Abstract
Objective To investigate the efficacy, safety, and associated mechanisms of injected ozonated saline in the treatment of VX2 tumors.
Methods A total of 90 rabbits bearing VX2 tumors on their left hind legs were randomly divided into three groups. The control group (A) received normal saline, while groups B and C received 20 μg/mL and 40 μg/mL O3/O2 ozonated saline, respectively. Rabbits were anesthetized and 2 mL of blood was drawn directly from the heart to measure serum concentrations of interleukin (IL-6) and tumor necrosis factor (TNF-α). The skin covering the VX2 tumor was cut in each rabbit and the maximum and vertical diameters of the tumors were measured under direct visualization. Several milliliters of saline, saline pre-treated with 20 μg/mL O3/O2, or saline pre-treated with 40 μg/mL O3/O2 were directly injected into the tumors of groups A, B, and C, respectively (injection volume (milliliter) =1/2 volume of the tumor, V = 1/2ab2). On days 4, 8 and 12 following treatment, 10 rabbits were randomly selected from each group for blood sample collection, and serum IL-6 and TNF-α were measured. The tumor growth rate was calculated by measuring the maximum and vertical diameters of the VX2 tumors under direct visualization. All selected rabbits were euthanized and the tumors, livers, and lungs were removed for pathological examination. The tumor necrosis rate was calculated by cutting the tumors into half along the longitudinal axis and measuring the maximum diameters of the intratumoral necrotic regions.
Results The average tumor volume in the three groups increased to different degrees at each time point; however, the average tumor growth rates in groups B and C were substantially lower than that in group A, exhibiting a statistically significant difference. The difference in the tumor growth rate between group B and group C was not statistically significant. The serum concentrations of IL-6 and TNF-α increased in the three groups at each time point, with larger increases occurring in groups B and C; however, the greater increases did not reach statistical significance. Although the diameters of the necrotic areas were larger in both groups B and C than that in group A, significant differences in necrotic area diameters were only found when comparing groups A and C on days 4 and 12 following treatment.
Conclusion Direct injection of different concentrations of ozonated saline into VX2 tumors significantly increased intratumoral necrosis and reduced the tumor growth rate. The associated mechanism may be partially mediated by IL-6 and TNF-α, as the serum concentrations of these molecules increased after the treatment.
Keywords: ozone, neoplasm, VX2 tumor, therapy, effectiveness
INTRODUCTION
Several studies have reported that ozone is an effective treatment for tumors (in vivo) and tumor cell lines (in vitro) (1-3). The clinical application of ozonated autohemotherapy effectively improves tumor hypoxia and sensitivity to chemotherapy (4). A previous study (5) demonstrated that ozone damages tumor ultrastructure; however, the use of ozone is limited due to its short half-life, strong inhalation toxicity, and the risk of air embolisms. Ozone is soluble in water, where it becomes more stable and safe while retaining its efficacy. Thus, ozonated water may be more appropriate for therapeutic purposes than ozone (6). Ozonated water has been largely used in the treatment of arthritis, infectious wounds, peritonitis, oral diseases, and gynecological diseases in clinical practice (7-9), but has rarely been utilized in experimental studies on tumors (10). This study aims to evaluate the efficacy of ozonated saline injected directly into VX2 tumors and explore the potential mechanisms involved in the effects of ozonated saline on VX2 tumors.
MATERIALS AND METHODS
Rabbit VX2 tumor model preparation
This study was approved by the ethics committee of Second Military Medical University. The tumor model was established using rabbit VX2 tumor modeling, described by Wei et al. (5) Ninety male New Zealand white rabbits, weighing 2.5–3 kg, were used for in vivo tumor modeling. The Naval Medical Research Institute of the Second Military Medical University in Shanghai provided and maintained all of the animals and all experimental procedures were performed at the Naval Medical Research Institute. Every rabbit was fed with 145–150 g fodder daily and some water, and was kept in a cage alone. The VX2 tumor strain was kindly provided by Zhongshan Hospital, Fudan University, Shanghai, and was maintained and passaged in rabbits. After anesthetizing each tumor-bearing rabbit with a 3% pentobarbital sodium solution (0.8–1 mL/kg) injected into the ear veins, the rabbit was placed on an operating bench in a supine position. The hair on the medial thigh of the left hind limb was then removed, Anerdian skin disinfection was performed, an incision was made in the tumor region and the flesh-like tumor tissue collected. The tumor tissues were washed twice in normal saline and cut into ~1 × 1 mm3 tissue blocks. The tissue blocks were then injected into the left hind-limb medial thigh muscles of the experimental rabbits. Subsequent experiments were conducted after the tumors grew to 1–2 cm in size.
Preparation of ozonated saline
Chilled normal saline (stored at 4°C) was used for the preparation of ozonated saline. An ozone generator from Humares GmbH (Bruchsal, Germany) was used to freshly prepare the ozonated normal saline one hour before use. Ozonated normal saline was made by constantly injecting the oxygen/ozone mixture into 30 mL of cold normal saline at ozone concentrations of 20 μg/mL or 40 μg/mL, at a speed of 3 L/min, at room temperature for 20 min. The ozonated saline was then stored at 4°C and utilized in later experiments at low temperatures.
Experimental grouping and sample collection
Ninety New Zealand white rabbits were randomly divided into three groups. Rabbits were anesthetized as described above, disinfected by iodophor for the skin of precordial region, and 2 mL of blood was collected from the heart. An incision was made through the skin in the region where the tumor was present and the maximum and vertical diameters of the tumor were measured using Vernier calipers. Low-temperature saline (Group A), 20 μg/mL low-temperature ozonated saline (Group B), or 40 μg/mL low-temperature ozonated saline (Group C) was administered into the tumors via multipoint puncture injection (dose = ½ of the tumor volume, V = ab2/2). The skin was sutured and disinfected and the animals were housed in the original cages. Ten rabbits from each group were randomly selected on days 4, 8, and 12 post-surgery, anesthetized as described above, disinfected as described previously, and 2 mL of blood was withdrawn from the heart, as above. An incision was made in the skin and the maximum and vertical diameters of the tumors were measured as before. The selected tumor-bearing rabbits were euthanized and tumor, liver, and lung tissues were collected. VX2 tumor morphology and color, as well as the presence, size, morphology, and number of subcutaneous daughter foci and the presence of liver and lung metastases were observed via the unaided eye.
Estimation of tumor volume, tumor growth rate (TGR), internal necrosis rate, interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α) concentration
Estimation of tumor volume and TGR
Tumor length and diameter on the day of treatment was used to calculate the tumor volume, which was defined as V0. The tumor length and diameter on the Xth day after treatment was used to calculate the tumor volume (VX). The formula VX/V0 × 100% was used to calculate the TGR of the tumor volume. A TRG > 100% was defined as tumor enlargement, and a TGR < 100% was defined as tumor shrinkage.
Estimation of the internal necrosis rate of the tumors
The residue-like tissue observed by the unaided eye was defined as necrotic tissue. The maximum (longest) diameter of the tumor specimen (D) and the maximum (longest) diameter of the internal necrotic tissue of the tumor (d) were measured after cutting along the maximum (longest) diameter of the tumor. The internal necrosis rate was calculated using the formula d/D × 100%.
Estimation and fold change calculation of serum IL-6 and TNF-α
The concentrations of IL-6 and TNF-α in all serum samples were measured using ELISA kits, according to the manufacturer’s instructions. These experiments were conducted by the Immunology Department of the Second Military Medical University. The concentrations of serum IL-6 and TNF-α were measured both pre-treatment and post-treatment to calculate the fold change of IL-6 and TNF-α. A value >1 and a value <1 represented an increase and decrease, respectively, in concentrations of IL-6 and TNF-α.
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis in this study. Measurement data are presented as the mean ± standard deviation. Fisher’s least significant difference (LSD) t-test was used for comparisons between groups and within groups. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Tumor growth rate
Table 1 demonstrates the tumor growth rates in the three groups of animals. Significant differences in tumor growth rates were found at different time points between the experimental groups and the control group (P = 0.000 at day 4; P = 0.012 at day 8; and P = 0.004 at day 12). No significant difference in tumor growth rate was found between the two experimental groups (Groups B and C; P > 0.05). On day 4, two tumors in group B and four tumors in group C had shrunk obviously, while the tumor volumes in group A were larger. On day 8, only one tumor (different from the ones that had shrunk before) in group C had shrunk, while the remaining tumors including the ones that originally shrunk in group C were enlarged. On day 12, all tumors including the ones that originally shrunk in the three groups were enlarged.
Table 1.
Average tumor growth rate (%).
| Group | Day 4 | Day 8 | Day 12 |
|---|---|---|---|
| Group A | 3.89±1.79 | 4.75±1.78 | 8.15±3.41 |
| Group B | 1.52±0.59 | 2.95±0.81 | 4.12±2.05 |
| Group C | 1.35±0.40 | 3.05±1.40 | 4.77±2.25 |
Changes of TGR As shown in Figure 1, the overall changes in tumor growth rate increased in all three groups during the experiment. The most obvious increase was observed in group A on days 8 and 12. The tumor growth rates in Groups B and C were similar on day 0–8, with the tumor volume growth rate in group C slightly higher than in group B on days 8–12.
Figure 1.
Changes of tumor growth rate.
Internal tumor necrosis rate
Table 2 shows the tumor necrosis rates in the different groups. The internal necrosis rates in groups B and C on days 4 and 12 were higher than in group A. The difference was statistically significant between group C and group A (P < 0.05); however, the differences were not statistically significant where comparing group C to group B or group A to group B (P > 0.05). The internal necrosis rates in groups B and C were higher on day 8 than in group A. However, there were no statistically significant differences in internal necrosis rates between the all groups on day 8 (P > 0.05). As shown in Figure 2, the internal necrosis rate of the tumors in group A (control group) demonstrated a linear increase; however, it was significantly lower compared with the two experimental groups B and C.
Table 2.
Internal necrosis rate of the tumors (%).
| Group | Day 4 | Day 8 | Day 12 |
|---|---|---|---|
| Group A | 0.38±0.22 | 0.48±0.28 | 0.59±0.26 |
| Group B | 0.55±0.30 | 0.57±0.33 | 0.69±0.25 |
| Group C | 0.70±0.14 | 0.66±0.12 | 0.83±0.06 |
Figure 2.
Changes of tumor necrosis rate.
Changes in IL-6 concentration
As shown in Table 3, the fold increase in IL-6 levels among the three groups at different time points was not statistically different (P > 0.05). On day 4, the increases in IL-6 concentration in the experimental groups were obviously higher than in the control group but there were no statistical significance. In addition, the IL-6 concentration in group C was slightly higher than in group B. On day 8, the increase in IL-6 concentration was similar among the three groups, while on day 12, the fold increase in IL-6 in group A was slightly higher than in groups B and C.
Table 3.
Fold increase in IL-6 concentration.
| Group | Day 4 | Day 8 | Day 12 |
|---|---|---|---|
| Group A | 0.98±0.27 | 1.12±0.32 | 1.30±0.43 |
| Group B | 1.04±0.42 | 1.13±0.63 | 1.25±0.36 |
| Group C | 1.14±0.28 | 1.12±0.17 | 1.19±0.52 |
Figure 3 demonstrates the overall variation in fold increase in IL-6 levels during the experiment. The fold increases in IL-6 were linear in groups A and B, while the fold increase in IL-6 was slightly reduced on days 5–8 but elevated in days 9–12 in group C. The fold increase in IL-6 was optimized in group C on day 4, and the fold increase in IL-6 was lowest in Group A on day 4. The fold increase in IL-6 was similar in all groups on day 8, while the fold increase in IL-6 was the lowest in group C and the highest in group A on day 12.
Figure 3.
Changes of IL-6 concentration.
Changes in TNF-α concentration
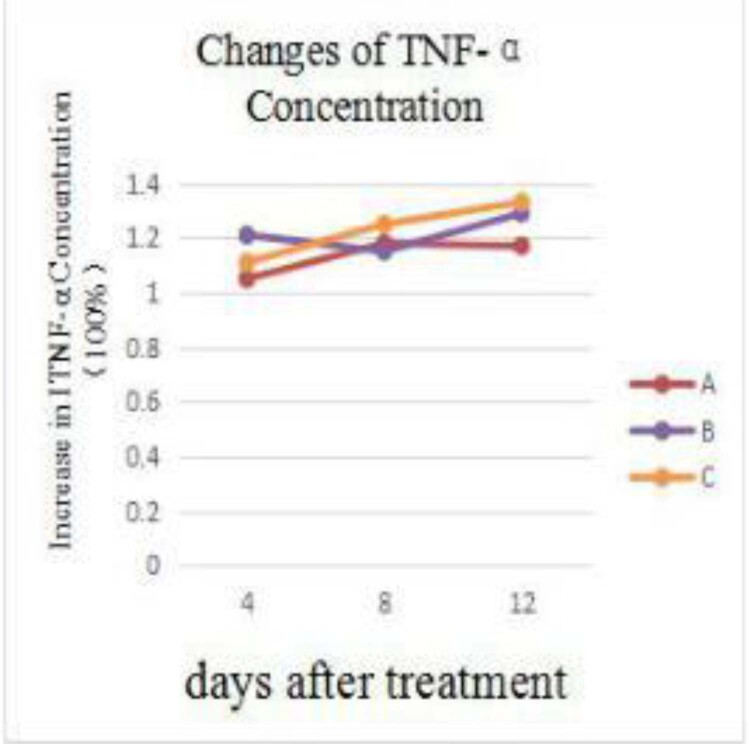
Table 4 shows the fold change in TNF-α concentration in the three groups. No significant differences in TNF-α concentration were found at the different time points among the three groups (P > 0.05). The fold increase in the TNF-α concentration was higher in the experimental groups (groups B and C) than in the control group (group A) on days 4 and 12.
Table 4.
Fold increase in TNF-α concentration tumor morphology and metastasis.
| Group | Day 4 | Day 8 | Day 12 |
|---|---|---|---|
| Group A | 1.05±0.17 | 1.18±0.38 | 1.17±0.27 |
| Group B | 1.21±0.48 | 1.15±0.58 | 1.29±0.37 |
| Group C | 1.11±0.23 | 1.25±0.19 | 1.33±0.47 |
Figure 4 demonstrates the variation in the TNF-α concentration fold increase, showing increases in both groups A and C at different time points, but a slight decrease in group B on days 5–8, which was even lower than the control group (group A) and reversed (increased again) on days 9–12.
Figure 4.
Changes of TNF-α concentration.
Tumor morphology and metastasis
Tumor metastasis
No tumor metastasis was found in any animal in any of the three groups on day 4. However, on day 8, two cases of lung metastasis and one case of ascites were found in group A and one case of lung metastasis was found in group B; no tumor metastasis was found in group C. On day 12, another one case of lung metastasis was found in group A, and one case of liver metastasis was found in groups B and C respectively. In group A, one case of cachexia was found on day 8 and three cases of cachexia were found on day 12. No cachexia was found in either groups B or C at any time point.
Subcutaneous daughter foci
Two cases of adjacent nodules (with approximate diameters of 0.4 cm) were found in group A prior to treatment and three cases of small nodules were found in group B (with diameters of 0.3 cm. 0.3 cm, and 0.4 cm) before treatment. Only one small nodule (approximately 0.4 cm in diameter) was found in group C prior to treatment. After treatment, the nodules and tumors in group A were fused, one nodule in group B disappeared, and no further growth or fusion of the other nodules was found.
DISCUSSION
Evaluation of the efficacy of ozonated saline for the treatment of VX2 tumors
A previous study by our group showed that ozone damages the internal structure of tumor cells; higher ozone concentrations corresponded to changes in tumor structure that were more obvious and an increase in internal tumor necrosis (5). Kuroda et al. (10) demonstrated that ozonated water significantly reduced the tumor growth rate and significantly increased the internal necrotic area of the tumors when compared with the control group treated with sterile distilled water. They also that the area of necrotic tumor cells became larger as the ozonated water concentration increased.
Similar results were found in this study. Although the tumor volumes in the three groups of animals had increased, the tumor growth rate in the experimental groups (groups B and C) were significantly lower than that in the control group (group A). In addition, there was less tumor metastasis and larger area of internal tumor necrosis in the two experimental groups compared to the control group (most significant on day 4). These results suggest that the ozonated saline significantly reduced tumor growth rate and increased tumor necrosis in a short period of time, and may even shrink or even eliminate tumor(s).
In the study by Kuroda et al. (10), sterile distilled water, which is hypotonic and causes cell swelling and disintegration when injected into muscles or directly into tumors, was used for the control group. Thus, the differences in the internal necrotic areas in the experimental and control groups were not significant in that study. In the current study, normal saline was used as the isotonic solution, which did not present the same problems as the low-permeability, sterile distilled water, suggesting that the conclusion of the present study is more reliable.
Previous studies (7, 10,11) have shown that the size of the tumor necrotic area depends and the ozone inhibition of tumor cell growth depend on the ozone concentration. In this study, the diameters of internal tumor necrosis in the experimental groups were significantly higher than those in the control group on day 4, post treatment. The larger diameter was more pronounced in the group (group C), which received the higher concentration of ozonated saline; however, the diameters of internal tumor necrosis were not statistically significantly different between groups B (low ozonated saline concentration) and C (high ozonated saline concentration). Tumor necrosis in the three groups became gradually more similar with prolonged treatment duration, suggesting that the 20 μg/mL ozone concentration reached the optimal tumoricidal effect and that the increased ozone concentration (40 μg/mL) did not further enhance this effect. We speculate that further experiments using a low concentration of ozonated saline may achieve the desired outcomes such as tumor reduction and/or increasing of intra-tumoral necrosis. In addition, low concentrations of ozonated saline cause less pollution than high concentrations. An accurate measurement of the concentration of dissolved ozone in ozonated saline could provide more information in terms of using relatively low, but effective concentrations of ozonated saline for tumor treatment; however, accurately detecting the concentration of ozone in water is difficult.
According to the observed tumor growth rates and internal necrosis conditions, the tumor growth rate on day 8 was significantly accelerated and the internal necrosis rate was decreased. This suggests that the local reinjection of ozonated saline may be necessary on one of the days between 4–8 to induce necrosis and decrease activity in tumor tissues, thereby producing the desired anticancer effect.
Preliminary evaluation of the mechanism of action of ozonated saline in the treatment of VX2 tumors
Tumor cells lacking antioxidants can be directly oxidized by the soluble ozone in ozonated water and its oxidation products, resulting in tumor tissue damage that is more severe with higher concentrations of ozone. A previous study by our group revealed that the direct injection of an O3/O2 mixture of different concentrations into VX2 tumors damaged the cellular membrane, mitochondria, endoplasmic reticulum, and nucleus, resulting in tumor cell death (5). When dissolved in water, ozone generates oxygen free radicals and promoted tumor cell apoptosis and mitochondrial damage. The reduction of the oxygen molecule into O2−, via electrons leaking from the damaged mitochondria, increases the damage to tumor cells and induces tumor cell apoptosis (9, 12,13). Reactive oxygen species (ROS) change the hypoxic tumor environment and inhibit tumor growth and metastasis. In addition, ROS increase the local oxygen content and regulate in vivo immune function; treatment outcomes are better with hypoxia that is more severe (7, 14-16). Dissolved ozone also reacts with unsaturated fatty acids and generates a relatively stable and strong oxidant, hydrogen peroxide (H2O2). A low H2O2 concentration activates a series of biochemical reactions (17) and ultimately causes cell death.
Dissolved ozone induces the release and activation of a variety of immune factors (18). TNF-α is the strongest cytokine against tumor activity and mediates cytotoxicity to achieve an anticancer effect through the signal transduction of its receptor. TNF-α induces the generation of oxygen free radicals, mediated by IL-8, while in turn, the oxygen free radicals promote the tumoricidal effects of TNF-α. This positive feedback system causes the immune mechanism to exert antitumor effects (11). Schulz and Bauer (19) reported that TNF-α induces oxygen free radicals to cause tumor cell apoptosis. Since the initial concentrations of survival factors are low in tumor cells, TNF-α is very effective. Mule et al. (20) demonstrated that IL-6 exhibits antitumor activity by activating an in vivo immune mechanism. In addition, IL-6 induces lymphokine-activated killer (LAK) cells and natural killer (NK) cells, which enhances the tumor cell-killing effect, and thereby promotes antibody synthesis and improves immunity in vivo (21). However, in the current study, no significant differences in the fold increases of IL-6 and TNF-α were found in the peripheral blood between the two experimental groups and the control group, suggesting that the dose of ozonated saline used in this study was relatively low. Furthermore, only one injection was administered in this study, which did not significantly affect the concentrations of immune factors in whole blood, but might have stimulated the local immune response so as to enhance the antitumor effect of ozonated saline. Thus, in future studies, the dose and/or number of injections should be increased in order to induce these immune reactions.
SUMMARY
In this study, ozonated saline increased the tumor necrotic area, induced the release of immune factors, improved the in vivo immune response, and slowed down the tumor growth rate, suggesting that a direct injection of ozonated saline into VX2 tumors inhibited the growth of transplanted VX2 tumors in rabbits.
Possible mechanisms for this may include 1) damage to tumor cell ultrastructure inducing tumor cell disintegration and apoptosis, resulting in a reduction in the tumor activity; 2) a change in the hypoxic environment of tumor cells, leading to a reduction in tumor growth; and/or 3) the activation of in vivo immune mechanisms and regulatory effects that indirectly triggered antitumor effects.
Conflict of interest:
The authors declare that they have no conflict of interest.
Funding:
This work was supported by the National Natural Science Foundation of China (81071245).
Ethical approval: This study was approved by the ethics committee of Second Military Medical University.
Informed consent: Not applicable.
REFERENCES
- 1.Schulz S, Häussler U, Mandic R, et al. Treatment with ozone/oxygen-pneumoperitoneum results in complete remission of rabbit squamous cell carcinomas. Int J Cancer. 2008;122:2360–2367. doi: 10.1002/ijc.23382. [DOI] [PubMed] [Google Scholar]
- 2.Sweet F, Kao MS, Lee SC, et al. Ozone selectively inhibits growth of human cancer cells. Science. 1980;209:931–933. doi: 10.1126/science.7403859. [DOI] [PubMed] [Google Scholar]
- 3.Kızıltan HŞ, Bayir AG, Yucesan G, et al. Medical ozone and radiotherapy in a peritoneal, Erlich-ascites, tumor-cell model. Altern Ther Health Med. 2015;21:24–29. [PubMed] [Google Scholar]
- 4.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- 5.Wei Q, Fang L, Yang JJ, et al. The changes of ultra-microstructure of transplanted VX-2 tumor caused by the injection of ozone into the tumor: an experimental study in rabbits. J Intervent Radiol. 2013;22:830–833. [Google Scholar]
- 6.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic Biol Med. 1995;19:935–941. doi: 10.1016/0891-5849(95)02033-7. [DOI] [PubMed] [Google Scholar]
- 7.Clavo B, Pérez JL, López L, et al. Ozone therapy for tumor oxygenation: a pilot study. Evid Based Complement Alternat Med. 2004;1:93–98. doi: 10.1093/ecam/neh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bocci VA. Scientific and medical aspects of ozone therapy: state of the art. Arch Med Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Azuma K, Mori T, Kawamoto K, et al. Anti-inflammatory effects of ozonated water in an experimental mouse model. Biomed Rep. 2014;2:671–674. doi: 10.3892/br.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda K, Azuma K, Mori T, et al. The safety and anti-tumor effects of ozonated water in vivo. Int J Mol Sci. 2015;16:25108–25120. doi: 10.3390/ijms161025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 12.Bocci V, Zanardi I, Travagli V. Ozone: a new therapeutic agent in vascular diseases. Am J Cardiovasc Drugs. 2011;11:73–82. doi: 10.2165/11539890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Katti SS, Chava VK. Effect of ozonised water on chronic periodontitis: a clinical study. J Int Oral Health. 2013;5:79–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 15.Selvendiran K, Kuppusamy ML, Ahmed S, et al. Oxygenation inhibits ovarian tumor growth by down-regulating STAT3 and cyclin-D1 expressions. Cancer Biol Ther. 2010;10:386–390. doi: 10.4161/cbt.10.4.12448. [DOI] [PubMed] [Google Scholar]
- 16.Calunga JL, Menéndez S, León R, et al. Application of ozone therapy in patients with knee osteoarthritis. Ozone Sci Eng. 2012;34:469–475. [Google Scholar]
- 17.Nagayoshi M, Fukuizumi T, Kitamura C, et al. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol. 2004;19:240–246. doi: 10.1111/j.1399-302X.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Bocci V, Zanardi I, Travagli V. Oxygen/ozone as a medical gas mixture: a critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res. 2011;1:6. doi: 10.1186/2045-9912-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz A, Bauer G. Selective effect of tumor necrosis factor on transformed versus nontransformed cells: nonselective signal recognition but differential target cell response. Anticancer Res. 2000;20:3435–3442. [PubMed] [Google Scholar]
- 20.Mule JJ, McIntosh JK, Jablons DM, et al. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990;171:629–636. doi: 10.1084/jem.171.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher G, Stimson WH, Findlay J, et al. Interleukin-6 enhances the induction of human lymphokine-activated killer cells. Cancer Immunol Immunother. 1990;31:49–52. doi: 10.1007/BF01742495. [DOI] [PMC free article] [PubMed] [Google Scholar]