Abstract
Radiopharmaceuticals are essential components of nuclear medicine and serve as one of the cornerstones of molecular imaging and precision medicine. They provide new means and approaches for early diagnosis and treatment of diseases. After decades of development and hard efforts, a relatively matured radiopharmaceutical production and management system has been established in China with high-quality facilities. This review provides an overview of the current status of radiopharmaceuticals on production and distribution, clinical application, and regulatory supervision and also describes some important advances in research and development and clinical translation of radiopharmaceuticals in the past 10 years. Moreover, some prospects of research and development of radiopharmaceuticals in the near future are discussed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-021-05615-6.
Keywords: Radiopharmaceuticals, Current status, Radionuclides, Theranostic, Future perspective
Introduction
The research and production of medical radioisotopes and radiopharmaceuticals in China began in the 1950s [1]. In 1958, the first heavy water reactor and the first accelerator were built and put into use in China at the China Institute of Atomic Energy (CIAE). The first batch of 33 radioisotopes including 24Na, 32P, 60Co, and 131I were successfully developed, thus initiating the undertakings of the production and application of radioisotopes and radiopharmaceuticals in China. Since the late 1950s, 131I has been widely used in the diagnosis of thyroid function and radionuclide therapy for hyperthyroidism and thyroid cancer, and 32P has been applied for the treatment of polycythemia vera and the control of cancerous pleural effusion and ascites, which pioneered the application of radioisotopes in clinical diagnosis and treatment in China.
Since the 1970s, China has committed to the development of the technologies on production of key medical radioisotopes such as 131I, 125I, 32P, 99Mo, and 99mTc. For example, two types of 99Mo/99mTc generators (fission and gel) were successively developed with stable manufacturing technique and established good manufacturing practice (GMP) facilities to meet the domestic market demand to a certain extent [2, 3]. Currently, there are five nuclear reactors in service in China for research purposes, and their associated facilities have been used for the production of medical radioisotopes such as 99Mo, 125I, 131I, 32P, 177Lu, 89Sr, and 153Sm. Among above five reactors, two of them are located in Beijing, and the other three are located in the Southwest China. However, because of various factors, only a small amount of 131I has been produced since 2008, and the supply of other reactor-produced medical radioisotopes has heavily relied on importing abroad. As a comparison, accelerator-produced radioisotopes have been largely developed in China. As of 2020, three 30 MeV cyclotrons and more than 120 small medical cyclotrons have been installed for the production of radionuclides such as 18F, 123I, and 64Cu.
The great progress on research and development of radiopharmaceuticals in China has been made during the course of more than half a century of growth [4]. Until now, over 40 radiopharmaceuticals involving 12 types of radionuclides including 99mTc, 18F, 131I, 125I, 153Sm, and 32P have been approved for commercial use by the National Medical Products Administration (NMPA). Out of the nearly 40 approved radiopharmaceuticals, 23 of them are 99mTc-labeled radiopharmaceuticals and associated kits, 1 is 18F-fluorodeoxyglucose ([18F]FDG), and 9 of them are therapeutic radiopharmaceuticals such as [131I]NaI oral solution (and/or capsules), [89Sr]SrCl2 injection, and 125I brachytherapy source which in contrast to other countries are regulatory categorized as radiopharmaceuticals in China.
At present, academic groups and associations that are engaged in the field of medical radioisotopes and radiopharmaceuticals in China mainly include the Chinese Isotope Society (CIS), the Chinese Nuclear and Radiochemistry Society (CNRS), the Chinese Society of Nuclear Medicine (CSNM), the China Isotope and Radiation Association (CIRA), and the Chinese Society of Radiopharmaceutical Science (CSRS). They have played a significant role in promoting the research, development, production, and clinical application of radiopharmaceuticals in China.
It should be noted that over the past few decades, a number of research institutes and universities in China have played an important role in conducting fundamental research, striving for technological progress as well as training talents and personnel in the fields of radiopharmaceuticals. These institutions include (in no particular order): (1) China Institute of Atomic Energy (Beijing); (2) Beijing Normal University (Beijing); (3) Chinese Academy of Medical Sciences (Beijing); (4) Shanghai Institute of Applied Physics (Chinese Academy of Sciences) (Shanghai); (5) Institute of High Energy Physics (Chinese Academy of Sciences) (Beijing); (6) China Academy of Engineering Physics (Sichuan Province); (7) Jiangsu Institute of Nuclear Medicine (Jiangsu Province); (8) Peking University (Beijing); (9) Fudan University (Shanghai); (10) Sichuan University (Sichuan Province); (11) Suzhou University (Jiangsu Province); and (12) Xiamen University (Fujian Province).
In June 2021, eight state departments including the China Atomic Energy Authority, the Ministry of Science and Technology, and the National Medical Products Administration jointly issued the Mid- and Long-term Development Plan (2021–2035) for Medical Isotopes. The Plan has deployed key tasks for the development of medical isotope-related industries including radiopharmaceuticals. It aims to advance the research and development of new radiopharmaceuticals, accelerate the realization of independent production and supply of medical isotopes, and promote the clinical application of radiopharmaceuticals. The Plan is of great significance and provides a roadmap in driving forward the development of radiopharmaceutical industry in China.
This review mainly focuses on the topic of the production and distribution, clinical application, regulatory supervision, research and development, and clinical translation of radiopharmaceuticals in China in the past 10 years, and it also discusses prospect of research and development on radiopharmaceuticals in China.
Production and distribution of radiopharmaceuticals
After decades of development, radiopharmaceutical industry in China has made great progress; the whole industry chain integrating research, production, sales, and application has been established; and the market scale has continued to grow [5]. At present, the companies engaged in production and sales of radiopharmaceuticals in China mainly include China Isotope & Radiation Corporation and its subsidiaries HighTech Atom Co., Ltd. (HTA) and Chengdu Gaotong Isotope Co., Ltd. (Chengdu Gaotong), Dongcheng Pharmaceutical Group Co., Ltd. and its subsidiary Dongcheng AMS Pharmaceutical, Jiangyuan Pharmaceutical Factory of Jiangsu Institute of Nuclear Medicine, and Beijing Shihong Pharmaceutical Co., Ltd. of Beijing Normal University. The radiopharmaceuticals commercially available in China are summarized in the Table 1. It indicates that only a limited number of radionuclides and radiopharmaceuticals are currently produced and supplied in China, and the demand of clinical nuclear medicine is not fully met yet.
Table 1.
Radiopharmaceuticals commercially available in China
| Radionuclide | Radiopharmaceuticals | Main suppliers |
|---|---|---|
| 131I | Sodium iodide [131I] oral solution ([131I]NaI) | HTA; Chengdu Gaotong; Department of Nuclear Physics and Chemistry, China Academy of Engineering Physics |
| Sodium iodide [131I] capsules for diagnostic use ([131I]NaI) | HTA | |
| Sodium iodide [131I] capsules for therapeutic use ([131I]NaI) | HTA | |
| Sodium iodohippurate [131I] injection ([131I]OIH) | Chengdu Gaotong | |
| Iodine [131I] metuximab injection ([131I]I-metuximab) | Chengdu Huasun Biotechnology | |
| 125I | Iodine [125I] brachytherapy source | HTA; Seeds Biological Pharmacy (Tianjin); ZHIBO Bio-Medical Technology (Beijing); Ningbo Junan Pharmaceuticals Technology; etc |
| 153Sm | Samarium [153Sm] lexidronam injection ([153Sm]Sm-EDTMP) | HTA |
| 18F | [18F]-Flurodeoxyglucose injection ([18F]FDG) | HTA; Nanjing Jiangyuan AMS Positron Research and Development; Jiangsu Huayi Technology; Shanghai Atom Kexing Pharmaceuticals |
| 32P | Sodium phosphate [32P] oral solution (mainly [32P]Na2HPO4) | HTA |
| 99mTc | Sodium pertechnetate [99mTc] injection ([99mTc]NaTcO4) | HTA; Chengdu Gaotong |
| Technetium [99mTc] bicisate injection ([99mTc]Tc-ECD) | HTA; Chengdu Gaotong; Shanghai Atom Kexing Pharmaceuticals; Guangzhou HTA Pharmaceutical; Shanghai GMS Pharmaceutical; Guangdong Ci Medicine | |
| Technetium [99mTc]-L,L-ethylenedicysteine injection ([99mTc]Tc-EC) | ||
| Technetium [99mTc] sestamibi injection ([99mTc]Tc-MIBI) | ||
| Technetium [99mTc] methylenediphosphonate injection ([99mTc]Tc-MDP) | ||
| Technetium [99mTc] etifenin injection ([99mTc]Tc-EHIDA) | ||
| Technetium [99mTc] phytate injection ([99mTc]Tc-Phytate) | ||
| Technetium [99mTc] pentetate injection ([99mTc]Tc-DTPA) | ||
| Technetium [99mTc] albumin aggregated injection ([99mTc]Tc-MAA) | ||
| Technetium [99mTc] dimercaptosuccinate injection ([99mTc]Tc-DMSA) | ||
| Technetium [99mTc] tetrofosmin injection ([99mTc]Tc-TF) | ||
| Technetium [99mTc] pyrophosphate injection ([99mTc]Tc-PYP) | ||
| 99Tc | Technetium [99Tc] methylenediphosphonate injection ([99Tc]Tc-MDP) | Chengdu Yunke Pharmaceuticals |
| 201Tl | Thallium [201Tl] chloride injection ([201Tl]TlCl) | HTA |
| 89Sr | Strontium [89Sr] chloride injection ([89Sr]SrCl2) | Chengdu Gaotong; Ningbo Junan Pharmaceuticals Technology; Shanghai Atom Kexing Pharmaceuticals |
| 223Ra | Radium [223Ra] chloride injection ([223Ra]RaCl2) | Bayer AG |
*Data from https://www.nmpa.gov.cn
Because of the short half-life of 99mTc (6.0 h) and 18F (109.7 min), medical institutions can prepare such radiopharmaceuticals on their own for nuclear medicine clinical application. A more common option is to rely on commercial radiopharmaceutical production centers (also known as centralized radiopharmacy) that meet GMP standards for instant radiolabeling, production and distribution. To date, there are around 50 centralized radiopharmacies built by large radiopharmaceutical companies such as HTA and Dongcheng AMS Pharmaceutical that are running in the large- and medium-sized cities in China for production and distribution of 99mTc-labeled radiopharmaceuticals and positron radiopharmaceuticals including [18F]FDG (refer to Fig. 1). In addition to these commercial centralized radiopharmacies, large medical institutions equipped with cyclotrons independently produce [18F]FDG and other 11C-, 15 N-, and 18F-labeled positron radiopharmaceuticals for clinical research in accordance with the requirements of Regulations on the Preparation of Positron Radiopharmaceuticals in Medical Institutions issued by the NMPA. As the nuclear medicine industry in China continues to grow, more centralized radiopharmacies need to be built to achieve extensive coverage and meet the pace of nuclear medicine development. It is estimated that by 2025, about 50 new centralized radiopharmacies will be built across the country, and the availability and accessibility of radiopharmaceuticals will be significantly improved by then.
Fig. 1.
The production and distribution network of radiopharmaceuticals in China. Centralized radiopharmacy refers to the production center for the diagnostic radiopharmaceuticals with radionuclides having shorter half-life, such as 99mTc and 18F. Radiopharmaceutical production base refers to the factory for the production of medical radioisotopes and the production of diagnostic and therapeutic radiopharmaceuticals labeled with radionuclides having longer half-life
In recent years, because of the successful application of targeted radiopharmaceuticals in the clinical diagnosis and treatment, the radiopharmaceuticals industry has gradually become the focus of attention among financial capitals and innovative pharmaceutical companies. Some traditional pharmaceutical companies, such as Dongcheng Pharmaceutical Co. Ltd., Sinotau Pharmaceuticals, and Hengrui Pharma, have begun to set foot in the radiopharmaceutical industry and started research, development, and production of radiopharmaceutical. This will further boost the development of the radiopharmaceutical industry in China.
Clinical applications of radiopharmaceuticals
In April 2020, the Chinese Society of Nuclear Medicine carried out a biennial national census on current status of nuclear medicine in China [6]. The number of nuclear imaging cameras and clinical nuclear medicine practices in China are illustrated in Fig. 2. The result highlights that as of December 2019, there are 1148 departments (sections) engaged in nuclear medicine practices, 770 (67.1%) of them have conducted radionuclide therapy. There are 427 sets of positron imaging equipment nationwide, including 23 sets of positron emission tomography–magnetic resonance imaging (PET-MRI) and 404 sets of PET-computerized tomography (CT). The number of single photon imaging equipment (including gamma camera, single photon emission computed tomography (SPECT), cardiac SPECT, SPECT-CT, and coincidence SPECT) amounts to 903 sets across the country. One hundred seventeen medical institutions are equipped with 120 medical cyclotrons for the preparation of [18F]-FDG and other positron radiopharmaceuticals.
Fig. 2.
The number and type of nuclear imaging cameras (a), categories of top 3 PET/CT + PET/MRI studies performed in the year of 2019 (b), categories of top 5 single photon studies performed in the year of 2019 (c), categories of top 5 radionuclide therapy performed in the year of 2019 (d)
In terms of PET/CT imaging, a total of 849,900 examinations were carried out in 2019, of which [18F]FDG PET accounted for the majority: tumor imaging (94.5%), central nervous system imaging (1.7%), and cardiovascular system imaging (0.6%). In addition, the total number of PET/MRI examinations was 14,095 in year 2019. With regard to the total number of SPECT examinations in year 2019, the figure stood at 2.51 million, up by 19.9% compared to that of 2017. SPECT mainly employs 99mTc-labeled radiopharmaceuticals. The top 5 SPECT examinations were skeletal system (account for 63.1%) using [99mTc]Tc-MDP, endocrine system (15.9%) using [131I]NaI and a few [99mTc]NaTcO4, urinary system (11.7%) using [99mTc]Tc-DTPA, circulatory system (4.2%) using [99mTc]Tc-MIBI and [99mTc]Tc-TF, and digestive system (1.9%) using [99mTc]Tc-EHIDA and [99mTc]Tc-Phytate.
At present, about 65% of the hospitals and/or medical institutions that use SPECT radiopharmaceuticals purchase 99mTc-labeled radiopharmaceuticals from the centralized radiopharmacies. Others purchase 99Mo/99mTc generators and 99mTc cold kits to prepare 99mTc-labeled radiopharmaceuticals on their own. With regard to positron pharmaceuticals which is mainly [18F]FDG, 333 hospitals and/or medical institutions purchase from radiopharmacies, while 119 hospitals and/or medical institutions prepare their own [18F]FDG. In addition, some medical institutions use their own cyclotrons to prepare positron radiopharmaceuticals such as [18F]NaF, [18F]F-PSMA derivatives, [18F]fluoromisonidazole ([18F]FMISO), 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine ([18F]F-DOPA), [18F]fluoro-ethyl-tyrosine ([18F]FET), [18F]fluorothymidine ([18F]FLT), [13 N]NH3.H2O, [15O]H2O, [11C]Acetate, [11C]Methionine, [11C]Choline, [11C]flumazenil ([11C]FMZ), [11C]Raclopride, [11C]methyl-N-2β-carbomethoxy-3β-(4-fluorophenyl) tropane ([11C]CFT), and [11C]-N-methylspiperone ([11C]NMSP) for the diagnosis and clinical research. Over the past few years, some medical institutions have also carried out research on the radiolabeling and clinical application of 68Ga-labeled positron radiopharmaceuticals such as [68Ga]Ga-DOTA-TATE, [68Ga]Ga-PSMA derivatives, and [68Ga]Ga-chemokine receptor 4 ([68Ga]Ga-CXCR4) by purchasing 68Ge/68Ga generators imported from abroad.
Up to now, there are 2544 beds in total dedicated to radionuclide therapy in all the medical institutions that carry out radionuclide therapy across China. Nearly 530,000 treatments have been conducted annually, among which 131I treatment of Graves’ hyperthyroidism accounts for 27.6%, and 90Sr/90Y applicator treatment for skin and eye diseases 25.5%, 99Tc-methylene bisphosphonate treatment for rheumatoid arthritis 18.6%, 131I treatment of differentiated thyroid cancer 15.9%, 32P application treatment 6.6%, radioactive seed interstitial implantation treatment 2.4%, and 89Sr for bone metastasis 2.0%. In recent years, some medical institutions have begun to use [223Ra]RaCl2 for the treatment of castrated prostate cancer patients with bone metastases, and some therapeutic radiopharmaceuticals such as [177Lu]Lu-DOTA-TATE, [177Lu]Lu-DOTA-TOC, and [177Lu]Lu-PSMA have also been prepared and investigated for the clinical research of neuroendocrine tumors and prostate cancer.
Radiopharmaceutical regulations and supervision
Radiopharmaceutical administration in China has gone through a long process of continuous improvement. In the 1950s to 1960s [7], radiopharmaceuticals were regulated as medical radioisotopes. It was not until 1974 that radiopharmaceuticals began to be regulated as pharmaceuticals. The 1977 edition of Chinese Pharmacopoeia included radiopharmaceuticals for the first time. In 1989, the State Council of China promulgated the Measures for the control of Radioactive Drugs, ushering in an era of law-based administration of radiopharmaceuticals in China. In 2017, the Measures for the control of Radioactive Drugs was revised. The provisions on the development, clinical research and approval, production, operation, import and export, packaging and transportation, and use of radiopharmaceuticals were provided. A radiopharmaceutical production and handling enterprise must hold the “Radiopharmaceutical Production License” and/or the “Radiopharmaceutical Handling License”. To be able to use radiopharmaceuticals, medical institutions must obtain a “Radiopharmaceutical Using License”.
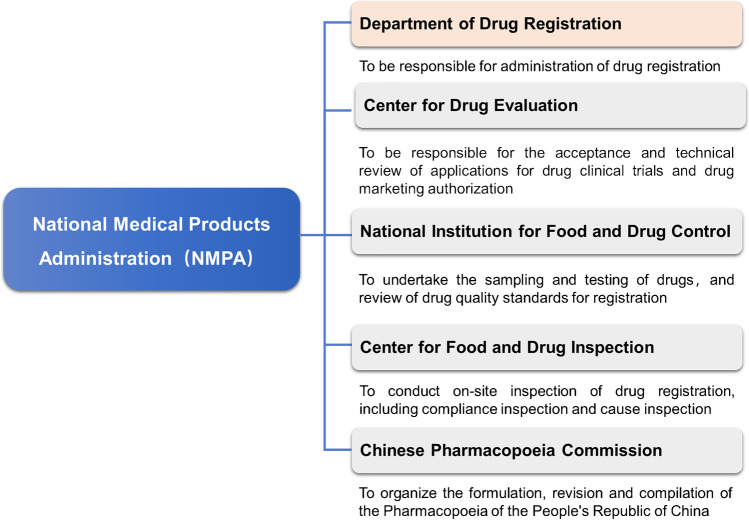
Currently, the state authority responsible for the supervision and registration of radiopharmaceuticals is the NMPA and its directly affiliated units, comprising mainly of the Department of Drug Registration, the Center for Drug Evaluation (CDE), the National Institutes for Food and Drug Control, Center for Food and Drug Inspection (CFDI), and the Chinese Pharmacopoeia Commission (refer to Fig. 3).
Fig. 3.
NMPA and its affiliated institutions responsible for the supervision and registration of radiopharmaceuticals in China
Radiopharmaceutical-related policies and regulations that are currently in effect include The Drug Administration Law of the People’s Republic of China (newly revised in 2019), Regulations for Implementation of the Drug Administration Law of the People’s Republic of China (newly revised in 2019), Measures for the Control of Radioactive Drugs, and Good Manufacturing Practice for Pharmaceutical Products (amended in 2010).
In 2006, the NMPA promulgated the Regulations on the Administration of the Preparation of Positron Radiopharmaceuticals by Medical Institutions, which regulates in explicit terms on the licensing conditions for the use and preparation of radiopharmaceuticals in medical institutions. The licenses are classified into four levels: a class I or a higher level license should be held for the use of radioimmunoassay kits for in vitro diagnosis; a class II or a higher level license should be held for the use of radiopharmaceuticals; a class III or a higher level license should be held for the preparation of radiopharmaceuticals; and a class IV license should be held for the development and related clinical research of new-type of radiopharmaceuticals, meanwhile a filing application should be submitted to the NMPA.
The introduction and use of a new type of radiopharmaceuticals without marketing authorization in medical institutions are mainly through the pathway of investigator-initiated clinical studies. Investigators should submit the complete preclinical study data to the Clinical Research Management Committee and Ethics Committee for approval of a limited number of patient clinical studies which are not for the purpose of seeking marketing authorization. Furthermore, the preparation of relevant radiopharmaceuticals in medical institutions shall be carried out in accordance with good manufacturing practice for pharmaceutical products, and the radiopharmaceuticals prepared in medical institutions for clinical studies are for local in-house use only.
In light of the fact that radiopharmaceuticals are usually with short physical half-life, the NMPA has successively issued the related documents such as Guidelines for Technetium [99mTc] Radiopharmaceutical Quality Control and Guidelines for Positron Radiopharmaceutical Quality Control. In 2019, the newly revised Drug Administration Law of the People’s Republic of China and Provisions of Drug Registration were issued, under which the marketing authorization holder (MAH) system was implemented. The regulation of clinical trials for new pharmaceuticals was reformed from the previous approval system into implied licensing. These revised regulations are also applicable to radiopharmaceuticals, presenting new opportunities to radiopharmaceutical innovation.
Since 2020, the NMPA has successively issued the Technical Guidelines for Clinical Evaluation of Diagnostic Radiopharmaceuticals and the Technical Guidelines for Non-clinical Research of Diagnostic Radiopharmaceuticals in order to promote and standardize the research, development, and clinical research of diagnostic radiopharmaceuticals in China.
Important advances in research and development of radiopharmaceuticals
Production of medical radioisotopes
In recent years, the preparation techniques of some major medical radioisotopes such as 99Mo (LEU, low enriched uranium), 177Lu, 64Cu, 123I, 89Zr, and 211At have been developed using reactors or cyclotrons, and some new progresses and breakthroughs have been achieved in China.
CIAE developed the production procedure of fission 99Mo with an emphasis on LEU target [8–10]. UO2 layer was electroplated on inner wall of a hollow stainless cylinder. After dissolution, radiochemical extraction, and further purification processes, the final 99Mo solution can meet the requirements for medical usage. CIAE also established the separation process of fission 99Mo using Al2O3 chromatography. The recovery yield of 99Mo was more than 90%, and the decontamination effectiveness of the impurities such as Ru, Sr, Zr, Te, and Cs was more than 99.99%, and more than 92% of 131I could be removed.
177Lu-labeled radiopharmaceuticals can be used in the treatment of primary and metastatic tumor and palliation of bone pain caused by metastases. In recent years, the continued improvement of 177Lu preparation technology has further promoted the development of 177Lu radiopharmaceuticals. Relying on China Mianyang Research Reactor (CMRR), China Academy of Engineering Physics has developed technology and production facilities for 177Lu, and it represents the first success in China to develop the production technology on no-carrier-added 177Lu [11]. Small batch production (curie level) was achieved, and the product has been used in clinical trials at some hospitals. In the meantime, Chengdu Gaotong has signed a technology licensing agreement with Isotopen Technologien München Group in Germany, and it will implement the development and GMP-grade production of no-carrier-added 177Lu of up to one hundred curies scale in China.
64Cu could form a perfect theranostic nuclide pair with 67Cu, and combining with its favorable properties makes 64Cu a radioisotope of choice for clinical application of PET with unique advantages. Based on Cyclone-30 cyclotron and after a series of improvements on production process, HTA has established an automated process for 64Cu production through 64Ni(p,n)64Cu reaction with batch capacity greater than 74 GBq and the radionuclide purity of the product greater than 99.9% [12, 13], and the product has been provided to a number of research institutions.
Also based on Cyclone-30 cyclotron, using high abundance (> 99.8%) 124Xe gas as target, HTA achieved the mass production of 123I via 124Xe(p,2n)123Cs(β+)123Xe(EC, β+)123I reaction, and the batch activity could reach 111 ~ 148 GBq, and the irradiation yield was greater than 296 MBq/(μA·h) [14]. 123I has been routinely produced and supplied by HTA domestically since 2017.
Based on CS-30 cyclotron, Sichuan University explored a simple and convenient method for radiochemical separation of 89Zr with no harmful substance [15]. The radionuclidic purity of separated 89Zr in the form of [89Zr]Zr-chloride was 99.99%, and the recovery rate was 85% ± 3%. The yields of 89Zr via the reaction of (p,n) or (d,2n) on Y target were also evaluated, and the latter one was proved to be more favorable for the production of 89Zr with a yield of 58 ± 4 MBq/μA·h.
Sichuan University also conducted research on the preparation of alpha particle emitting radioisotope 211At using CS30 cyclotron via 209Bi(α,2n)211At reaction [16]. Bismuth target was prepared by electroplating method. Using a homemade high-temperature dry distillation still, around 200 MBq of 211At was isolated, with the ratio of 211Po/211At less than 10−8.
Research and development of radiopharmaceuticals
Over the last decade, tremendous progress has been made in the development of novel radiopharmaceuticals by a large number of universities, hospitals, and research institutions in China. Numerous publications in this topic have become available in the literature, including a recent manuscript in Nature [17] and increasing number of publications in EJNMMI [18–24]. The trajectory has displayed a major upward trend over the last decade, which clearly showed that this is a highly vibrant field. A comprehensive summary of the progress in the development of novel radiopharmaceuticals in China over the last decade is far beyond the scope of this review article, and interested authors are referred to these excellent review articles for more details [4, 25]. Below, we will only briefly mention a few representative tracers and therapeutic radiopharmaceuticals for various applications in cancer and other diseases.
Central nervous system imaging agents
Potential radiotracers for β-amyloid plaque and Tau protein in Alzheimer’s disease
Patients with Alzheimer’s disease (AD) present with both extracellular amyloid-β (Aβ) plaques and intracellular tau-containing neurofibrillary tangles in the brain [26]. Therefore, Aβ plaques and Tau protein are important pathological features of AD, which are considered to be the important targets for early diagnosis of AD.
Cui et al. reported the design and synthesis of a series of PET probes for Aβ plaque imaging [27–32]. Among them, [18F]Florbetazine, which has a new molecular structure of dihydrazone, demonstrates suitable pharmacokinetic properties including high initial brain uptake and fast brain clearance in rodents and non-human primates, and it also shows comparative affinity compared to the Food and Drug Administration (FDA) approved [18F]Florbetapir and [18F]Florbetaben [27]. Xu et al. reported a new Aβ plaque radiotracer [18F]DRKXH1 (5-(4-(6-(2-[18]fluoroethoxy)ethoxy)imidazo[1,2-alpha]pyridin-2-yl)phenyl), whose distribution volume ratios value is higher than that of [18F]Florbetapir (1.29 ± 0.05 vs. 1.05 ± 0.08) [20]. The AD patients have high retention in cortical regions, while healthy control subjects have uniformly low radioactivity uptake.
A chiral 2-fluoromethyl-1,2-ethylenediol side chain was attached to the 2-phenylquinoxaline backbone to increase hydrophilicity of the compound, thereby improving the binding affinity and selectivity of the probe toward Tau tangles on β-amyloid plaques (Aβ). Quantitative binding assays with AD homogenates show that the probes (R)-1-fluoro-3-(4-(6-(methylamino)quinoxalin-2-yl)-phenoxy)propan-2-ol ((R)-5) and (S)-1-(4-(6-(dimethylamino)quinoxalin-2-yl)phenoxy)-3-fluoropropan-2-ol ((S)-16) have high affinity (Ki = 4.1 and 10.3 nM, respectively) and high selectivity (30.5-fold and 34.6-fold, respectively) for Tau tangles [33]. In addition, they display sufficient blood–brain barrier penetration (7.06% and 10.95% ID/g, respectively) and suitable brain kinetics (brain2 min/brain60 min = 10.1, 6.5, respectively) in normal mice. These results demonstrate that (R)-[18F]5 and (S)-[18F]16 are promising PET probes for Tau tangles imaging.
Potential radiotracers for imaging of sigma-1 receptor
Sigma-1 (σ1) receptors are proved to be related to brain dysfunction and tumors as well as heart failure. Development of specific radiotracers for σ1 receptor imaging may provide useful diagnostic tools for investigation of their pathophysiology [34].
Jia’s group designed and synthesized a range of new 18F-labeled compounds that show potential for imaging σ1 receptor, including benzylpiperazine derivatives such as 1-(4-18F-fluorobenzyl)-4-((tetrahydrofuran-2-yl)methyl)piperazine [35], 4-phenylpiperidine-4-carbonitrile derivatives [36], 1,4-dioxa-8-azaspiro[4,5]decane derivatives [37], and 1-oxa-8-azaspiro[4.5]decane derivatives [38]. [18F]FBFP was synthesized in one step from an iodonium ylide precursor, and it possesses higher regional non-displaceable binding potential (BPND) values across the brain regions compared with (S)-[18F]fluspidine [39]. [18F]FBFP displays high brain uptake and suitable tissue kinetics for quantitative analysis in cynomolgus monkeys.
Myocardial perfusion imaging agents
Myocardial perfusion imaging is a well-established non-invasive method for diagnosing coronary artery disease.
Recently, Zhao et al. reported the optimization of biodistribution properties of radiotracers [99mTc]TcCl(CDO)(CDOH)2B-R] (CDOH2 = cyclohexanedione dioxime) using different boronate caps [40]. Among these 11 new 99mTc-labeled radiotracers, [99mTc]Tc-3SPboroxime [R = 3SP; 3-(methylsulfonyl)pyridine] shows the most promising characteristics as an optimal heart imaging agent. The SPECT image quality with [99mTc]Tc-3SPboroxime in SD rats is better than that with [99mTc]Tc-teboroxime. High heart uptake and long myocardial retention of 99mTc-3SPboroxime have been confirmed in swine models [41].
Zhang et al. reported three novel 18F-labeled pyridaben analogues (Fmpp1, Fmpp2, and Fmpp3) for potential myocardial perfusion imaging [42]. In the whole-body PET/CT images of mini-swine, [18F]Fmpp2 shows excellent initial heart standardized uptake value (SUV) (7.12 at 5 min p.i.) and good retention (5.75 at 120 min p.i.). The heart/liver SUV ratios are 4.12, 5.42, and 5.99 at 30, 60, and 120 min after injection, respectively. The favorable biological properties of [18F]Fmpp2 suggest that it is worth further investigation.
Tumor imaging agents
Prostate-specific membrane antigen targeting radiotracers
Prostate-specific membrane antigen (PSMA) is over-expressed on the surface of the most of prostate cancer cells, and this expression of PSMA increases in low differentiated, metastatic, and androgen-independent prostate cancer cells. Therefore, PSMA is a promising target for diagnosis and therapy of prostate cancer [43].
In an effort to seek novel agents targeting PSMA, based on oxalyldiaminopropionic acid (ODAP), 16 ligands with structural modifications in PSMA S1′ binding pocket were synthesized and evaluated for PSMA inhibition by Duan et al. (S)-3-(carboxyformamido)-2-(3-(carboxymethyl)ureido) propanoic acids prove to be potent PSMA ligands with Ki values ranging from 0.08 to 8.98 nM [44]. Twelve ODAP-urea-based ligands were synthesized and radiolabeled with 68 Ga [22]. [68 Ga]Ga-P137 with the side chain of naphthalenyl can image PSMA in xenograft models and humans, with lower bladder accumulation to the Glu-Urea-based agent, [68 Ga]Ga-PSMA-617.
Liu et al. reported a novel PSMA inhibitor, 6-hydrazinonicotinate–amino-caproic acid-lysine-urea-glutamate (HYNIC-ALUG), which was labeled with 99mTc [45]. Preliminary clinical results show that this probe can be used to guide surgical procedures to remove more metastatic lymph nodes, and it can be of great value for response assessment and prediction of the effectiveness of treatment in metastatic castration resistance prostate cancer (mCRPC) patients after long-term abiraterone treatment.
Based on the Glu-Ureido-Lys binding motif, three 18F-labeled PSMA tracers with a more lipophilic quinoline functional spacer were designed, synthesized, evaluated, and compared with [18F]DCFPyL by Zhang et al. [46]. There is no significant correlation between the renal elimination and the lipophilicity of the tracers in all species. However, the more lipophilic of the tracer was, the more radioactivity accumulated in the liver of primate and human, and the fewer radioactivity was to be excreted to the bladder with urine. The screened tracer [18F]8c, with a Ki value of 4.58 nM, displays notable low bladder retention and demonstrates good imaging properties in patients with prostate cancer.
Intergrin αvβ3 targeting radiotracers
The integrin αvβ3 is highly expressed in neovascular epithelium of various tumors, which is a highly potential target for tumor diagnosis and therapy, and tripeptide sequence of arginine-glycine-aspartic acid (RGD) is the specific ligand which can be combined to integrin αvβ3 [47].
Researchers in China have made great effort on the exploration of RGD peptides labeled with [18F]AlF, 68Ga, and 99mTc, such as [18F]F-Alfatide injection [48], [68Ga]Ga-cycratide [49], [68Ga]Ga-NOTA-PEG3-β-Glu-RGD [50], [99mTc]Tc-3PRGD2 [51], and [99mTc]Tc-RWY [52]. Among them, [18F]F-Alfatide injection used for the diagnosis of tumor lymph metastasis is carrying out phase II study, and [99mTc]Tc-3PRGD2 injection used for benign and malignant diagnosis of lung tumors and diagnosis of lymph node metastasis is in the phase III study.
PD-1/PD-L1 targeting radiotracers
In recent years, along with the clinical success of tumor immunotherapy, it is very important to screen the beneficiaries, predict the efficacy of drugs, and guide the clinical treatment during the immunotherapy. Accurate detection of PD-1/PD-L1 can be used to screen patients most likely to be responsive to PD-1/PD-L1 immunotherapy and to distinguish reactive tumors from refractory tumors at an early stage [53].
Using a single-domain antibody, NM-01, against PD-L1, radiolabeled site specifically with 99mTc for SPECT, Xing et al. conducted early phase I study in non-small cell lung cancer, which demonstrates that 99mTc-labeled anti-PD-L1-single-domain antibody SPECT/CT imaging is safe and associated with acceptable dosimetry [54]. Tumor uptake is readily visible against background tissues, particularly at 2 h when the primary tumor-to-blood-pool ratios (1.24 to 3.53 (mean, 2.22)) correlate with PD-L1 immunohistochemistry results (r = 0.68, P = 0.014).
Miao et al. reported the radiosynthesis of the small molecule PD-L1 inhibitor [18F]LN via 18F-19F isotope exchange reaction [55]. [18F]LN was achieved with a high radiochemical purity above 95%. PET reveals that [18F]LN enters into PD-L1 expressing tumor site and visualizes the outline of tumor. And tumor uptake reaches the maximum (1.96 ± 0.27%ID/g) at 15 min in the positive group, 2.2-fold higher than that of the negative or the blocked groups.
Huang et al. reported the construction of a novel heavy chain-only antibody named Nb6 with high affinity for hPD-L1 [56]. [124I]I-anti-hPD-L1 Nb6 and [64Cu]Cu-NOTA-Nb6 were prepared respectively for PET to screen patients with malignant tumors such as osteosarcoma and lung adenocarcinoma [56, 57]. JS001 (toripalimab) is a humanized IgG monoclonal antibody which strongly inhibits PD1. Huang et al. tested the new immuno-PET probe [124I]I-JS001 to verify its potential in PD1-positive tumors patients [58]. Yang’s group also conducted a first-in-human study of [68Ga]Ga-NOTA-WL12 (a PD-L1-binding peptide radiotracer) demonstrating the safety and feasibility of the tracer for the detection of the tumor PD-L1 expression levels [59].
99mTc-labeled glucose derivatives
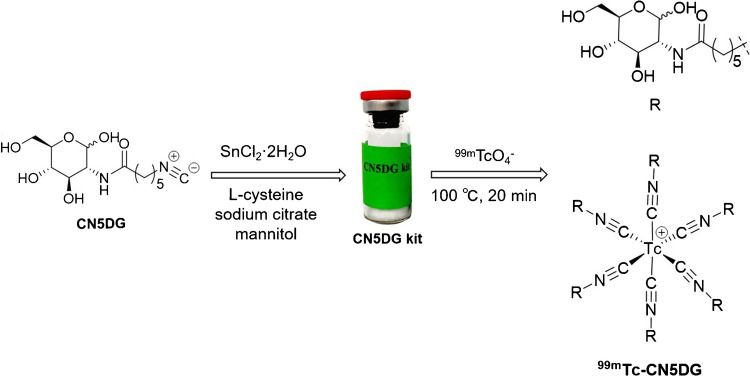
To seek novel 99mTc-labeled glucose derivatives as tumor imaging agents, Zhang’s group reported the synthesis and evaluation of a series of 99mTc-labeled D-glucosamine derivatives with isonitrile as a coordinating group; among them, [99mTc]Tc-CN7DG shows the highest tumor uptake and tumor-to-background ratios in the biodistribution and SPECT/CT studies in mice bearing A549 tumor xenografts [60]. [99mTc]Tc-CN5DG could be readily prepared by using a CN5DG kit (Fig. 4) and was further studied in U87MG, HCT-116, PANC-1, and TE-1 tumor xenografts mice models to verify its potential application for imaging of different kinds of tumors (Fig. 5) [61, 62]. The biodistribution data shows that the tumor/muscle ratios (from 4.08 ± 0.42 to 9.63 ± 3.53) and tumor/blood ratios (from 17.18 ± 7.40 to 53.17 ± 16.16) of [99mTc]Tc-CN5DG in four tumor models are high. These results demonstrate that [99mTc]Tc-CN5DG may become a broad-spectrum SPECT probe for tumor imaging. Phase I clinical trial of this agent is in progress.
Fig. 4.
Preparation of [99mTc]Tc-CN5DG and the chemical structure of CN5DG and [99mTc]Tc-CN5DG.
Reproduced from Translational Oncology, Vol. 14/1, Zhang X et al. [61, 62], Evaluation of 99mTc-CN5DG as a broad-spectrum SPECT probe for tumor imaging, 100,966, Copyright (2021), with permission from Elsevier
Fig. 5.
SPECT/CT images of [99mTc]Tc-CN5DG in nude mice bearing U87 MG(A), HCT-116(B), PANC-1(C) and TE-1(D) tumor xenografts at 2 h after intravenous injection.
Reproduced from Translational Oncology, Vol. 14/1, Zhang X et al. [61, 62], Evaluation of 99mTc-CN5DG as a broad-spectrum SPECT probe for tumor imaging, 100,966, Copyright (2021), with permission from Elsevier
Amino acids based radiotracers
Liu’s group developed 18F-labeled boramino acid derivatives [18F]Ala-BF3 and [18F]Gln-BF3 for tumor imaging [63, 64]. PET study demonstrates that these probes show remarkable and selective tumor uptake in BGC-823 and 4T1 xenografts, respectively. 18F-trifluorobborate-derived tyrosine (denoted as [18F]FBY), a PET tracer with favorable dosimetry profile and pharmacokinetics, was also developed, which shows potential to assay large neutral amino acid transporter type-1 (LAT-1) expression in glioma patients and may provide imaging guidance for further boron neutron capture therapy of gliomas [65]. [18F]FBQ-C2 was designed by adding two more methylene groups to the side chain of 18F-fluoroboronoglutamine ([18F]FBQ). [18F]FBQ-C2 shows greater in vivo stability than that of 18F-(2S,4R)4-fluoroglutamine ([18F]FGln) and [18F]FBQ, and it can be used to perform [82].
Tang’s group reported a new amino acid tracer N-(2-[18F]-fluoropropionyl)-L-glutamate ([18F]FPGLU) [66], which seems to be a better potential PET tracer than [18F]FDG for brain glioma imaging with good visualization and ability to assess the tumor activity, and they also confirmed that excitatory amino acid carrier 1 is an important transporter of [18F]FPGLU in oncologic PET.
Other tumor imaging agents
Besides the tumor imaging agents mentioned above, other tumor targeting radiotracers for imaging of hypoxia and a variety of tumor receptors have also been extensively explored which including: radiolabeled nitroimidazole derivatives for hypoxia imaging such as [64Cu]Cu-BMS181321 [67], [64Cu]Cu-BMS2P2 [68], and [18F]FDG-2NNC2ON [69]; 68Ga- and [18F]AlF-labeled NOTA-MAL-Cys39-exendin-4 for the detection of glucagon-like peptide 1 receptor (GLP-1R)-positive tumor [70, 71]; 18F-labeled ethisterone derivative [18F]FPTT for imaging of progesterone receptor-positive breast cancer [72]; 18F-labeled isonicotinamide-based radioligands for investigating the glycogen synthase kinase-3β (GSK-3β) levels [73]; [18F]FP-Lys-GE11 for imaging of the epidermal growth factor receptor (EGFR) over expressed tumor [74]; [18F]-5-fluoro-N-(2-(Diethylamino)ethyl)picolinamide ([18F]5-FPN) for the detection of metastatic lymph node and metastatic pulmonary lesions of melanoma [75]; [99mTc]Tc-HYNIC-Polypeptide-PEG11-Tz(1,2,4,5-tetrazine) and cetuximab-TCO for the pretargeted imaging of human colon cancer tumor [76]; [68Ga]Ga-NOTA-Nb1053 for visualizing multiple myeloma niduses [19]; and [99mTc]Tc-HYNIC-H10F for imaging of HER2-positive tumors [77].
Imaging agents for other diseases
Some radiotracers targeting other diseases have also been developed including a purinergic receptor P2X7 antagonist [18F]F-PTTP for the detection of inflammation [78], [99mTc]Tc-GlcNAc-PEI (N-acetylglucosamine (GlcNAc) conjugated polyethylenimine (PEI)) specifically interacts with desmin and vimentin expressed on activated hepatic stellate cells (HSCs) for assessing liver fibrosis [79], [18F]FPGal based on isotonicamide for the imaging of asialoglycoprotein receptor (ASGPR) related liver disease [80], and [68Ga]Ga-HZ20 for the quantitative analysis of angiotensin converting enzyme 2 (ACE2) expression in novel coronavirus sensitive organs [81].
The representative tracers mentioned above are summarized in Table 2.
Table 2.
The representative tracers under research and development in China
| Disease | Molecular targets | Radiopharmaceuticals | Stage | Reference |
|---|---|---|---|---|
| Alzheimer's disease | Amyloid-β plaques | [18F]Florbetazine, [18F]DRKXH1 | Clinical | Cui [27], Xu [20] |
| Tau protein | (R)-[18F]5 & (S)-[18F]16 | Preclinical | Zhou [33] | |
| Brain dysfunction | Sigma-1 receptors | [18F]FBFP | Preclinical | Jia [39] |
| Coronary artery disease | [99mTc]Tc-3SPboroxime; [18F]Fmpp2 | Preclinical | Zhao [40, 41], Zhang [42] | |
| Tumor | PSMA |
ODAP-based ligands, [99mTc]Tc-HYNIC-ALUG [18F]8c (with quinoline spacer) |
Preclinical Clinical |
Zhang [46] |
| Intergrin αvβ3 |
[18F]F-Alfatide, [99mTc]Tc-3PRGD2 [68Ga]Ga-cycratide, [99mTc]Tc-RWY [68Ga]Ga-NOTA-PEG3-β-Glu-RGD |
Phase III Clinical Preclinical |
Ma [50] |
|
| PD-1/PD-L1 |
99mTc-labeled anti-PD-L1-single-domain antibody [18F]LN, [124I]I-anti-hPD-L1 Nb6 [64Cu]Cu-NOTA-Nb6, [124I]I-JS001 [68Ga]Ga-NOTA-WL12 |
Phase I Preclinical Preclinical Clinical |
Xing [54] Yang [59] |
|
| Glucose | [99mTc]Tc-CN7DG; [99mTc]Tc-CN5DG | Clinical | Gan [60], Zhang[61] | |
| Amino acid |
[18F]Ala-BF3, [18F]Gln-BF3, [18F]FBQ-C2, [18F]FPGLU [18F]FBY |
Preclinical Clinical |
Liu [63], Li [64], Chen [82], Tang [66] Li [65] |
|
| Hypoxia | [64Cu]Cu-BMS181321, [64Cu]Cu-BMS2P2 [18F]FDG-2NNC2ON | Preclinical |
Yang [69] |
|
| GLP-1R | 68Ga and [18F]AlF-labeled NOTA-MAL-Cys39-exendin-4 | Preclinical | Xu [70], Zhang [71] | |
| Progesterone receptor | [18F]FPTT | Preclinical | Gao [72] | |
| GSK-3β | 18F-Labeled isonicotinamide-based radioligands | Preclinical | Zhong [73] | |
| EGFR | [18F]FP-Lys-GE11 | Preclinical | Li [74] | |
| Melanin | [18F]5-FPN | Preclinical | Wang [75] | |
| Pretargeting | [99mTc]Tc-HYNIC-polypeptide-PEG11-Tz(1,2,4,5-tetrazine) and cetuximab-TCO | Preclinical | Qiu [76] | |
| CD38 | [68Ga]Ga-NOTA-Nb1053 | Preclinical | Wang [19] | |
| HER2 | [99mTc]Tc-HYNIC-H10F | Preclinical | Wu [77] | |
| Inflammation | P2X7 | [18F]F-PTTP | Preclinical | Fu [78] |
| Liver fibrosis | Activated HSCs | [99mTc]Tc-GlcNAc-PEI | Preclinical | Zhang [79] |
| Liver disease | ASGPR | [18F]FPGal | Preclinical | Sun [80] |
| Coronavirus | ACE2 | [68Ga]Ga-HZ20 | Clinical | Zhu [81] |
*Preclinical: radiotracers are under stage of animal study; clinical: radiotracers are under clinical research with healthy volunteers or patients
Phase I–III: radiotracers are under different stages of clinical trials
Therapeutic radiopharmaceuticals
Since [177Lu]Lu-DOTA-TATE was successively approved by European Medicines Agency (EMA) and FDA for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs), and along with the positive results obtained from phase III clinical trial of [177Lu]Lu-PSMA-617 developed by Novartis for the treatment of metastatic castration-resistant prostate cancer (mCRPC), 177Lu-labeled radiopharmaceuticals has attracted increasing attention in radionuclide therapy.
Zhu’s group has carried out the clinical translational researches of [177Lu]Lu-DOTA-EB-TATE, a radiolabeled somatostatin analog modified by Evans blue [83]. In addition, the group is also conducting a clinical study of [177Lu]Lu-EB-PSMA.
Radiolabeling, kit formulation, and animal biodistribution of [177Lu]Lu-EDTMP and [177Lu]Lu-DOTMP have been investigated [84, 85]. Shanghai Huashan Hospital conducted the first clinical study of 177Lu-labeled radiopharmaceuticals in China which is [177Lu]Lu-EDTMP for the pain palliation of bone metastases [86]. Ma et al. conducted radiolabeling study and preliminary biological evaluation of [177Lu]Lu-Rituximab [87]. Liu’s group developed a highly tumor-selective anti-PD-L1 (αPD-L1) antibody, and subsequent radioimmunotherapy with [177Lu]Lu-αPD-L1 antibody showed that this 177Lu-labeled antibody could successfully upregulate antitumor immunity in the tumor microenvironment and turn “cold” tumors “hot” for immunotherapy [88]. Liu et al. investigated 177Lu-labeled panitumumab and cetuximab (conjugated with DOTA), and the results suggested that both177Lu-labeled antibodies were promising for targeted therapy of EGFR-positive tumors especially for those that are resistant to antibody-based immunotherapy [89].
Alpha particles have high linear energy transfer (LET) and moderate path length, giving them an effective range of less than 10 cell diameters, which means cancer cells can be significantly damaged while causing minimal toxicity to the surrounding healthy cells. This renders targeted alpha therapy significant advantages, and it thus has become the hotspot of nuclear medicine research [90].
Based on homemade 211At, several research works on 211At-labeled agents have been carried out. Using N-succinimidyl-5-(tributylstannyl)-3-pyridinecarboxylate (SPC) as a bi-functional linker [91], a one-step method was used for the preparation of [211At]At-SPC-VP2 peptide [92]. The following research suggests that [211At]At–SPC-VP2 has good in vivo stability and shows potential for targeted cancer radiotherapy.
First investigation of the possible use of 211At-labeled octreotide as a potential alpha-radionuclide therapeutic agent for non-small cell lung cancer cell treatment was reported by Yu’s group [93]. [211At]At-SPC-octreotide was prepared by indirect method, and it demonstrates much more lethal effect than the control groups (PBS, octreotide, and free 211At) and a radiation dose-dependent apoptosis-inducing ability.
Radiopharmaceuticals under commercial clinical development or market approval process
In recent years, along with the technological advancement in the research and development of radiopharmaceuticals, and also with the introduction of NMPA's policy to encourage new drug creation, a lot of domestic and foreign radiopharmaceutical companies have been stepping up efforts on radiopharmaceuticals registration application in China. A batch of new radiopharmaceuticals have entered either the approval process or the clinical trials or marketing approval stage, and some of these radiopharmaceuticals are expected to be approved and serve the patients clinically in the near future. The current status of radiopharmaceutical application and approval by the NMPA in China is listed in Table 3.
Table 3.
Radiopharmaceuticals under commercial clinical development in China
| Drug name | Company name | Status | Indications |
|---|---|---|---|
| [18F]F-Alfatide injection | Jiangsu Shimeikang Pharmaceutical Co., Ltd | Phase III | Tumor diagnosis |
|
[18F]Flutemetamol injection |
General Electric Pharmaceutical (Shanghai) Co., Ltd | Phase I–II | Diagnosis of Alzheimer’s disease related to β-amyloid neuritic plaques |
|
[18F]APN-1607 injection |
Suzhou Xinxu Pharmaceutical Co., Ltd | Phase III | Diagnosis and evaluation of neurodegenerative diseases including Alzheimer’s disease and cognitive impairment related to Tau protein pathology |
| [18F]Florbetaben injection | Tianjin HTA Isotope Medicine Co., Ltd | Phase III | Diagnosis of Alzheimer’s disease related to β-amyloid neuritic plaques |
| [18F]NaF injection | Nanjing Jiangyuan AMS Positron Research and Development Co., Ltd | Phase III | Diagnosis of bone metastasis of malignant tumor |
| HTA Co., Ltd | Phase III | ||
| [18F]Florbetapir injection | Nanjing Jiangyuan AMS Positron Research and Development Co., Ltd | Phase II | Diagnosis of Alzheimer’s disease related to β-amyloid neuritic plaques |
| [99mTc]Tc-3PRGD2 injection | Foshan Ruidiao Pharmaceutical Co., Ltd | Phase III | Diagnosis of benign and malignant lung tumors and lung lymph node metastasis |
| [99mTc]Tc-CNDG injection | Beijing Shihong Drug Development Center | Phase I | Diagnosis of benign and malignant tumors |
| [99mTc]Tc-GSA injection | Beijing Shihong Drug Development Center | Phase III | Diagnose the function and morphology of the liver |
| [123I]Ioflupane injection | GE Pharmaceutical (Shanghai) Co., Ltd | Phase III | Diagnosis of Parkinson’s syndrome |
| [90Y]-Glass microspheres | Fangen (Tianjin) Pharmaceutical Development Co., Ltd | Phase III | Therapy of unresectable primary liver cancer |
| [90Y]-resin microspheres | Grandpharma (China) Co., Ltd | Phase II | Therapy of inoperable primary liver cancer |
| [131I]MIBG injection | HTA Co., Ltd | Phase III | Diagnosis of neuroblastoma and pheochromocytoma |
| [177Lu]Lu-DOTA-TATE injection | Advanced Accelerator Applications S.A.; Jingding pharmaceutical research and development (Shanghai) Co., Ltd | Phase III | Therapy of neuroendocrine tumor |
| [188Re]Re-HEDP injection | Yantai Dongcheng Pharmaceutical Group Co., Ltd | Phase II | Palliation of bone pain caused by bone metastases |
| [131I]I-Actuximab injection | Shanghai Haikang Chinese Medicine Technology Development Co., Ltd | Phase I | Therapy of advanced malignant solid tumor |
*Data from http://www.cde.org.cn
Future perspectives
In the past 10 years, major progress has been made in the research and development of diagnostic and therapeutic pharmaceuticals for cancer, cardiovascular system and central nervous system diseases, immunotherapy inflammation, and infection. Some radiopharmaceuticals are in the stage of clinical translation or marketing authorization. However, the radiopharmaceuticals currently in clinical application in China are mainly generic drugs, and the gap compared with developed countries is still quite significant, especially in the area of research and development and clinical translation of therapeutic radiopharmaceuticals. Proceeding from the actual situation in China, the important future trends may be including the following aspects: (1) production of medical radionuclides using both reactors and accelerators; (2) development of new targeted radiopharmaceuticals; and (3) improvement of radiopharmaceuticals supervision.
In order to meet the basic requirements of the development of diagnostic and therapeutic radiopharmaceuticals, the production of the most important medical radionuclides such as 99Mo, 131I, 177Lu, and 123I needs to be emphasized in China. In addition, novel theranostic radionuclides pairs and alpha radionuclides such as 123/124I, 64/67Cu, 44/47Sc, 68Ge/68Ga, 89Zr, 211At, 225Ac, and 223Ra need to be paid more attention.
As an indispensable tool of molecular imaging, the future focus should be on the development of new positron radiopharmaceuticals including central nervous system receptor-binding radiopharmaceuticals with high affinity and selectivity and peptide-based imaging radiopharmaceuticals. The recent major advancements in solid-state detector technology have led to dramatic improvements of the sensitivity and spatial resolution of SPECT cameras with a concomitant sharp shortening of acquisition times. The SPECT imaging is still very active and can efficiently continue to complement PET diagnostic in routine clinical studies. Because of China’s large size and uneven development of nuclear medicine, 99mTc radiopharmaceuticals is expected to remain the mainstream of diagnostic radiopharmaceuticals for a long time, and thus, more new categories of 99mTc radiopharmaceuticals need to be explored and developed.
The theranostic radiopharmaceutical which is an integral part of nuclear medicine to achieve precision medicine will be a major future trend. The combination of 123I/131I radiopharmaceuticals already has found a well-recognized clinical application. By comparison, 68Ga/177Lu, 64Cu/67Cu, 44Sc/47Sc, and other radionuclide pairs and combinations, as well as a number of new targets, especially certain “broad-spectrum” targets such as FAP and PSMA, have emerged as research focus in recent years. Many antibody based biotargets that have been clinically applied, such as PD-L1, CEA, MUC1, and HER2, also provide multiple options for the development of related radiopharmaceuticals, for example, the use of 89Zr/64Cu-labeled antibodies for radioimmunoimaging, and the use of 177Lu/90Y/131I-labeled antibodies for radioimmunotherapy.
Furthermore, attention should also be paid to the unique advantages and promising prospects of targeted alpha therapy. The development and clinical application of alpha radiopharmaceuticals such as 225Ac- and 211At-labeled radiopharmaceuticals should be promoted and advanced.
The past few years have witnessed a great number of revisions and improvements in relevant laws and regulations to better accommodate the characteristics of radiopharmaceuticals. Still, more rules and regulations that are both applicable and feasible need to be introduced, and the evaluation mechanism for radiopharmaceuticals remains to be further improved so as to provide better policy guarantee to the clinical trials and industrialization of new radiopharmaceuticals.
As stated above, the issuing of the Mid- and Long-term Development Plan (2021–2035) for Medical Isotopes will promote the clinical application of radiopharmaceuticals, meanwhile, to advance the research and development of new radiopharmaceuticals. We expect that through all the unremitting efforts put into the area, radiopharmaceuticals will play a crucial role in the improvement and enhancement of the quality of human life and health care in China.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express gratitude to Mr. Zhihao Song, Xuhu Huang, Hailong Zhao, Kai Wen, Xiangyu Qin, Weihua Cheng, Chengwei Ma, and Tianwei Luo for the help of literature search, and to Ms. Jinqi Zhou for revising the language of the manuscript.
Author contribution
JD conceptualized and designed the study; HYL and YYS performed literature search; JH and HYL performed articles selection and drafted the paper; YYS and JD critically commented the paper; all the authors critically revised the paper and approved the submitted version of the manuscript.
Availability of data and material
The manuscript represents valid work, and neither this manuscript nor one with substantially similar content under the same authorship has been published or is being considered for publication elsewhere.
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
This manuscript is submitting to the China special issue
This article is part of the Topical Collection on Radiopharmacy
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang J, Luo Z. The progress of radioisotope technology and application in China. Engineering Sciences. 2008;6(2):19–31. http://www.cnki.com.cn/Article/CJFDTotal-ENSC200802003.htm. Accessed 8 Nov 2021.
- 2.Liang J, Wu Y, Luo Z. Development of radioisotope preparation technology at CIAE. Atomic Energy Science and Technology. 2020;54, Suppl.:177–84 (in Chinese). http://www.aest.org.cn/CN/Y2020/V54/Izengkan/177. Accessed 8 Nov 2021.
- 3.Zhang H, Luo S, Liu G, Zhong Z. Isotope technologies in INPC: state of the art and perspective. Journal of Isotopes. 2011;24, Suppl.(Suppl):116–20 (in Chinese). http://www.tws.org.cn/CN/Y2011/V24/I增刊/116. Accessed 8 Nov 2021.
- 4.Jia H, Liu B. Radiopharmaceuticals in China: current status and prospects. Radiochim Acta. 2014;102(1–2):53–67. doi: 10.1515/ract-2014-2100. [DOI] [Google Scholar]
- 5.Zhang J, Du J. Preparation of radiopharmaceuticals in china: current status and prospects. Journal of Isotopes. 2019;32(3):178–85. doi: 10.7538/tws.2019.32.03.0178. [DOI] [Google Scholar]
- 6.Chinese Society of Nuclear Medicine. A brief report on the results of the national survey of nuclear medicine in 2020. Chin J Nucl Med Mol Imaging. 2020;40(12):747–9 (in Chinese). 10.3760/cma.j.cn321828-20201109-00403. [DOI]
- 7.Xia Z, Luo Z. Review of the evaluation of radiopharmaceuticals. Chin J Nucl Med Mol Imaging. 2006;26(1):49–50 (in Chinese). http://rs.yiigle.com/CN321828200601/162190.htm. Accessed 8 Nov 2021.
- 8.Liang J, Shen Y, Wu Y, Xiang X, Yu N, Guo S, et al. Development of simulated process for medical fission 99Mo production using electroplating UO2 target with low enriched uranium. Journal of Isotopes. 2018;31(3):165–72 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2018.31.03.0165.
- 9.Luo Z, Wu Y, J L. Methods for production of medical radioisotope 99Mo. Journal of Isotopes. 2018;31(3):129–42 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2018.31.03.0129.
- 10.Huang W, Liang J, Wu Y, Yu N, Xiang X. Investigation on separation of 99Mo from low enriched uranium target by Al2O3 chromatography. Journal of Isotopes. 2021;34(1):54–60 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2021.34.01.0054.
- 11.Peng S, Yang Y, Xie X, Qian D. Current status and prospects of reactor produced medical radioisotopes in China. Chin Sci Bull. 2020;65(32):3526–37. doi: 10.1360/TB-2020-0374. [DOI] [Google Scholar]
- 12.Shen Y, Chen Y, Liang J, Qiao L, Deng X, Li G, et al. Preparation of Ni target for cyclotron-produced 64Cu by electrodeposition. Journal of Isotopes. 2013;26(1):38–41 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2013.26.01.0038 ..
- 13.Chen Y, Liang J, Li G, Deng X, Qiao L, Shen Y, et al. Chemical separation of 64Cu from irradiated Ni target by anion exchange method. Journal of Isotopes. 2012;25(3):144–8 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2012.25.03.0144. Accessed 8 Nov 2021.
- 14.Deng X, Zhao Z, Zhang B, Liu Y, Liu S, Li M, et al. Development of 124Xe gas target system for production of high purity 123I. Atomic Energy Science and Technology. 2016;50(07):1324–8 (in Chinese). https://www.cnki.com.cn/Article/CJFDTotal-YZJS201607028.htm . Accessed 8 Nov 2021.
- 15.Tang Y, Li S, Yang Y, Chen W, Wei H, Wang G, et al. A simple and convenient method for production of 89Zr with high purity. Appl Radiat Isot. 2016;118:326–330. doi: 10.1016/j.apradiso.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Liu N, Yang Y, Jin J, Lin R, Cao Y, Liao J, et al. Preparation of radioactive isotopes by CS-30 cyclotron and their applications. Journal of Isotopes. 2012;25(3):189–92 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2012.25.03.0189.
- 17.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579(7799):421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Li L, Wang Z, Shi J, Hu Z, Gao S, et al. Imaging and monitoring HER2 expression in breast cancer during trastuzumab therapy with a peptide probe 99mTc-HYNIC-H10F. Eur J Nucl Med Mol Imaging. 2020;47(11):2613–2623. doi: 10.1007/s00259-020-04754-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Chen Y, Hou YN, Liu Q, Zhang D, Zhao H, et al. ImmunoPET imaging of multiple myeloma with [68Ga]Ga-NOTA-Nb1053. Eur J Nucl Med Mol Imaging. 2021;48(9):2749–2760. doi: 10.1007/s00259-021-05218-1. [DOI] [PubMed] [Google Scholar]
- 20.Xu M, Guo J, Gu J, Zhang L, Liu Z, Ding L, et al. Preclinical and clinical study on [18F]DRKXH1: a novel β-amyloid PET tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Liu C, Zhang Z, Zhang N, Guo X, Xia L, et al. 64Cu-PSMA-BCH: a new radiotracer for delayed PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05426-9. [DOI] [PubMed] [Google Scholar]
- 22.Duan X, Cao Z, Zhu H, Liu C, Zhang X, Zhang J, et al. 68Ga-labeled ODAP-Urea-based PSMA agents in prostate cancer: first-in-human imaging of an optimized agent. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05486-x. [DOI] [PubMed] [Google Scholar]
- 23.Ding J, Zhang Y, Wen J, Zhang H, Wang H, Luo Y, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism. Eur J Nucl Med Mol Imaging. 2020;47(11):2656–2665. doi: 10.1007/s00259-020-04722-0. [DOI] [PubMed] [Google Scholar]
- 24.Xie Q, Liu T, Ding J, Zhou N, Meng X, Zhu H, et al. Synthesis, preclinical evaluation, and a pilot clinical imaging study of [18F]AlF-NOTA-JR11 for neuroendocrine neoplasms compared with [68Ga]Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05249-8. [DOI] [PubMed] [Google Scholar]
- 25.You L, Guo Z, Zhang X. Current status and prospects of radiopharmaceuticals for diagnosis. Journal of Isotopes. 2017 30(4):292–306 (in Chinese). http://www.tws.org.cn/CN/Y2017/V30/I4/292. Accessed 8 Nov 2021.
- 26.Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23(10):1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui M, Zhou K, Shen L, Deng X. Dihydrazone compounds having high affinity to Aβ protein and Tau protein, derivatives thereof and use thereof. China Patent, CN109704988A, 20190503 (in Chinese). https://www.incopat.com/detail/init2?formerQuery=wE2KAomDT2iaiJrWYUfKOmr4kAd0KKkg&local=zh. Accessed 8 Nov 2021.
- 28.Cui M, Wang X, Yu P, Zhang J, Li Z, Zhang X, et al. Synthesis and evaluation of novel 18F labeled 2-pyridinylbenzoxazole and 2-pyridinylbenzothiazole derivatives as ligands for positron emission tomography (PET) imaging of β-amyloid plaques. J Med Chem. 2012;55(21):9283–9296. doi: 10.1021/jm300973k. [DOI] [PubMed] [Google Scholar]
- 29.Song J, Peng X, Li L, Yang F, Zhang X, Zhang J, et al. Al18F-NODA benzothiazole derivatives as imaging agents for cerebrovascular amyloid in cerebral amyloid angiopathy. ACS Omega. 2018;3(10):13089–96. 10.1021/acsomega.8b01120. [DOI] [PMC free article] [PubMed]
- 30.Yang Y, Wang X, Yang H, Fu H, Zhang J, Zhang X, et al. Synthesis and monkey-PET study of (R)- and (S)-18F-labeled 2-arylbenzoheterocyclic derivatives as amyloid probes with distinctive in vivo kinetics. Mol Pharm. 2016;13(11):3852–3863. doi: 10.1021/acs.molpharmaceut.6b00643. [DOI] [PubMed] [Google Scholar]
- 31.Song J, Zhang X, Zhao Y, Yang H, Zhang J, Zhang X, et al. (R)- and (S)-18F-labeled 2-arylbenzofurans with improved pharmacokinetics as β-amyloid imaging probes. Eur J Med Chem. 2017;134:271–280. doi: 10.1016/j.ejmech.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhou K, Guo W, Cui M. 18F-labeled 2-phenylbenzoheterocycles with chiral dihydroxyl side chains as β-amyloid imaging probes. Biorg Med Chem. 2021;29:115884. doi: 10.1016/j.bmc.2020.115884. [DOI] [PubMed] [Google Scholar]
- 33.Zhou K, Yang F, Li Y, Chen Y, Zhang X, Zhang J, et al. Synthesis and evaluation of fluorine-18 labeled 2-phenylquinoxaline derivatives as potential tau imaging agents. Mol Pharm. 2021;18(3):1176–1195. doi: 10.1021/acs.molpharmaceut.0c01078. [DOI] [PubMed] [Google Scholar]
- 34.Banister SD, Manoli M, Kassiou M. The development of radiotracers for imaging sigma (σ) receptors in the central nervous system (CNS) using positron emission tomography (PET) J Labelled Compd Radiopharmaceut. 2013;56(3–4):215–224. doi: 10.1002/jlcr.3010. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Xie F, Ye J, Deuther-Conrad W, Cui B, Wang L, et al. 1-(4-[18F]Fluorobenzyl)-4-[(tetrahydrofuran-2-yl)methyl]piperazine: a novel suitable radioligand with low lipophilicity for imaging σ1 receptors in the brain. J Med Chem. 2017;60(10):4161–4172. doi: 10.1021/acs.jmedchem.6b01723. [DOI] [PubMed] [Google Scholar]
- 36.Ye J, Wang X, Deuther-Conrad W, Zhang J, Li J, Zhang X, et al. Synthesis and evaluation of a 18F-labeled 4-phenylpiperidine-4-carbonitrile radioligand for σ1 receptor imaging. J Labelled Compd Radiopharmaceut. 2016;59(9):332–339. doi: 10.1002/jlcr.3408. [DOI] [PubMed] [Google Scholar]
- 37.Xie F, Bergmann R, Kniess T, Deuther-Conrad W, Mamat C, Neuber C, et al. 18F-Labeled 1,4-dioxa-8-azaspiro[4.5]decane derivative: synthesis and biological evaluation of a σ1 receptor radioligand with low lipophilicity as potent tumor imaging agent. J Med Chem. 2015;58(14):5395–407. 10.1021/acs.jmedchem.5b00593. [DOI] [PubMed]
- 38.Tian J, He Y, Deuther-Conrad W, Fu H, Xie F, Zhang Y, et al. Synthesis and evaluation of new 1-oxa-8-azaspiro[4.5]decane derivatives as candidate radioligands for sigma-1 receptors. Biorg Med Chem. 2020;28(14):115560. doi: 10.1016/j.bmc.2020.115560. [DOI] [PubMed] [Google Scholar]
- 39.Jia H, Cai Z, Holden D, He Y, Lin S-f, Li S, et al. Positron emission tomography imaging evaluation of a novel 18F-labeled sigma-1 Receptor radioligand in cynomolgus monkeys. ACS Chem Neurosci. 2020;11(11):1673–81. 10.1021/acschemneuro.0c00171. [DOI] [PubMed]
- 40.Zhao Z-Q, Liu M, Fang W, Liu S. Sulfonyl-Containing boronate caps for optimization of biological properties of 99mTc(III) Radiotracers [99mTcCl(CDO)(CDOH)2B-R] (CDOH2 = Cyclohexanedione Dioxime) J Med Chem. 2018;61(1):319–328. doi: 10.1021/acs.jmedchem.7b01412. [DOI] [PubMed] [Google Scholar]
- 41.Xi X, Wang L, Hsu B, Zhao Z, Liu S, Fang W. 99mTc-3SPboroxime: a neutral 99mTc(III) radiotracer with high heart uptake and long myocardial retention. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02087-3. [DOI] [PubMed] [Google Scholar]
- 42.Mou T, Zhao Z, You L, Li Y, Wang Q, Fang W, et al. Synthesis and evaluation of 18F-labeled pyridaben analogues for myocardial perfusion imaging in mice, rats and chinese mini-swine. Sci Rep. 2016;6(1):33450. doi: 10.1038/srep33450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmett L. Changing the goal posts: prostate-specific membrane antigen targeted theranostics in prostate cancer. Semin Oncol Nurs. 2020;36(4):151052. doi: 10.1016/j.soncn.2020.151052. [DOI] [PubMed] [Google Scholar]
- 44.Duan X, Liu F, Kwon H, Byun Y, Minn I, Cai X, et al. (S)-3-(Carboxyformamido)-2-(3-(carboxymethyl)ureido)propanoic acid as a novel PSMA targeting scaffold for prostate cancer imaging. J Med Chem. 2020;63(7):3563–76. 10.1021/acs.jmedchem.9b02031. [DOI] [PubMed]
- 45.Liu C, Zhu Y, Su H, Xu X, Zhang Y, Song S, et al. Preliminary results of targeted prostate-specific membrane antigen imaging in evaluating the efficacy of a novel hormone agent in metastatic castration-resistant prostate cancer. Cancer Med. 2020;9(10):3278–3286. doi: 10.1002/cam4.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Wu Y, Zeng Q, Xie T, Yao S, Zhang J, et al. Synthesis, preclinical evaluation, and first-in-human PET study of quinoline-containing PSMA tracers with decreased renal excretion. J Med Chem. 2021;64(7):4179–95. 10.1021/acs.jmedchem.1c00117. [DOI] [PubMed]
- 47.Sani S, Messe M, Fuchs Q, Pierrevelcin M, Laquerriere P, Entz-Werle N, et al. Biological relevance of RGD-integrin subtype-specific ligands in cancer. ChemBioChem. 2021;22(7):1151–1160. doi: 10.1002/cbic.202000626. [DOI] [PubMed] [Google Scholar]
- 48.Mi B, Yu C, Pan D, Yang M, Wan W, Niu G, et al. Pilot prospective evaluation of 18F-alfatide II for detection of skeletal metastases. Theranostics. 2015;5(10):1115–21. https://www.thno.org/v05p1115.htm. Accesssed 8 Nov 2021. [DOI] [PMC free article] [PubMed]
- 49.Feng X, Wang Y, Lu D, Xu X, Zhou X, Zhang H, et al. Clinical translation of a 68Ga-labeled integrin αvβ6-targeting cyclic radiotracer for PET imaging of pancreatic cancer. J Nucl Med. 2020;61(10)1461-7. 10.2967/jnumed.119.237347. [DOI] [PMC free article] [PubMed]
- 50.Ma H, Liu S, Zhang Z, Tang G, Yuan G, Zhao J, et al. Preliminary biological evaluation of 68Ga-labeled cyclic RGD dimer as an integrin αvβ3-targeting radiotracer for tumor PET imaging. J Radioanal Nucl Chem. 2019;321(3):857–865. doi: 10.1007/s10967-019-06654-y. [DOI] [Google Scholar]
- 51.Yu X, Wu Y, Liu H, Gao L, Sun X, Zhang C, et al. Small-animal SPECT/CT of the Progression and recovery of rat liver fibrosis by using an integrin αvβ3–targeting radiotracer. Radiology. 2015;279(2):502–512. doi: 10.1148/radiol.2015150090. [DOI] [PubMed] [Google Scholar]
- 52.Gao S, Jia B, Feng G, Dong C, Du H, Bai L, et al. First-in-human pilot study of an integrin α6-targeted radiotracer for SPECT imaging of breast cancer. Signal Transduct Target Ther. 2020;5(1):147. doi: 10.1038/s41392-020-00266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing Y, Chand G, Liu C, Cook GJR, O’Doherty J, Zhao L, et al. Early Phase I study of a 99mTc-labeled anti–programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non–small cell lung cancer. J Nucl Med. 2019;60(9):1213. doi: 10.2967/jnumed.118.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miao Y, Lv G, Chen Y, Qiu L, Xie M, Lin J. One-step radiosynthesis and initial evaluation of a small molecule PET tracer for PD-L1 imaging. Bioorg Med Chem Lett. 2020;30(24):127572. doi: 10.1016/j.bmcl.2020.127572. [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Zhu H, Li G, Xie Q, Yang X, Xu X, et al. Construction of Anti-hPD-L1 HCAb Nb6 and in situ 124I labeling for noninvasive detection of PD-L1 expression in human bone sarcoma. Bioconj Chem. 2019;30(10):2614–2623. doi: 10.1021/acs.bioconjchem.9b00539. [DOI] [PubMed] [Google Scholar]
- 57.Jiang J, Zhang M, Li G, Liu T, Wan Y, Liu Z, et al. Evaluation of 64Cu radiolabeled anti-hPD-L1 Nb6 for positron emission tomography imaging in lung cancer tumor mice model. Bioorg Med Chem Lett. 2020;30(4):126915. doi: 10.1016/j.bmcl.2019.126915. [DOI] [PubMed] [Google Scholar]
- 58.Huang H, Zhu H, Xie Q, Tian X, Yang X, Feng F, et al. Evaluation of 124I-JS001 for hPD1 immuno-PET imaging using sarcoma cell homografts in humanized mice. Acta Pharmaceutica Sinica B. 2020;10(7):1321–1330. doi: 10.1016/j.apsb.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X, Jiang J, Yang X, Liu T, Ding J, Nimmagadda S, et al. First-in-human evaluation of a PD-L1-binding peptide radiotracer in non-small cell lung cancer patients with PET. J Nucl Med. 2021:jnumed.121.262045. 10.2967/jnumed.121.262045. [DOI] [PMC free article] [PubMed]
- 60.Gan Q, Zhang X, Ruan Q, Fang Sa, Zhang J. 99mTc-CN7DG: a highly expected SPECT imaging agent of cancer with satisfactory tumor uptake and tumor-to-nontarget ratios. Mol Pharm. 2021;18(3):1356–63. 10.1021/acs.molpharmaceut.0c01177. [DOI] [PubMed]
- 61.Zhang X, Ruan Q, Jiang Y, Gan Q, Zhang J. Evaluation of 99mTc-CN5DG as a broad-spectrum SPECT probe for tumor imaging. Transl Oncol. 2021;14(1):100966. doi: 10.1016/j.tranon.2020.100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Ruan Q, Duan X, Gan Q, Song X, Fang S, et al. Novel 99mTc-labeled glucose derivative for single photon emission computed tomography: a promising tumor imaging agent. Mol Pharm. 2018;15(8):3417–24. 10.1021/acs.molpharmaceut.8b00415. [DOI] [PubMed]
- 63.Liu H, Han Y, Li J, Qin M, Fu Q, Wang C, et al. 18F-alanine derivative serves as an ASCT2 marker for cancer imaging. Mol Pharm. 2018;15(3):947–54. 10.1021/acs.molpharmaceut.7b00884. [DOI] [PubMed]
- 64.Li C, Liu H, Duan D, Zhou Z, Liu Z. Preclinical study of an 18F-labeled glutamine derivative for cancer imaging. Nucl Med Biol. 2018;64–65:34–40. doi: 10.1016/j.nucmedbio.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Kong Z, Chen J, Li J, Li N, Yang Z, et al. 18F-Boramino acid PET/CT in healthy volunteers and glioma patients. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05212-7. [DOI] [PubMed] [Google Scholar]
- 66.Sun A, Liu S, Tang X, Pan Q, Zhang Z, Ma H, et al. N-(2–18F-fluoropropionyl)-l-glutamate as a potential oncology tracer for PET imaging of glioma. Appl Radiat Isot. 2021;168:109530. doi: 10.1016/j.apradiso.2020.109530. [DOI] [PubMed] [Google Scholar]
- 67.Luo Z, Zhu H, Lin X, Hong Y, Xiao S, Zhang Q, et al. Radio-labelling and micro-PET study of 64Cu Labelled PnAO-1-(2-nitroimidazole) for hypoxia imaging. Chemical Journal of Chinese Universities. 2015;36(1):87–92. doi: 10.7503/cjcu20140438. [DOI] [Google Scholar]
- 68.Luo Z, Zhu H, Lin X, Chu T, Luo R, Wang Y, et al. Synthesis and radiolabeling of 64Cu-labeled 2-nitroimidazole derivative 64Cu-BMS2P2 for hypoxia imaging. Bioorg Med Chem Lett. 2016;26(5):1397–1400. doi: 10.1016/j.bmcl.2016.01.077. [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Wang F, Zhu H, Yang Z, Chu T. Synthesis and bioevaluation of novel [18F]FDG-conjugated 2-nitroimidazole derivatives for tumor hypoxia imaging. Mol Pharm. 2019;16(5):2118–2128. doi: 10.1021/acs.molpharmaceut.9b00075. [DOI] [PubMed] [Google Scholar]
- 70.Xu Q, Zhu C, Xu Y, Pan D, Liu P, Yang R, et al. Preliminary evaluation of [18F]AlF-NOTA-MAL-Cys39-exendin-4 in insulinoma with PET. J Drug Targeting. 2015;23(9):813–820. doi: 10.3109/1061186X.2015.1020808. [DOI] [PubMed] [Google Scholar]
- 71.Zhang P, Zhao Z, Zhang L, Wu W, Xu Y, Pan D, et al. [68Ga]Ga-NOTA-MAL-Cys39-exendin-4, a potential GLP-1R targeted PET tracer for the detection of insulinoma. Nucl Med Biol. 2019;74–75:19–24. doi: 10.1016/j.nucmedbio.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Gao F, Peng C, Zhuang R, Guo Z, Liu H, Huang L, et al. 18F-labeled ethisterone derivative for progesterone receptor targeted PET imaging of breast cancer. Nucl Med Biol. 2019;72–73:62–69. doi: 10.1016/j.nucmedbio.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Zhong Y, Yang S, Cui J, Wang J, Li L, Chen Y, et al. Novel 18F-labeled isonicotinamide-based radioligands for positron emission tomography imaging of glycogen synthase kinase-3β. Mol Pharm. 2021;18(3):1277–1284. doi: 10.1021/acs.molpharmaceut.0c01133. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Hu K, Liu W, Wei Y, Sha R, Long Y, et al. Synthesis and evaluation of [18F]FP-Lys-GE11 as a new radiolabeled peptide probe for epidermal growth factor receptor (EGFR) imaging. Nucl Med Biol. 2020;90–91:84–92. doi: 10.1016/j.nucmedbio.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Li M, Zhang Y, Zhang F, Liu C, Song Y, et al. Detection of melanoma metastases with PET—comparison of 18F–5-FPN with 18F–FDG. Nucl Med Biol. 2017;50:33–38. doi: 10.1016/j.nucmedbio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Qiu L, Tan H, Lin Q, Si Z, Mao W, Wang T, et al. A pretargeted imaging strategy for immune checkpoint ligand Pd-L1 expression in tumor based on bioorthogonal Diels-Alder click chemistry. Mol Imag Biol. 2020;22(4):842–853. doi: 10.1007/s11307-019-01441-3. [DOI] [PubMed] [Google Scholar]
- 77.Wu Y, Li L, Dong C, Wang F. Small-animal SPECT/CT imaging with 99mTc-HYNIC-H10F detects HER2 expression in mice bearing human breast cancers. J Nucl Med. 2016;57(supplement 2):1162. https://jnm.snmjournals.org/content/57/supplement_2/1162. Accessed 8 Nov 2021.
- 78.Fu Z, Lin Q, Hu B, Zhang Y, Chen W, Zhu J, et al. P2X7 PET Radioligand 18F-PTTP for differentiation of lung tumor from inflammation. J Nucl Med. 2019;60(7):930. doi: 10.2967/jnumed.118.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D, Zhuang R, Guo Z, Gao M, Huang L, You L, et al. Desmin- and vimentin-mediated hepatic stellate cell-targeting radiotracer 99mTc-GlcNAc-PEI for liver fibrosis imaging with SPECT. Theranostics. 2018;8(5):1340–9. https://www.thno.org/v08p1340.htm. Accessed 8 Nov 2021. [DOI] [PMC free article] [PubMed]
- 80.Sun P, Zhu Y, Han Y, Hu K, Huang S, Wang M, et al. Radiosynthesis and biological evaluation of an fluorine-18 labeled galactose derivative [18F]FPGal for imaging the hepatic asialoglycoprotein receptor. Bioorg Med Chem Lett. 2020;30(12):127187. doi: 10.1016/j.bmcl.2020.127187. [DOI] [PubMed] [Google Scholar]
- 81.Zhu H, Zhang H, Zhou N, Ding J, Jiang J, Liu T, et al. Molecular PET/CT profiling of ACE2 expression in vivo: implications for infection and outcome from SARS-CoV-2. Adv Sci. 2021;8(16):2100965. doi: 10.1002/advs.202100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Li C, Hong H, Liu H, Wang C, Xu M, et al. Side chain optimization remarkably enhances the in vivo stability of 18F-labeled glutamine for tumor imaging. Mol Pharm. 2019;16(12):5035–41. 10.1021/acs.molpharmaceut.9b00891. [DOI] [PubMed]
- 83.Liu Q, Zang J, Sui H, Ren J, Guo H, Wang H, et al. Peptide receptor radionuclide therapy of late-stage neuroendocrine tumor patients with multiple cycles of 177Lu-DOTA-EB-TATE. J Nucl Med. 2021:62(3):386-92. 10.2967/jnumed.120.248658. [DOI] [PMC free article] [PubMed]
- 84.Deng X, Li H, Ye Z, Guo H, Li F, Luo Z. Radiolabeling and biodistribution of 177Lu-EDTMP and 177Lu-DOTMP. Journal of Isotopes. 2009;22(2):71–5 (in Chinese). http://www.tws.org.cn/CN/volumn/volumn_1150.shtml#1. Accessed 8 Nov 2021.
- 85.Li H, Liang J, Xiang X, Deng X, Zheng D, Luo H. Biodistribution and imaging study on 177Lu-EDTMP prepared by kit method. Journal of Isotopes. 2010;23(2):65–70 (in Chinese). http://www.tws.org.cn/CN/10.7538/tws.2010.23.02.0065. Accessed 8 Nov 2021.
- 86.Yuan J, Liu C, Liu X, Wang Y, Kuai D, Zhang G, et al. Efficacy and safety of 177Lu-EDTMP in bone metastatic pain palliation in breast cancer and hormone refractory prostate cancer: a phase ii study. Clin Nucl Med. 2013;38(2):88-92. 10.1097/RLU.0b013e318279bf4d. [DOI] [PubMed]
- 87.Ma X, Zhang J, Li H, Liang J, Yang Y, Yang C. Preparation and preliminary biological evaluation of 177Lu labelled rituximab. Journal of Isotopes. 2014;27(2):98–103 (in Chinese). http://www.tws.org.cn/CN/Y2014/V27/I2/98. Accessed 8 Nov 2021.
- 88.Ren J, Xu M, Chen J, Ding J, Wang P, Huo L, et al. PET imaging facilitates antibody screening for synergistic radioimmunotherapy with a 177Lu-labeled αPD-L1 antibody. Theranostics. 2021;11(1):304–315. doi: 10.7150/thno.45540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Ma T, Liu H, Jin Z, Sun X, Zhao H, et al. 177Lu-labeled antibodies for EGFR-targeted SPECT/CT Imaging and radioimmunotherapy in a preclinical head and neck carcinoma model. Mol Pharm. 2014;11(3):800–807. doi: 10.1021/mp4005047. [DOI] [PubMed] [Google Scholar]
- 90.Targeted alpha therapy working g. targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncology. 2018;4(12):1765–72. 10.1001/jamaoncol.2018.4044. [DOI] [PubMed]
- 91.Liu N, Yang Y, Zan L, Liao J, Jin J. Astatine-211 labeling of insulin: synthesis and preliminary evaluation in vivo and in vitro. J Radioanal Nucl Chem. 2007;272(1):85–90. doi: 10.1007/s10967-006-6781-8. [DOI] [Google Scholar]
- 92.Liu W, Ma H, Tang Y, Chen Q, Peng S, Yang J, et al. One-step labelling of a novel small-molecule peptide with astatine-211: preliminary evaluation in vitro and in vivo. J Radioanal Nucl Chem. 2018;316(2):451–456. doi: 10.1007/s10967-018-5780-x. [DOI] [Google Scholar]
- 93.Zhao B, Qin S, Chai L, Lu G, Yang Y, Cai H, et al. Evaluation of astatine-211-labeled octreotide as a potential radiotherapeutic agent for NSCLC treatment. Biorg Med Chem. 2018;26(5):1086–1091. doi: 10.1016/j.bmc.2018.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The manuscript represents valid work, and neither this manuscript nor one with substantially similar content under the same authorship has been published or is being considered for publication elsewhere.