Abstract
Purpose: The present study retrospectively analyzed thesafety and efficacy of computed tomography (CT)-guided cryoablationin the treatment ofunresectable or recurrent advanced colorectal cancer, which did not respond well to or experienced progression with radiotherapy or chemotherapy.
Materials and Methods: From January 2013 to April 2015, 31 lesions in 27 patients (16 males, 11 females; mean age of 57.2 years) with pelvic unresectableadvanced or recurrent colorectal cancer were included in the study. The tumor diameter was approximately 3.37 ±1.41 cm. The primary tumor included 25 rectal cancers, 1 sigmoid colon adenocarcinoma, and 1 ileocecal mucinous adenocarcinoma. Cryoablation was performed with 17-gauge cryoprobes and monitored by 64-slice spiral CT. Follow-up was carried out by contrast-enhanced magnetic resonance imaging (MRI). The treatment efficacy was evaluated by symptom palliation, decreased carcinoembryonic antigen (CEA) serum level, and tumor response.
Results: The cryoablation procedure was well-tolerated in all patients without major complications or procedure-related mortality. Long-term complications included abscess formation (1 patient), skin frostbite and post-sacrum antrum formation (1 patient). Pain relief was satisfactory in patients with perineal pain (P<0.001), and the median time of pain relief was 3.0 months. Complete ablations were obtained in 22 lesions of 18 patients, while 9 lesions in 9 patients underwent incomplete ablation. The median time to local recurrence for lesions with complete ablations was 15.0 months, and that to the progression of tumors with incomplete ablation was 4.0 months.
Conclusion: CT-guided cryoablation is a minimally invasive, safe, and effective therapeutic option for unresectableadvanced or recurrent colorectal cancer. The treatment is well-tolerated by patients, and pain relief is achieved rapidly.
Keywords: cryoablation, colorectal cancer, pelvic recurrence, pelvic cancer
INTRODUCTION
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide. About 132,700 new cases of large bowel cancer are diagnosed annually, of which 93,090 are colon, and the remaining are rectal cancers (1). Although surgery remains the primary treatment for colorectal cancer, radiotherapy and chemotherapy are also critical supplementary treatments. The results of CRC surgery or chemo-radiotherapy are limited due to the development of local recurrence that is a great challenge for the treatment. However, no standard care is available for patients with advanced CRC, who failed the second-line treatment. In the case of patients who did not respond well to or experienced progression with radiotherapy or chemotherapy, various forms of physical ablation treatments controlled the local symptoms and complications.
In the past two decades, the ablative management of tumors has been acceptedcomprehensively, and several thermal and nonthermal ablation modalities are available (2). Compared to thermal ablation, cryoablation has unique advantages. Owing to the ability of well-visualized ice ball on computed tomography (CT) imaging, an alleviated effect, controllable ablation zone, and cryoablation are potential alternatives for treatment strategies in colorectal cancer. The study retrospectively evaluated the clinical results of CT-guided cryoablation in the treatment of patients with unresectable advanced or recurrent colorectal cancer.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center. From January 2013 to April 2015, 27 consecutive patients with a histologically confirmed diagnosis of advanced colon cancer (TNM stage IIIb, IIIc, or IV, 7th Edition) were treated using cryoablation. The inclusion criteria of the patients were as follows: local recurrence after radical resection treated with adjuvant chemotherapy and unresectable abdominopelvic metastases after chemotherapy or radiotherapy within 12 months. The inclusion criteria for cryoablation were as follows: age ≥20 years; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; neutrophil count ≥1.5×109/L; platelet count ≥75×109/L; prothrombin activity ≥50%, hemoglobin level ≥90 g/L; total bilirubin ≤1.5-fold of the upper limit of normal (ULN); aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase ≤2.5-fold of the ULN; serum creatinine ≤1.5-fold of the ULN; provide signed informed consent. All patients underwent recent (<2 weeks) contrast-enhanced CT or magnetic resonance imaging (MRI) documentation of the target tumor (3). Imaging findings, especially tumor size and its correlation to adjacent structures, were used to plan the ablative procedure.
Patients with any of the following conditions were excluded from the study: peritoneal metastases causing obstruction or imminent obstruction; synchronous liver or lung metastasis; a bleeding diathesis or coagulopathy; international normalized ratio ≤1.5 within 2 weeks before treatment; long-term treatment with high-dose aspirin (≥325 mg/day); uncontrolled active infection.
CT-guided percutaneous cryoablation
Cryoablation was performed under local anesthesia on an inpatient basis by physicians with >5-year experience. Before the procedure, 10 mg diazepam was administered by intramuscular injection as basal sedation, supplemented with 5 mg analgesic morphine as required. Patients underwent continuous pulse oximetry and Electrocardiography (ECG). The Philips 64-slice spiral CT (120 kV, 250 mA, and 3-mm thickness; Philips Healthcare, Andover, MA, USA) was used for imaging guidance, localization, and intraoperative real-time monitoring of the ablation procedures in order to avoid spreading the injured extension into the surrounding critical structures.
A tabletop argon gas-based cryoablation apparatus (Precise Cryoablation System; Galil Medical, Yokneam, Israel) with 17-gauge cryoprobes was used to perform the procedure. The cryoprobes were placed into the lesion within 1.5 cm from the tumor edge, but <2 cm interval between the probes (4). The number and type of probes used were dependent on the preoperative tumor volume in order to ensure producing precise ice balls that encompassed the target tumor. However, the invasion of the adjacent structures such as the urinary bladder, uterus, vagina, seminal vesicle, or small bowel should be under intensive focus. After the cryoprobes had been placed, the cryosurgery system was initiated to begin rapid freezing. A double freeze-thaw cycle (15 min/time: freezing for 10 min and thawing for 5 min) was performed according to the standard protocols. An additional cycle was necessary if the ablation zone was not satisfied, followed by repeat ablations if the carcinoembryonic antigen (CEA)-levels were elevated, and tumor progression or recurrence was noted on CT scan or MRI.
Postoperative follow-up
The follow-up was performed from day 1 after the final cryoablation until the patient died or was lost to follow-up that ranged from 15–34 (average, 19±5.4) months. The follow-up and staging were carried out by contrast-enhanced MRI at the 1-month interval after treatment. CT or MRI was also performed as required when procedure-related complications were suspected. Positron emission computed tomography (PET) scan was used selectively. Physical examination, laboratory assessments such as carcinoembryonic antigen (CEA) levels, and blood routine test were conducted every month in all patients.
Evaluation of safety and efficacy
Complications were defined as major life-threatening complications requiring active treatment or minor complications requiring only symptomatic treatment according to the Society of Interventional Radiology Clinical Practice Guidelines (3, 5). The treatment efficacy was confirmed by symptom palliation, decreased CEA serum level, and tumor response. The degree of pain palliation was recorded using the numerical rating scale (NRS) (6). A multidisciplinary colon cancer team in the hospital confirmed the treatment response. Low-density ice ball encompassing the whole tumor on CT during the procedure indicated complete ablation that did not demonstrate any enhancement in the ablation zone on the contrast-enhanced MRI 1-month after treatment. Local tumor recurrence was defined as the presence of irregular focal soft-tissue enhancement on follow-up imaging after >1 month of treatment. The progression of tumor was defined as increased size in at least 20% of the total diameters according to the modified Response Evaluation Criteria for Solid Tumors (mRECST) (7).
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD)and qualitative data as frequency and rate. The statistical analysis was performed using the statistical software SPSS 23.0 (Chicago, IL, USA). The NRS scores or the level of CEA between pre- and post-ablation were compared by Wilcoxon’s signed rank test. The survival analysis of pain relief, as well as, local recurrent and tumor progression were estimated by Kaplan–Meier method. P<0.05 was considered to indicate a statistically significant difference.
RESULTS
Patients’ baseline characteristics
A total of 27consecutive patients (16 males and 11 females), with a mean age of 57.2 ± 10.7 (range, 33–75) years were included in the study. Twenty-four patients presented solitary lesions, and 3 patients were treated for more than one tumor, with a total of 31 tumors. The tumor diameter was 1.2–6.3 (mean, 3.37 ± 1.41) cm. The primary tumor included 25 rectal cancers, 1 sigmoid colon adenocarcinoma, and 1 ileocecal mucinous adenocarcinoma. Of the 25 rectal cancers, 4 cases were well-differentiated adenocarcinoma, 1 was melanoma, and 1 was a neuroendocrine neoplasm, while the remaining 19 cases (76%) were moderate-to-low differentiated adenocarcinomas. Twenty-six (96.3%) patients exhibited pelvic recurrence after curative resection, and only 1 case presented an unresectable advancedcolorectal cancer. The median recurrence time after resection was 19.5 (range, 3–121) months. The abdominoperineal resection (Miles operation) was performed on 20 patients, low anterior resection (Dixon operation) on 5 patients, and ileocecectomy on 1 patient. Two patients underwent palliative care combined with adjuvant chemotherapy as an initial treatment. Subsequently, after recurrence, all patients underwent radiotherapy or chemotherapy, and the lesions failed to respond to chemoradiotherapy. Prior to cryoablation, 7 patients were asymptomatic, while 14 experienced perineum pain, 4 had single lower limbnumbness, and 2 showed urinary symptoms such as frequent urination, or dysuria. The patients’ baseline characteristics are summarized in Table 1.
Table 1.
Characteristics of patients (n=27).
| Characteristic | N (%) |
|---|---|
| Age (years) | 57.2 ± 10.7 (33–75) |
| sex | |
| male | 16 (59.3) |
| female | 11 (40.7) |
| ECOG performance status | |
| PS 0 | 10 (37.0) |
| PS 1 | 17 (63.0) |
| Primary tumor | |
| Rectal cancer | 25 (92.6) |
| Sigmoid colon cancer | 1 (3.7) |
| Ileocecal cancer | 1 (3.7) |
| Number of tumors | 31/27 |
| Solitary | 24 |
| Multiple | 3 |
| Tumor location | |
| anterior sacral | 16/14 (51.6) |
| perineum | 9/9 (29.0) |
| iliac region | 6/4 (19.4) |
| Major symptom | |
| perineum pain | 14 (51.9) |
| single lower limb numbness | 4 (14.8) |
| urinary symptoms | 2 (7.4) |
| asymptomatic | 7 (25.9) |
ECOG: Eastern Cooperative Oncology Group
Complications
The median number of cryoprobes was 3 (range, 1–14), that of the freezing time for each patient was 20 (range, 20–30) min, and the median rewarming time was 10 (range, 10–15) min. The cryoablation procedure was well-tolerated in all patients without major complications or procedure-related mortality (Fig. 1). Seven patients complained about neuralgia or dull pains during the ablation procedure; however, ablation was paused, and 5 mg morphine was administered to control pain only in 4 patients. Plain CT was performed immediately after ablation did not demonstrate any procedure-related complications.
Figure 1.
A 42-year-old male with rectal adenocarcinoma underwent Abdominoperineal resection and chemoradiotherapy 13 months before recurrent. a) CT-guided percutaneous cryoablation was performed with 7 cryoprobes placed into the anterior sacral tumor. b) The low density ice ball covering whole tumor on CT scan immediately after ablation procedure. c) Axial contrast-enhancement T1-weighted MRI showing pelvic recurrent before cryoablation. d) The treated lesion was complete ablated with lack of enhancement on MRI one month after cryoablation.
In the 3 days after cryotherapy, dysuria and urinary retention were observed in 3 patients (11.1%), and neuralgia, lower limb paralysis, and walking function impairment emerged in 2 patients (7.4%). Moreover, 4 patients (14.8%) had fever (body temperature up to 39 ℃) during hospitalization. The short-term complications were transient and managed with antibiotics, analgesia, and supportive treatment. Long-term complications included abscess formation (1 patient required percutaneous drainage), skin frostbite, and post-sacrum antrum formation (1 patient). Other major complications were not noted in the current study, all complications are summarized in Table 1.
Efficacy
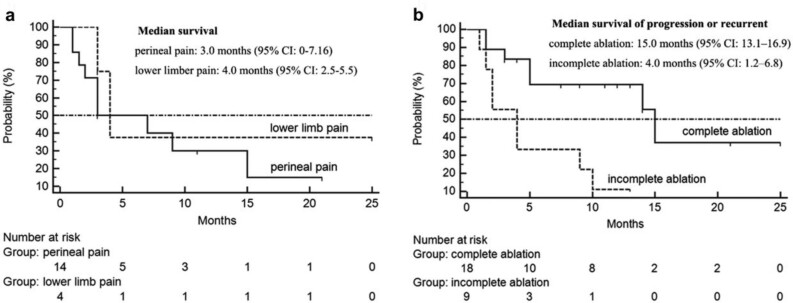
Before cryoablation, 14 patients (51.9%) had perineal pain with median NRS score 6.5 (range, 4–9), while 4 patients (14.8%) had single low limb numbness and sciatic pain with median NRS score 4 (range, 2–6). One week after treatment, the median NRS score was 3 (range, 0–6) and 2 (range, 1–4), respectively. All patients experienced relief from painful symptoms with reduced use of analgesics. The pain relief was unsatisfactory in patients with lower limb numbness and sciatic pain (P=0.059) as compared to those with perineal pain (P<0.001, Table 3). The tumors associated with perineal pain were localized at the anterior sacral and liable to complete ablation. Of these, only 2 patients, with index tumor infiltrate to urinary bladder or close to perineum presented a reduced NRS score <2. The median time of pain relief was 3.0 months [95% confidence interval (CI): 0–7.16] in patients with perineal pain and4.0months (95% CI: 2.5–5.5) in patients with lower limb pain (Fig. 2a).
Table 2.
Post-cryoablation adverse events (n=27).
| Symptom | N (%) |
|---|---|
| Severe pain | 4 (14.8%) |
| High fever | 4 (14.8%) |
| Symptom of urinary system | 3 (11.1%) |
| Symptom of nerve | 2 (7.4%) |
| Abscess formation | 1 (3.7%) |
| Skin frostbite | 1 (3.7%) |
| Post-sacrum antrum formation | 1 (3.7%) |
Table 3.
Efficacy of pain control (n=27).
| Pain | No. (%) | NRS pre-cryoablation | NRS post-ablation | P |
|---|---|---|---|---|
| Perineal pain | 14 (51.9) | 6.5 (4–9) | 3 (0–6) | 0.001 |
| Sciatic pain | 4 (14.8) | 4 (2–6) | 2 (1–4) | 0.059 |
Figure 2.
The survival curves of pain relief, local recurrent and tumor progression. a) Survival of pain relief in patients with lower limb pain and perineal pain. b) Probability of progression in patients with incomplete ablation and recurrent in patients with complete ablation after cryoablation.
The low-density ice ball encompassing the whole tumor on CT during treatment was observed in 27 lesions (23 patients), 4 patients with large lesions adjacent to the urinary bladder, and perineum skin that accepted incomplete ablation. However, complete ablations were obtained in 22 lesions of 18 patients that were confirmed by MRI 1 month post-cryoablation (Fig. 1d). A total of 9 lesions were incomplete with irregular enhancement of the tumor margin as observed by MRI, of which, 3 tumors were close to iliac vessels. A total of 15 patients displayed increased CEA levels before treatment (normal value, 5 ng/mL); of these, 11 showed significantly decreased levels as a result of cryoablation (P=0.005).
During the 11.0 ± 7.8-month follow-up, 3 (11.1%) patients died, 1 (3.7%) was lost to follow-up, and 23 (85.2%) were alive. Five (18.5%) alive patients were free of disease. The median time to progression of the tumor with incomplete ablation was 4.0 months (95% CI: 1.2–6.8). Moreover, the median time to local recurrence in lesions with complete ablation was 15.0 months (95% CI: 13.1–16.9) (Fig. 2b). Of the 9 patients, who could not obtain achieve tumor ablation, 2 presented lung metastasis at the end of the follow-up. In 3 patients, repeated cryoablation was performed to treat the local recurrences. Also, percutaneous transarterial embolization was performed through the internal iliac artery for further treatment in 5 patients with tumor progression.
DISCUSSION
In recent years, significant improvements have been observed in the treatment of patients with colon and rectal cancers. Combined with neoadjuvantor adjuvant chemotherapy, the indications of curative surgery were extended, and advanced tumors including liver or lung metastasis could convert to resection, which provided additional benefit to those patients. However, local recurrence always occurs within 5 years after primary treatment, especially within the first 2-3 postoperative years, and is usually close to the pelvic side walls and internal iliac vessels (8, 9). Moreover, several advanced tumors respond to radiotherapy or chemotherapy, supportive care, palliative treatment, or symptom control. In this study, we described our experience of utilizing cryoablationin pelvic recurrence orunresectable advanced colorectal cancer after the previous failure with chemoradiotherapy.
Cryoablation causes the formation of ice crystals within the cells, inducing membrane rupture and cell death via cellular dehydration and local tissue ischemia. Furthermore, low temperature can lead to microvascular contraction, leading to ischemic necrosis of the tumor cells. In the current study, the target lesions that were primarily fixed to the pelvic wall or urinary system were not resectable, rather associated with systemic or local complications, such as pain, kinetic abnormalities (walking or sitting impairment), lower limb edema, hematuresis, or dysuria (6, 10). For these patients, image-guided ablation could be considered as a local treatment. Furthermore, ablation therapy was regarded as a promising method for tumor regression, for which, recent evidence suggested survival advantage as compared to the best supportive care (6). Few studies have explored the application of radiofrequency ablation (RFA) to pelvic recurrence of rectal cancer (9, 11). Belfiore et al. reported that 14 unresectable patients with recurrent rectal cancer received RFA treatment, 11 patients achieved satisfactory pain reduction, and 10 patients showed locoregional control of tumor progression (12). RFA combined with surgical debulking was also feasible in locally advanced abdominopelvic malignancies (13, 14). Although a majority of the patients in these studies showed adequate pain palliation and tumor necrosis, while analgesia management was frequently needed during intolerable pain. In addition, heat-based ablation rapidly created coagulation necrosis that caused irreversible damage. Nevertheless, cryoablation exhibited specific advantages on tolerability and safety as compared to RFA (11). Since the margin of the freeze zone showed low-density changes on CT imaging, physicians can assess of the ablated region exactly during the procedure, thereby avoiding damage to the adjacent vulnerable structures. In the current study, 1 patient had skin frostbite due to the close distance between the lesion and haunch skin, while the neural functional injury referring to the cryoablation zone was transient and would be regenerated over time. The neurological symptoms were recorded in only 2 patients. Hence, cryoablation is safe in treatment lesions involving pelvic nerve plexus.
In addition, cryoablation efficiently controlled pain and improved the related disability and quality of life (15). Patients with pelvic recurrence of rectal carcinoma exhibited a poor quality of life due to painful symptoms. In this study, a total of 18patients (66.7%) suffered pain before treatment, and the primary objective of treatment for these patients was to control pain that was palliated significantly 1 week after cryoablation as measured by NRS. Moreover, the median time of pain control was >3 months, which suggested significant improvement in the quality of life.
Although complete ablation was obtained in only18 patients, the cases without complete lesion ablation also achieved satisfactory pain control and local tumor control. In these patients, the ablation zones encompassed >90% of the target lesions that sufficiently avoided severe complications. At the end of follow-up, 8/9 patients, who did not obtain complete tumor ablation, showed tumor progression; of these 3 underwent repeat cryoablation and 5 accepted further transarterial chemotherapy and embolization.
Nevertheless, the present study had several limitations. This was a retrospective study with small cohort and short-term follow-up, and no control group was included. Thus, we could not analyze the overall survival. In addition, the clinical stage of colorectal cancer in this study was inhomogeneous, and the locations of the target lesions were variable. Only patients without obviously invading adjacent structures were included in the present study, which limited the sample size that might potentially underestimate the incidence of complications. Nonetheless, the distance between the lesion andadjacent major structures should be measured accurately before cryoablation. The potentially curative effect of cryoablation in the management of patients with pelvic malignancy necessitates furtherrandomized controlled trials.
Cryoablation seemed to be an attractive alternative for pelvic recurrence or metastasis with satisfying tumor regression and symptoms control.
Conflict of interest:
The authors declare that they have no conflict of interest.
Funding:
This study was supported by grants from the National Natural Sciences Foundation of China (No. 81501562) and the National Key Research and Development Program of China (No. 2016YFC0106203).
Ethical approval: This retrospective study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center.
Informed consent: Institutional review board approval was obtained for this study, with waiver of informed consent for retrospective review of our clinical database.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 5.Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–191. doi: 10.1016/j.jvir.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Mylona S, Karagiannis G, Patsoura S, et al. Palliative treatment of rectal carcinoma recurrence using radiofrequency ablation. Cardiovasc Intervent Radiol. 2012;35:875–882. doi: 10.1007/s00270-011-0320-x. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Watanabe H, Sone M, et al. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16–22. doi: 10.3109/03009734.2012.729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun HR, Lee LJ, Park JH, et al. Local recurrence after curative resection in patients with colon and rectal cancers. Int J Colorectal Dis. 2008;23:1081–1087. doi: 10.1007/s00384-008-0530-0. [DOI] [PubMed] [Google Scholar]
- 9.Lefevre JH, Parc Y, Lewin M, et al. Radiofrequency ablation for recurrent pelvic cancer. Colorectal Dis. 2008;10:781–784. doi: 10.1111/j.1463-1318.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 10.Colibaseanu DT, Dozois EJ, Mathis KL, et al. Extended sacropelvic resection for locally recurrent rectal cancer: can it be done safely and with good oncologic outcomes? Dis Colon Rectum. 2014;57:47–55. doi: 10.1097/DCR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 11.Ohhigashi S, Watanabe F. Radiofrequency ablation is useful for selected cases of pelvic recurrence of rectal carcinoma. Tech Coloproctol. 2003;7:186–191. doi: 10.1007/s10151-003-0033-5. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore G, Tedeschi E, Ronza FM, et al. CT-guided radiofrequency ablation in the treatment of recurrent rectal cancer. AJR Am J Roentgenol. 2009;192:137–141. doi: 10.2214/AJR.07.2649. [DOI] [PubMed] [Google Scholar]
- 13.Gajdos C, Macdermott T, McCarter MD, et al. Combined thermal-surgical ablation of locally advanced abdominopelvic malignancies. Ann Surg Oncol. 2011;18:1267–1273. doi: 10.1245/s10434-010-1467-4. [DOI] [PubMed] [Google Scholar]
- 14.Spiliotis J, Hadjicostas P, Rogdakis A, et al. Management of advanced abdominopelvic tumors with combined radiofrequency ablation and surgical debulking. Dig Surg. 2008;25:188–190. doi: 10.1159/000140687. [DOI] [PubMed] [Google Scholar]
- 15.Simon CJ, Dupuy DE. Image-guided ablative techniques in pelvic malignancies: radiofrequency ablation, cryoablation, microwave ablation. Surg Oncol Clin N Am. 2005;14:419–431. doi: 10.1016/j.soc.2004.11.005. [DOI] [PubMed] [Google Scholar]