Abstract
Background
Several studies report the role of Regulatory T-cells (Tregs) in the pathophysiology of pregnancy adverse outcomes.
Objective
The aim of this systematic review and meta-analysis was to determine whether there is an association between regulatory T cell levels and pregnancy adverse outcomes (PAOs), including pre-eclampsia and preterm birth (PTB).
Method
Literature searches were conducted in PubMed/MEDLINE, Embase, and Cochrane CENTRAL databases. Inclusion criteria were original articles (clinical trials, case-control studies and cohort studies) comparing Tregs, sampled from the decidua or maternal blood, in healthy pregnant women versus women with pre-eclampsia or PTB. The outcome was standardised mean difference (SMD) in Treg numbers. The tau-squared (Tau²), inconsistency index (I²), and chi-squared (χ²) test quantified heterogeneity among different studies. Analyses were performed in RevMan software V.5.4.0 for Mac using a random-effects model with outcome data reported with 95% confidence intervals (CI). This study was prospectively registered with PROSPERO (CRD42020205469). PRISMA guidelines were followed.
Results
From 4,085 unique studies identified, 36 were included in qualitative synthesis, and 34 were included in quantitative synthesis (meta-analysis). In total, there were 1,783 participants in these studies: healthy controls=964, pre-eclampsia=759, PTB=60. Thirty-two studies compared Tregs in healthy pregnant women and women with pre-eclampsia, and 30 of these sampled Tregs from peripheral blood showing significantly higher Treg numbers in healthy pregnancies (SMD; 1.46; 95% CI, 1.03–1.88; I²=92%). Four studies sampled Tregs from the maternal decidua showing higher Tregs in healthy pregnancies (SMD, 0.76; 95% CI, -0.13–1.65; I²=84%). No difference was found in the number of Tregs between early versus late pre-eclampsia (SMD,-1.17; 95% CI, -2.79–0.44; I²=94%). For PTB, two studies compared Tregs sampled from the peripheral blood with a tendency for higher Tregs in healthy pregnancies but this did not reach significance (SMD, 2.18; 95% CI, -1.34–5.70; I²=96%). Subcohort analysis using Treg analysis (flow cytometry vs. qPCR vs. immunofluorescence tissue staining) showed similar associations.
Conclusion
Lower Tregs in pregnancy, sampled from the maternal peripheral blood, are associated with pre-eclampsia. There is a need for further studies to confirm a relationship between low Tregs and PTB. As the precise mechanisms by which Tregs may mediate pre-eclampsia and PTB remain unclear, further fundamental research is necessary to elucidate the underlying processes and highlight the causative link.
Systematic Review Registration
PROSPERO, identifier CRD42020205469.
Keywords: regulatory T cells (Tregs), pregnancy, high blood pressure (hypertension), pre-eclampsia, pre-term birth (PTB), pregnancy adverse outcomes (PAO)
Introduction
Preterm Birth
Preterm birth (PTB) is defined by the World Health Organization (WHO) as birth prior to 37 weeks of gestation (1), further subdivided into extreme, very and moderate-to-late preterm occurring prior to 28 weeks, 28 to 32 weeks, and 32 to 37 weeks respectively. PTB has become the leading cause of perinatal morbidity and mortality in developed countries (2) and despite an overall decline in perinatal mortality, preterm infants face increased short-term morbidity and long-term neurodevelopmental, respiratory, and gastrointestinal complications (2).
The complex heterogeneity of PTB is due to its varying aetiology and pathogenesis, which result in idiopathic premature activation of the labour process, with or without pathological insults (2). Approximately 50% of PTBs are due to preterm labour (PTL), uterine contractions before 37 weeks’ gestation that may or may not progress to delivery (i.e., PTB), with intact membranes or preterm premature rupture of membranes (PPROM, 25%) (3). Up to 40% of these cases are due to intrauterine infection. Other causes include inflammation, vascular disease, uterine overdistension, placental abruption or hormonal disruptions (4–6). 25% involve induced labours or caesarean deliveries as a result of maternal or fetal indications (7). Due to effects on placental blood supply and intrauterine growth, pre-eclampsia is a prime example of such an indication, accounting for up to 20% of PTBs (8).
Pre-eclampsia
Pre-eclampsia affects 3-5% of pregnancies, with incidence increasing due to a higher prevalence of risk factors including maternal obesity, older maternal age and diabetes mellitus (9). Pre-eclampsia is diagnosed in the presence of hypertension after 20 weeks’ gestation accompanied by maternal acute kidney injury, liver dysfunction, neurological symptoms, haemolysis or thrombocytopenia, or fetal growth restriction (10). Further risk factors include first pregnancy, hypertensive disease in previous pregnancies and co-morbidities including autoimmune and renal disease. Proposed pathways of pre-eclampsia suggest immunological factors of genetic and environmental origin are involved in the pathogenesis (11).
Early and late-onset pre-eclampsia, defined as onset before 34 weeks of gestation and at or after 34 weeks respectively. These are important to differentiate as different pathogenic mechanisms and outcomes are implicated – whilst shallow trophoblast invasion is common in early onset type (12), exaggerated inflammatory responses may play a role in the development of late-onset disease (12). Furthermore, aspirin treatment prevents early onset pre-eclampsia but not late onset disease (13). Thus, it is important to compare Treg numbers between these subtypes as they may represent different disease entities (12).
Clinical diagnosis requires proteinuria, new-onset hypertension or signs of end-organ damage. The American College of Obstetricians and Gynaecologists (ACOG) (14) suggest diagnosis in the presence of proteinuria and new-onset hypertension, or new-onset hypertension with thrombocytopenia, renal insufficiency, impaired liver function or pulmonary oedema. Classification of severe disease involves severe hypertension (systolic BP ≥ 160mmHg, diastolic BP ≥ 110mmHg or both) or signs of end-organ damage.
Tregs in Pregnancy
Regulatory T-cells (Tregs) are a specialised subset of immunosuppressive cells defined by the expression of lineage-defining transcription factors FOXP3, CD25 and low or absent CD127 expression (15), subdivided into thymic/natural Tregs (nTregs) and peripherally induced Tregs (iTregs), which are thought to control autoimmune responses and mucosal immunity, respectively (16). Immunosuppressive function is primarily exerted by direct cell-cell interactions with the target cell, consumption of interleukin-2 (IL-2) and the release of anti-inflammatory molecules14. In addition to ensuring tolerance to self and non-inherited antigens (17), Tregs are essential in inducing transplantation tolerance (18–20).
During pregnancy, Tregs prevent rejection of the semi-allogeneic fetus by the maternal immune system (21, 22). Decidual Tregs, including nTregs and iTregs (23), create a tolerogenic microenvironment through the production of soluble factors such as IL-10 (22). In healthy pregnancies, great diversity in Treg populations exists in both peripheral blood and at the maternal-fetal interface (22). High levels of CD25hiFOXP3+ Tregs are found in decidual tissues21 and both FOXP3+ and FOXP3- Tregs are increased in the peripheral blood of pregnant women (22). The composition of fetal cells and maternal immune cells changes throughout gestation (22). T-cell frequencies increase during gestation, with local and systemic Treg expansion reaching its maximum in the 2nd trimester (21). As labour progresses, the proportions of decidual Tregs once again decreases (24).
In PAO, maternal Tregs are altered (16). Treg maldistribution or functional impairment has been reported in implantation failure, miscarriage and pre-eclampsia (21, 22, 25, 26). In primary unexplained infertility, expression of FOXP3 mRNA is decreased in the uterine endometrium (27). In pre-eclampsia, Treg percentages are lower than in healthy pregnancies (28–30) and associated with spiral artery adaptation and defective maternal blood flow to the placenta (30). A reduction of decidual CD4+CD25HI FOXP3+ and HELIOS+ Tregs is observed in miscarriages (21–23, 31).
Impaired peripheral Treg signalling has also been found (22). Signalling pathways implicated in modulating T-cell function during pregnancy include the IL-2–dependent STAT5ab signalling pathways (32), the PD1-PDL1 pathway (23) and the TIM-3 pathway (24). Fetal Tregs are also implicated (33), however, this systematic review and meta-analysis focuses on maternal Tregs only.
Objective
Through this systematic review and meta-analysis, we aimed to determine whether there is an association between regulatory T cell levels and PAOs, including pre-eclampsia and preterm birth.
2 Materials and Methods
Design
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (34). The review was prospectively registered with PROSPERO (CRD42020205469).
Outcomes
Primary outcome was standardised mean difference (SMD) in Treg numbers between healthy pregnant women and women with pre-eclampsia or PTB. The measures used to identify these differences include Tregs expressing CD4+/CD25+/CD127low or CD4+/CD125+/FOXP3+ sampled from the peripheral blood or maternal decidua.
Eligibility Criteria
Eligible for inclusion were original articles including clinical trials, case-control studies and cohort studies that examined the association between maternal Tregs in human pregnancy, sampled from the decidua or maternal blood, and the onset of pre-eclampsia and PTB. Studies were selected that compared these maternal co-morbidities with healthy age-matched pregnant individuals as control. No restrictions were made regarding population characteristics, such as age, ethnicity or setting. Studies examining fetal Tregs and studies that did not sample Tregs from the maternal blood or decidua were excluded. Duplicate studies were excluded from total counts.
Information Sources and Search Strategy
Three reviewers (SG, KSR and MP) searched PubMed/MEDLINE, Embase and Cochrane CENTRAL for eligible articles published between August 1st, 2010 and August 1st, 2020 using search terms specific for ‘maternal’ OR ‘fetal’ ‘regulatory T-cells’ AND ‘pregnancy’, ‘pre-eclampsia’, ‘preterm birth’, OR ‘miscarriage’ ( Appendix 1 ). Results were restricted by article type (see Appendix 1 for detailed search strategies), language (English), and species (Human).
Selection Process
For each article, title, abstract and full-text screening was performed independently by one of three reviewers (SG, KSR and MP). Screening results were reviewed by a senior author in the study (PS). Discrepancies were resolved through discussion in which a senior author was consulted (PS).
Data Collection Process and Data Items
For each article, data was extracted independently by one of four reviewers (SG, KSR, MP, ASVC) using a predefined data extraction form. Results were reviewed by a senior author in the study (PS). From each study, information was extracted regarding study design, location, population, participant demographics, baseline characteristics, details of intervention and control, interventions and attrition rate. Miscellaneous information, e.g. method of delivery, antenatal steroid use was also recorded.
Quality Assessment
A modified version (35) of the Newcastle–Ottawa Scale (NOS) (36) ( Supplementary Table 4 ) was used to assess methodological quality of included studies. Studies were judged based on selection, comparability and outcome, with a maximum of 3, 2 and 2 stars, respectively, equating to a total score ranging from zero (worst) to 7 (best). ≥ 6 stars indicated high quality, 4–5 moderate quality and a high risk of bias and <4 indicated a very high risk of bias. Quality assessment was undertaken independently by two reviewers (KSR and MP), and inter-rater reliability was assessed.
Statistical Analysis
We estimated the SMD in Treg numbers, sampled from the decidua and peripheral blood, of healthy pregnant women versus pregnant women with pre-eclampsia or PTB along with 95% confidence intervals (CI) under a random-effects (RE) model using Review Manager Version 5.4 (V.5.4.0) for Mac.
We used the tau-squared (Tau2), inconsistency index (I2), and chi-squared (χ²) test to quantify heterogeneity among different studies. Heterogeneity as defined by I2 was considered to be minor if 0% to 40%, moderate if 30% to 60%, substantial if 50% to 90% and considerable if 75% to 100%. The percent heterogeneity was interpreted in the context of the magnitude of the effect size and the strength of evidence surrounding the heterogeneity (37). Potential publication bias was tested using the rank correlation test of funnel plot asymmetry [Begg’s test (38) and Egger’s test (39)].
Results
Search Results
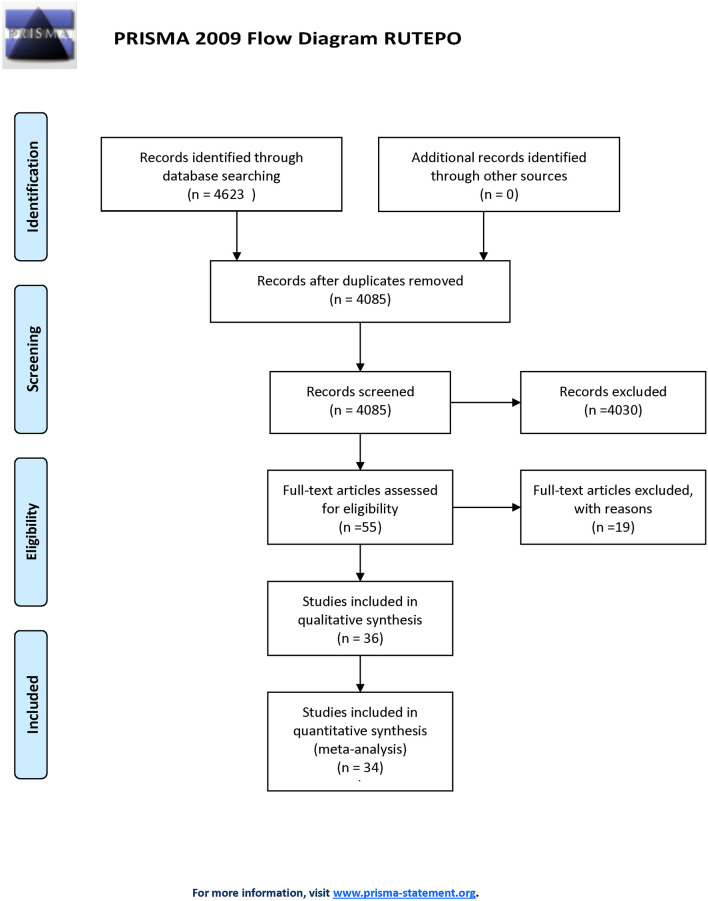
Figure 1 outlines the study selection process following PRISMA guidelines (40). The initial search identified 4,085 unique articles. 55 articles underwent full-text screening, with 36 studies included in qualitative synthesis and 34 in quantitative synthesis (meta-analysis). Treg populations between healthy pregnant women and pregnant women with pre-eclampsia was compared in 32 studies, 30 of which sampled Tregs from maternal peripheral blood ( Supplementray Table 1 ). Four studies sample Tregs from maternal decidua ( Supplementary Table 2 ), whilst an additional two studies compared Tregs, sampled from the peripheral blood, between healthy pregnant women and pregnant women who underwent PTL and PTB ( Supplementary Table 3 ).
Figure 1.
PRISMA flowchart of study selection.
Characteristics of Studies Included in the Meta-Analysis (Quantitative Synthesis)
Supplementary Tables 1–3 feature the characteristics of included articles. Included studies were published between 2009 and 2019. Total sample sizes (including cases and controls) ranged from 20 to 108 pregnant subjects. Studies spanned 5 continents (Asia=19, Australia=1, Europe=9, North America=3 and South America=2), 12 countries (Australia=1, Bosnia and Herzegovina=1, Brazil=2, China=13, Czech Republic=1, Germany=1, Hungary=4, Iran=5, Japan=1, Mexico=1, Poland=2 and USA=2) and represented 1,783 participants (healthy controls=964, pre-eclampsia=759, PTB=60) of African American, Asian, Black, Caucasian, Hispanic, Latin and Persian ethnicity/race. The mean and median ages of women across studies ranged between 26.0 and 35.5 years. Treg analysis was typically performed in the second and third trimester of gestation. Most studies used flow cytometry (n=28) as the method of Treg analysis, while few reports using qPCR (n=5) and immunofluorescence tissue staining (n=1). Six studies considered and reported BMI measurements between cases and controls as an important confounder, and six studies considered and reported smoking status. All studies identified gestational age at the time of Treg analysis as a confounder, and 24 out of 34 studies (71%) considered gestational age at delivery. Birth weight was reported in 24 out of 34 studies (71%). A selection of different Treg markers were used in each study to identify Treg populations with CD4+, CD25+, FOXP3+ as well as CD4+, CD25+, CD127low being the most common Treg marker combinations ( Supplementary Tables 1–3 ). Gestational age at delivery is missing from 20 studies ( Supplementary Table 1 ). Use of corticosteroids is not mentioned in 26 studies ( Supplementary Tables 1–3 ).
Meta-Analysis Findings (Quantitative Synthesis)
Lower Number of Tregs in Peripheral Blood in the Peripheral Blood of Women Who Develop Pre-Eclampsia
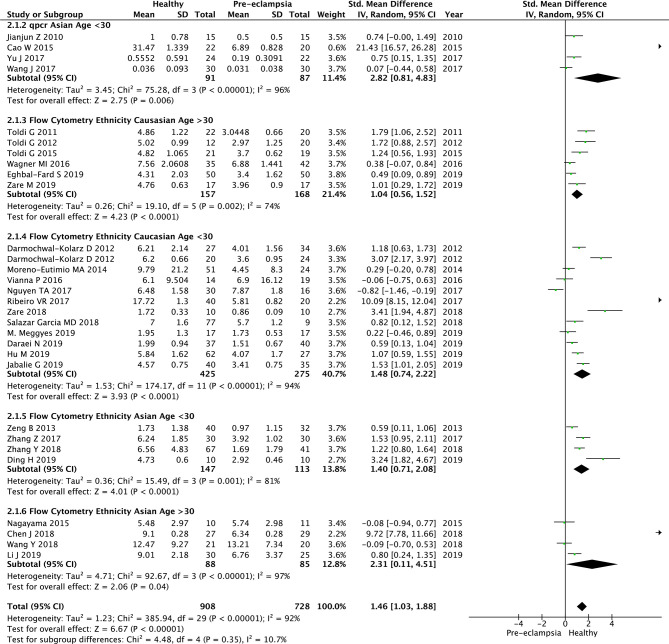
30 studies were included in analysis exploring the association of pre-eclampsia and Treg populations in the peripheral blood. Twenty-six studies used flow cytometry to analyse Tregs populations (41–64) and four used qPCR (65–68). We analysed these two groups separately and combined them ( Figure 2 ). In the qPCR group, the patients were matched for ethnicity and age group (Asian, <30 years old). The SMD of Treg numbers in the peripheral blood of healthy pregnant women compared to pregnant women with pre-eclampsia was 2.82 (95% CI, 0.81–4.83; I2 = 96%; 4 studies), with healthy women reporting significantly higher Treg numbers in two studies and non-significant difference in two studies ( Figure 2.1.2 ).
Figure 2.
Standardized mean difference of T regulatory cell numbers in the peripheral blood of healthy pregnant women and women with pre-eclampsia, in subgroups according to ethnicity, age and method of analysis. CI, confidence interval; SD, standard deviation; Std. Mean Difference, standardised mean difference; IV, inverse variance.
The overall SMD of Treg numbers in the peripheral blood of healthy pregnant women (using flow cytometry and qpcr) compared to pregnant women with pre-eclampsia was 1.46 (95% CI, 1.03–1.88; I2 = 92%; 30 studies), with healthy women reporting significantly higher Treg numbers overall ( Figure 2 ). We performed subgroup analysis based on their ethnic background and age. Testing for subgroup differences did not reveal any significant results (P=0.35, I2 = 10.7%) ( Figure 2.1.2 , 2.1.3, 2.1.4, 2.1.5, 2.1.6). In addition changing the method of analysis from SMD to Mean Difference (MD) showed a MD of 2.49 (95% CI, 1.41-3.57; I2 = 100%; 30 studies, data not shown). Testing for subgroup differences using MD did not show any significant results (P=0.14, I2-41.9%, data not shown). In addition, we also performed subgroup analysis based on the year of publication. We divided the studies to the ones published before (n=10) and after 2015 (n=20). Testing for subgroups differences based on the year of publication did not show any significant results (P=0.66, I2 = 0%, data not shown).
Tregs in the Decidua of Women With Pre-Eclampsia and Healthy Women
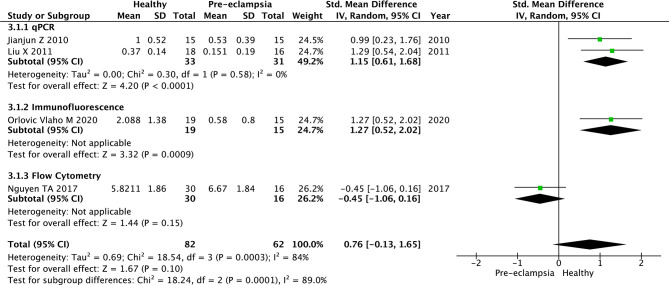
Four studies were included in the analysis to determine association of pre-eclampsia and Treg populations in the maternal decidua. Two (69, 70) used qPCR to analyse Treg populations, one (71) used immunofluorescence tissue staining and one used flow cytometry (43). We analysed these three subgroups separately and further analysis combined all four studies ( Figure 3 ). For the qPCR group (69, 70), the SMD in Treg numbers in the decidua of healthy pregnant women compared to pregnant women with pre-eclampsia was 1.15 (95% CI, 0.61–1.68; I2 = 0%; 2 studies), with healthy pregnant women reporting significantly higher Treg numbers in both studies ( Figure 3.1.1 ). The immunofluorescence tissue staining study (71) also reported significantly higher Tregs in healthy pregnant women (SMD, 1.27; 95% CI, 0.52–2.02) ( Figure 3.1.2 ). Flow cytometry (43) showed no significant difference in Treg numbers between healthy pregnancies and women with pre-eclampsia (SMD, -0.45; 95% CI, -1.06–0.16) ( Figure 3.1.3 ). Analysing qPCR, immunofluorescence tissue staining and flow cytometry studies together, the SMD of Treg numbers in the decidua of healthy pregnant women compared to pregnant women with pre-eclampsia was 0.76 (95% CI, -0.13–1.65; I2 = 84%; 4 studies), with healthy women reporting higher Treg numbers in 3 studies (65, 70, 71) and non-significant difference in 1 study (43). This result should be interpreted with caution as the 95% CI crosses the null value.
Figure 3.
Standardized mean difference of T regulatory cell numbers in the decidua of healthy pregnant women and women with pre-eclampsia. CI, confidence interval; SD, standard deviation; Std. Mean Difference, standardised mean difference; IV, inverse variance.
The Number of Tregs in Women With Early Pre-Eclampsia Are Similar to the Ones in Late Pre-Eclampsia
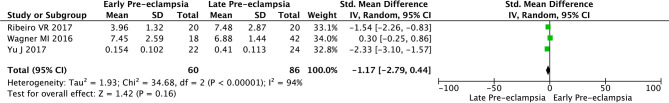
We identified studies, n=3 (44, 63, 68), which reported the number of Tregs separately in early versus late pre-eclampsia.
The SMD in Treg numbers in the peripheral blood of pregnant women with late pre-eclampsia compared to pregnant women with early pre-eclampsia was -1.17 [95% CI, -2.79–0.44; I2 = 94%; 3 studies (44, 63, 68)], a non-significant difference with the 95% CI crossing the null value ( Figure 4 ).
Figure 4.
Standardized mean difference of T regulatory cell numbers in the maternal blood of women with early and late pre-eclampsia. CI, confidence interval; SD, standard deviation; Std. Mean Difference, standardised mean difference; IV, inverse variance.
The Number of Tregs in Women Who Develop PTL and Had PTB Are Similar to Healthy Women
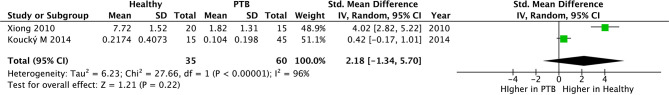
Only two studies (72, 73) reporting the association of PTB and Tregs in peripheral blood were included in the analysis. Both studies measured Tregs with flow cytometry. The SMD of Treg numbers in the peripheral blood of healthy pregnant women compared to pregnant women who underwent PTB was 2.18 (95% CI, -1.34–5.70; I2 = 96%; 2 studies), with healthy pregnant women reporting higher Treg numbers overall in one study (72) and a non-significant difference in the second study (73) ( Figure 5 ). However, the 95% CI crosses the null value.
Figure 5.
Standardized mean difference of T regulatory cell numbers in the peripheral blood of healthy pregnant women and women who underwent preterm birth (PTB). PTB, preterm birth; CI, confidence interval; SD, standard deviation; Std. Mean Difference, standardised mean difference; IV, inverse variance.
Heterogeneity of the Studies
Heterogeneity was considerable in all meta-analyses, the 95% prediction intervals for individual studies crossing the null value ( Figures 2–5 ). I2 was 92% and 84% for the 30 and 4 respective studies investigating pre-eclampsia by sampling Tregs from maternal peripheral blood and decidua, respectively. Similarly, the I2 was 96% for the two studies investigating PTB by sampling Tregs from maternal peripheral blood. Where I2 could be estimated within subgroup analyses, age, ethnicity, year of publication and by the method of Treg analysis (flow cytometry vs. qPCR vs. immunofluorescence tissue staining), it remained considerable for all except the qPCR subgroup analysis of 2 studies (65, 70) investigating pre-eclampsia by sampling Tregs from the maternal decidua (I2 = 0%) - 95% prediction intervals of neither of these studies crossed the null value ( Figure 3.1.1 ). In the flow cytometry, ethnicity Caucasian, age >30 subgroup, of 6 studies (41, 52, 53, 56, 62, 63) the heterogeneity was relatively lower that the rest of the subgroups (I2 = 74%) with only one study (63) crossing the null value. Heterogeneity should be considered as a confounder when interpreting the significance of results, particularly in relation to the analysis of pre-eclampsia studies sampling Tregs from the maternal decidua ( Figure 3 ) and PTB studies sampling Tregs from the maternal peripheral blood ( Figure 5 ) as the 95% CI crossed the null value in the end outcome of these analyses. This was not true for the analysis of pre-eclampsia studies sampling Tregs from the maternal peripheral blood ( Figure 2 ).
Publication Bias
A funnel plot was used to graphically evaluate articles for publication bias. This was tested using the rank correlation test of funnel plot asymmetry [Begg’s test (38) and Egger’s test (39)]. SMD values were plotted against standard error (SE). Data from the 30 studies seemed to be roughly symmetrically distributed in an inverted funnel-shaped area ( Figure 6 ).
Figure 6.
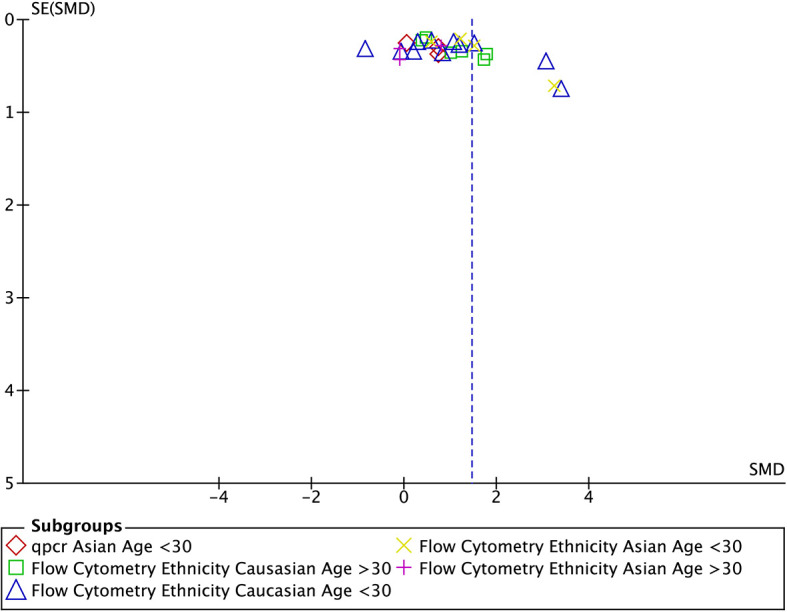
Funnel plot for studies looking at the number of Tregs in the maternal blood included in the subgroup meta-analysis (n=30). SE, standard error; SMD, standardised mean difference.
Quality Assessment
The overall quality rating of the studies included in the meta-analysis was moderate, representing a high risk of bias. NOS scores ranged between 3–6 with a median score of 4 out of 7 ( Supplementray Table 5 ). Quality ratings were impaired by poor population representativeness of the exposed cohort (due to hospital-based sampling), and inadequate follow up (<1 month after labour). Comparability was limited in several studies that did not adjust for all confounding variables (age, race, smoking and interpregnancy interval). The IRR was 100% regarding the assessment of ‘selection’ and ‘outcomes’ criteria; however, there were inter-rater discrepancies in the assessment of ‘comparability’ in 13 out of 36 studies (IRR=64%).
Discussion
Findings
Our meta-analysis suggests that lower Treg cell numbers may be a potential independent risk factor for PAO, including pre-eclampsia and potentially PTL. Overall, healthy pregnant women have significantly higher Treg numbers than pregnant women with pre-eclampsia, evident by an SMD of 1.46 (95% CI, 1.03–1.88; I2 = 92%; 30 studies) when sampling Tregs from the peripheral blood. In studies sampling Tregs from the maternal decidua, healthy pregnant women had higher, but non-significantly higher Treg numbers compared to women with pre-eclampsia, evident by an SMD of 0.76 (95% CI, -0.13–1.65; I2 = 84%; 4 studies). This might be due to poor phenotyping of the decidual tissue. Healthy pregnant women also have a non-significant numerically higher Treg numbers compared to pregnant women who undergo PTL, with an SMD of 2.18 (95% CI, -1.34–5.70; I2 = 96%; 2 studies) when sampling Tregs from the peripheral blood. Similar trends are observed in subcohort analysis when studies are grouped by the method of Treg analysis.
This is supported by previous research by Han et al (74) and Schober et al (75) who found an association between impaired Treg function and pre-eclampsia (74) and PTL (75). Han et al. (74), using high-dimensional mass cytometry immunoassay, suggests that specific aspects of peripheral immune system dynamics may be disrupted in preeclamptic pregnancies. Furthermore, Schober et al. (75), using flow cytometry, found that the suppressive activity of CD4+CD127low+/-CD25+-Treg cells was strongly diminished in PTL women and, to a lesser extent, in spontaneously term labouring women compared to term non-labouring women. This reduction in suppressive activity was due to Treg-cell deficiency but not due to CD4+-responder T (Tresp) cell resistance, with CD4+-T cells significantly reduced in term and preterm labouring women (75). There is a need for additional research, using tightly phenotyped PTL groups, before confirming any relationship and methods used by Han et al. and Schober et al. may be worthwhile.
Strengths
We identified and screened over 4,000 unique articles, the meta-analysis therefore including participants across five continents of different ethnicity, adding to the representability and generalizability of findings. We found similar results, in terms of Treg associations with healthy and adverse pregnancy outcomes, across studies that sampled Tregs from different sites (maternal decidua and maternal peripheral blood) as well as studies using other methods of Treg analysis (flow cytometry, qPCR and immunofluorescence tissue staining), increasing robustness of findings. Performance of subcohort analysis by age, ethnicity, year of publication, site of sampling and method of Treg analysis further supports the strength of association across different research conditions. Most studies attempted to adjust for confounding variables.
Limitations
Participant numbers were limited, especially for the PTB cohort (1739 pregnant women including 944 healthy controls, 735 pre-eclampsia, 60 PTB). Furthermore, each separate study had a small number of patients in each group. Indeed, only two studies investigating PTB were included in quantitative analysis; both sampling Tregs from maternal peripheral blood, none sampling decidua. In pre-eclampsia, only four studies tested Tregs from the maternal decidua, utilising three different methods of Treg analysis, with the flow cytometry study producing opposing findings to studies utilising qPCR or immunofluorescence tissue staining. Antenatal corticosteroids have been shown to alter T cell trafficking and cytokine production (76), yet use of corticosteroids prior to blood sampling was omitted in most studies (supplementary data). Additionally, whilst Treg cells are classified into naïve and effector Tregs, which express weak and powerful immunoregulation respectively, we did not discriminate between the two. Comparing Treg populations in early onset versus late onset pre-eclampsia is also important given the distinct underlying pathogenic processes (44, 63, 68, 77). In our cohort only 3 studies reported separately the Treg numbers in early versus late pre-eclampsia. In addition a subgroup analysis based on BMI would have been ideal since there is evidence the BMI alters the number of Tregs in obese individuals (78). Unfortunately only 7 studies reported BMI in their results, which was between 22-28 and no subgroup analysis could have been done ( Supplementary Tables 1–3 ).
Methodological quality of studies was low to moderate. Nevertheless, all studies were included in the analysis regardless of quality assessment, none excluded based on high risk of bias. It may have been prudent to repeat the analysis, excluding studies with low NOS quality rating. Equally, meta-analyses could have been repeated after excluding studies with high heterogeneity (I2) in which the 95% prediction intervals crossed the null value (Figs. 2-5). Overall, there was considerable heterogeneity across analyses. Indeed, this limited the significance of results in three of the meta-analyses, specifically for pre-eclampsia studies sampling Tregs from the maternal decidua and PTB studies sampling Tregs from the maternal peripheral blood as well as in the early versus late comparison ( Figure 4 ). Assessing relative risk (RR) or odds ratio (OR) in addition to SMD may have demonstrated a stronger level of association to support outcomes.
Clinical Significance
Future research could investigate the potential of monitoring Treg numbers in peripheral blood of pregnant women as a possible biomarker to assess the risk of PAO, stratifying patients with high-risk pregnancies. Future preclinical and clinical models could investigate strategies to increase Treg numbers in pregnant women as a candidate therapeutic approach. Both of these suggestions, however, remain at a hypothesis stage and require further systematic evaluation.
Conclusion
This meta-analysis suggests an association between lower T-regulatory cell numbers and risk for pre-eclampsia and potentially for PTL (28, 29). Importantly, correlation does not imply causation and possibility of an underlying mechanism causing both low Treg numbers and pre-eclampsia and PTL must be considered. As the precise mechanisms by which Tregs may mediate pre-eclampsia and PTL remain unclear, further research is necessary to elucidate the underlying processes and highlight the causative link.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
PS, SG, MP, KR, and AS conceptualized the topic and structure of the systematic review. SG, MP, KR, and AS drafted and revised the manuscript. AE, NM, KD, CS, GL, RT, KN, and PS provided expert opinion, edited, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
PS is funded by an NIHR Clinical Lectureship (CL-2018-17-002). This study was funded by the Fetal Medicine Foundation (KHN,AE&NM) (registered charity 1037116), Tommy’s (RT&KD) (registered charity number 1060508) and the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London (IS-BRC-1215–20006).
Author Disclaimer
The views expressed in this Article are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Appendix 1 Search Terms
1. PubMed
Theme 1: Maternal T Cells in Pregnancy
((maternal) OR (mother)) AND ((regulatory T cells) OR (Tregs)) AND (pregnancy)
Theme 2: Fetal T Cells in Pregnancy
((Fetal) OR (Fetal)) AND ((Regulatory T Cells) OR (Tregs) OR (Regulatory T Lymphocyte)) AND (Pregnancy)
Theme 3: Adverse Outcomes
((Preeclampsia) OR (preterm birth) OR (miscarriage)) AND ((Regulatory T cells) OR (Tregs) OR (Regulatory T lymphocyte)) AND (pregnancy)
2. Embase
Theme 1: Maternal T Cells in Pregnancy
((maternal OR mother) AND ‘regulatory t lymphocytes’ OR ‘regulatory t cells’ OR ‘tregs’/exp OR tregs) AND (‘pregnancy’/exp OR pregnancy)
Theme 2: Fetal T Cells in Pregnancy
((((Fetal OR Fetal) AND ‘Regulatory T Cells’ OR Tregs OR Regulatory T Lymphocyte) AND Pregnancy))
Theme 3: Adverse Outcomes
((preeclampsia) OR ‘preterm birth’ OR ‘preterm labor’ AND ‘tregs’) AND (‘pregnancy’)
3. Cochrane
Theme 1: Maternal T Cells in Pregnancy
(maternal):ti,ab,kw OR (mother):ti,ab,kw AND (Tregs):ti,ab,kw OR (T regulatory lymphocytes):ti,ab,kw OR (T regulatory cells):ti,ab,kw AND (pregnancy):ti,ab,kw
Theme 2: Fetal T Cells in Pregnancy
(Fetal):ti,ab,kw OR (Fetal):ti,ab,kw AND (Tregs):ti,ab,kw OR (Regulatory T Cells):ti,ab,kw OR (Regulatory T Lymphocytes):ti,ab,kw AND (Pregnancy):ti,ab,kw
Theme 3: Adverse Outcomes
(preeclampsia):ti,ab,kw OR (preterm birth):ti,ab,kw OR (miscarriage):ti,ab,kw AND (Tregs):ti,ab,kw OR (regulatory T lymphocyte):ti,ab,kw OR (regulatory T cells):ti,ab,kw AND (pregnancy):ti,ab,kw
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.737862/full#supplementary-material
References
- 1. Preterm Birth. Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed April 26, 2021).
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and Causes of Preterm Birth. Lancet (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moutquin J. Classification and Heterogeneity of Preterm Birth. BJOG Int J Obstet Gynaecol (2003) 110:30–3. doi: 10.1016/S1470-0328(03)00021-1 [DOI] [PubMed] [Google Scholar]
- 4. Agrawal V, Hirsch E. Intrauterine Infection and Preterm Labor. Semin Fetal Neonatal Med (2012) 17:12–9. doi: 10.1016/j.siny.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lockwood CJ. The Diagnosis of Preterm Labor and the Prediction of Preterm Delivery. Clin Obstet Gynecol (1995) 38:675–87. doi: 10.1097/00003081-199538040-00002 [DOI] [PubMed] [Google Scholar]
- 6. Romero R, Espinoza J, Kusanovic J, Gotsch F, Hassan S, Erez O, et al. The Preterm Parturition Syndrome. BJOG Int J Obstet Gynaecol (2006) 113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibai B, Dekker G, Kupferminc M. Pre-Eclampsia. Lancet (2005) 365:785–99. doi: 10.1016/S0140-6736(05)17987-2 [DOI] [PubMed] [Google Scholar]
- 8. Duley L. The Global Impact of Pre-Eclampsia and Eclampsia. Semin Perinatol (2009) 33:130–7. doi: 10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 9. Lisonkova S, Joseph KS. Incidence of Preeclampsia: Risk Factors and Outcomes Associated With Early- Versus Late-Onset Disease. Am J Obstet Gynecol (2013) 209:544.e1–e12. doi: 10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 10. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive Disorders of Pregnancy. Hypertension (2018) 72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803 [DOI] [PubMed] [Google Scholar]
- 11. Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin J Am Soc Nephrol (2016) 11:1102–13. doi: 10.2215/CJN.12081115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-Eclampsia. Lancet (2010) 376:631–44. doi: 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 13. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin Versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med (2017) 377:613–22. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 14. American College of Obstetricians and Gynecologists . Gestational Hypertension and Preeclampsia. Obstet Gynecol (2020) 135:1492–5. doi: 10.1097/AOG.0000000000003892 [DOI] [PubMed] [Google Scholar]
- 15. Rackaityte E, Halkias J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front Immunol (2020) 11:588. doi: 10.3389/fimmu.2020.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller D, Gershater M, Slutsky R, Romero R, Gomez-Lopez N. Maternal and Fetal T Cells in Term Pregnancy and Preterm Labor. Cell Mol Immunol (2020) 17:693–704. doi: 10.1038/s41423-020-0471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burt TD. Fetal Regulatory T Cells and Peripheral Immune Tolerance In Utero : Implications for Development and Disease. Am J Reprod Immunol (2013) 69:346–58. doi: 10.1111/aji.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velásquez-Lopera MM, Eaton VL, Lerret NM, Correa LA, Decresce RP, García LF, et al. Induction of Transplantation Tolerance by Allogeneic Donor-Derived CD4(+)CD25(+)Foxp3(+) Regulatory T Cells. Transpl Immunol (2008) 19:127–35. doi: 10.1016/j.trim.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 19. van der Net JB, Bushell A, Wood KJ, Harden PN. Regulatory T Cells: First Steps of Clinical Application in Solid Organ Transplantation. Transpl Int (2016) 29:3–11. doi: 10.1111/tri.12608 [DOI] [PubMed] [Google Scholar]
- 20. Romano M, Tung SL, Smyth LA, Lombardi G. Treg Therapy in Transplantation: A General Overview. Transpl Int (2017) 30:745–53. doi: 10.1111/tri.12909 [DOI] [PubMed] [Google Scholar]
- 21. Tsuda S, Nakashima A, Shima T, Saito S. New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front Immunol (2019) 10:573. doi: 10.3389/fimmu.2019.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krop J, Heidt S, Claas FHJ, Eikmans M. Regulatory T Cells in Pregnancy: It Is Not All About Foxp3. Front Immunol (2020) 11:1182. doi: 10.3389/fimmu.2020.01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salvany-Celades M, van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, et al. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep (2019) 27:2537–2547.e5. doi: 10.1016/j.celrep.2019.04.109 [DOI] [PubMed] [Google Scholar]
- 24. Miller D, Gershater M, Slutsky R, Romero R, Gomez-Lopez N. Maternal and Fetal T Cells in Term Pregnancy and Preterm Labor. Cell Mol Immunol (2020) 17:693–704. doi: 10.1038/s41423-020-0471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered Decidual DC-SIGN+ Antigen-Presenting Cells and Impaired Regulatory T-Cell Induction in Preeclampsia. Am J Pathol (2012) 181:2149–60. doi: 10.1016/j.ajpath.2012.08.032 [DOI] [PubMed] [Google Scholar]
- 26. Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic Increase in the Ratio Between Foxp3 + and IL-17-Producing CD4 + T Cells in Healthy Pregnancy But Not in Preeclampsia. J Immunol (2009) 183:7023–30. doi: 10.4049/jimmunol.0901154 [DOI] [PubMed] [Google Scholar]
- 27. Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, et al. Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, But Not in Preeclampsia, in Humans. Front Immunol (2018) 9:1934. doi: 10.3389/fimmu.2018.01934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of Peripheral Blood and Decidual CD4+ CD25 Bright Regulatory T Cells in Pre-Eclampsia. Clin Exp Immunol (2007) 149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quinn KH, Parast MM. Decidual Regulatory T Cells in Placental Pathology and Pregnancy Complications. Am J Reprod Immunol (2013) 69:533–8. doi: 10.1111/aji.12077 [DOI] [PubMed] [Google Scholar]
- 30. Robertson SA, Green ES, Care AS, Moldenhauer LM, Prins JR, Louise Hull M, et al. Therapeutic Potential of Regulatory T Cells in Preeclampsia-Opportunities and Challenges. Front Immunol (2019) 10:478. doi: 10.3389/fimmu.2019.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-Positive Functional Regulatory T Cells Are Decreased in Decidua of Miscarriage Cases With Normal Fetal Chromosomal Content. J Reprod Immunol (2015) 107:10–9. doi: 10.1016/j.jri.2014.09.053 [DOI] [PubMed] [Google Scholar]
- 32. Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, Van Gassen S, et al. An Immune Clock of Human Pregnancy. Sci Immunol (2017) 2:eaan2946. doi: 10.1126/sciimmunol.aan2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rackaityte E, Halkias J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front Immunol (2020) 11:588. doi: 10.3389/fimmu.2020.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Weight Loss Instead of Weight Gain Within the Guidelines in Obese Women During Pregnancy: A Systematic Review and Meta-Analyses of Maternal and Infant Outcomes. PloS One (2015) 10:e0132650. doi: 10.1371/journal.pone.0132650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the Quality of Studies in Meta-Analyses: Advantages and Limitations of the Newcastle Ottawa Scale. World J Meta-Analysis (2017) 5:80. doi: 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- 37. Ryan R. Planning the Analysis at Protocol Stage. Cochrane Consum Commun Rev (2013) 2016:2–9. [Google Scholar]
- 38. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toldi G, Rigó J, Stenczer B, Vásárhelyi B, Molvarec A. Increased Prevalence of IL-17-Producing Peripheral Blood Lymphocytes in Pre-Eclampsia. Am J Reprod Immunol (2011) 66:223–9. doi: 10.1111/j.1600-0897.2011.00987.x [DOI] [PubMed] [Google Scholar]
- 42. Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The Predominance of Th17 Lymphocytes and Decreased Number and Function of Treg Cells in Preeclampsia. J Reprod Immunol (2012) 93:75–81. doi: 10.1016/j.jri.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 43. Nguyen TA, Kahn DA, Loewendorf AI. Maternal—Fetal Rejection Reactions Are Unconstrained in Preeclamptic Women. PloS One (2017) 12:e0188250. doi: 10.1371/journal.pone.0188250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribeiro VR, Romao-Veiga M, Romagnoli GG, Matias ML, Nunes PR, Borges VTM, et al. Association Between Cytokine Profile and Transcription Factors Produced by T-Cell Subsets in Early- and Late-Onset Pre-Eclampsia. Immunology (2017) 152:163–73. doi: 10.1111/imm.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Z, Liu H, Shi Y, Xu N, Wang Y, Li A, et al. Increased Circulating Th22 Cells Correlated With Th17 Cells in Patients With Severe Preeclampsia. Hypertens Pregnancy (2017) 36:100–7. doi: 10.1080/10641955.2016.1239737 [DOI] [PubMed] [Google Scholar]
- 46. Salazar Garcia MD, Mobley Y, Henson J, Davies M, Skariah A, Dambaeva S, et al. Early Pregnancy Immune Biomarkers in Peripheral Blood may Predict Preeclampsia. J Reprod Immunol (2018) 125:25–31. doi: 10.1016/j.jri.2017.10.048 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Zhao L, Wang D, Xu Y, Gao H, Tan W, et al. Contribution of Regulatory T�cells to Immune Tolerance and Association of microRNA−210 and Foxp3 in Preeclampsia. Mol Med Rep (2018) 19:1150–8. doi: 10.3892/mmr.2018.9733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zare M, Doroudchi M, Gharesi-Fard B. Altered Frequencies of CD4+ CD25+ Foxp3+ and CD8+ CD25+ Foxp3+ Regulatory T Cells in Pre-Eclampsia. Iran J Allergy Asthma Immunol (2018) 17:540–7. [PubMed] [Google Scholar]
- 49. Wang Y, Liu Y, Shu C, Wan J, Shan Y, Zhi X, et al. Inhibition of Pregnancy-Associated Granulocytic Myeloid-Derived Suppressor Cell Expansion and Arginase-1 Production in Preeclampsia. J Reprod Immunol (2018) 127:48–54. doi: 10.1016/j.jri.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 50. Daraei N, Ghafourian M, Ghadiri A, Amari A, Najafian M, Rokhafrooz S. Evaluation of Exhausted Regulatory T Cells in Preeclampsia. Iran J Immunol (2019) 16:163–9. doi: 10.22034/IJI.2019.80259 [DOI] [PubMed] [Google Scholar]
- 51. Ding H, Dai Y, Lei Y, Wang Z, Liu D, Li R, et al. Upregulation of CD81 in Trophoblasts Induces an Imbalance of Treg/Th17 Cells by Promoting IL-6 Expression in Preeclampsia. Cell Mol Immunol (2019) 16:302–12. doi: 10.1038/s41423-018-0186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A, et al. The Imbalance of Th17/Treg Axis Involved in the Pathogenesis of Preeclampsia. J Cell Physiol (2019) 234:5106–16. doi: 10.1002/jcp.27315 [DOI] [PubMed] [Google Scholar]
- 53. Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vásárhelyi B, et al. The Frequency of Peripheral Blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ Regulatory T Cells in Normal Pregnancy and Pre-Eclampsia. Am J Reprod Immunol (2012) 68:175–80. doi: 10.1111/j.1600-0897.2012.01145.x [DOI] [PubMed] [Google Scholar]
- 54. Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, et al. Decreased Maternal Serum Acetate and Impaired Fetal Thymic and Regulatory T Cell Development in Preeclampsia. Nat Commun (2019) 10:3031. doi: 10.1038/s41467-019-10703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li J, Huang L, Wang S, Zhang Z. The Prevalence of Regulatory T and Dendritic Cells is Altered in Peripheral Blood of Women With Pre-Eclampsia. Pregnancy Hypertens (2019) 17:233–40. doi: 10.1016/j.preghy.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 56. Zare M, Namavar Jahromi B, Gharesi-Fard B. Analysis of the Frequencies and Functions of CD4+CD25+CD127low/neg, CD4+HLA-G+, and CD8+HLA-G+ Regulatory T Cells in Pre-Eclampsia. J Reprod Immunol (2019) 133:43–51. doi: 10.1016/j.jri.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 57. Meggyes M, Miko E, Lajko A, Csiszar B, Sandor B, Matrai P, et al. Involvement of the PD-1/PD-L1 Co-Inhibitory Pathway in the Pathogenesis of the Inflammatory Stage of Early-Onset Preeclampsia. Int J Mol Sci (2019) 20:583. doi: 10.3390/ijms20030583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Darmochwal-Kolarz D, Saito S, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. Apoptosis Signaling is Altered in CD4 +CD25 +FoxP3 + T Regulatory Lymphocytes in Pre-Eclampsi. Int J Mol Sci (2012) 13:6548–60. doi: 10.3390/ijms13066548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zeng B, Kwak-Kim J, Liu Y, Liao A-H. Treg Cells Are Negatively Correlated With Increased Memory B Cells in Pre-Eclampsia While Maintaining Suppressive Function on Autologous B-Cell Proliferation. Am J Reprod Immunol (2013) 70:454–63. doi: 10.1111/aji.12154 [DOI] [PubMed] [Google Scholar]
- 60. Moreno-Eutimio MA, Tovar-Rodríguez JM, Vargas-Avila K, Nieto-Velázquez NG, Frías-De-León MG, Sierra-Martinez M, et al. Increased Serum Levels of Inflammatory Mediators and Low Frequency of Regulatory T Cells in the Peripheral Blood of Preeclamptic Mexican Women. BioMed Res Int (2014) 2014:1–8. doi: 10.1155/2014/413249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nagayama S, Ohkuchi A, Shirasuna K, Takahashi K, Suzuki H, Hirashima C, et al. The Frequency of Peripheral Blood CD4 + FoxP3 + Regulatory T Cells in Women With Pre-Eclampsia and Those With High-Risk Factors for Pre-Eclampsia. Hypertens Pregnancy (2015) 34:443–55. doi: 10.3109/10641955.2015.1065884 [DOI] [PubMed] [Google Scholar]
- 62. Toldi G, Vásárhelyi ZE, Rigó J, Orbán C, Tamássy Z, Bajnok A, et al. Prevalence of Regulatory T-Cell Subtypes in Preeclampsia. Am J Reprod Immunol (2015) 74:110–5. doi: 10.1111/aji.12380 [DOI] [PubMed] [Google Scholar]
- 63. Wagner MI, Jöst M, Spratte J, Schaier M, Mahnke K, Meuer S, et al. Differentiation of ICOS + and ICOS – Recent Thymic Emigrant Regulatory T Cells (RTE T Regs ) During Normal Pregnancy, Pre-Eclampsia and HELLP Syndrome. Clin Exp Immunol (2016) 183:129–42. doi: 10.1111/cei.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vianna P, Mondadori AG, Bauer ME, Dornfeld D, Chies JAB. HLA-G and CD8+ Regulatory T Cells in the Inflammatory Environment of Pre-Eclampsia. Reproduction (2016) 152:741–51. doi: 10.1530/REP-15-0608 [DOI] [PubMed] [Google Scholar]
- 65. Jianjun Z, Yali H, Zhiqun W, Mingming Z, Xia Z. ORIGINAL ARTICLE: Imbalance of T-Cell Transcription Factors Contributes to the Th1 Type Immunity Predominant in Pre-Eclampsia. Am J Reprod Immunol (2009) 63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x [DOI] [PubMed] [Google Scholar]
- 66. Cao W, Wang X, Chen T, Zhu H, Xu W, Zhao S, et al. The Expression of Notch/Notch Ligand, IL-35, IL-17, and Th17/Treg in Preeclampsia. Dis Markers (2015) 2015:1–9. doi: 10.1155/2015/316182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang J, Wen ZQ, Cheng X-Y, Mei TY, Chen Z-F, Su L-X. siRNA-Mediated Knockdown of T-Bet and Rorγt Contributes to Decreased Inflammation in Pre-Eclampsia. Mol Med Rep (2017) 16:6368–75. doi: 10.3892/mmr.2017.7348 [DOI] [PubMed] [Google Scholar]
- 68. Yu J, Qian L, Wu F, Li M, Chen W, Wang H. Decreased Frequency of Peripheral Blood CD8 + CD25 + FoxP3 + Regulatory T Cells Correlates With IL-33 Levels in Pre-Eclampsia. Hypertens Pregnancy (2017) 36:217–25. doi: 10.1080/10641955.2017.1302470 [DOI] [PubMed] [Google Scholar]
- 69. Jianjun Z, Yali H, Zhiqun W, Mingming Z, Xia Z. ORIGINAL ARTICLE: Imbalance of T-Cell Transcription Factors Contributes to the Th1 Type Immunity Predominant in Pre-Eclampsia. Am J Reprod Immunol (2009) 63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x [DOI] [PubMed] [Google Scholar]
- 70. Liu X, Liu Y, Ding M, Wang X. Reduced Expression of Indoleamine 2,3-Dioxygenase Participates in Pathogenesis of Preeclampsia via Regulatory T Cells. Mol Med Rep (2011) 4:53–8. doi: 10.3892/mmr.2010.395 [DOI] [PubMed] [Google Scholar]
- 71. Orlovic Vlaho M, Tomic V, Vukojevic K, Vasilj A, Pejic R, Lesko J, et al. CD25+FOXP3+ and CD4+CD25+ Cells Distribution in Decidual Departments of Women With Severe and Mild Pre-Eclampsia: Comparison With Healthy Pregnancies. Am J Reprod Immunol (2020) 84:1–8. doi: 10.1111/aji.13281 [DOI] [PubMed] [Google Scholar]
- 72. Xiong H, Zhou C, Qi G. Proportional Changes of CD4+CD25+Foxp3+ Regulatory T Cells in Maternal Peripheral Blood During Pregnancy and Labor at Term and Preterm. Clin Investig Med (2010) 33:422. doi: 10.25011/cim.v33i6.14594 [DOI] [PubMed] [Google Scholar]
- 73. Koucký M, Malíčková K, Cindrová-Davies T, Germanová A, Pařízek A, Kalousová M, et al. Low Levels of Circulating T-Regulatory Lymphocytes and Short Cervical Length Are Associated With Preterm Labor. J Reprod Immunol (2014) 106:110–7. doi: 10.1016/j.jri.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 74. Han X, Ghaemi MS, Ando K, Peterson LS, Ganio EA, Tsai AS, et al. Differential Dynamics of the Maternal Immune System in Healthy Pregnancy and Preeclampsia. Front Immunol (2019) 10:1305. doi: 10.3389/fimmu.2019.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and Preterm Labor: Decreased Suppressive Activity and Changes in Composition of the Regulatory T-Cell Pool. Immunol Cell Biol (2012) 90:935–44. doi: 10.1038/icb.2012.33 [DOI] [PubMed] [Google Scholar]
- 76. Whirledge S, Cidlowski JA. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol Metab (2017) 28:399–415. doi: 10.1016/j.tem.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin Versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med (2017) 377:613–22. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 78. Wagner N-M, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, et al. Circulating Regulatory T Cells Are Reduced in Obesity and may Identify Subjects at Increased Metabolic and Cardiovascular Risk. Obesity (2013) 21:461–8. doi: 10.1002/oby.20087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.