integrated with the inputs from a national report (in England and Wales) to mimic the effect of PR on outcomes (see Table A1). 8 The report showed that admission rates, bed days, and mortality rates (within 180 days after referral to PR) are higher in patients who were assessed but did not enroll to PR and who enrolled but did not complete PR compared to those who were assessed, enrolled and completed PR. Thus, the variation in the rates and bed days (eg, those who completed their PR program versus those who do not complete) were captured.
Three different scenarios and volumes of referral were tested as recommended by the COPD team. The impact of referring 10% (Scenario 1), 20% (Scenario 2), and 30% (Scenario 3) more patients to PR were evaluated in terms of operational and health economics aspects. The model considers the enrolment rate and completion rate, 57 which are the subjects of concern for the PR program, as there are multiple sessions for patients to attend. In addition, patients may stop the program model due to a need for an emergency admission or stay.
4.2. Model outputs
Our DES model is able to produce variety of results around operational and patient outcome. The key results of baseline and policy scenarios (referring more patients to PR) are presented and explained accordingly.
Firstly, referring more patients to PR (by 10%, 20%, and 30%) resulted in an increase in PR, as expected (see Table 1). In the baseline, 485 patients among 1540 patients (the COPD population during the simulation period) were referred to the PR program, and 204 patients completed the program. The number of patients referred to PR increased to 635 (Scenario 3), and completed cases increased to 267 patients (around 30% increase). The resource use (eg, staff, venue) in PR also increased accordingly for each scenario.
TABLE 1.
PR activity results
| Number of Patients | Baseline | Scenario 1 (10% increase) | Scenario 2 (20% increase) | Scenario 3 (30% increase) |
|---|---|---|---|---|
| Referred to PR | 485 (461, 509) | 535 (508, 562) | 585 (556, 614) | 635 (609, 661) |
| Assessed for PR | 335 (319, 351) | 369 (351, 388) | 404 (384, 423) | 438 (420, 456) |
| Enrolled to PR | 286 (273, 300) | 316 (300, 331) | 345 (329, 362) | 375 (360, 389) |
| Completed PR | 204 (194, 213) | 225 (214, 236) | 246 (234, 258) | 267 (256, 277) |
Figures in brackets are the 95% confidence intervals.
Abbreviation: PR, pulmonary rehabilitation.
The tested scenarios provided a small but important reduction in the number of admissions, death, and bed days (see Table 2). In addition, the increase in the number of patients completing PR sessions and the reduction in hospitalizations had positive impacts on total QALYs of patients. In the baseline scenario, 1098 inpatient admissions utilized 6284 bed days, and 175 patients died over the simulation period of 3 years. In comparison, the policy could potentially save the life of 3.56 people (in a COPD population of 1540), avoid 4.9 admissions and 137 bed days in addition to gaining 5.53 QALY value (see Scenario 3).
TABLE 2.
The impact of increased use of PR on patient outcomes
| The change against baseline | Scenario 1 (10% increase) | Scenario 2 (20% increase) | Scenario 3 (30% increase) |
|---|---|---|---|
| Change in QALYs | +1.98 | +3.28 | +5.53 |
| Change in No of Admissions | −1.63 | −3.27 | −4.90 |
| Change in Bed Days | −45.77 | −91.54 | −137.31 |
| Change in Deaths | −1.19 | −2.37 | −3.56 |
Abbreviations: PR, pulmonary rehabilitation; QALYs, Quality‐adjusted life years.
The benefits may seem to be small, but it is from only one hospital/district. There are 195 PR services COPD patients in the UK, which mean that the intervention at the national level could potentially have a major impact on admissions (ie, preventing 100s of admissions as well as freeing 1000s of beds). Similarly, other positive effects, for example, QALYs, could be applicable after disseminating or introducing the policy at the local and national level.
4.3. Cost‐benefit analysis
Here, the cost‐benefit analysis‐oriented results of the scenarios are presented with the explanation of the calculations. Mortality cost, which considers the expected earnings (ie, wage and pension) of a patient until a certain age, if s/he stayed alive, are calculated using the formula for lifetime earnings mentioned above. As a result of the PR intervention, the cost of death to society reduced to £684 340 (Scenario 3) from £697 926 (baseline) as fewer patients died (see Table 3). Similarly, there is a slight decrease in cost of the disease, including direct and indirect costs, from £14 371 399 to £14 349 245.
TABLE 3.
The monetary results of the scenarios
| Categories | Baseline | Scenario 1 (10% increase) | Scenario 2 (20% increase) | Scenario 3 (%30 increase) |
|---|---|---|---|---|
| Mortality cost | £697 926 | £693 205 | £688 749 | £684 340 |
| Cost of disease | £14 371 399 | £14 363 700 | £14 356 434 | £14 349 245 |
| Decrease in morbidity cost a | — | £125 874 | £208 787 | £351 772 |
| Decrease in mortality cost a | — | £4722 | £9178 | £13 586 |
| Decrease in cost of disease a | — | £7699 | £14 965 | £22 154 |
Against baseline.
Total QALYs of the patients were compared between the baseline and the selected scenarios. For example, after scenario 2, about 3.28 QALYs were gained against baseline (see Table 2). Thus, the decrease in morbidity cost was found as £208 787 in Table 3 by simply multiplying the increase in QALYs with the monetary value of a QALY (3.28 × £63 668 = £208 787).
Then, avoidable disease cost was calculated to see the net effect and benefit of the scenarios (see Table 4). The avoidable disease cost means that the amount of the costs associated with the management of COPD patients that could have been saved by implementing the scenario. It is estimated by summing the decrease in mortality cost, cost of the disease, and morbidity cost against the baseline for each scenario. For example, the avoidable cost of COPD disease was found to be £232 930 in Scenario 2 (20% increase), illustrating the overall reduction in total avoidable disease costs. This represents the potential benefits of the new policy.
TABLE 4.
Cost‐benefit analysis of the scenarios
| Categories | Scenario 1 (10% increase) | Scenario 2 (20% increase) | Scenario 3 (%30 increase) |
|---|---|---|---|
| Avoidable disease cost | £138 294 | £232 930 | £387 512 |
| New intervention cost | £23 786 | £39 119 | £65 574 |
| Total net benefits | £114 508 | £193 810 | £321 938 |
| Net benefits per patient | £74.32 | £125.79 | £208.94 |
| Benefit‐cost ratio | 5.81 | 5.95 | 5.91 |
Benefit‐cost ratio values are indicated in bold.
On the other hand, new intervention cost indicates the cost of the policy due to the increase in the usage of PR services (staff, equipment, venue, etc). New intervention cost increased as more patients are referred to PR for Scenario 1, 2, and 3 from about £23 000 to £65 000. Total net benefits are calculated by subtracting new intervention cost from the avoidable disease cost. For example, total net benefits for Scenario 2 are £193 810. The cost of the intervention clearly outweighed by its benefit for all scenarios.
Next, total net benefits were divided by the number of COPD patients in the system/hospital. The net benefits per patient were estimated as £125.79 (for Scenario 2). Lastly, the scenarios were evaluated, via benefit‐cost ratio (BCR), if it is worthwhile (cost‐efficient) for implementation. The ratio is calculated by dividing the avoidable disease cost by new intervention cost, thus 5.81, 5.95, and 5.91 for the scenarios, respectively. As the resulting numbers were greater than one, the policy was found to be cost‐effective for all scenarios.
As a result, the health economic model proved that all tested scenarios are cost‐effective and provide benefit to patients and society. Sensitivity analysis, where the selected variables in the model were changed, is given in Appendix S4. The BCR is substantially higher than 1 in all scenarios and in sensitivity analysis, even if more usage of PR has a small effect on mortality, admission, and length of stay.
5. DISCUSSION
PR is an evidence‐based intervention known to improve outcomes in COPD, both in meta‐analysis of randomized controlled trials 9 , 10 , 28 , 30 , 31 and in real‐world data from the UK national audit program. 8 NACAP data also highlight inadequate referral, both from primary care and following a hospitalized exacerbation. 8 Improved access to PR is a component of the current NHS long‐term plan. Increasing referrals to PR will increase demand on PR services, which will therefore require expansion and the case must be made at the payer level (eg, Clinical Commissioning Group (CCG) in England).
For the first time, we developed a detailed model for COPD services across secondary and community care, with input parameters that can be adjusted to local data. In comparison with other cost‐benefit analysis relating to PR, this study evaluated the effectiveness using a stochastic approach, developing a novel operational level simulation model. The model then integrated a comprehensive health economic model that calculates the expected net benefits of strategies as well as the direct and indirect costs of COPD.
The health economic model consists of an adapted version of a combined mathematical equation that calculates the expected lifetime earnings of individuals in case of death. Note that the lifetime earnings approach was embedded within a simulation model for the first time. For COPD context, the equation was updated, and the value of the notions was calculated. Furthermore, the financial worth of morbidity was estimated based on the monetary value of a QALY.
A specific strategy (increased referral to PR), which improves patient outcomes and reduces the risk of mortality, admission, and LOS, was chosen to carry out a cost‐benefit analysis using the simulation model. Each scenario was found to be cost‐effective and associated with improvement in patient and operational outcomes, having a BCR substantially higher than 1 (at least 5). The intervention benefits society by raising QoL and life expectancy, as well as reducing the burden on patients and society, and releasing medical resources to other conditions. The scenarios were still cost‐effective after sensitivity analyses. The cutoff value of the PR program was found to be more than five times higher than the current cost.
The results showed that more usage of PR represents good use of NHS resources for people with COPD. The model will enable COPD services to assess the impact of increasing PR usage not just on patient outcomes (which is well documented), but on resource usage too, for example, on admission, readmissions, and bed days.
More importantly, the scenarios inform capacity planning, resource re‐allocations, planning, and scheduling activities to cope with the demand to PR. Thus, it requires financial investment, for example, in nurses, physiotherapist, physical space, administrative, and planning activities. In the study, assumptions on key measures, such as demand, resource usage, disease severity transition and mortality rate, budgeting, were mainly avoided by using real historical data (HES), data from the hospital setting, and the experiments and reports in the literature.
The power of DES is the ability to capture the full patient pathway under uncertainty with a variety of constraints (eg, resources, capacity, waiting time, and queuing). The processes only start when all the required resources are available. COPD services are highly complex with many variables/constraints, and the ability to deal with complex constraints simply does not exist in any statistical model or other modeling approaches. National Institute for Health and Care Excellence has also recognized DES as a valid methodology simulating complex patient pathways such as COPD. 58 , 59
Our model captured demand, resource, capacity, disease progression, readmission dynamics, and financial implications, not just on one aspect of the service but in its entirety (outpatient, A&E, and inpatient services). Thus, we have the power to test the knock‐on effect of an intervention (or series of interventions simultaneously). Although the tested scenarios have a difference by a fixed rate (10%, 20%, and 30% increase), the monetary results and cost‐benefit analysis (see Tables 3 and 4) presented variation and stochastic effect on each scenario. Moreover, this model reflected a fluctuation in BCRs, illustrating that Scenario 2 (20% increase) is a better option with a higher BCR than Scenario 3 (30% increase), that is, 5.95 and 5.91, respectively. These estimates demonstrate the necessity of a stochastic operational model as well as its distinction compared to statistical or Excel‐based models.
This model is useful for assessing the impact of changes that have short/long‐term effects on patients. It could equally be well applied to alternative scenarios, such as vaccination, early diagnosis/screening, or more specialized interventions such as lung volume reduction, which are also associated with mortality and/or morbidity. Even a small change in the outcomes can provide a remarkable benefit to society and patients. This hidden information can be revealed with the usage of health economic model.
In case of data availability, the benefits of PR and the rates (attendance and completion) can be adjusted depending on patient`s age, disease severity, and disease history. Also, the possibility of service cancelation (eg, due to an epidemic, sudden unavailability of staff or venue) can be easily included in the model.
Finally, the study provided detailed and useful quantitative information which could be used to support evidence‐based decision making processes for changes in the policies at the local level (hospital or CCGs) or in the wider context (NHS or DoH). DES with HES is not widely used for cost‐benefit analysis purposes, yet this study has demonstrated that useful information can be extracted for decision makers and health services to assess the efficiency and the cost‐effectiveness of interventions in terms of re/admissions, mortality, morbidity, and LOS. The approach can be adapted to other diseases or conditions. In summary, the model will provide evidence and help decision making process in the event of possible policy changes at local or national level.
Supporting information
Author matrix
Appendix S1‐S4
Figure S1
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: We would like to thank the following health care professionals for allocating their precious time and valuable input to build the COPD pathway: Dr Swapna Mandal, Stephen Byrd, Anthony Geraets, Ailsa Carmichael, Lisa Wordsworth, Abigail Greenwell (all Royal Free London NHS Foundation Trust); Travis Edwards, Bianca Nwaneri (all Central and North West London NHS Foundation Trust).
Yakutcan U, Demir E, Hurst JR, Taylor PC, Ridsdale HA. Operational Modeling with Health Economics to Support Decision Making for COPD Patients. Health Serv Res. 2021;56:1271–1280. 10.1111/1475-6773.13652
REFERENCES
- 1. WHO . Chronic respiratory diseases ‐ Chronic obstructive pulmonary disease (COPD). World Health Organization (WHO). 2019. http://www.who.int/respiratory/copd/en. Accessed 23 August, 2019.
- 2. ONS . Deaths from asthma and COPD, England and Wales, 2001 to 2017 by occurrence. Office for National Statistics (ONS). 2017. https://www.ONS.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/009602deathsfromasthmaandcopdenglandandwales2001to2017byoccurrence. Accessed 18 March, 2019.
- 3. Rothnie KJ, Su B, Newson R, Quint JK, Soljak M. COPD prevalence model for small populations: Technical Document produced for Public Health England. National Heart and Lung Institute and Department of Primary Care & Public Health SoPH, Imperial College London; 2016. [Google Scholar]
- 4. Trueman D, Woodcock F, Hancock E. Estimating the Economic Burden of Respiratory Illness in the UK. British Lung Foundation; 2017. [Google Scholar]
- 5. Purdy S, Griffin T, Salisbury C, Sharp D. Prioritizing ambulatory care sensitive hospital admissions in England for research and intervention: a Delphi exercise. Primary Health Care Res Develop. 2010;11(1):41‐50. [Google Scholar]
- 6. OECD/EU . Health at a Glance: Europe 2016 – State of Health in the EU Cycle. Paris: OECD Publishing. 2016. https://ec.europa.eu/health/sites/health/files/state/docs/health_glance_2016_rep_en.pdf. Accessed 10 February, 2018. [Google Scholar]
- 7. NHS England . Next steps on the NHS five year forward view. 2017. https://www.england.nhs.uk/wp‐content/uploads/2017/03/NEXT‐STEPS‐ON‐THE‐NHS‐FIVE‐YEAR‐FORWARD‐VIEW.pdf. Accessed 15 October, 2018.
- 8. Steiner M, McMillan V, Lowe D, et al. Pulmonary rehabilitation: beyond breathing better. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: outcomes from the clinical audit of pulmonary rehabilitation services in England 2015. Results and data analysis. London RCP. 2017. https://www.rcplondon.ac.uk/projects/outputs/pulmonary‐rehabilitation‐beyond‐breathing‐better. Accessed 5 August, 2018.
- 9. Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta‐analysis. Chest. 2019;156(1):80‐91. [DOI] [PubMed] [Google Scholar]
- 10. Rubí M, Renom F, Ramis F, et al. Effectiveness of pulmonary rehabilitation in reducing health resources use in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(3):364‐368. [DOI] [PubMed] [Google Scholar]
- 11. NHS England . The NHS Long Term Plan. 2019. [Google Scholar]
- 12. Wang S, Roshanaei V, Aleman D, Urbach D. A discrete event simulation evaluation of distributed operating room scheduling. IIE Trans Healthcare Syst Eng. 2016;6(4):236‐245. [Google Scholar]
- 13. Shenoy ES, Lee H, Ryan EE, et al. A discrete event simulation model of patient flow in a general hospital incorporating infection control policy for Methicillin‐Resistant Staphylococcus aureus (MRSA) and Vancomycin‐Resistant Enterococcus (VRE). Med Decis Making. 2018;38(2):246‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elkhuizen SG, Das SF, Bakker PJM, Hontelez JAM. Using computer simulation to reduce access time for outpatient departments. Qual Safety Health Care. 2007;16(5):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duguay C, Chetouane F. Modeling and improving emergency department systems using discrete event simulation. Simulation. 2007;83(4):311‐320. [Google Scholar]
- 16. Standfield L, Comans T, Raymer M, O’Leary S, Moretto N, Scuffham P. The efficiency of increasing the capacity of physiotherapy screening clinics or traditional medical services to address unmet demand in orthopaedic outpatients: a practical application of discrete event simulation with dynamic queuing. Appl Health Econ Health Policy. 2016;14(4):479‐491. [DOI] [PubMed] [Google Scholar]
- 17. Getsios D, Marton JP, Revankar N, et al. Smoking cessation treatment and outcomes patterns simulation: a new framework for evaluating the potential health and economic impact of smoking cessation interventions. Pharmacoeconomics. 2013;31(9):767‐780. [DOI] [PubMed] [Google Scholar]
- 18. Arrospide A, Rue M, van Ravesteyn NT, et al. Evaluation of health benefits and harms of the breast cancer screening programme in the Basque Country using discrete event simulation. BMC Cancer. 2015;15(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chemweno P, Thijs V, Pintelon L, Van Horenbeek A. Discrete event simulation case study: diagnostic path for stroke patients in a stroke unit. Simul Model Pract Theory. 2014;48:45‐57. [Google Scholar]
- 20. Demir E, Southern D, Rashid S, Lebcir R. A discrete event simulation model to evaluate the treatment pathways of patients with cataract in the United Kingdom. BMC Health Serv Res. 2018;18(1):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Lay A, Despiegel N, François C, Duru G. Can discrete event simulation be of use in modelling major depression? Cost Eff Resour Alloc. 2006;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harper PR, Sayyad M, de Senna V, Shahani AK, Yajnik C, Shelgikar K. A systems modelling approach for the prevention and treatment of diabetic retinopathy. Eur J Oper Res. 2003;150(1):81‐91. [Google Scholar]
- 23. Simpson KN, Strassburger A, Jones WJ, Dietz B, Rajagopalan R. Comparison of Markov model and discrete‐event simulation techniques for HIV. Pharmacoeconomics. 2009;27(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 24. Lebcir R, Demir E, Ahmad R, Vasilakis C, Southern D. A discrete event simulation model to evaluate the use of community services in the treatment of patients with Parkinson’s disease in the United Kingdom. BMC Health Serv Res. 2017;17(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan F, Reifsnider O, Zheng Y, et al. Modeling clinical outcomes in prostate cancer: application and validation of the discrete event simulation approach. Value Health. 2018;21(4):416‐422. [DOI] [PubMed] [Google Scholar]
- 26. Karnon J, Afzali HHA. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. Pharmacoeconomics. 2014;32(6):547‐558. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Zhao Q, Li W, Zhao X, Li K. The cost‐effectiveness of pulmonary rehabilitation for COPD in different settings: a systematic review. Appl Health Econ Health Policy. 2020. 10.1007/s40258-020-00613-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28. Griffiths TL, Phillips C, Davies S, Burr ML, Campbell I. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax. 2001;56(10):779‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golmohammadi K, Jacobs P, Sin DD. Economic evaluation of a community‐based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung. 2004;182(3):187‐196. [DOI] [PubMed] [Google Scholar]
- 30. Reina‐Rosenbaum R, Bach JR, Penek J. The cost/benefits of outpatient‐based pulmonary rehabilitation. Arch Phys Med Rehabil. 1997;78(3):240‐244. [DOI] [PubMed] [Google Scholar]
- 31. Gillespie P, O'Shea E, Casey D, et al. The cost‐effectiveness of a structured education pulmonary rehabilitation programme for chronic obstructive pulmonary disease in primary care: the PRINCE cluster randomised trial. BMJ Open. 2013;3(11):e003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atsou K, Crequit P, Chouaid C, Hejblum G. Simulation‐based estimates of the effectiveness and cost‐effectiveness of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease in France. PLoS One. 2016;11(6):e0156514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haesum LKE, Soerensen N, Dinesen B, et al. Cost‐utility analysis of a telerehabilitation program: a case study of COPD Patients. Telemedicine e‐Health. 2012;18(9):688‐692. [DOI] [PubMed] [Google Scholar]
- 34. Cox M, O'Connor C, Biggs K, et al. The feasibility of early pulmonary rehabilitation and activity after COPD exacerbations: external pilot randomised controlled trial, qualitative case study and exploratory economic evaluation. Health Technol Assess. 2018;22(11):1‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA. A randomised 2 x 2 trial of community versus hospital pulmonary rehabilitation, followed by telephone or conventional follow‐up. Health Technol Assess. 2010;14(6): i‐v, vii‐xi, 1‐140. [DOI] [PubMed] [Google Scholar]
- 36. Burge AT, Holland AE, McDonald CF, et al. Home‐based pulmonary rehabilitation for COPD using minimal resources: An economic analysis. Respirology. 2019;25(2):183‐190. [DOI] [PubMed] [Google Scholar]
- 37. NHS Digital . Hospital Episode Statistics (HES). 2019. https://digital.nhs.uk/data‐and‐information/data‐tools‐and‐services/data‐services/hospital‐episode‐statistics. Accessed 29 September, 2020.
- 38. Stone R, McMillan V, Mortier K, et al. COPD: Working together. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Clinical audit of COPD exacerbations admitted to acute hospitals in England and Wales 2017. Data analysis and methodology. London RCP. 2018. https://www.rcplondon.ac.uk/projects/outputs/copd‐working‐together‐clinical‐audit‐2017. Accessed 5 March, 2020.
- 39. GOLD . Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. The Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2018. https://goldcopd.org/wp‐content/uploads/2017/11/GOLD‐2018‐v6.0‐FINAL‐revised‐20‐Nov_WMS.pdf. Accessed 12 December, 2018
- 40. Atsou K, Chouaid C, Hejblum G. Simulation‐based estimates of effectiveness and cost‐effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLoS One. 2011;6(9):e24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. NHS Digital . Hospital Episode Statistics (HES). 2019. https://digital.nhs.uk/data‐and‐information/data‐tools‐and‐services/data‐services/hospital‐episode‐statistics. Accessed 29 September, 2020.
- 42. Marno P, Bryden C, Bird W, Watkin HA. How different measures of cold weather affect chronic obstructive pulmonary disease (COPD) hospital admissions in London. Eur Respir Rev. 2006;15(101):185. [Google Scholar]
- 43. Suissa S, Dell AS, Ernst P. Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McAllister DA, Morling JR, Fischbacher CM, MacNee W, Wild SH. Socioeconomic deprivation increases the effect of winter on admissions to hospital with COPD: retrospective analysis of 10 years of national hospitalisation data. Primary Care Respiratory J. 2013;22(3):296‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Demir E. A decision support tool for predicting patients at risk of readmission: a comparison of classification trees, logistic regression, generalized additive models, and multivariate adaptive regression splines. Decis Sci. 2014;45(5):849‐880. [Google Scholar]
- 46. Obi J, Mehari A, Gillum R. Mortality related to chronic obstructive pulmonary disease and co‐morbidities in the united states, a multiple causes of death analysis. COPD: J Chronic Obstruct Pulmon Dis. 2018;15(2):200‐205. [DOI] [PubMed] [Google Scholar]
- 47. Patokos T. Lives in the hands of economists: a critical review of the main methodologies used to derive the value of a statistical life. Environ Econ. 2010. [Google Scholar]
- 48. Johannesson M. The willingness to pay for health changes, the human‐capital approach and the external costs. Health Policy. 1996;36(3):231‐244. [DOI] [PubMed] [Google Scholar]
- 49. Thomas G. A cost‐benefit analysis of the immunisation of children against respiratory syncytial virus (RSV) using the English Hospital Episode Statistics (HES) data set. Eur J Health Econ. 2018;19(2):177‐187. [DOI] [PubMed] [Google Scholar]
- 50. Hussey G, Lasser M, Reekie W. The costs and benefits of a vaccination programme for Haemophilus influenzae type 8 disease. S Afr Med J. 1995;85(1):20‐25. [PubMed] [Google Scholar]
- 51. Rice DP, Miller LS. Health economics and cost implications of anxiety and other mental disorders in the United States. Br J Psychiatry. 1998;173(S34):4‐9. [PubMed] [Google Scholar]
- 52. Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow‐up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jansson S‐A, Backman H, Stenling A, Lindberg A, Rönmark E, Lundbäck B. Health economic costs of COPD in Sweden by disease severity–has it changed during a ten years period? Respir Med. 2013;107(12):1931‐1938. [DOI] [PubMed] [Google Scholar]
- 54. Hollmann M, Garin O, Galante M, Ferrer M, Dominguez A, Alonso J. Impact of influenza on health‐related quality of life among confirmed (H1N1) 2009 patients. PLoS One. 2013;8(3):e60477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ryen L, Svensson M. The willingness to pay for a quality adjusted life year: a review of the empirical literature. Health Econ. 2015;24(10):1289‐1301. [DOI] [PubMed] [Google Scholar]
- 56. NICE . Chronic obstructive pulmonary disease in adults. NICE quality standard QS10. Updated Feb 2016. National Institute for Health and Care Excellence (NICE). 2016. https://www.nice.org.uk/guidance/qs10/chapter/Quality‐statement‐5‐Pulmonary‐rehabilitation‐after‐an‐acute‐exacerbation. Accessed 18 April, 2019.
- 57. NACAP . National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme (NACAP): Outcomes from the Pulmonary Rehabilitation COPD Audit 2015 Findings and quality improvement. 2017. https://www.rcplondon.ac.uk/projects/outputs/pulmonary‐rehabilitation‐beyond‐breathing‐better. Accessed 17 September, 2019.
- 58. Davis S, Stevenson M, Tappenden P, Wailoo A. NICE DSU Technical support document 15: Cost‐effectiveness modelling using patient‐level simulation. Rep BY Decis Support UNIT. 2014. [PubMed]
- 59. Pitt M, Monks T, Chalk D. NICE Guideliness TSU Interim methods guide for developing service guidance 2013: Appendix 2: Service Delivery Operational Research Methods. 2013. https://www.nice.org.uk/Media/Default/About/what‐we‐do/NICE‐guidance/NICE‐guidelines/Clinical‐guidelines/Interim‐methods‐guide‐for‐developing‐service‐guidance‐2013‐appendix‐2.pdf. Accessed 29 January, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author matrix
Appendix S1‐S4
Figure S1


