Abstract
Stem cell-derived exosomes (SC-EXO) was an emerging therapeutic agent in regenerative medicine. Intratunical injection of SC-EXO is considered as a prospective approach for erectile dysfunction (ED) treatment. However, high vascularization of cavernous body makes effective retention a major challenge for SC-EXO intratunical injection. In this study, a Polydopamine nanoparticles (PDNPs) incorporated poly (ethylene glycol)-poly(ε-caprolactone-co-lactide) (PDNPs-PELA) thermosensitive hydrogels were fabricated by a facile in situ polymerization for intratunical administration of adipose stem cell-derived exosomes (EXO). The hydrogels exhibited sol-gel transition at body temperature. Moreover, the in-situ polymerization of PDNPs using poly (ethylene glycol)-poly(ε-caprolactone-co-lactide) (PELA) block copolymer as a template was found to be more stable dispersion in the gel system. After being encapsulated into the hydrogel, EXO shows sustained release behavior within two weeks. In vivo animal experiments revealed that exosomes released from hydrogel lead to the healing of endothelial cells and neurons, increase of the cavity's pressure, thereby restoring the erectile function. In particular, since the PDNPs in thermosensitive gels have excellent photoacoustic performance, the hydrogel can be accurately delivered into the tunica albuginea by the guidance of real-time photoacoustic imaging. These results suggest that the as-prepared PDNPs-PELA has a promising future as an injectable exosome carrier for ED treatment.
Keywords: Photoacoustic imaging, Exosomes, Thermosensitive gel, Intratunical injection, Erectile dysfunction
Graphical abstract

Highlight
-
•
A temperature-sensitive hydrogel with photoacoustic activity was developed for intratunical injection by in-situ polymerization.
•The exosomes encapsulated in the hydrogel can be slowly released and effectively restore damaged nerve and vascular endothelial cells.
•The injection guided by photoacoustic images realizes accurate puncture and real-time filling detection of hydrogel in the corpus cavernosum.
1. Introduction
Erectile dysfunction (ED) is a highly prevalent health problem that seriously constrain life quality of elderly men and their partners [1]. Oral phosphodiesterase type 5 inhibitors (PDE5-Is) are currently the first line therapeutic option for ED treatment [2]. However, it is not effective in up to 30% of ED patients [3]. Moreover, long-term PDE5-Is administration may lead to severe headache and hearing loss [4]. Accumulating evidence indicate the Stem cell-derived exosomes (SC-EXO) hold great promise for the restoration of erectile capacity in injured tissue. Wei et al. found that the adipose stem cell-derived exosomes (EXO) could restore the erectile function in type 2 diabetic rats [5]. Zhao further reveal that EXO can effectively improve erectile function in a rat arterial injury model [6].
At present, intratunical injection was an effective administration route for exosomes in ED treatment [7]. However, due to the abundant blood flow in the cavernous body, the exosomes are difficult to retain at the injection site and will be quickly cleared [8]. To address this issue, some hydrogels have been exploited to load exosomes for a sustained therapeutic effect. For example, Lin et al. [9] developed a kind of hyaluronic acid-based hydrogel. This hydrogel is crosslinked under the initiation of ultraviolet light. After being loaded with SC-EXO, it can accelerate the repair of cartilage defects. Mao [10] and Li et al. [11] respectively fabricated two thermo-sensitive hydrogels systems for the in situ delivery of stem cell exosomes. These hydrogels are versatile injectable and can effectively prevent the exosomes from being cleared by circulating fluids at the injection site. They showed the increasing efficiency of treatment and patient compliance. However, those hydrogels are not satisfactory for intratunical injection. Their gel time is generally more than 10 min, which might be diluted by the blood after injection. Furthermore, the polymers in those hydrogels are not easily degradable after exosome release. This may lead a corpora cavernosa occlusion and increase the risk of penis necrosis.
Temperature sensitive poly (ethylene glycol)-based block copolymer is an injectable and degradable hydrogel material. By adjusting the proportion of monomers in the polymer backbone, a hydrogel with a low critical temperature of about twenty-eight degrees can be obtained [12]. The hydrogel is injected in vivo in a sol state at low temperatures (normally less than 10 °C), and then undergoes a phase transformation towards the excitation of body temperature to form a gel. It can quickly form a hydrogel within 3 min after injection, thereby reducing the flushing effect of the blood. In addition, this hydrogel can be completely degraded into PEG and small molecule fragments within one month. This makes it easy to be expelled from the corpus cavernosum, which is conducive to the restoration of erectile function.
From a clinical perspective, accurate intratunical injection is the key to ED treatment. Since the corpus cavernous is rich in blood vessels, the traditional puncture method increases the risk of bleeding and edema. Moreover, the tunica albuginea is subcutaneous tissues so that it is difficult to determine its puncture location and depth by visual observation [13]. Traditional intratunical increase the risk of cavernous hemorrhage and arteriovenous fistula. The photoacoustic imaging can clearly distinguish the position and structure of the tunica albuginea [14]. It provides a new strategy for precise intratunical injection. Moreover, the angle and depth of puncture could be monitored and adjusted in real time during the injection [15]. This would make hydrogel more uniformly distributed in the tunica albuginea, therefore enhancing the likelihood of treatment success.
Herein, we constructed a polydopamine nanoparticles (PDNPs) incorporated poly (ethylene glycol)-poly(ε-caprolactone-co-lactide) (PELA) temperature-sensitive hydrogel and used it to encapsulate stem cell-derived exosomes. PDNPs were selected as biocompatible photoacoustic contrast agents [16], while PELA hydrogels were used to control the release of exosomes. The as-prepared hydrogel was expected to be accurately injected into the tunica albuginea under the guidance of photoacoustic images, followed by sustained release of exosomes in it. The rheology and sol-gel transition of the hydrogel were investigated. In addition, its cytocompatibility was evaluated by Cell Counting Kit-8 (CCK-8) and lactate dehydrogenase assay (LDH). The exosome release profile from the hydrogel was quantitatively studied. The intratunical injection of the composite hydrogel guided by photoacoustic images was verified in vivo. At last, the treatment effect of exosome-loaded composite hydrogels on rats ED model were studied by immunohistochemistry and intrasinus pressure detection. We highlighted the in vivo biocompatibility and ED restoration effect of PDNPs-PELA, which aimed at providing a substantial contribution towards accurate treatment of ED.
2. Materials and methods
2.1. Materials
Poly (ethylene glycol) (PEG), Caprolactone (CL), Dopamine hydrochloride were purchased from Sigma-Aldrich. Stannous octoate (95%) and D, l-lactide (LA) was bought from Acros Organics and distilled from calcium hydride (CaH2) under nitrogen before use. Sodium hydroxide was obtained from Sinopharm Chemical Reagent. CCK-8 kit was from Dojindo. LDH kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Dulbecco's modified Eagle's medium high glucose (DMEM), fetal bovine serum (FBS) penicillin, streptomycin as well as phosphate buffer saline (PBS) were received from Gibco (Grand Island, USA). Sprague-Dawley rats with 6–8 weeks old were bought from SLAC Laboratory Animal, Co., Ltd. (Shanghai, China). The water used in all experiments was purified using a Milli-Q water purification process (Millipore, Bedford, MA) with a resistivity at 18.2 MΩ. All other chemical compounds were of analytical grade from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Cell culture and exosomes extraction
Adipose-derived mesenchymal stem cells were purchased from Lonza (USA). Cells with three passages were used for exosome extraction. The cells were maintained in DMEM medium with 10% FBS at 37 °C in a humidified atmosphere of 5% CO2. The medium was changed every two days. When cells reached 80% confluence, it would be passaged by trypsinization. The medium was changed to serum-free medium before extracting exosomes. After next two days of culture, the supernatant was collected, and exosomes were extracted by differential centrifugation as described previously [17]. Briefly, 100 ml of cell supernatant was centrifuged at 2000 g for 20 min to remove cell, and then centrifuged at 15,000 g for 30 min to remove cell debris. Exosomes were pelleted by centrifugation at 110,000 g for 70 min at 4 °C. The obtained exosomes were stored in a −80° for subsequent test.
2.3. Synthesis of temperature-sensitive block copolymers
PELA triblock copolymer was synthesized by ring-opening polymerization of CL and LA in the presence of PEG using stannous octoate as a catalyst [18]. Briefly, 29.5 g of PEG was heated in a vacuum (less than 800 Pa) at 115 °C for 1 h to eliminate trace amounts of water. Subsequently, the distilled CL (32.2 g) and LA (32.0 g) were sequentially added to the cooled flask. Finally, a solution of stannous octoate (0.3% by mass of the reactant) dissolved in toluene was added to the system. The reaction was carried out at 140 °C for 15 h under the protection of dry nitrogen. The product was washed three times with 80 °C hot water precipitation for purification, then lyophilized and stored at −20 °C before use.
2.4. Preparation of PDNPs incorporated hydrogels
The hydrogel loaded with PDNPs was synthesized using in-situ polymerization. First, 10 g of PELA was dissolved in 40 ml of ultrapure water to form a uniform and transparent solution. Subsequently, 180 mg of dopamine hydrochloride was added. After complete dissolution, 0.38 ml of 1 M NaOH solution was added to the solution to trigger the polymerization. After 2 h of polymerization, the pH value was adjusted to 7.0 with 1 M HCl solution to terminate the reaction. The composite then purified by dialysis at 4 °C for 72 h. The dialyzed solution was lyophilized for 72 h to obtain PDNPs-PELA.
2.5. Characterization
The structure of the polymer was confirmed by Bruker NMR spectrometer (using deuterated DMSO as the solvent). The chemical groups of the composite are characterized by fourier transform infrared spectroscopy (FTIR, PerkinElmer model 1600-FTIR). The rheological properties of the gel were determined by a rheometer (Discovery Hybrid Rheometer (HR2), TA instruments.). The sol-gel transition of the PDNPs-PELA solution is demonstrated by flip test. The morphology of PDNPs in the gel was characterized by FEI Tecnai F20 transmission electron microscope. Photoacoustic imaging was captured using the Vevo LAZR photoacoustic-micro-ultrasound imaging system (FUJIFILM VisualSonics, Toronto, Canada) with a 21 MHz center frequency probe. The particle size was measured using DLS (Malvern, Zetasizer Nano ZS90).
2.6. In vitro degradation of the hydrogel
500 μl of 20% PDNPs-PELA gel was added to a 3 ml sample bottle. The weight of the gel is obtained and marked as W1. Samples were then incubated at 37 °C for 5 min. After the gel has formed, 2 ml of 37 °C PBS with 200 units lipase (from thermomyces lanuginosus) was added to the sample vials. Subsequently, the sample vial was placed on the shaker (500 rpm) with temperature at 37 °C. At different time points, the added PBS was aspirated and the gel was weighted as W2. The degradation rate of the gel is calculated according to the following formula:
2.7. In vitro cytotoxicity assay
The in vitro cytotoxicity was evaluated using Cell Counting Kit 8 (CCK-8) and lactate dehydrogenase kit analysis. The cell viability of smooth muscle cells (A7R5 from Shanghai Cell Bank, Chinese Academy of Sciences), nerve cells (PC12 cell from ATCC) and vascular endothelial cells (HUVEC form ATCC) treated with different PDNPs-PELA concentrations was investigated. Cell culture media and culture conditions according to supplier's instructions. In this experiment, a cell suspension was seeded in a 96-well plate at a density of 4000 cells per well. After 24 h of proliferation, cells were treated with different amounts of PDNPs-PELA in the range of 10–320 μg/ml (10, 20, 40, 80, 160, 320 μg/ml). After 1 and 3 days of incubation, cell viability was measured by CCK-8 and lactate dehydrogenase, respectively. The viability of the cells was calculated as percentage of the untreated control.
2.8. In vitro release of exosome
To test the retention of exosomes in PDNPs-PELA hydrogels, in vitro release profile was investigated. In this experiment, a commercially ELISA kit (ExoElisa) purchased from System Biosciences (SBI) (Mountain View, CA, USA) was used to determine the concentration of exosomes in the samples. Firstly, 150 μg exosomes were dissolved in 100 μL PDNPs-PELA gel (20% wt/v in PBS solution) form an exosomes-loaded PDNPs-PELA hydrogel (EXO@PDNPs-PELA). Afterwards, the EXO@PDNPs-PELA was added to a 1.5 ml centrifuge tube and incubated at 37 °C for 2 min to allow gel formation. The formed gel was then immersed in 0.5 ml pre-thermostated PBS at 37 °C. Finally, the centrifuge tube was placed on a 37 °C shaker and shaken at 200 rpm. 50 μl of supernatant was collected at different time points for concentration measurement. Each time after the 50 μl sample was removed, fresh pre-warmed (37 °C) 50 μl PBS was added to equilibrate the entire dissolution volume.
2.9. The ED model of rat
Male Sprague Dawley rats (6–8 weeks old) were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. Animals were maintained under standard light-dark cycle (06.00–18.00). All operation complied with US National Research Council's Guide for the Care and Use of Laboratory Animals, the US Public Health Services Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals. The experimental protocol was reviewed and approved by the Institutional Ethics Committee of Shanghai Ninth People's Hospital. Animals were randomly divided into five groups of 6 animals each. Group was detailed described as follows: Control (n = 6, sham operation), PBS (n = 6, treated by PBS injection), PELA (n = 6, treated by PELA hydrogel injection), AD-SC (n = 6, treated by AD-SC exosome injection), EXO@PDNPs-PELA (n = 6, treated by EXO@PDNPs-PELA injection). After anesthetized with 2% isoflurane inhalation with an isoflurane delivery system (Viking Medical, Medford, NJ), the rat was put on isothermal heat pad. Hair on the abdomen was shaved and disinfected with iodophor. Through a low abdominal midline incision, the cavernous nerves were exposed on bilateral dorsolateral lobes of the prostate and crushed with fine surgical forceps for 2 min. A rubber band was applied at the base of the penis to block blood flow. Then total 0.2 ml hydrogel was injected into the corpus cavernous under ultrasonographic guidance. The elastic tourniquet was kept for 120 s after injection to induce the hydrogel formation.
2.10. Erectile function evaluation
The right carotid artery, crus penis and bilateral cavernous nerves were exposed in an unanesthetized condition. Two 25-gauge catheters filled with 250 U/ml heparin solution were separately inserted into carotid artery and crus penis to record the intracavernous pressure (ICP) and real-time carotid arterial pressure (RT-AP) simultaneously. Cavernous nerve was stimulated by an electrode hook at intervals of 5 min (3 times/per side). The stimulus parameters were as follows: 1.5 mA, 20 Hz, pulse width 0.2 ms, and total duration 60s. At the end of testing, the penis tissues were harvested for immunofluorescence staining and Western Blot analysis [19].
2.11. Histology and immunohistochemistry
Tissues collected from rat penis were subjected to histological examination to investigate the distribution of hydrogel in the cavernous body of the penis. The tissue was washed with a PBS three times and then fixed at 4% formalin overnight. Subsequently, the fixed implants were dehydrated in a series of graded alcohols, embedded in paraffin, and sectioned at a thickness of 3 μm (Leica RM 2265). The sections were placed on a slide, stained with haematoxylin & eosin (H&E) and Masson's trichrome blue (Masson), and observed by a light microscope (Leica Microsystems, Germany).
For immunohistochemistry, the tissues were immersed in OCT (Sakura Finetek, Torrance, CA, USA) and frozen immediately using liquid nitrogen. Sections were cut at a thickness of 5 mm. Immunofluorescence staining and Western Blot was performed as previously described [20]. The primary and secondary antibodies were the same as our previous report [20]. The nerve fibers were analyzed by the ratio of positive nNOS counts to DAPI. The smooth muscle and endothelial staining were analyzed based on the positively stained area to DAPI. Immunofluorescence or protein band images were analyzed with Image-Pro Plus 5.1 (Media Cybernetics, Bethesda, MD, USA.
2.12. Statistical analysis
All the data were given as the mean ± standard deviation. Statistical differences were analyzed using one-way analysis of variance (ANOVA) with LSD post hoc tests. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Characterization of the PDNPs-PELA
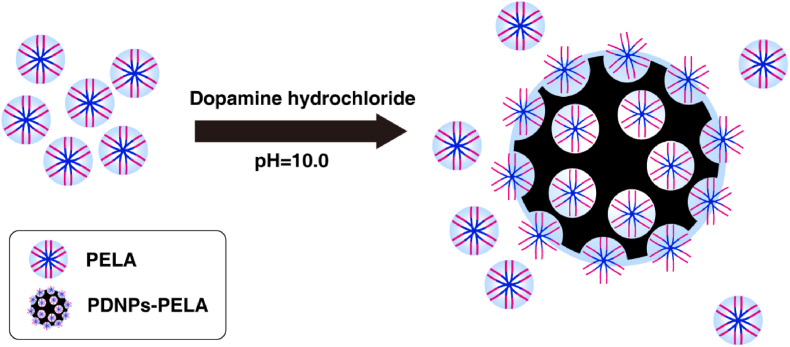
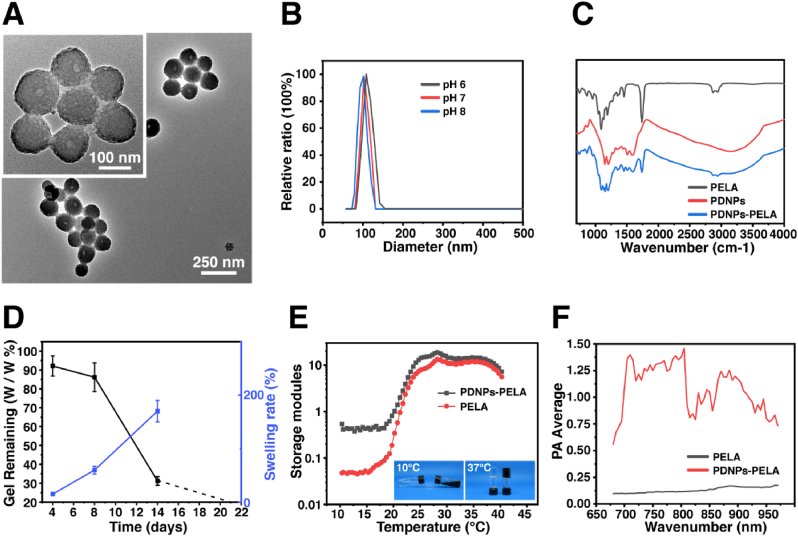
The preparation process of PDNPs-PELA is shown in Fig. 1. PELA is synthesized by ring-opening polymerization. In this study, in-situ polymerization was used to synthesize polydopamine in PELA solutions. At room temperature, the PELA block copolymer self-assembles into micelles in solution. The micelles were employed as soft templates for the polymerization of dopamine in alkaline condition. This process is similar to the synthesis of mesoporous polydopamine reported in the previous article [21]. The difference is, as a template, PELA was not removed after polymerization. It remained in the polydopamine nanoparticles. The prepared polydopamine showed a similar structure to mesoporous polydopamine under TEM (Fig. 2A), which verified the template function of PELA during the synthesis of polydopamine. The PDNPs-PELA particle size is around 120 nm measured by dynamic light scattering in different pH (Fig. 2B), which is consistent with the results of TEM image. More importantly, PELA in the PDNPs improved the nanoparticles dispersion stability. Fig. S-1 illustrates the dispersion stability of as-prepared nanoparticles. Polydopamine nanoparticles (synthesized in the traditional method [22] mixed with PELA solution (20% PBS solution) as a control. It was found that stratification became visible after standing for 12 h in direct blend PDNPs-PELA hydrogel. However, at the same concentration, in-situ synthesized polydopamine showed long-term solution stability. Even after 1 week of storage, no obvious delamination was observed.
Fig. 1.
Schematic illustration of the formation of PDNPs-PELA in-situ polymerization.
Fig. 2.
Physicochemical characterization of PDNPs-PELA: (A) TEM image. (B) Particle size distribution of PDNPs-PELA in different pH. (C) FTIR spectra. (D) Swelling ratio and percentage of weight loss in the degradation of PDNPs-PELA. (E) Storage modulus (G′) of PDNPs-PELA as a function of temperature. (F) Photoacoustic signals under wavelength scanning.
The chemical composition of PDNPs-PELA was characterized by FTIR (Fig. 2C). A pure PDNPs shows typical C C (1590 cm−1) and C–N (1207 cm−1). However, the PDNPs formed in PELA showed a new C O (1735 cm−1) peak, which indicated that the PDNPs was synthesized by using PELA as soft template. This result is consistent with the results of transmission electron microscopy, suggesting the successful in situ polymerization of dopamine under the guidance of PELA micelles. The degradation behavior of the gel is showed in Fig. 2D. The weight of the PDNPs-PELA hydrogel decreased by 100% in one month. It also undergoes swelling accompanied by a degradation process. After one month of degradation, the gel swelled by 170%.
The thermal sensitivity of the PDNPs-PELA was investigated by rheological test. The result was shown in Fig. 2E. The storage modulus of all groups increased decreases with increasing temperature due to the sol-gel transition. As the temperature approach body temperature (37 °C), the storage modulus the blank PELA gel was 12.1 Pa. At the same temperature, the storage modulus of PDNPs-PELA gel is 14.2 Pa. In addition to rheological tests, traditional tube flip test is also used to determine sol-gel transition of the hydrogel. The experimental results are shown in the inset of the rheological curve (Fig. 2E). As can be seen from the photo, when the temperature increased from 4 °C to 37 °C, the fluidity of the PDNPs-PELA solution gradually lost, and a sol-gel transition occurred at 37 °C, thus forming a stable hydrogel.
Photoacoustic characterizations of PDNPs-PELA were assessed with near infrared laser excitation ranged from 680 nm to 980 nm. The results are shown in Fig. 2F. The ultrasonic signal of PELA is between 0.11 and 0.18 during the test. In contrast, PDNPs-PELA showed a significantly increased ultrasound signal and have the strongest ultrasonic signal (1.47) under irradiation of 805 nm. It is worth noting that although the PDNPs-PELA gel has photoacoustic activity, the modulus of the gel did not change significantly under near-infrared light irradiation (Fig. S-2). The incorporation of PDNPs improves the photoacoustic performance and maintain the thermo-sensitivity of the hydrogel, thereby facilitating subsequent in vivo application.
3.2. In vitro cytotoxicity assays
The biocompatibility of PDNPs-PELA composite was investigated by CCK-8 and LDH in different cell lines. Fig. 3A and B shows the metabolic activity of cells at different exposure concentrations of PDNPs-PELA after 24 h and 48 h incubation. All experimental concentrations have no effect on cell viability. The results of the LDH experiment (Fig. 3C and D) show that after treatment with different concentrations of PDNPs-PELA, no significant LDH increase was found. This reveals that PDNPs-PELA has good cell compatibility and low cytotoxicity in vitro.
Fig. 3.
Cell viability measured by CCK-8 after (A) 24h and (B) 48h incubation with different PDNPs-PELA concentration. Lactate dehydrogenase (LDH) activity assay after (A) 24h and (B) 48h incubation with different PDNPs-PELA concentration. No statistical differences were found at p < 0.01.
3.3. Exosome identification and in vitro release profile
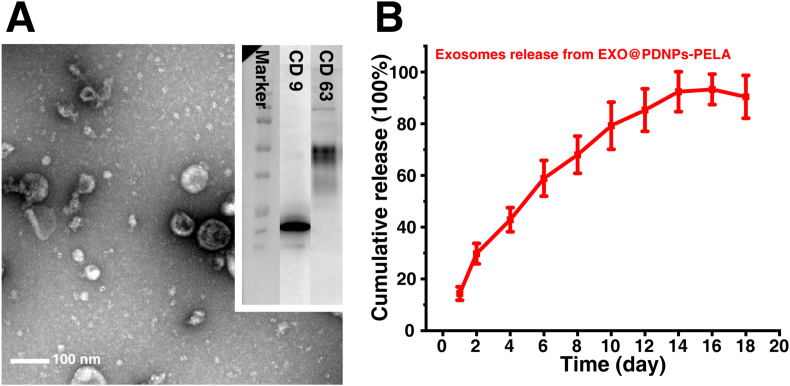
The characterization of exosomes is shown in Fig. 4A. TEM image show typical exosome vesicle structures. The size of the extracted exosomes by TEM is between 100 nm and 200 nm, which is agree with previous literature [23]. Western blot analysis confirmed the presence of specific exosome markers CD63 and CD9. These results revealed the successful extraction of adipose-derived mesenchymal stem cell exosomes. Fig. 4B present the release profile of exosomes in PDNPs-PELA. The cumulative release of exosomes gradually increased during the first two weeks (from 14.4 ± 2.6% to 92.5 ± 5.7%) and then remained relatively stable. Since the test was performed at 37 °C, it was a schematic representation of the release behavior of exosomes in PDNPs-PELA in vivo. This result indicates that the PDNPs-PELA was capable of providing sustained release of exosome and thus an optimal vehicle for next in vivo ED treatment.
Fig. 4.
(A) Transmission electron microscope images and marker western blotting of exosome. (B) Quantification of exosome release from PDNPs-PELA. The AD-SC from PDNPs-PELA was not significant different (p < 0.01) after 12 days release.
3.4. Photoacoustic image-guided injection in the tunica albuginea
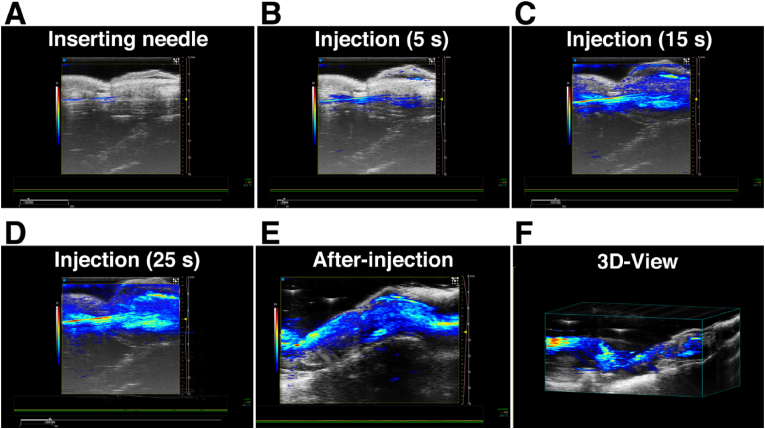
Fig. 5 shows a typical photoacoustic image of the PA-guided intratunical injection. The needle can be clearly traced through the photoacoustic image due to the high photoacoustic performance of EXO@PDNPs-PELA (Fig. 5A). This facilitates the fine location adjustment of the needle, thereby improving the accuracy of puncture. After needle enter into the penile tunica albuginea, the injection of EXO@PDNPs-PELA was started. Fig. 5B–D illustrated the entire injection process. From 5 s to 25 s in the injection, the photoacoustic signal area gradually extends to the whole corpora cavernosa, which indicated that the EXO@PDNPs-PELA suspension was fully filled under the tunica albuginea. The gel would form in 3 min after the injection due to a thermo-responsive sol-gel transition (Fig. 5E). After 24 h of gel formation, a three-dimensional (3D) photoacoustic scan of the injection site was performed (Fig. 5F). A banded 3D photoacoustic image is clearly observed around the corpus cavernosum. This means the gel formation in the tunica albuginea. Furthermore, there is no significant signal intensity difference in any site of the image, indicating that the EXO@PDNPs-PELA hydrogel is evenly distributed and in close apposition under the tunica albuginea.
Fig. 5.
In vivo photoacoustic imaging-guided intratunical injection. (A) The needle was punctured into tunica albuginea under the guidance of photoacoustic images. (B–D) EXO@PDNPs-PELA was injected and evenly distributed in tunica albuginea. (E) EXO@PDNPs-PELA formed a gel after 3 min injection. (F) A representative 3D reconstructed image of (E).
As can be seen from Fig. 6, the cavernous tissue structure retained normal and no necrotic tissue was found during the test. The hydrogel could exist stably one week after injection. After two weeks of injection, only a small amount of hydrogel remained under the tunica albuginea as the hydrogel degradation. In the third week, no residue of material was found in tunica albuginea. Tissue organization and cell morphology in the injection site has no apparent change compared with normal corpus cavernous tissue (Fig. S-3). In particular, the decreased optical density of the gel was detected over time after injection, indicating that effective elimination of degradation fragments in vivo.
Fig. 6.
Histological test of penile (macroscope) tunica albuginea (microscope, inset) after EXO@PDNPs-PELA injection.
3.5. In vivo ED treatment
The experimental process of rats ED treatment was clearly shown in Fig. 7A. Modeling of rat ED was synchronized with the intratunical injection. The intrasinus pressure were measured in the third week after injection, followed by the collection of penile tissue. From Fig. 7B, a significant decrease of MICP/MAP value in ED groups (0.27 ± 0.04) confirmed the successful establishment of the ED model. Exosome injection group present an improvement in erectile function with increased MICP/MAP value of 0.45 ± 0.06. This is well agreed with previous report [20]. Moreover, there is no difference between the PELA and PBS groups, indicating that the PELA has no therapeutic effect on erectile function recovery. The best erectile recovery ability was found in EXO@PDNPs-PELA group, where the MICP/MAP value (0.60 ± 0.07) is closest to that of the control (0.77 ± 0.05).
Fig. 7.
(A) Schematic illustration of animal experiment. (B) Intrasinus pressure of ED rat in different treatment group.
3.6. Immunofluorescence and Western blot
Immunofluorescence and Western blot test were employed to investigate the recovery of erectile function at the cytology and protein levels, respectively. Fig. 8A shows the immunofluorescence of the penis tissue with different functional markers. α-SMA is an important protein for smooth muscle function maintenance. Since the atrophy of cavernous muscles, the fluorescence intensity of ED model group (9.9 ± 1.48) decreased significantly compared to the control group (30.2 ± 3.34). The expression of α-SMA in exosomes group (17.3 ± 2.90) and EXO@PDNPs-PELA group (24.1 ± 3.47) was higher than that in PBS and PELA, indicating that exosomes are effective therapeutic agent of ED. It can also be found that the α-SMA fluorescence signal in the EXO@PDNPs-PELA group is stronger than that in the exosome group. This result verified the enhanced exosome efficiency of EXO@PDNPs-PELA on muscle function. eNOS is a functional neurotransmitter released by cavernous sinus vascular endothelium. It can regulate the erectile function of the cavernous body and thus was an important functional marker of the vascular endothelium. nNOS is a signaling molecule in nerve tissue, which is mainly responsible for the transmission of external stimuli in the penis. The data trend of eNOS and nNOS(Fig. 5B) in different treatment groups is the same as α-SMA. The increase of eNOS and nNOS indicates that the function of the damaged smooth muscle and nervous system has returned to normal. Since this repair will not be reversed even after the treatment is completed, it can be considered as a permanent restoration of erectile function. Fig. 5C shows the WB band of the above protein. The results are in agreement with the fluorescence staining results. The consistency of the expression changes among these markers comprehensively illustrates the potential application of EXO@PDNPs-PELA in the treatment of ED.
Fig. 8.
(A) Immunofluorescence analysis of penis tissue in different group. (B) Statistical results of fluorescence intensity. (C) Western blot of α-SMA, eNOS, nNOS in different group.
4. Discussion
As an easily available SC-EXO, adipose-derived mesenchymal stem cell-derived exosomes (EXO) have shown therapeutic effects on ED in several studies [6,24,25]. The target site of exosome therapies for ED is the cavernous body. The complicated cavity structure and abundant blood flow make the delivery and enrichment of exosomes in tunica albuginea very difficult. PDNPs-PELA temperature-sensitive hydrogel was used to encapsulate exosomes. The gelation of temperature-sensitive hydrogels mainly depends on the temperature rise. At room temperature, the system exhibits fluidity similar to liquid and has good injectability. After the injection, as the temperature rises, it gradually undergoes sol-gel transition and forms a stable hydrogel. During this process, the suspension can still maintain a certain fluidity, so that it can better fit the irregular cavity gap under the tunica albuginea. More importantly, the final storage modulus of the formed gel is around 15 Pa, which is lower than that of cavernous tissue (1200 Pa). This means gel filling the cavity is softer than the surrounding tissue and does not affect the normal bending and swelling behavior of the corpus cavernosum. The daily release of exosomes in the gel is between 10 and 20 μg, which is in a therapeutic dose range of the ED treatment. In addition, the introduction of PEG fragments in the polymer improves the degradation performance of the gel. Compared with previously reported gels [10,11], the prepared gel can be completely degraded after two weeks of injection, thereby reducing the risk of necrosis caused by long-term occupation of corpus cavernosum. The acid generated by the degradation of the gel may stimulate cells to produce temporary inflammation. However, they can be metabolized by the cell, so it will not cause permanent damage to the tissue. These results imply that PEG-based polyester temperature-sensitive polymer is a promising exosome carrier for repair and regeneration of irregular soft tissues.
Accurate puncture into target site is a challenge for in situ injection [[9], [10], [11],26]. In this study, photoacoustic imaging was used to guide in situ intratunical injection. During the puncture, the ultrasound image provides a clear structure and location of the penis sponge, while the injection needle filled with contrast agent shows a strong photoacoustic signal. Precise entry into cavernous cavity of the penis is easy to achieve by adjusting the position and angle of the injection needle in real time under the photoacoustic image. Polydopamine nanoparticles were synthesized in situ in PELA solution as a contrast agent. PELA is a block copolymer that exists in the form of micelles at room temperature in water. Dopamine uses these micelles as templates to generate polydopamine with mesoporous structure. PELA fills the pores of polydopamine nanoparticles, increasing their dispersibility and stability. The PELA that does not participate in the polymerization of dopamine in the solution is mainly responsible for the gel formation under temperature stimulation. This in-situ polymerization method provides a new route for the preparation of polydopamine nanoparticle composites and expands its scope of application.
EXO shows a series of pharmacological effects in vivo, including increased vascular endothelial activity, enhanced neuron survival, stimulated extracellular matrix remodeling and limited local inflammation. The combined effect rescues damaged cells, reduces tissue damage, and finally accelerates the recovery of erectile function. However, the abundant blood flow in the cavernous body is not conducive to maintaining the therapeutic concentration of exosomes. In this study, the tight fit of the hydrogel to the cavernous cavity extended the retention of the exosomes in the cavernous body. The sustained release of exosomes from the gel increases the concentration of exosomes in the corpus cavernosum. In addition, no penile necrosis was found in all gel injection groups, indicating that the prepared gel had good compatibility with corpus cavernosum. This implies that such exosome-loaded hydrogels have the potential to treat other tissue defects with rich blood flow.
5. Conclusion
In this study, Polydopamine nanoparticle-incorporated PELA was successfully synthesized by a in situ polymerization. The physicochemical properties, biocompatibility, and erectile function recovery effects were scrutinized via a series of in vitro and in vivo studies. PDNPs exhibits mesoporous structure through PELA micelle-template inducing reaction. Moreover, PDNPs synthesized in this way also showed improved dispersion and stability in the PBS solution due to the amphiphilicity of PELA. 20% PDNPs-PELA suspension undergoes a body temperature triggered sol-gel transition and then give the largest photoacoustic signal under 805 nm infrared light irradiation. With the help of these properties, in-situ injection of tunica albuginea under the guidance of photoacoustic imaging can be accurately performed. Adipose-derived mesenchymal stem cell-derived exosomes were successfully extracted as an effective therapeutic component of ED and achieved sustained release within two weeks in PDNPs-PELA hydrogel. In vivo ED treatment experiments found that a single injection of EXO@PDNPs-PELA inhibited the apoptosis of cavernous smooth muscle cells, promoted the recovery of cavernous sinus vascular endothelial cells, and thus accelerated the recovery of erectile function. This study revealed that the PDNPs-PELA hydrogel prepared by in-situ polymerization can be an ideal exosome carrier with sustained release and real-time photoacoustic imaging guidance injection for ED treatment.
Declaration of competing interest
The authors declare no competing financial interest.
CRediT authorship contribution statement
Li Liang: Conceptualization, Writing – original draft. Yi Shen: Methodology, Software. Zhifeng Dong: Validation, Formal analysis. Xin Gu: Investigation, Resources, Data curation, Writing – review & editing.
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (81700086), Shanghai Municipal Commission of Health and Family Planning Foundation (20134087).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.07.024.
Contributor Information
Zhifeng Dong, Email: zhifengdong6@126.com.
Xin Gu, Email: guyiran08612@163.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Sanchez-Cruz J., Cabrera-Leon A., Martın-Morales A., Fernandez A., Burgos R., Rejas J. Male erectile dysfunction and health-related quality of life. Eur. Urol. 2003;44:245–253. doi: 10.1016/s0302-2838(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 2.Burnett A.L., Nehra A., Breau R.H., Culkin D.J., Faraday M.M., Hakim L.S., Heidelbaugh J., Khera M., McVary K.T., Miner M.M. Erectile dysfunction: AUA guideline. J. Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J., Zhang R., Yang Z., Lee J., Liu Y., Tian J., Qin X., Ren Z., Ding H., Chen Q. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur. Urol. 2013;63:902–912. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Yafi F.A., Sharlip I.D., Becher E.F. Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. Sexual medicine reviews. 2018;6:242–252. doi: 10.1016/j.sxmr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen F., Zhang H., Wang Z., Ding W., Zeng Q., Liu W., Huang C., He S., Wei A. Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J. Sex. Med. 2017;14:1084–1094. doi: 10.1016/j.jsxm.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Zhao S., Luo L., Wang J., Zhu Z., Xiang Q., Deng Y., Zhao Z. Mesenchymal stem cell‐derived exosomes ameliorate erection by reducing oxidative stress damage of corpus cavernosum in a rat model of artery injury. J. Cell Mol. Med. 2019;23:7462–7473. doi: 10.1111/jcmm.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castiglione F., Hedlund P., Van der Aa F., Bivalacqua T.J., Rigatti P., Van Poppel H., Montorsi F., De Ridder D., Albersen M. Intratunical injection of human adipose tissue–derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur. Urol. 2013;63:551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z., Han X., Ouyang X., Fang J., Huang X., Wei H. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics. 2019;9:6354. doi: 10.7150/thno.34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., Bao C., Xie Z., Lin Q., Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., Xu H., Lei B., Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K., Zhao X., Chen X., Wei Y., Du W., Wang Y., Liu L., Zhao W., Han Z., Kong D. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl. Mater. Interfaces. 2018;10:30081–30091. doi: 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- 12.He C., Kim S.W., Lee D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Contr. Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Hu K.-N., Burks C., Christy W.C. Fibrosis of tunica albuginea: complication of long-term intracavernous pharmacological self-injection. J. Urol. 1987;138:404–405. doi: 10.1016/s0022-5347(17)43164-8. [DOI] [PubMed] [Google Scholar]
- 14.Nomura J.T., Sierzenski P.R. Ultrasound diagnosis of penile fracture. J. Emerg. Med. 2010;38:362–365. doi: 10.1016/j.jemermed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Jokerst J.V., Thangaraj M., Kempen P.J., Sinclair R., Gambhir S.S. Photoacoustic imaging of mesenchymal stem cells in living mice via silica-coated gold nanorods. ACS Nano. 2012;6:5920–5930. doi: 10.1021/nn302042y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L.-S., Cong Z.-X., Cao J.-B., Ke K.-M., Peng Q.-L., Gao J., Yang H.-H., Liu G., Chen X. Multifunctional Fe3O4@ polydopamine core–shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano. 2014;8:3876–3883. doi: 10.1021/nn500722y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capello M., Vykoukal J.V., Katayama H., Bantis L.E., Wang H., Kundnani D.L., Aguilar-Bonavides C., Aguilar M., Tripathi S.C., Dhillon D.S. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-018-08109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z., Ni J., Chen L., Yu L., Xu J., Ding J. Biodegradable and thermoreversible PCLA–PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials. 2011;32:4725–4736. doi: 10.1016/j.biomaterials.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Gu X., Thakker P.U., Matz E.L., Terlecki R.P., Marini F.C., Allickson J.G., Lue T.F., Lin G., Atala A., Yoo J.J. Dynamic changes in erectile function and histological architecture after intracorporal injection of human placental stem cells in a pelvic neurovascular injury rat model. J. Sex. Med. 2020;17:400–411. doi: 10.1016/j.jsxm.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Gu X., Shi H., Matz E., Zhong L., Long T., Clouse C., Li W., Chen D., Chung H., Murphy S. Long‐term therapeutic effect of cell therapy on improvement in erectile function in a rat model with pelvic neurovascular injury. BJU Int. 2019;124:145–154. doi: 10.1111/bju.14631. [DOI] [PubMed] [Google Scholar]
- 21.Wu D., Duan X., Guan Q., Liu J., Yang X., Zhang F., Huang P., Shen J., Shuai X., Cao Z. Mesoporous polydopamine carrying manganese carbonyl responds to tumor microenvironment for multimodal imaging‐guided cancer therapy. Adv. Funct. Mater. 2019;29:1900095. [Google Scholar]
- 22.Zhao H., Zeng Z., Liu L., Chen J., Zhou H., Huang L., Huang J., Xu H., Xu Y., Chen Z. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale. 2018;10:6981–6991. doi: 10.1039/c8nr00838h. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Jin K., Gao L., Zhang Z., Li F., Zhou F., Zhang L. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2:1800021. [Google Scholar]
- 24.Zhu L., Huang X., Yu W., Chen H., Chen Y., Dai Y. Transplantation of adipose tissue‐derived stem cell‐derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50 doi: 10.1111/and.12871. [DOI] [PubMed] [Google Scholar]
- 25.Li M., Lei H., Xu Y., Li H., Yang B., Yu C., Yuan Y., Fang D., Xin Z., Guan R. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6:927–935. doi: 10.1111/andr.12519. [DOI] [PubMed] [Google Scholar]
- 26.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.